Abstract
Background
Atrial fibrillation (AF) has been shown to be independently associated with an increased risk of myocardial infarction (MI) in a predominantly middle‐aged population; however, this association has not been examined in older populations.
Hypothesis
AF is associated with MI in older adults.
Methods
A total of 4608 participants (85% white, 40% male) from the Cardiovascular Health Study without evidence of baseline coronary heart disease were included in this analysis. AF cases were identified during the yearly study electrocardiogram, a self‐reported history of a physician diagnosis, or by hospitalization data. Incident MI was identified using medical records with local and central adjudication. Cox regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between AF and incident MI.
Results
A total of 434 (9.4%) participants had evidence of AF before incident MI. Over a median follow‐up of 12.2 years, a total of 797 (17.3%) participants developed MI. In a multivariable Cox proportional hazards analysis adjusted for socio‐demographics, cardiovascular risk factors, and potential confounders, AF was associated with an increased risk of MI (HR: 1.7, 95% CI: 1.4‐2.2). A significant interaction was detected by race, with black (HR: 3.1, 95% CI: 1.7‐5.6) AF participants having an increased risk of MI compared with whites (HR: 1.6, 95% CI: 1.2‐2.1; P interaction = 0.030).
Conclusions
AF is associated with an increased risk of MI in a population of older adults.
Introduction
Coronary heart disease (CHD) is the cause of 1 in 6 deaths in the United States, and its prevalence is projected to increase considerably in the coming decades.1 These projections are largely attributable to the aging United States population and the disproportionately high prevalence of CHD within this group.2 Similarly, the prevalence of atrial fibrillation (AF) is projected to double by the year 2050 due to the increased prevalence among the elderly and those with known cardiovascular disease risk factors.3, 4
Recently, data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study have shown that AF is independently associated with an increased risk of myocardial infarction (MI).5 However, this association has not been examined in older populations. Due to the increased prevalence of both conditions in the elderly, AF potentially is strongly associated with incident MI in this population. Therefore, the purpose of this study was to examine the association of AF with incident MI in the Cardiovascular Health Study (CHS), a population‐based study of predominantly older adults.
Methods
Study Population
Details of CHS have been previously described.6 Briefly, CHS is a prospective, population‐based cohort study of risk factors for CHD and stroke in individuals 65 years and older. A total of 5888 participants with Medicare eligibility in the United States were recruited from 4 field centers in the following locations: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. Subjects were followed with semiannual contacts, alternating between telephone calls and surveillance clinic visits. CHS clinic exams ended in June 1999, and since that time 2 yearly phone calls to participants were used to identify events and collect data. The institutional review board at each site approved the study. Written informed consent was obtained from participants at enrollment. A longitudinal cohort study was used to examine the association between AF and incident MI. Participants were excluded if any of the following criteria were met: prevalent CHD was reported, baseline covariate data were missing, or follow‐up data were missing. Prevalent CHD was determined by a report of a physician diagnosis of angina pectoris, MI, coronary artery bypass graft surgery, or percutaneous transluminal coronary angioplasty before the initial evaluation.
Covariates
Participant characteristics were collected during the initial CHS interview and questionnaire. Age, sex, race, income, and education were self‐reported. Annual income was dichotomized at $25 000 and education was dichotomized at “high school or less.” Smoking was defined as current or ever smoker. Participants' blood samples were obtained after a 12‐hour fast at the local field center. Measurements of total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), plasma glucose, and high‐sensitivity C‐reactive protein (hs‐CRP) were used in this analysis. Diabetes mellitus (DM) was defined as a self‐reported history of a physician diagnosis, a fasting glucose value ≥126 mg/dL, or by the current use of insulin or oral hypoglycemic medications. Blood pressure was measured for each participant in the seated position and systolic measurements were used in this analysis. The use of aspirin, statins, and antihypertensive medications was self‐reported. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. Identical electrocardiographs (MAC PC, Marquette Electronics Inc., Milwaukee, WI) were used at all clinic sites and resting, 10‐second standard simultaneous 12‐lead electrocardiograms (ECG) were recorded in all participants. All ECGs were processed in a central laboratory (initially at Dalhousie University, Halifax, NS, Canada; and later at the EPICARE Center, Wake Forest School of Medicine, Winston‐Salem, NC). The ECGs were first processed by the Dalhousie ECG program and were reprocessed for the present study using the 2001 version of the GE Marquette 12‐SL program (GE, Milwaukee, WI). The methodology and prevalence of ECG abnormalities in CHS have been previously reported.7
Atrial Fibrillation
Baseline AF cases were identified during the initial study ECG or through a self‐reported history of a physician diagnosis. Cases of AF also were identified during the annual study ECGs that were performed annually until 1999. Additionally, hospitalization discharge data were used to identify AF cases using International Classification of Diseases codes 427.31 and 427.32. Hospital diagnosis codes for AF ascertainment have been shown to have a positive predictive value of 98.6%.8 AF cases that were identified after the baseline study visit were included if they occurred prior to a diagnosis of MI.
Coronary Heart Disease Events
The CHS Cardiovascular Events Committee reviewed and classified all CHD events, and specific criteria, including the adjudication process, have been previously described.9 Briefly, suspected events were adjudicated according to standard criteria by a physician review panel using medical records and interviews with the physician, participant, or a proxy informant. Additionally, Medicare claims data were searched to ascertain potential missed events. The algorithm for classifying MI was based on elements of the medical history, cardiac enzymes, and ECG readings. MI was defined as definite or probable nonfatal MI and/or definite fatal MI. Definite MI included participants with ≥1 of the following: an evolving diagnostic ECG pattern, a diagnostic ECG pattern and abnormal cardiac enzymes, cardiac chest pain and abnormal enzymes, and either an evolving ST‐T pattern or an equivocal ECG pattern. Incident CHD events were defined as 1 of the following: fatal or nonfatal MI, angina pectoris without MI, coronary revascularization procedures (angioplasty and coronary artery bypass graft surgery), or other fatal CHD events. Criteria for angina required that the participant had all of the following: report of symptoms (chest pain, chest tightness, or shortness of breath), a physician diagnosis of angina, and to have received medical treatment for angina. During the adjudication process, center investigators presented the medical history, symptoms, course, and outcome of each event. Classification of each endpoint was proposed using standardized criteria. Quality‐control measures included re‐adjudication of samples of previously reviewed cases, focusing on those where the International Classification of Diseases codes for an endpoint were absent on the hospital record but an incident endpoint was present. Members of the adjudication committee were blinded to the cases. To avoid overlap in conditions occurring in individual participants, only first‐time events were used.
Statistical Analysis
Categorical variables were reported as frequency and percentage, whereas continuous variables were recorded as mean ± standard deviation. Statistical significance for categorical variables was tested using the χ2 method and the Wilcoxon rank‐sum procedure was used for continuous variables. Follow‐up time was defined as the time between AF diagnosis until one of the following: MI, death, loss to follow‐up, or end of follow‐up (July 1, 2008). Incidence rates for MI were computed in units of 1000 person‐years. Kaplan‐Meier estimates were used to compute cumulative incidence of MI by AF and the difference in estimates was compared using the log‐rank procedure.10 Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between AF and incident MI. Multivariable models were constructed as follows: Model 1 adjusted for age, sex, race, education, and income; Model 2 adjusted for Model 1 covariates plus smoking status, systolic blood pressure, DM, body mass index, total cholesterol, HDL‐C, aspirin, statin, antihypertensive medications, and log(hs‐CRP). We tested for interactions between our main effect variable and age (dichotomized at 80 years), sex, and race (white vs black). In a secondary analysis, we examined the association of AF with incident CHD events. The test statistic of Grambsch and Therneau was used to check the proportional hazards assumption and this assumption was not violated.11 Statistical significance was defined as P < 0.05. SAS version 9.3 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Of the 5888 participants from the original CHS cohort, 69 participants missing follow‐up data were excluded. Of those that remained, 945 participants with baseline CHD and 266 with missing baseline covariate data also were excluded. A total of 4608 participants (85% white, 40% male) with complete data were used in this analysis.
A total of 434 (9.4%) participants had evidence of AF before incident MI. Of these, 218 (51%) were identifed at baseline, and the remaining AF cases were detected during subsequent study ECGs or through AF hospitalizaiton data. Baseline characteristics stratifed by AF are shown in Table 1. Persons with AF were more likely to be older, male, and to report using antihypertensive medications compared with persons without AF. Those with AF had lower values for total cholesterol and higher values for systolic blood pressure and log(hs‐CRP) than those without AF.
Table 1.
Baseline Characteristics of Study Participants (N = 4608)
| Characteristic | AF, n = 434 | No AF, n = 4174 | P Valuea |
|---|---|---|---|
| Age, years | |||
| 65–70 (%) | 152 (35) | 1865 (45) | |
| 71–74 (%) | 110 (25) | 999 (24) | |
| 75–80 (%) | 116 (27) | 909 (22) | |
| >80 (%) | 56 (13) | 401 (9.6) | 0.0006 |
| Male sex (%) | 194 (45) | 1649 (40) | 0.036 |
| White (%) | 381 (88) | 3530 (85) | 0.075 |
| High school or less (%) | 238 (55) | 2372 (57) | 0.43 |
| Annual income < $25 000 (%) | 272 (63) | 2629 (63) | 0.90 |
| BMI, mean (SD), kg/m2 | 27 (4.3) | 27 (4.1) | 0.93 |
| Current or former smoker (%) | 228 (53) | 2190 (52) | 0.98 |
| DM (%) | 65 (15) | 605 (14) | 0.79 |
| SBP, mean (SD), mm Hg | 141 (20) | 140 (21) | 0.038 |
| Total cholesterol, mean (SD), mg/dL | 201 (37) | 213 (39) | <0.0001 |
| HDL‐C, mean (SD), mg/dL | 54 (16) | 55 (16) | 0.19 |
| Antihypertensive medication use (%) | 222 (51) | 1671 (40) | <0.0001 |
| Statin use (%) | 8 (1.8) | 69 (1.7) | 0.77 |
| Aspirin use (%) | 133 (31) | 1222 (29) | 0.55 |
| Log(hs‐CRP), mean (SD), mg/L | 1.0 (1.0) | 0.91 (1.0) | 0.0099 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; SBP, systolic blood pressure; SD, standard deviation.
Statistical significance for continuous data was tested using the Wilcoxon rank‐sum procedure and categorical data was tested using the χ2 test.
Over a median follow‐up of 12.2 years, a total of 797 (17.3%) participants developed MI. The incidence rate for MI was higher among participants with AF (incidence rate per 1000 person‐years: 25.5, 95% CI: 20.5‐31.6) compared with those without AF (incidence rate per 1000 person‐years: 13.9, 95% CI: 12.9‐14.9). Unadjusted cumulative incidence for MI by AF is shown in Figure 1 (log‐rank P < 0.0001). In a multivariable Cox proportional hazards analysis, AF was associated with a 70% increased risk of MI (P < 0.0001; Table 2). A significant interaction was detected by race, with black AF participants having an increased risk of MI compared with whites (Table 2).
Figure 1.
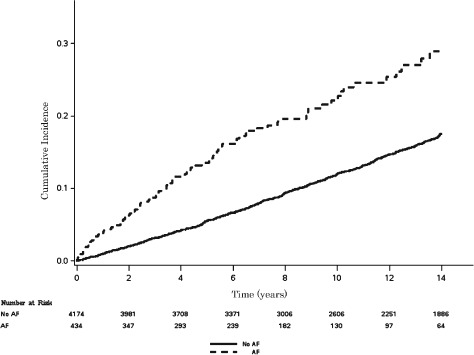
Cumulative incidence of MI by AF. Incidence curves are statistically different (log‐rank P < 0.0001). Abbreviations: AF, atrial fibrillation; MI, myocardial infarction.
Table 2.
Risk of MI by AF Stratified by Age, Sex, and Race
| Subgroup | Events/No. at Risk | Model 1a, HR (95% CI) | P Value | Model 2b, HR (95% CI) | P Value | Interaction P Valuec |
|---|---|---|---|---|---|---|
| All | 797/4608 | 1.8 (1.4‐2.2) | <0.0001 | 1.7 (1.4‐2.2) | <0.0001 | — |
| Age, years | ||||||
| ≤80 | 727/4151 | 1.9 (1.5‐2.4) | <0.0001 | 1.8 (1.4‐2.3) | <0.0001 | |
| >80 | 70/457 | 1.8 (0.98‐3.5) | 0.059 | 1.7 (0.91‐3.3) | 0.092 | 0.84 |
| Sex | ||||||
| F | 390/2765 | 2.0 (1.4‐2.8) | <0.0001 | 1.9 (1.4‐2.7) | 0.0002 | |
| M | 407/1843 | 1.6 (1.2‐2.2) | 0.0027 | 1.6 (1.2‐2.2) | 0.0041 | 0.54 |
| Race | ||||||
| White | 694/3911 | 1.6 (1.3‐2.1) | 0.0002 | 1.6 (1.2‐2.1) | 0.0003 | |
| Black | 103/697 | 3.3 (1.9‐5.9) | <0.0001 | 3.1 (1.7‐5.6) | 0.0003 | 0.030 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; F, female; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; M, male; MI, myocardial infarction; SBP, systolic blood pressure.
Adjusted for age, sex, race, education, and income.
Adjusted for model 1 covariates plus smoking status, SBP, DM, BMI, total cholesterol, HDL‐C, aspirin, statins, antihypertensive medications, and log(hs‐CRP).
Interactions tested using model 2.
Similar results were obtained when CHD events were used as the main outcome. A total of 1711 (37%) CHD events occurred during the study period. The incidence rate for CHD was higher among participants with AF (incidence rate per 1000 person‐years: 68.5, 95% CI: 59.5‐78.8) than those without AF (incidence rate per 1000 person‐years: 32.2, 95% CI: 30.6‐33.8). Unadjusted cumulative incidence of CHD by AF is shown in Figure 2 (log‐rank P < 0.0001). AF was associated with a 90% increased risk of CHD events (P < 0.0001; Table 3). A significant interaction was detected by sex, with female AF participants having an increased risk of CHD compared with males (Table 3).
Figure 2.
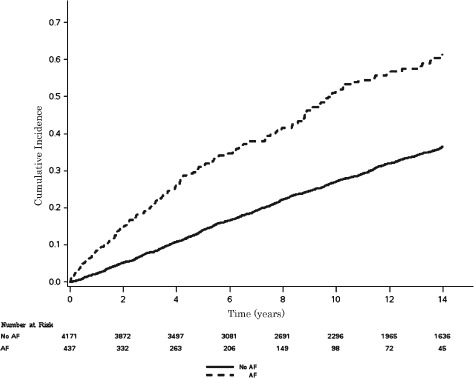
Cumulative incidence of CHD by AF. Incidence curves are statistically different (log‐rank P < 0.0001). Abbreviations: AF, atrial fibrillation; CHD, coronary heart disease.
Table 3.
Risk of Coronary Heart Disease by AF Stratified by Age, Sex, and Race
| Subgroup | Events/No. at Risk | Model 1a, HR (95% CI) | P Value | Model 2b, HR (95% CI) | P Value | Interaction P Valuec |
|---|---|---|---|---|---|---|
| All | 1711/4608 | 2.0 (1.7‐2.3) | <0.0001 | 1.9 (1.6‐2.2) | <0.0001 | — |
| Age, years | ||||||
| ≤80 | 1537/4151 | 2.2 (1.9‐2.6) | <0.0001 | 2.1 (1.8‐2.5) | <0.0001 | |
| >80 | 174/457 | 1.6 (1.1‐2.5) | 0.020 | 1.6 (1.1‐2.4) | 0.028 | 0.088 |
| Sex | ||||||
| F | 898/2765 | 2.4 (2.0‐3.0) | <0.0001 | 2.3 (1.8‐2.8) | <0.0001 | |
| M | 813/1843 | 1.6 (1.3‐2.0) | <0.0001 | 1.6 (1.3‐2.0) | <0.0001 | 0.021 |
| Race | ||||||
| White | 1462/3911 | 1.9 (1.6‐2.2) | <0.0001 | 1.8 (1.6‐2.2) | <0.0001 | |
| Black | 249/697 | 2.8 (1.9‐4.2) | <0.0001 | 2.5 (1.7‐3.9) | <0.0001 | 0.27 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; F, female; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; M, male; MI, myocardial infarction; SBP, systolic blood pressure.
Adjusted for age, sex, race, education, and income.
Adjusted for model 1 covariates plus smoking status, SBP, DM, BMI, total cholesterol, HDL‐C, aspirin, statins, antihypertensive medications, and log(hs‐CRP).
Interactions tested using model 2.
Discussion
In this analysis from CHS, AF was independently associated with incident MI. A significant interaction was detected by race, with black AF participants having an increased risk for MI compared with whites. Additionally, we observed an increased risk of incident CHD with AF. Our results strengthen the argument that AF is associated with MI and CHD.
The high prevalence of CHD events in persons with AF was first explored in the Reduction of Atherothrombosis for Continued Health (REACH) Registry, in which persons with AF were observed to have higher rates of fatal and nonfatal cardiovascular outcomes.12 Additionally, data from REGARDS have shown that AF is associated with an increased risk of MI (HR: 1.70, 95% CI: 1.26‐2.30).5 We observed a similar risk for incident MI in the older population of CHS and also an increased risk for CHD events. In REGARDS, interactions were observed for race and sex, with the association between AF and MI being stronger for women compared with men and for blacks compared with whites. In this study from CHS, we confirm the differential association of AF with MI by race. A differential association also was observed for sex, but the interaction did not reach statistical significance. However, a significant interaction by sex for CHD events was observed, with women having a stronger association than men. Potentially, the association of AF with MI does not differ by sex in the elderly, but we cannot exclude that our study was underpowered to detect such a difference. Nonetheless, our study adds REACH and REGARDS results showing that AF is associated with cardiovascular outcomes and confirms this association in the older population of CHS.
Explanations for the association between AF and MI include common risk factors that predispose to the simultaneous development of both conditions (eg, DM, hypertension, obesity, congestive heart failure).13 However, our results remained significant after adjustment for these potential confounders. AF and MI also are associated with increased levels of inflammation, and dysfunctional regulation of this process may explain our findings.14, 15 Persons with AF potentially have an increased risk of coronary thromboembolism that increases the risk of MI. For example, autopsy data of persons with coronary artery embolism have shown that AF is associated with this condition.16 Additionally, alterations in hemodynamics related to poor rate control among participants with AF may precipitate MI or CHD events (eg, demand ischemia).
Our results also suggest that a bidirectional relationship between AF and MI exists, with each condition predisposing to the development of the other. Data from the Framingham Heart Study have shown that prior MI increases one's risk for developing AF.13, 17 Additionally, participants with MI in the Manitoba Follow‐Up Study were more likely to develop AF than persons without MI.18 MI also has been incorporated into the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) AF risk score to identify individuals at risk for AF.19 The results observed in this study and those reported previously confirm that a reverse relationship exists.5 Similar observations have been observed among persons with AF and chronic kidney disease.20 Common pathophysiologic processes such as inflammation have been suggested to explain the bidirectional relationship between AF and kidney dysfunction, and similar processes also may link AF with MI.5 Further research is needed to investigate the underlying mechanisms of our findings and to discover the pathophysiologic link that predisposes those with AF to the development of MI, and vice versa.
The prevalence of both AF and CHD are increased in older persons, and both conditions are projected to increase in the coming decades.2, 3 Along with the increases in the prevalence of both AF and CHD, a substantial financial burden will be placed on the health care system, with a projected 61% increase in the cost of cardiovascular care by the year 2030.21 The expected population growth among individuals 65 years and older largely explains these projections. Therefore, the identification of new CHD risk factors, such as AF, in elderly populations and the development of preventive strategies remain a top priority for public health officials to reduce the burden of CHD in this population and decrease the future financial strain on the health care system. However, due to the wide spectrum of arrhythmias that encompass a diagnosis of AF (eg, fibrillation, flutter), further research is needed to determine which patients with AF will benefit from early CHD screening before the implementation of widespread programs.
Study Limitations
This study should be interpreted in the context of several limitations. AF cases were identified by participant‐reported history, study ECG data, and hospital discharge records. Potentially, paroxysmal cases of AF were missed due to the time‐dependent nature of this condition. However, we do not know of any reason to suggest that the resulting bias, if any, would have been differential in nature, rather than merely reducing effect estimates toward the null. Additionally, although the adjudication of CHD events in CHS was quite detailed, misclassification of the endpoints studied is possible. The association between AF and MI may vary by MI subtype (eg, non–ST‐elevation MI), and this was not recorded in the CHS. Several potential confounders were included in our multivariable models that likely influenced the development of CHD, but similar to other epidemiological studies, we acknowledge that residual confounding remains a possibility. For example, the duration and severity of these potential confounders were unable to be fully accounted for in our models (eg, duration of hypertension, glucose control in patients with DM, and continued tobacco use). Furthermore, our sensitivity analyses may have been underpowered to detect differences in the predetermined subgroups due to the limited sample size of CHS.
Conclusion
We have shown that AF is associated with incident MI and CHD in a population of predominantly older adults. With the growing elderly population in the United States, the identification of CHD risk factors and targeted preventive measures remain a top priority for future public health initiatives.
This article was prepared using Cardiovascular Health Study (CHS) research materials obtained from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the CHS or the NHLBI.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Lloyd‐Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. [DOI] [PubMed] [Google Scholar]
- 2. Odden MC, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124:827.e5–833.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 4. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 7. Furberg CD, Manolio TA, Psaty BM, et al; Cardiovascular Health Study Collaborative Research Group . Major electrocardiographic abnormalities in persons aged 65 years and older (the Cardiovascular Health Study). Am J Cardiol. 1992;69:1329–1335. [DOI] [PubMed] [Google Scholar]
- 8. Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 9. Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. [DOI] [PubMed] [Google Scholar]
- 10. Gray RJ, Tsiatis AA. A linear rank test for use when the main interest is in differences in cure rates. Biometrics. 1989;45:899–904. [PubMed] [Google Scholar]
- 11. Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 12. Goto S, Bhatt DL, Rother J, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–863, 863.e2. [DOI] [PubMed] [Google Scholar]
- 13. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 14. Hatzinikolaou‐Kotsakou E, Tziakas D, Hotidis A, et al. Relation of C‐reactive protein to the first onset and the recurrence rate in lone atrial fibrillation. Am J Cardiol. 2006;97:659–661. [DOI] [PubMed] [Google Scholar]
- 15. Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin‐6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. [DOI] [PubMed] [Google Scholar]
- 16. Prizel KR, Hutchins GM, Bulkley BH. Coronary artery embolism and myocardial infarction. Ann Intern Med. 1978;88:155–161. [DOI] [PubMed] [Google Scholar]
- 17. Lloyd‐Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 18. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. [DOI] [PubMed] [Google Scholar]
- 19. Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watanabe H, Watanabe T, Sasaki S, et al. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009;158:629–636. [DOI] [PubMed] [Google Scholar]
- 21. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. [DOI] [PubMed] [Google Scholar]


