Abstract
The first transcatheter aortisc valve replacement (TAVR) was performed in 2002, and has been proven beneficial in inoperable and high‐risk patients for open heart surgery. Stroke occurrence after TAVR, both periprocedure and at follow‐up, has not been well described. We sought to review incidence, pathophysiology, predictors, prognosis, and current preventive strategies of cerebrovascular accidents (CVAs) after TAVR. Studies were selected from a Medline search if they contained clinical outcomes data after TAVR. Acute and subacute CVAs after TAVR have been reported in 3% to 6% of patients. Approximately 45% of CVAs occur within 2 days after TAVR; 28% between 3 and 10 days; 4% between 10 and 30 days; and 10.5% occur from 1 month to 2 years. Clinically silent cerebral embolisms have been reported, with an incidence greatly exceeding that of overt CVAs. Underlying pathophysiologic mechanisms for CVAs can be broadly categorized into embolic and nonembolic causes, as well as procedural and postprocedural (early and late). Important predictors of early CVAs are small aortic valve area, atrial fibrillation, and balloon postdilation, whereas late CVAs are mostly influenced by chronic atrial fibrillation, prior cerebrovascular disease, and transapical approach. Following stroke, patients exhibit increased morbidity and mortality. A multilevel approach for the prevention of CVAs includes improved interventional techniques, embolic protection devices, antithrombotic treatment, close monitoring, and aggressive management of modifiable risk factors. Technology advances notwithstanding stroke morbidity and mortality remains steady. The significance of silent cerebral embolism on prognosis remains uncertain, and optimal medical treatment during and after TAVR should be further investigated.
Introduction
Severe aortic stenosis (AS) is the most common valvular disease in the elderly, with an estimated prevalence of 2% to 4% in the general population.1 Although surgical aortic valve replacement (SAVR) remains the mainstay of treatment for severe AS, a transcatheter technique has evolved in the last decade enabling high‐surgical‐risk or inoperable patients with severe AS to undergo transcatheter aortic valve replacement (TAVR).2, 3, 4 Cerebrovascular accidents (CVAs) are one of the major adverse events following surgical or transcatheter AVR in the acute, subacute and remote postoperative period. The aim of this article is to provide insights on existing data regarding CVAs after with TAVR.
Incidence and Timing of Events
The risk of CVA is inherently related to both patient‐based and procedure‐related risks. The variability of CVA rates among studies might be due to study design, sample size, methodology, and patient and site‐specific factors, as well as different event ascertainment and definitions (Figure 1). In an effort to eliminate discrepancies, the Valve Academic Research Consortium (VARC) has determined a set of standardized CVA‐related definitions (Table 1) that have been utilized since their publication.5
Figure 1.
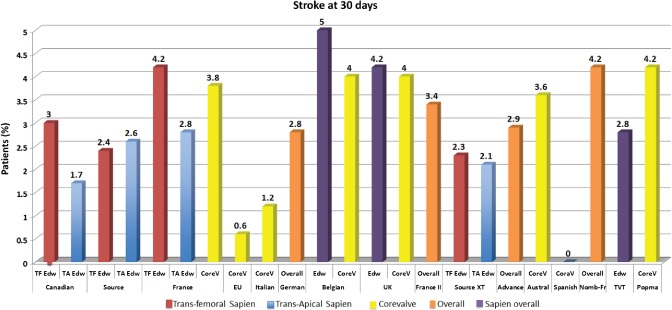
Registry‐based incidence of major and minor stroke after TAVR in current clinical practice. Abbreviations: ACC, American College of Cardiology; TAVR, transcatheter aortic valve replacement; TVT, TransValvular Therapy registry. Data are from Rodes‐Cabau et al.,6 Thomas et al.,7 Eltchaninoff et al.,8 Piazza et al.,9 Tamburino et al.,41 Zahn et al.,10 Bosmans et al.,11 Moat et al,12 Gilard et al.,13 Avanzas et al.,14 Nombela‐Franco et al.,36 Mack et al.,57 Popma et al.21
Table 1.
| Diagnostic Criteria |
|---|
| Acute episode of a focal or global neurological deficit with ≥1 of the following: change in the level of consciousness, hemiplegia, hemiparesis, numbness or sensory loss affecting one side of the body, dysphasia or aphasia, hemianopia, amaurosis fugax, or other neurological signs or symptoms consistent with stroke. |
| Stroke: Duration of a focal or global neurological deficit ≥24 hours; OR <24 hours if available neuroimaging documents a new hemorrhage or infarct; OR the neurological deficit results in death. |
| TIA: Duration of a focal or global neurological deficit <24 hours; any variable neuroimaging does not demonstrate a new hemorrhage or infarct. |
| No other readily identifiable nonstroke cause for the clinical presentation (eg, brain tumor, trauma, infection, hypoglycemia, peripheral lesion, pharmacological influences) to be determined by or in conjugation with the designated neurologist. |
| Confirmation of the diagnosis by ≥1 of the following: |
| Neurologist or neurosurgical specialist |
| Neuroimaging technique (CT scan or brain MRI), but stroke may be diagnosed on clinical grounds alone. |
| Stroke Definitions (VARC 2012) |
| Disabling stroke: An mRS score of ≥2 at 90 days and an increase in ≥1 mRS category from an individual's prestroke baseline. |
| Nondisabling stroke: An mRS score of 2 at 90 days or one that does not result in an increase in ≥1 mRS category from an individual's prestroke baseline. |
| Former Stroke Definitions (VARC 2011) |
| TIA: |
| New focal neurological deficit with rapid symptom resolution (usually 1–2 hours), always within 24 hours. |
| Neuroimaging without tissue injury |
| Stroke (diagnosis as above, preferably with positive neuroimaging study): |
| Minor: mRS score of 2 at 30 and 90 daysb |
| Major: mRS score ≥2 at 30 and 90 days |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; TIA, transient ischemic attack; VARC, Valve Academic Research Consortium.
aPatients with nonfocal global encephalopathy will not be reported as having a stroke without unequivocal evidence based on neuroimaging studies.
bModified Rankin Score assessments should be made by qualified individuals according to a certification process. If there is discordance between the 30‐day and 90‐day modified Rankin Scores, a final determination of major vs minor stroke will be adjudicated by the neurology members of the clinical events committee.
In the landmark Placement of Aortic Transcatheter Valves (PARTNER) trial, in cohort A investigating the balloon expandable SAPIEN valve (Edwards Lifesciences, Irvine, CA), patients with very high surgical risk (Society of Thoracic Surgeons score of 11.8% ± 3.5%) were randomized to either TAVR or SAVR, with similar rates of the combined endpoints of “mortality or stroke” and mortality alone. However, rates of adverse neurologic events were higher in the TAVR group throughout the study (Table 2).2 Similarly, TAVR was associated with a higher risk of CVAs in the PARTNER cohort B when compared with inoperable patients treated without AVR.3 In the recently published randomized controlled trial of the self‐expandable CoreValve bioprosthesis (Medtronic, Minneapolis, MN) by Adams et al, patients with high surgical risk (mean Society of Thoracic Surgeons score 7.4%) underwent either TAVR or SAVR. Patients treated with TAVR (83% iliofemoral access) had a numerically lower stroke incidence at 30 days and 1 year. In comparison to the PARTNER trial, stroke incidence in the CoreValve trial was similar in TAVR patients but occurred at a 3‐fold rate in the SAVR group4; cross‐trial comparisons are always challenging, as the 2 populations have vast differences.
Table 2.
Stroke Incidence in Major Randomized Controlled Trials
| 30 Days | 1 Year | 2 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Balloon‐Expandable Valve (SAPIEN) | Surgery, N = 351 | TAVR, N = 348 | P Value | Surgery, N = 351 | TAVR, N = 348 | P Value | Surgery, N = 351 | TAVR, N = 348 | P Value | |
| PARTNER Cohort A | TIA | 1 (0.3) | 3 (0.9) | 0.33 | 4 (1.5) | 8 (2.6) | 0.32 | 5 (2.0) | 10 (3.6) | 0.26 |
| Minor stroke | 1 (0.3) | 3 (0.9) | 0.34 | 2 (0.7) | 3 (0.9) | 0.84 | 14 (4.9) | 24 (7.7) | 0.17 | |
| Major stroke | 7 (2.1) | 13 (3.8) | 0.2 | 8 (2.4) | 17 (5.1) | 0.07 | ||||
| Balloon‐Expandable Valve (SAPIEN) | Standard Tx, N = 179 | TAVR, N = 179 | P Value | Standard Tx, N = 179 | TAVR, N = 179 | P Value | Standard Tx, N = 179 | TAVR, N = 179 | P Value | |
| PARTNER Cohort B | TIA | 0 | 0 | — | 0 | 1 (0.6) | 0.37 | NA | NA | NA |
| Minor stroke | 1 (0.6) | 3 (1.7) | 0.62 | 1 (0.6) | 4 (2.2) | 0.37 | 8 (5.5) | 22 (13.8) | 0.01 | |
| Major stroke | 2 (1.1) | 9 (5.0) | 0.06 | 7 (3.9) | 14 (7.8) | 0.18 | ||||
| Self‐Expandable Valve (CoreValve) | Surgery, N = 357 | TAVR, N = 390 | P Value | Surgery, N = 357 | TAVR, N = 390 | P Value | Surgery, N = 357 | TAVR, N = 390 | P Value | |
| Adams et al | TIA | 1 (0.3) | 3 (0.8) | 0.36 | 5 (1.6) | 6 (1.6) | 0.93 | NA | NA | NA |
| Minor stroke | 12 (3.4) | 4 (1.0) | 0.03 | 20 (6.0) | 11 (3.0) | 0.05 | NA | NA | NA | |
| Major stroke | 11 (3.1) | 15 (3.9) | 0.55 | 23 (7.0) | 22 (5.8) | 0.59 | NA | NA | NA | |
Abbreviations: NA, not applicable; PARTNER, Placement of Aortic Transcatheter Valves; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack; Tx, therapy.
In a recent meta‐analysis including randomized clinical trials along with observational studies, Khatri et al analyzed data from 16 063 patients who underwent TAVR with the commercially available valves in the United States (Edwards SAPIEN valve and CoreValve). Overall, the early stroke rate (<30 days) was as low as 2.9% (95% confidence interval [CI]: 2.4‐3.4) and CVA rates did not differ significantly according to valve type (SAPIEN 2.9% vs CoreValve 3.6%, P = not significant).15
Time‐Related Aspects
Multiple studies have shown that CVA incidence after TAVR peaks in the immediate postoperative period, with a steady decline over the following months (Figure 2). In a study recruiting 253 patients after TAVR, Tay et al showed that 87% of CVAs occurred within 2 months, and half of them within 24 hours of the procedure.16 Out of a total of 12 major strokes in the first month in PARTNER, 58% happened within 2 days and 17% occurred between days 3 and 5, with the remaining until day 10.17 Notably, whereas the early event rate in the transfemoral (TF) TAVR patients was higher than in the AVR group, transapical (TA) TAVR had a slightly lower early incidence compared with AVR, but a more prolonged period (2 weeks) of early events, suggesting an ostensibly different pathophysiology between the 2 TAVR approaches.
Figure 2.
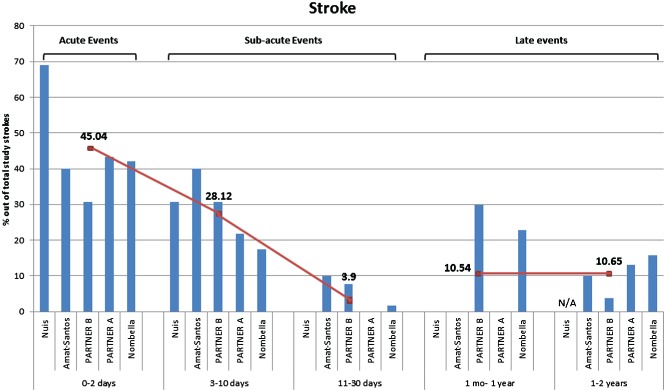
Timing of stroke after TAVR with approximately 2 years follow‐up. Each bar‐chart represents the percentage of strokes out of the total individual study strokes. Abbreviations: PARTNER, Placement of Aortic Transcatheter Valves; TAVR, transcatheter aortic valve replacement. Data are from Amat‐Santos et al,34 Nuis et al,35 PARTNER A3 and B,17 and Nombela‐Franco et al.36
Brain Imaging
Concern has been expressed about silent events occurring during or after valve implantation, regardless of valve type and approach. A handful of studies have estimated their incidence by diffusion‐weighted magnetic resonance imaging (DW‐MRI) and subsequent clinical effect (Table 3). In the first study, conducted by Kahlert et al, 32 patients undergoing TF‐TAVR were compared with 21 SAVR patients. Repeat MRI postprocedure revealed novel hyperintense cerebral lesions in 86% of the Edwards SAPIEN and 80% of CoreValve patients, compared with only 48% in the SAVR group. Lesions in the TAVR group were multiple, small, and bilaterally spread, suggesting their embolic nature, whereas surgical patients had fewer lesions but they were greater in volume. Intriguingly, however, the vast majority of lesions had no remaining signal in follow‐up MRI and cognitive function remained unaffected.18 In a multicenter study, Rodés‐Cabau et al compared the incidence of new ischemic lesions in patients undergoing TAVR with the transfemoral vs the transapical approach using DW‐MRI (baseline and within 6 days). Among 60 patients, almost two‐thirds had evidence of new lesions. The majority (76%) had multiple lesions, with a median of 3 (range, 1–31). No difference was found between the groups. Except for 2 apparent strokes, all of the patients were unaffected.19
Table 3.
Clinically Silent Cerebral Embolism Assessed With DW‐MRI: Summary of Studies Available
| % of Patients With New Cerebral Lesions (n) | Mean No. of Infarcts Per Patient | Mean Lesion Volume and SD, cm3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | All | TF | TA | All | TF | TA | TF | TA | Mean Total Volume and SD, cm3 |
| Fairbairn et al, 201229 | 77% (24/31) | 77% (24/31) | 4.2 ± 6.5 | 2.05 ± 3.5 | |||||
| Kahlert et al, 201018 | 84.4% (27/32) | 84.4% (27/32) | 4.0 (2.1–6.0) | 0.081 (0.06–0.10) | 0.32 | ||||
| Ghanem et al, 201061 | 72.7% (16/22) | 72.7% (16/22) | 3.4 ± 5.1 | 4.3 ± 14.9 | |||||
| Astarci et al, 201062 | 91.5% (32/35) | 90% (19/21) | 93% (13/14) | 5.9 ± 6.8 | 6.6 ± 7.1 | 0.475 | 2.170 | 2. | |
| Rodés‐Cabau et al, 201119 | 68% (41/60) | 66% (19/29) | 71% (22/31) | 3 (2–8) | 3 (1–7) | 4 (2–9) | |||
| Average estimate | 78.2% | 4.1 | 2.4 ± 9.2 | ||||||
Abbreviations: DW‐MRI, diffusion‐weighted magnetic resonance imaging; SD, standard deviation; TA, transapical; TF, transfemoral.
With advancing technology in instrumentation and valve devices, as well as accumulated experience in centers performing TAVR, one would expect better overall outcomes and fewer complications. Van Mieghem et al analyzed the temporal trends (2005 to 2011) in outcomes after TF‐TAVR among 3 propensity‐matched consecutive cohorts. Moving from initial to last cohort, there were significantly fewer major vascular complications, life‐threatening bleedings, and major bleeding. However, stroke, minor stroke, or TIA rates remained temporally consistent throughout the 3 cohorts.20 Another recent prospective nonrandomized trial using the self‐expandable CoreValve in high‐risk patients has found approximately the same stroke rate (2.3%) at 30 days of follow‐up.21
This illustrates that, although they occur at a relatively low rate, CVAs after TAVR constitute a central window of opportunity for improvement, necessitating a better understanding of the causative mechanisms.
Pathophysiology
The time distribution of strokes is inherently correlated to the underlying pathophysiology. Strokes occurring in the acute (<24 hours) and subacute early (<30 days) post‐TAVR period are strongly related to procedural factors, whereas late events (1 to 12 months) are mostly connected to patient and disease factors.22 Retrograde crossing of a stenotic aortic valve during diagnostic catheterization results in new focal cerebral lesions in 22% of patients.23 Initial balloon aortic valvuloplasty (BAV) not only results in the fracture of valvular tissue leading to embolism of overlying calcium deposits, but also increases the risk of thrombogenic complications. Stenotic valves, unlike normal ones, host large amounts of localized tissue factor and thrombin covered by vascular endothelium. By means of endothelial denudation and fracture of the valve, BAV exposes these factors to blood circulation, which in turn triggers coagulation cascades and platelet activation, resulting in increased inflammation and recurrent thrombogenicity.24
The interaction of the newly deployed stent valve with the aortic annulus over the displaced natural valve can cause additional embolic debris. It has been suggested that the balloon‐expandable SAPIEN valve produces emboli during positioning of the valve on the annulus, whereas the self‐expanding CoreValve does so during valve deployment, as manifested by simultaneous transcranial Doppler studies.25, 26 There are currently no conclusive data suggesting differences in the stroke rate of the 2 valves, but a recent randomized trial showed a numerical difference in stroke incidence at 30 days between the balloon and self‐expandable valves (5.8% vs 2.6%, P = 0.33).27 Although these results are based on small event numbers and could be due to chance, they could also point to another potential, yet unconfirmed, mechanism of ischemic injury during TAVR. Hypoperfusion may occur during BAV and balloon‐expandable valve deployment due to repeated rapid ventricular pacing, which is necessary for positioning and deployment and results in transiently reduced cardiac output. This technique may therefore induce ischemia to watershed areas localized in the border zones between the territories of 2 major arteries in the brain, where cerebral blood flow may be additionally impaired due to decreased washout of dislodged microemboli.28
Individual stroke risk is also influenced by age and aortic atheroma extent.29, 30 Consequently, ascending aorta instrumentation may confer an additional risk for embolic events. The TA approach offers an alternative in patients hosting a high degree of aortic atheroma with potential reduction of the risk caused by aorta manipulation and anterograde valve access. However, the TA approach is also limited by the risk of air embolisms, given the large catheter used and the direct communication of the left ventricle to the external air space.19
In a recently conducted study, Van Mieghem et al examined the histopathology of embolic debris captured in an embolic protection device following TAVR. Macroscopic debris was found in 30 (75%) of patients; out of those, 27% had amorphous calcium or valve tissue likely to originate from degenerated aortic leaflets, and 43% had evidence of collagenous tissue coming from either the valve or the aortic wall. Importantly, about half (55%) of patients had thrombotic tissue debris.31 Possible mechanisms beyond those already described could be prosthetic valve surface exposure, flow turbulence, and exposure of stent struts to the circulation.32 Blood stasis in the perivalvular space “outside” the metallic stent of a small or underexpanded prosthetic valve where the irregularly crushed native aortic valve cusps exist could also generate thrombi with subsequent events.
A further central mechanism of acute and early CVAs is preexisting (chronic) or new‐onset atrial fibrillation (NOAF). New‐onset AF is a common complication after cardiac surgery, with inflammatory factors acting as mediators. Patients undergoing TAVR are mainly octogenarians, representing a population with an even higher baseline risk for NOAF due to diastolic dysfunction and left atrial enlargement as a consequence of aortic stenosis.22 Instrumentation during the TAVR procedure is another likely mechanism of NOAF.
Risk Factors for Cerebrovascular Accident
Based on these pathophysiologic mechanisms, plausible assumptions can be made about risk factors associated with CVA (Table 4). In the PARTNER cohort A, being in the TAVR treatment group and having smaller aortic valve area index were both associated with increased risk of neurologic events during the early (<30 days) phase. In the long term, functional impairment (New York Heart Association class) and history of stroke within 6 to 12 months prior to TAVR emerged as significant risk factors. The single strongest late risk factor was the “non‐TF candidate” (ie, assigned to the TA stratum), with a 2.3‐fold adjusted hazard for stroke, which should be interpreted as an indicator of the patient's burden of comorbidities.17 In the same context, prior cerebrovascular disease independently confers a 4‐fold hazard for CVAs after SAVR at a median follow‐up of 455 days.16
Table 4.
Adjusted Risk Factors of Early and Late CVAs
| Risk Factor | Estimated HR |
|---|---|
| For early CVAs | |
| NOAF34, 35, 36 | 2.27–4.40 |
| Smaller aortic valve area17 | 11.8 |
| Balloon postdilation36 | 1.94 |
| Valve dislodgement/embolization36 | 4.36 |
| Severely calcified aorta36 | 2.26 |
| For late CVAs | |
| Chronic AF36 | 1.44–2.84 |
| Prior stroke within 6–12 months2 | 1.93 |
| Non–TF‐TAVR candidate2 | 2.30 |
| PVD36 | 2.02 |
| CVD36 | 2.04 |
| Age (per 1‐year increase)36 | 1.03 |
Abbreviations: AF, atrial fibrillation; CVAs, cerebrovascular accidents; CVD, cerebrovascular disease; HR, hazard ratio; NOAF, new‐onset atrial fibrillation; PVD, peripheral vascular disease; TF‐TAVR, transfemoral‐transcatheter aortic valve replacement.
Atrial fibrillation, both preexisting and new onset, has been identified as an important predictor for stroke. In a recent review, NOAF has been reported in up to one‐third of patients after TAVR, with prevalence varying among studies due to different approaches and baseline characteristics.33 Besides left atrial enlargement and transapical approach, little is known about the risk factors of NOAF after TAVR.34 Its onset most usually coincides with the immediate postprocedural period. Not surprisingly then, Nuis et al found in 214 TF‐TAVR patients that NOAF had an independent 4‐fold increased risk of in‐hospital stroke.35 Similarly, Nombella‐Franco et al and Amat‐Santos et al found that NOAF predicts a 2‐fold to 5‐fold increased 30‐day hazard for CVAs, respectively. However NOAF, as opposed to chronic AF, was not a predictor of mortality.34, 36 It seems though that NOAF also has a sustained effect by increasing late (>30 days) CVAs risk by 2‐ to 4.3‐fold hazard.36 Preexisting AF with a reported incidence range between 16% and 40% has an almost 3‐fold adjusted hazard for clinical stroke beyond 30 days after TAVR.36 The significance of even short transient NOAF regarding early CVA, as well as late silent recurrent and late CVA, is unclear.
Furthermore, balloon postdilation (odds ratio [OR]: 1.94) and valve embolization/dislodgement (OR: 4.36) have been found predictive of CVA within 30 days and of periprocedural events, respectively. Interestingly, whereas initial reports showed that the transfemoral TAVR approach might be associated with an increased stroke rate, newer evidence suggests that this is not the case.19, 36, 37 In a recent multicenter study conducted in Europe, 882 patients undergoing TF‐TAVR and TA‐TAVR were compared. Both approaches had similar stroke rates within 30 days, after adjustment for baseline differences (OR: 0.87, 95% CI: 0.11‐7.49, P = 0.91). However, the transapical approach appears to be a predictive factor for NOAF. This may be held accountable for the failure of this approach to render a lower risk of acute and subacute stroke.
Risk factors for late CVAs point to the natural risk of stroke among elderly people undergoing TAVR rather than to procedural risk factors. Preexisting AF was found to be a predictive factor for late CVAs. Invariably, previous cerebrovascular and peripheral vascular disease, reflecting the diffuse atherosclerotic load of this population, were also found to affect stroke risk.17, 36 It is evident that traditional risk factors of stroke, such as age, female sex, hypertension, diabetes mellitus, left ventricle dysfunction, and smoking, are primarily responsible in this time period after the TAVR procedure.38
Prognosis and Cognitive Trajectory in Patients Suffering From Stroke After TAVR
Stroke has been consistently associated with increased mortality in patients undergoing TAVR. In PARTNER cohort A, the observed mortality after CVA was higher than in patients without this complication.17 In a substudy, Miller et al described the time variation of increased mortality hazard after CVA and found that in patients undergoing TF‐TAVR, mortality risk was maximal immediately after the neurologic event followed by a continuous decrease over the first year, eventually matching the expected background hazard. Patients undergoing TA‐TAVR had a lower immediate mortality hazard, then followed the same pattern as patients undergoing TF‐TVR. Collectively, peri‐interventional stroke has been associated with an approximate 2‐ to 6‐fold increase in hospital mortality, a 3‐ to 12‐fold increase in mortality at 30 days, and a 2‐ to 16‐fold increase in long‐term mortality.2, 36, 39, 40, 41, 42, 43 Nonetheless, PARTNER A reported similar occurrence of “death or CVA” for SAVR and TAVR (8.2% vs 6.9% at 30 days, P = 0.52, 28% vs 26.5% at 1 year, P = 0.68).
The impact of clinically silent cerebral embolism after TAVR on cognitive and daily functionality is contentious. The prospective Rotterdam Scan Study showed in a large cohort that the presence of silent brain infarcts at baseline produces a >2‐fold risk of dementia, irrespective of subcortical atrophy or white‐matter lesions severity, and is also associated with a steeper decline in cognitive function.44 The same association was found in a study examining cerebral ischemic lesions and neurocognitive decline after cardiac surgery within 6 weeks after the procedure.45 Conversely, Ghanem et al found in their study of 111 patients with silent lesions that 91% of them had preserved cognitive function after the first 2 years post‐TAVR and only 6 (5.4%) had early cognitive decline.46 These results should be cautiously interpreted, as the short follow‐up period and the small patient sample may not be sensitive to late cognitive decline.
Preventive and Therapeutic Measures
Preventive and protective measures and strategies should be implemented to curb the incidence of CVAs. Underlying mechanisms and the timing of the events should dictate the strategy followed, which can be categorized as early and late event prevention.
Mechanical factors should be targeted for stroke‐rate improvement. Reducing the size of the valve apparatus and the associated catheters could lead to fewer vascular complications and less scraping of a densely calcific aorta.47 A minimal technique that interferes less with the aortic wall and bypasses predilation of the stenotic valve has been proposed to reduce calcium emboli.48 Multimodality imaging prior to TAVR is warranted to achieve correct sizing of the annulus and avoid malposition, underexpansion of the valve, as well as needless procedural maneuvers. Similarly to carotid artery stenting, cerebral protection devices have been developed and designed to fit the aortic arch or the anonymous and common carotid arteries.49 Their efficacy remains to be evaluated in prospective trials. These devices have been developed to avert cerebral embolism either by means of filtration (Claret Montage Device, Claret Medical Inc., Santa Rosa, CA; and EMBOL‐X, Edwards Lifesciences) or diversion (Embrella Embolic Deflector, Edwards Lifesciences; and TriGuard Cerebral Protection Device, Keystone Heart, Caeserea, Israel) of debris away from the cerebral circulation while maintaining normal cerebral perfusion.50 Depending on aortic‐arch anatomy, these devices fit to different anatomic landmarks providing protection by covering innominate or left carotid artery ostia. Safety, feasibility, and efficacy are currently being tested in ongoing trials. At the EuroPCR 2013 meeting, Rodés‐Cabau presented the yet unpublished results of the Prospective Outcome Study in Patients Undergoing TAVI to Examine Cerebral Ischemia (PROTAVI‐C) trial, which investigated the safety and efficacy of the Embrella device. The initial findings were, however, disappointing, as cerebral microembolization continued to occur during device insertion and valve positioning. Another trial, the DEFLECT‐I trial, is evaluating the safety and performance of the TriGuard device. In preliminary data of the first 20 patients, new DW‐MRI lesions were apparent in 70% of them. In the currently recruiting DEFLECT‐III (NCT02070731) trial, a prospective randomized device evaluation in the same patient population will be provided. Whether the routine use of embolic‐protection devices has a place in patients undergoing TAVR remains to be elucidated.
Antithrombotic treatment is believed to be a cornerstone for the prevention of ischemic CVAs during and after TAVR. Although TAVR procedures have been performed for more than a decade, little is known about optimal antiplatelet and anticoagulation therapy. Current recommendations for antithrombotic agents and strategies for TAVR are presented in Table 4 and are not based on large randomized controlled studies. Thus, there is an unmet need for better antithrombotic therapies, given the fact that major stroke has not declined significantly over time. In PARTNER, heparin was used for procedural anticoagulation (5000 IU bolus loading dose) with a target of activated clotting time >250 seconds, whereas guidelines recommend a target time of 300 seconds. Similarly, dual antiplatelet therapy (loading dose, maintaining dose, duration) after TAVR has not been explicitly defined. PARTNER recommendation was 75 to 100 mg of daily aspirin, a 300‐mg clopidogrel loading dose, and 75 mg QD for 6 months following TAVR.2, 3 However, clopidogrel duration or loading dose are not specifically defined in guidelines, and lately the general usefulness of clopidogrel on top of aspirin in TAVR patients has been questioned.51, 52 In 2 studies comparing DAPT to mono‐antiplatelet therapy (aspirin or clopidogrel), DAPT did not reduce the incidence of new CVAs but was associated with a significantly higher rate of major and life‐threatening bleeding complications.52, 53 Because it is unclear whether thrombi produced during and after TAVR are of platelet or thrombin‐based origin, the latter may not favor clopidogrel as an effective agent in these patients.
Controversy also exists for patients with a history of preexisting AF. No consensus or evidence from trials exists regarding treatment with triple therapy, warfarin with 1 antiplatelet medication, or warfarin alone, although American and Canadian guidelines discourage the use of triple therapy (Table 5).51, 54
Table 5.
Recommendations for Antithrombotic Agents and Strategies After TAVR
| ACC/AHA/STS51 | ESC63 | CCS Statement54 | |
|---|---|---|---|
| Procedural | Unfractionated heparin | ||
| Goal ACT: 300 sec | |||
| Reversal with protamine recommended | |||
| Postprocedural | ASA 81 mg indefinitely | ASA or clopidogrel indefinitely | Indefinite low‐dose ASA + P2Y12 for 1 to 3 months |
| Clopidogrel 75 mg for 3 to 6 months | ASA and clopidogrel early after TAVI | If OAC is indicated, avoid triple Tx unless definite indication exists | |
| If VKA indicated, no clopidogrel | If VKA indicated, no antiplatelet Tx |
Abbreviations: ACC, American College of Cardiology; ACT, activated clotting time; AHA, American Heart Association; ASA, acetylsalicylic acid (aspirin); CCS, Canadian Cardiovascular Society; ESC, European Society of Cardiology; OAC, oral anticoagulant; P2Y12, thienopyridine; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; TAVR, transcatheter aortic valve replacement; Tx, therapy; VKA, vitamin K antagonists.
The direct thrombin inhibitor bivalirudin has been proven efficacious in BAV patients and is currently compared with procedural heparin during TAVR. Bivalirudin is evaluated for its assumed beneficial bleeding profile, but concerns over reversibility of its activity exist in the case of life‐threatening bleeding/vascular complications (Effect of Bivalirudin on Aortic Valve Intervention Outcomes [BRAVO] trial).55, 56 Additionally, it is a strictly procedural agent (parenteral administration) that may only impact acute events.
The clustering of thromboembolic risk factors in TAVR populations such as renal failure (10%), AF (40%), severe chronic obstructive pulmonary disease (15%), coronary artery disease (70%), peripheral vascular disease (30%), and moderate to severe mitral regurgitation (30%) could imply that long‐term anticoagulation therapy (beyond 12 months) after TAVR is of value. Whether such a strategy would provide benefit is currently unknown.57
Importantly however, >50% of postprocedural strokes are of a likely thromboembolic nature. To that end, the prescription of a mid‐term (6 to 12 months) anticoagulation might play a significant role in the reduction of subacute and late CVAs.58 Currently, anticoagulation treatment after TAVR is only recommended if other indications for anticoagulation exist.
A further stroke‐prevention initiative could be a multistep approach for the avoidance of NOAF, including patient risk stratification comprising prior AF, echocardiographic evidence of dilated atrium, diastolic dysfunction and atrial thrombi, and avoidance of triggers such as myocardial injury, electrolyte imbalance, and volume overload, as well as pharmacologic measures such as β‐blockers, amiodarone, angiotensin‐converting enzyme inhibitors, and angiotensin II receptor blockers.22
Triple therapy after TAVR should be avoided in these patient cohorts with a high inherent bleeding risk. Furthermore, data show no difference in stroke rates in mono antiplatelet vs dual antiplatelet therapy, and the combination of 1 oral anticoagulant with 1 antiplatelet has recently showed better safety results without an excess of ischemic events in comparison with triple therapy in AF patients undergoing percutaneous coronary intervention.59 The exact regimen and duration remain to be determined in a prospective manner. Modifiable risk factors of late CVAs, such as hypertension, diabetes, dyslipidemia, and smoking, should undergo aggressive treatment attempts.
Conclusion
The incidence of stroke has a pronounced impact on morbidity and mortality after TAVR but has remained relatively stable over time despite major technology advances and constitutes the single most important outcome metric to improve in TAVR. Clinical silent cerebral embolisms are frequent, but their impact on patient prognosis remains unclear. A better understanding of responsible pathophysiologic mechanisms will lead to improved preventive measures, and optimal medical treatment during and after TAVR must be further studied.
Disclosures: Roxana Mehran, MD, has received institutional research‐grant support from The Medicines Company, Bristol‐Myers Squibb/sanofi‐aventis, Lilly/Daiichi‐Sankyo, Regado Biosciences, and STENTYS; has consulted for Abbott Vascular, AstraZeneca, Boston Scientific, Covidien, CSL Behring, Janssen (Johnson & Johnson), Maya Medical, and Merck; has served on advisory boards of Covidien, Janssen Pharmaceuticals, and sanofi‐aventis; and is an equity shareholder of 25,000 shares for Endothelix, Inc. George Dangas, MD, has received consulting fees/honoraria from Johnson & Johnson, sanofi‐aventis, Covidien, The Medicines Company, Merck, CSL Behring, AstraZeneca, Medtronic, Abbott, Bayer, Boston Scientific, Osprey Medical, and GE Healthcare; and has performed research for and/or received research grants from sanofi‐aventis, BMS, and Daiichi‐Sankyo/Eli Lilly partnership.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Kodali SK, Williams MR, Smith CR, et al. Two‐year outcomes after transcatheter or surgical aortic‐valve replacement. N Engl J Med. 2012;366:1686–1695. [DOI] [PubMed] [Google Scholar]
- 3. Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic‐valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. [DOI] [PubMed] [Google Scholar]
- 4. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 5. Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 6. Rodes‐Cabau, J. et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. [DOI] [PubMed] [Google Scholar]
- 7. Thomas, M. et al. Thirty‐day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62–79. [DOI] [PubMed] [Google Scholar]
- 8. Eltchaninoff, H. et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–197. [DOI] [PubMed] [Google Scholar]
- 9. Piazza, N. et al. Procedural and 30‐day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1‐year following CE mark approval. EuroIntervention. 2008;4:242–249. [DOI] [PubMed] [Google Scholar]
- 10. Zahn, R. et al. Transcatheter aortic valve implantation: first results from a multi‐centre real‐world registry. Eur Heart J. 2011;32:198–204. [DOI] [PubMed] [Google Scholar]
- 11. Bosmans, J.M. et al. Procedural, 30‐day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg. 2011. [DOI] [PubMed] [Google Scholar]
- 12. Moat, N.E. et al. Long‐term outcomes after transcatheter aortic valve implantation in high‐risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–2138. [DOI] [PubMed] [Google Scholar]
- 13. Gilard, M. et al. Registry of transcatheter aortic‐valve implantation in high‐risk patients. N Engl J Med. 2012;366:1705‐1715. [DOI] [PubMed] [Google Scholar]
- 14. Avanzas, P. et al. Percutaneous implantation of the CoreValve self‐expanding aortic valve prosthesis in patients with severe aortic stenosis: early experience in Spain. Rev Esp Cardiol. 2010;63:141–148. [DOI] [PubMed] [Google Scholar]
- 15. Khatri PJ, Webb JG, Rodés‐Cabau J, et al. Adverse effects associated with transcatheter aortic valve implantation: a meta‐analysis of contemporary studies. Ann Intern Med. 2013;158:35–46. [DOI] [PubMed] [Google Scholar]
- 16. Tay EL, Gurvitch R, Wijesinghe N, et al. A high‐risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2011;4:1290–1297. [DOI] [PubMed] [Google Scholar]
- 17. Miller DC, Blackstone EH, Mack MJ, et al. Transcatheter (TAVR) versus surgical (AVR) aortic valve replacement: occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg. 2012;143:832.e13–843.e13. [DOI] [PubMed] [Google Scholar]
- 18. Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion‐weighted magnetic resonance imaging study. Circulation. 2010;121:870–878. [DOI] [PubMed] [Google Scholar]
- 19. Rodés‐Cabau J, Dumont E, Boone RH, et al. Cerebral embolism following transcatheter aortic valve implantation: comparison of transfemoral and transapical approaches. J Am Coll Cardiol. 2011;57:18–28. [DOI] [PubMed] [Google Scholar]
- 20. Van Mieghem NM, Chieffo A, Dumonteil N, et al. Trends in outcome after transfemoral transcatheter aortic valve implantation. Am Heart J. 2013;165:183–192. [DOI] [PubMed] [Google Scholar]
- 21. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self‐expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–1981. [DOI] [PubMed] [Google Scholar]
- 22. Stortecky S, Windecker S. Stroke: an infrequent but devastating complication in cardiovascular interventions. Circulation. 2012;126:2921–2924. [DOI] [PubMed] [Google Scholar]
- 23. Omran H, Schmidt H, Hackenbroch M, et al. Silent and apparent cerebral embolism after retrograde catheterisation of the aortic valve in valvular stenosis: a prospective, randomised study. Lancet. 2003;361:1241–1246. [DOI] [PubMed] [Google Scholar]
- 24. Marechaux S, Corseaux D, Vincentelli A, et al. Identification of tissue factor in experimental aortic valve sclerosis. Cardiovasc Pathol. 2009;18:67–76. [DOI] [PubMed] [Google Scholar]
- 25. Erdoes G, Basciani R, Huber C, et al. Transcranial Doppler‐detected cerebral embolic load during transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2012;41:778–784. [DOI] [PubMed] [Google Scholar]
- 26. Kahlert P, Al‐Rashid F, Dottger P, et al. Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation. 2012;126:1245–1255. [DOI] [PubMed] [Google Scholar]
- 27. Abdel‐Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon‐expandable vs self‐expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311:1503–1514. [DOI] [PubMed] [Google Scholar]
- 28. Hynes BG, Rodés‐Cabau J. Transcatheter aortic valve implantation and cerebrovascular events: the current state of the art. Ann N Y Acad Sci. 2012;1254:151–163. [DOI] [PubMed] [Google Scholar]
- 29. Fairbairn TA, Mather AN, Bijsterveld P, et al. Diffusion‐weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart. 2012;98:18–23. [DOI] [PubMed] [Google Scholar]
- 30.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The French Study of Aortic Plaques in Stroke Group. N Engl J Med. 1996;334:1216–1221. [DOI] [PubMed] [Google Scholar]
- 31. Van Mieghem NM, Schipper ME, Ladich E, et al. Histopathology of embolic debris captured during transcatheter aortic valve replacement. Circulation. 2013;127:2194–2201. [DOI] [PubMed] [Google Scholar]
- 32. Sun JC, Davidson MJ, Lamy A, et al. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet. 2009;374:565–576. [DOI] [PubMed] [Google Scholar]
- 33. Mok M, Urena M, Nombela‐Franco L, et al. Clinical and prognostic implications of existing and new‐onset atrial fibrillation in patients undergoing transcatheter aortic valve implantation. J Thromb Thrombolysis. 2013;35:450–455. [DOI] [PubMed] [Google Scholar]
- 34. Amat‐Santos IJ, Rodés‐Cabau J, Urena M, et al. Incidence, predictive factors, and prognostic value of new‐onset atrial fibrillation following transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59:178–188. [DOI] [PubMed] [Google Scholar]
- 35. Nuis RJ, Van Mieghem NM, Schultz CJ, et al. Frequency and causes of stroke during or after transcatheter aortic valve implantation. Am J Cardiol. 2012;109:1637–1643. [DOI] [PubMed] [Google Scholar]
- 36. Nombela‐Franco L, Webb JG, de Jaegere PP, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation. 2012;126:3041–3053. [DOI] [PubMed] [Google Scholar]
- 37. van der Boon RM, Marcheix B, Tchetche D, et al. Transapical versus transfemoral aortic valve implantation: a multicenter collaborative study. Ann Thoracic Surg. 2014;97:22–28. [DOI] [PubMed] [Google Scholar]
- 38. Wolf PA, D'Agostino RB, Belanger AJ, et al. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. [DOI] [PubMed] [Google Scholar]
- 39. Eggebrecht H, Schmermund A, Voigtlander T, et al. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta‐analysis of 10 037 published patients. EuroIntervention. 2012;8:129–138. [DOI] [PubMed] [Google Scholar]
- 40. Zahn R, Gerckens U, Linke A, et al. Predictors of one‐year mortality after transcatheter aortic valve implantation for severe symptomatic aortic stenosis. Am J Cardiol. 2013;112:272–279. [DOI] [PubMed] [Google Scholar]
- 41. Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. [DOI] [PubMed] [Google Scholar]
- 42. Muñoz‐García AJ, del Valle R, Trillo‐Nouche R, et al. The Ibero‐American transcatheter aortic valve implantation registry with the CoreValve prosthesis: early and long‐term results. Int J Cardiol. 2013;169:359–365. [DOI] [PubMed] [Google Scholar]
- 43. Stortecky S, Windecker S, Pilgrim T, et al. Cerebrovascular accidents complicating transcatheter aortic valve implantation: frequency, timing and impact on outcomes. EuroIntervention. 2012;8:62–70. [DOI] [PubMed] [Google Scholar]
- 44. Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 45. Barber PA, Hach S, Tippett LJ, et al. Cerebral ischemic lesions on diffusion‐weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–1433. [DOI] [PubMed] [Google Scholar]
- 46. Ghanem A, Kocurek J, Sinning JM, et al. Cognitive trajectory after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2013;6:615–624. [DOI] [PubMed] [Google Scholar]
- 47. Van Mieghem NM, Tchetche D, Chieffo A, et al. Incidence, predictors, and implications of access site complications with transfemoral transcatheter aortic valve implantation. Am J Cardiol. 2012;110:1361–1367. [DOI] [PubMed] [Google Scholar]
- 48. Grube E, Naber C, Abizaid A, et al. Feasibility of transcatheter aortic valve implantation without balloon pre‐dilation: a pilot study. JACC Cardiovasc Interv. 2011;4:751–757. [DOI] [PubMed] [Google Scholar]
- 49. Ghanem A, Kocurek J, Sinning JM, et al. Novel approaches for prevention of stroke related to transcatheter aortic valve implantation. Expert Rev Cardiovasc Ther. 2013;11:1311–1320. [DOI] [PubMed] [Google Scholar]
- 50. Freeman M, Barbanti M, Wood DA, et al. Cerebral events and protection during transcatheter aortic valve replacement [published online ahead of print February 19, 2014.] Catheter Cardiovasc Interv. doi: 10.1002/ccd.25457. [DOI] [PubMed] [Google Scholar]
- 51. Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200–1254. [DOI] [PubMed] [Google Scholar]
- 52. Ussia GP, Scarabelli M, Mulè M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2011;108:1772–1776. [DOI] [PubMed] [Google Scholar]
- 53. Durand E, Blanchard D, Chassaing S, et al. Comparison of two antiplatelet therapy strategies in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2014;113:355–360. [DOI] [PubMed] [Google Scholar]
- 54. Webb J, Rodés‐Cabau J, Fremes S, et al. Transcatheter aortic valve implantation: a Canadian Cardiovascular Society position statement. Can J Cardiol. 2012;28:520–528. [DOI] [PubMed] [Google Scholar]
- 55. Kini A, Yu J, Cohen MG, et al. Effect of bivalirudin on aortic valve intervention outcomes study: a two‐centre registry study comparing bivalirudin and unfractionated heparin in balloon aortic valvuloplasty. EuroIntervention. 2014;10:312–319. [DOI] [PubMed] [Google Scholar]
- 56. Sergie Z, Lefèvre T, Van Belle E, et al. Current periprocedural anticoagulation in transcatheter aortic valve replacement: could bivalirudin be an option? Rationale and design of the BRAVO 2/3 studies. J Thromb Thrombolysis. 2013;35:483–493. [DOI] [PubMed] [Google Scholar]
- 57. Mack MJ, Brennan J, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the united states. JAMA. 2013;310:2069–2077. [DOI] [PubMed] [Google Scholar]
- 58. Mérie C, Køber L, Skov Olsen P, et al. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications, and bleeding. JAMA. 2012;308:2118–2125. [DOI] [PubMed] [Google Scholar]
- 59. Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open‐label, randomised, controlled trial. Lancet. 2013;381:1107–1115. [DOI] [PubMed] [Google Scholar]
- 60. Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. [DOI] [PubMed] [Google Scholar]
- 61. Ghanem A, Müller A, Nähle CP, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion‐weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55:1427–1432. [DOI] [PubMed] [Google Scholar]
- 62. Astarci P, Glineur D, Kefer J, et al. Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and trans‐apical approaches using Edwards Sapiens valve. Eur J Cardiothorac Surg. 2011;40:475–479. [DOI] [PubMed] [Google Scholar]
- 63. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012;42:S1–S44. [DOI] [PubMed] [Google Scholar]


