Abstract
We aimed to evaluate serum levels of serum amyloid‐A (SAA) both in the diagnosis and monitoring the treatment response of necrotizing enterocolitis (NEC). Forty‐five preterm neonates were enrolled in the study, including 15 infants with NEC, 15 with sepsis, and 15 healthy preterm infants. Pre‐ and posttreatment serum SAA levels were measured. Among patients with NEC, 11 had stage 1 and 4 had stage 2 disease according to the modified Bell's staging criteria. Baseline SAA levels of the infants with NEC were significantly higher than controls (P=0.013) and were significantly lower than those with sepsis (P=0.004). When infants with stage 1and stage 2 NEC were analyzed separately, baseline SAA levels of the infants with stage 2 NEC were significantly higher than controls (P=0.027) than those with stage 1 NEC (P=0.018), but similar to those with sepsis. There was a trend that baseline SAA levels were also correlated with the Bell stage (r=0.501, P=0.057). Posttreatment SAA levels significantly decreased in infants with sepsis (P=0.002). Pre‐ and posttreatment SAA levels were similar in patients with stage 1 and 2 NEC. In conclusion, SAA rises in early stages of NEC and may aid in diagnosis as a serum marker. J. Clin. Lab. Anal. 25:233–237, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: necrotizing enterocolitis, preterm, serum amyloid A
INTRODUCTION
Necrotizing enterocolitis (NEC) is one of the major causes of morbidity and mortality in the neonatal intensive care units (NICU). Incidence of NEC is 0.72/1,000 live births per year, most of them being born at <37 weeks of gestation 1. Mortality rates are also high, between 15 and 30% in various series. Furthermore, long‐term complications, including poor growth, cerebral palsy, vision and hearing impairment and other neurodevelopmental disorders, can be seen in affected newborns 1. Prevention, diagnosis, and treatment of NEC are an active area of research owing to the significant mortality and morbidity.
Initial clinical findings of NEC may include delicate signs of feeding intolerance, abdominal distention/tenderness, delayed gastric emptying, vomiting, apnea, bradycardia, and lethargy. Because these symptoms are generally not specific for NEC and can be seen in other diseases of the newborn, additional tests are needed for the establishment of NEC diagnosis 2. Abdominal radiographs may demonstrate multiple dilated bowel loops, pneumatosis intestinalis. Portal venous gas and gallbladder gas are indicative of a serious disease. Pneumoperitoneum indicates a bowel perforation. Computed tomography scanning may be used to demonstrate pneumatosis or the site of perforation 2, 3. Various other laboratory findings have been investigated previously for the early diagnosis of NEC, such as leucopenia, thrombocytopenia, high ratios of immature to total leukocyte counts, metabolic acidosis, hyponatremia, high C‐reactive protein (CRP) values, and hyperglycemia 4, 5, 6, 7.
Serum amyloid‐A (SAA) is an acute‐phase reactant that rises in acute inflammatory conditions. SAA is synthesized in the liver upon stimulation by proinflammatory cytokines, such as tumor necrosis factor‐ and interleukin‐6 (IL‐6) 8. SAA and CRP have both increased in many disease states associated with inflammation. Moreover, SAA has been suggested to be more sensitive and specific than CRP in various inflammatory conditions 9, 10. Previously, only one study assessed the diagnostic value of SAA levels in NEC. Ng et al. developed the ApoSAA score, which was computed from plasma apolipoprotein CII and des‐arginine variant of SAA concentrations and was effective in identifying sepsis/NEC cases 11. As exaggerated inflammatory response to gastrointestinal injury remains the leading hypothesis in the pathophysiology of NEC 12, 13, we sought to investigate the utility of major inflammatory markers, i.e., SAA, CRP, and IL‐6 in the diagnosis of NEC. We aimed to evaluate serum levels of SAA both in the diagnosis and monitoring the treatment response of NEC.
PATIENTS AND METHODS
This study with a case–control design was performed between March 2007 and September 2007 in the NICU in Zekai Tahir Burak Maternity Teaching Hospital in Ankara, Turkey. Forty‐five preterm neonates were included in the study. Initially, 15 patients with NEC were included, according to the following criteria: gestational week <35 weeks, Bell's stage I‐III NEC diagnosis, no congenital malformations. The diagnosis of NEC was made based on clinical and radiological findings and graded according to the modified Bell's staging criteria. All infants were re‐evaluated at 48 hr after disease onset to determine the diagnosis of NEC, because clinical findings of NEC are nonspecific. As SAA is a well‐established acute phase reactant in sepsis, 15 infants with sepsis were also included in the study as positive controls. Sepsis diagnosis was made in the presence of clinical and biochemical signs as well as positive blood cultures. Fifteen healthy preterm infants, who did not have a severe illness, sepsis, or NEC, and who were hospitalized for feeding purposes, were also included as a negative control group. In all patients, peripheral venous blood samples were collected. Serum was separated from blood cells by centrifugation at 4,000 cycles per minute. All samples were stored at −70°C until analyzed. Serum SAA levels of the subjects were measured with enzyme‐linked immunosorbent assay method (Biosource Hu SAA, CA). Upper detection limit for SAA was 600 mg/l. CRP and IL‐6 levels were only measured in the patient groups, but not controls, using particle‐enhanced immunoturbidimetric assay for CRP (CRP latex HS, Cobas, IN), and chemiluminescent sequential immunometric assay for IL‐6 (Immulite 1000 systems, Los Angeles, CA). SAA levels of the infants with NEC and sepsis were also measured in the posttreatment period after complete clinical resolution.
Statistical analysis was performed with SPSS software version 13.0 and statistical significance was set at P less than 0.05. Kruskal–Wallis and Mann–Whitney U test was used to compare independent variables, Wilcoxon test for dependent variables, Spearman test for correlations, and Chi‐square test for comparison of ratios. The study was approved by the institutional review board of the hospital and informed consent was obtained from the parents of the participants.
RESULTS
Forty‐five premature infants were involved in the study. Patient characteristics are shown in Table 1. Gestational ages, postnatal ages, birth weights, and gender distribution were similar between infants with NEC and those in other groups. Among patients with NEC, 11 had stage 1 (suspected NEC) and 4 had stage 2 disease according to the modified Bell's staging criteria. Table 2 shows baseline SAA, CRP, and IL‐6 levels of the infants in three groups.
Table 1.
Baseline Characteristics of the Infantsa
| NEC (n=15) | Sepsis (n=15) | Control (n=15) | P | |
|---|---|---|---|---|
| Gestational age (weeks) | 30 (29–33) | 32 (30–33) | 32 (30–32) | P>0.05 |
| Birth weight (grams) | 1,510 (1,290–1,710) | 1,400 (1,230–1,680) | 1,490 (1,310–1,740) | P>0.05 |
| Gender (male/female) | 10/4 | 9/6 | 7/8 | P>0.05 |
| Postnatal age (days) | 10 (7–26) | 13 (7–24) | 7 (14–9) | P=0.024 |
| NEC Stage 1A 1B 2A |
9 2 4 |
– | – |
aValues are median (interquartile range) or number.
Table 2.
SAA, CRP, and IL‐6 Levels of the Infantsa
| NEC Stage I (n=11) | NEC Stage II (n=4) | Sepsis (n=15) | Control (n=15) | P | |
|---|---|---|---|---|---|
| SAA (mg/l) | 40.6 (13.2–600) | 152.2 (20.2–600) | 600 (192.30–600.00) | 6.65 (1.00–120.13) | P<0.001b |
| CRP (mg/ml) | 0.8 (0.16–5.00) | 11.8 (1.64–30.1) | 16.2 (3.90–32.40) | – | P<0.001c |
| IL‐6 (mg/ml) | 14.2 (5.50–25.0) | 32.0 (28.0–97.3) | 268 (26.05–1,000) | – | P=0.004c |
aValues are median (interquartile range).
bKruskal–Wallis test.
cMann–Whitney U test.
Baseline SAA levels of the infants with NEC were significantly higher than controls (P=0.013) and were significantly lower than those with sepsis (P=0.004). When infants with stage 1 and stage 2 NEC were analyzed separately, baseline SAA levels of the infants with stage 2 NEC were significantly higher than controls (P=0.027) and than those with stage 1 NEC (P=0.018), but similar to those with sepsis (P=0.357) (Table 2). The distribution of baseline SAA levels of the groups is demonstrated in Figure 1.
Figure 1.
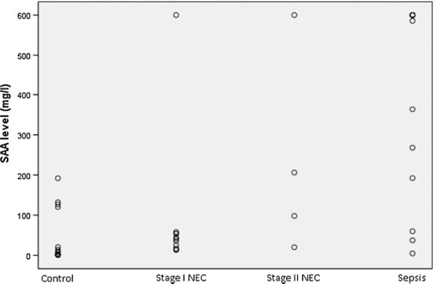
Baseline SAA levels of the patients with NEC, sepsis, and controls.
Among all patients, baseline SAA levels were correlated with CRP and IL‐6 levels (r=0.506, P=0.004 for CRP; r=0.437, P=0.026 for IL‐6). There was a trend that baseline SAA levels were also correlated with the Bell stage (r=0.501, P=0.057). Posttreatment SAA levels significantly decreased in infants with sepsis (P=0.002). Pre‐ and posttreatment SAA levels were similar in patients with stage 1 and 2 NEC (Table 3).
Table 3.
Pre‐ and Post Treatment SAA Levels of the Patients With NEC or Sepsis
| Pretreatment SAA (mg/l) | Posttreatment SAA (mg/l) | P a | |
|---|---|---|---|
| NEC stage 1 | 40.6 (13.2–600) | 16.3 (3.6–562.4) | 0.594 |
| NEC stage 2 | 152.2 (20.2–600) | 104.7 (25.9–562.4) | 0.273 |
| Sepsis | 600 (4.7–600) | 11.3 (1.0–393.8) | 0.002 |
Data show median (interquartile range) values.
aWilcoxon test.
DISCUSSION
In this study, we found that baseline SAA levels of the infants with NEC were significantly higher than healthy preterms. Moreover, baseline SAA levels were correlated with NEC stage. Together, these data suggest that SAA may have a potential role in diagnosis of the patients with NEC.
SAA proteins are involved in acute phase responses; these are the immediate early‐host responses to inflammation. The human SAA gene family maps to chromosome 11. Liver is the major site of synthesis, although extrahepatic synthesis macrophages, endothelial cells adipocytes, and smooth muscle cells have been reported 8, 14. The exact function of SAA is not known. It has immunomodulatory actions, aids in tissue regeneration by activating collagenases, acts as a chemoattractant for monocytes, T lymphocytes, mast cells, and neutrophils 8. SAA is a sensitive acute phase reactant both in the diagnosis and monitoring of inflammatory and infectious diseases. Elevated SAA levels are reported in various infectious and inflammatory disorders, including viral and bacterial infections, sepsis, rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel diseases, lymphoma, and solid cancers 15, 16, 17, 18. Studies in patients with chronic obstructive pulmonary disease, acute coronary syndromes, and acute appendicitis revealed SAA to aid better in diagnosis and a better predictor of outcomes 10, 19, 20.
SAA is most widely studied in sepsis in newborns. In one study, SAA has been investigated in early‐onset sepsis. When compared with CRP, SAA was more sensitive at onset of sepsis, rose earlier, and returned faster to normal values in those infants who recovered. They concluded that it can be used as a reliable tool for detection of neonatal early‐onset sepsis in full‐term infants 17. It was also reported as a useful inflammatory marker during late‐onset sepsis in preterm infants 21. For this reason, we included patients with sepsis as positive controls. Consistent with the literature, we also observed high levels of SAA in infants with sepsis that has decreased significantly after the treatment. SAA levels of the infants with NEC were not as high as those with sepsis. This may be because all our patients with diagnosis of NEC had early‐stage disease, i.e., either stage I or II. When stage I and II patients were analyzed separately, patients with stage II NEC and those with sepsis had similar SAA levels, whereas those with stage I NEC had lower SAA levels. Nevertheless, SAA levels of the infants with NEC were higher than controls even in early stages. Thus, it can be possible to use SAA as a serum marker to aid in the early diagnosis of NEC when the clinical signs are subtle. A recent study used the proteomic approach to identify potential biomarkers in the diagnosis of sepsis/NEC in preterm infants. Among 180 proteomic peaks obtained in patients with NEC/sepsis by quantitative proteomics profiling, Ng et al. identified 25 peaks that were significantly higher and 40 peaks that were significantly lower in the sepsis/NEC group compared with nonsepsis infants. Multivariate logistic regression analysis identified plasma apolipoprotein CII (P=0.011) and SAA (P=0.007) as the only two nonredundant biomarkers. Thus, immunoassay concentrations of plasma apoC2 and SAA were used to construct an equation for calculating the “ApoSAA score,” which was the diagnostic score used for identification of sepsis and/or NEC cases. The diagnostic value of the ApoSAA score was subsequently validated by the independent case–control cohort as well as in a prospective cohort study. In this study, the ApoSAA score has also provided a definitive strategy for safe and effective use of antimicrobial therapy for suspected preterm infants. The authors proposed that this triage strategy would enable neonatologists to prescribe antibiotics rationally and to judiciously allocate resources by targeting those who truly require urgent treatment and intensive care 11.
Ng et al. found SAA levels returned to normal after recovery of NEC 11. In our study, baseline and posttreatment levels of SAA were similar in patients with both stage 1 and stage 2 NEC. However, 11 of 15 patients with NEC had median 76% reduction in SAA levels after recovery. Therefore, we feel that we could not demonstrate the difference between pre‐ and posttreatment SAA levels because of the limited number of patients. Studies with a higher number of patients may show the value of SAA on follow‐up of patients with NEC.
In conclusion, SAA rises in early stages of NEC and may provide early diagnosis. The role of SAA in follow‐up of these patients and whether SAA was more sensitive than other inflammatory markers should be investigated in larger patient series. Preliminary results in this study deserve further studies with more patients to search the utility of SAA in diagnosis and follow‐up of the patients with NEC.
REFERENCES
- 1. Llanos AR, Moss ME, Pinzon MC, Dye T, Sinkin RA, Kendig JW. Epidemiology of neonatal necrotising enterocolitis: A population‐based study. Paediatr Perinat Epidemiol 2002;16:342–349. [DOI] [PubMed] [Google Scholar]
- 2. Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Curr Opin Infect Dis 2003;16:349–355. [DOI] [PubMed] [Google Scholar]
- 3. Kanto WP Jr, Hunter JE, Stoll BJ. Recognition and medical management of necrotizing enterocolitis. Clin Perinatol 1994;21:335–346. [PubMed] [Google Scholar]
- 4. Hallstrom M, Koivisto AM, Janas M, Tammela O. Laboratory parameters predictive of developing necrotizing enterocolitis in infants born before 33 weeks of gestation. J Pediatr Surg 2006;41:792–798. [DOI] [PubMed] [Google Scholar]
- 5. O'Neill JA Jr, Holcomb GW. Jr Surgical experience with neonatal necrotizing enterocolitis (NNE). Ann Surg 1979;189:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ververidis M, Kiely EM, Spitz L, Drake DP, Eaton S, Pierro A. The clinical significance of thrombocytopenia in neonates with necrotizing enterocolitis. J Pediatr Surg 2001;36:799–803. [DOI] [PubMed] [Google Scholar]
- 7. Hutter JJ Jr, Hathaway WE, Wayne ER. Hematologic abnormalities in severe neonatal necrotizing enterocolitis. J Pediatr 1976;88:1026–1031. [DOI] [PubMed] [Google Scholar]
- 8. Gabay C, Kushner I. Acute‐phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–454. [DOI] [PubMed] [Google Scholar]
- 9. Mayer JM, Raraty M, Slavin J, et al. Serum amyloid A is a better early predictor of severity than C‐reactive protein in acute pancreatitis. Br J Surg 2002;89:163–171. [DOI] [PubMed] [Google Scholar]
- 10. Bozinovski S, Hutchinson A, Thompson M, et al. Serum amyloid A is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:269–278. [DOI] [PubMed] [Google Scholar]
- 11. Ng PC, Ang IL, Chiu RW, et al. Host‐response biomarkers for diagnosis of late‐onset septicemia and necrotizing enterocolitis in preterm infants. J Clin Invest 2010;120:2989–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grave GD, Nelson SA, Walker WA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res 2007;62:510–514. [DOI] [PubMed] [Google Scholar]
- 13. Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 2008;32:70–82. [DOI] [PubMed] [Google Scholar]
- 14. Upragarin N, Landman WJ, Gaastra W, Gruys E. Extrahepatic production of acute phase serum amyloid A. Histol Histopathol 2005;20:1295–1307. [DOI] [PubMed] [Google Scholar]
- 15. Rosenthal CJ, Sullivan LM. Serum amyloid A to monitor cancer dissemination. Ann Intern Med 1979;91:383–390. [DOI] [PubMed] [Google Scholar]
- 16. Niederau C, Backmerhoff F, Schumacher B, Niederau C. Inflammatory mediators and acute phase proteins in patients with Crohn's disease and ulcerative colitis. Hepatogastroenterology 1997;44:90–107. [PubMed] [Google Scholar]
- 17. Arnon S, Litmanovitz I, Regev RH, Bauer S, Shainkin‐Kestenbaum R, Dolfin T. Serum amyloid A: an early and accurate marker of neonatal early‐onset sepsis. J Perinatol 2007;27:297–302. [DOI] [PubMed] [Google Scholar]
- 18. Jung SY, Park MC, Park YB, Lee SK. Serum amyloid A as a useful indicator of disease activity in patients with ankylosing spondylitis. Yonsei Med J 2007;48:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kosuge M, Ebina T, Ishikawa T, et al. Serum amyloid A is a better predictor of clinical outcomes than C‐reactive protein in non‐ST‐segment elevation acute coronary syndromes. Circ J 2007;71:186–190. [DOI] [PubMed] [Google Scholar]
- 20. Lycopoulou L, Mamoulakis C, Hantzi E, et al. Serum amyloid A protein levels as a possible aid in the diagnosis of acute appendicitis in children. Clin Chem Lab Med 2005;43:49–53. [DOI] [PubMed] [Google Scholar]
- 21. Arnon S, Litmanovitz I, Regev R, et al. Serum amyloid A protein is a useful inflammatory marker during late‐onset sepsis in preterm infants. Biol Neonate 2005;87:105–110. [DOI] [PubMed] [Google Scholar]


