Abstract
Many laboratories rely on dedicated nephelometers and turbidimeters for the measurement of serum proteins. There are, however, a number of chemistry analyzers that offer open channel configurations for end‐user applications. We developed and validated 14 human serum protein assays (α1‐antitrypsin, α2‐macroglobulin, albumin, apolipoproteins AI and B, complement components 3 and 4, haptoglobin, immunoglobulins A, G, and M, orosomucoid, transferrin, and transthyretin) on the Roche cobas® c 501. We obtained excellent precision at low, normal, and high physiologic concentrations of each protein (within‐run imprecision CVs ≤2.5%, total imprecision CVs ≤3.6%). Linearity for each method was within 5% of the expected value throughout the calibration range, and method comparison studies to commercial assays from Roche or Siemens were in good agreement (r>0.975). We observed no significant interference from bilirubin (up to 414 mg/l), hemoglobin (up to 8.9 g/l), triglyceride (up to 28 g/l), or rheumatoid factor (up to 3,930 IU/ml). Calibration was stable for at least 14 days. The instrument's small reaction cell allowed us to conserve nearly 60% of our specimen and reagent volume compared with our previous system. These newly developed assays provide precise and accurate results with high throughput, but without the associated cost of a dedicated instrument. J. Clin. Lab. Anal. 25:52–60, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: acute‐phase proteins, serum globulins, immunoproteins, albumin, apolipoproteins, nephelometry, turbidimetry
INTRODUCTION
The measurement of specific proteins in human physiological fluids by nephelometric and turbidimetric techniques has improved considerably over time 1, 2, 3, 4, 5, 6, 7. These improvements include advances in antibody purification techniques, enhancements in instrument design and function, and production of new reference materials based on global standardization initiatives 8, 9, 10, 11. Collectively, these changes have resulted in more reliable tests and helped to reduce among‐laboratory variance 12, 13.
Laboratories are faced with a multitude of issues when considering the purchase of a new system. These include ease of instrument operation, breadth of test menu, interface options, and cost, to name but a few. For research‐oriented laboratories, the availability of user‐programmable parameters is another variable in the decision‐making process. The purpose of this study was to develop assays for specific serum proteins with clinical relevance, which enhances the role of protein electrophoresis. This report describes the performance characteristics for 14 serum protein assays (α1‐antitrypsin (α1AT), α2‐macroglobulin (α2M), albumin (Alb), apolipoproteins (apo) AI and B, complement components 3 and 4 (C3, C4), haptoglobin (Hpt), immunoglobulins A, G, and M, orosomucoid (Oro), transferrin (Tf), and transthyretin (Ttr)) developed for use on the Roche cobas® c 501.
MATERIALS AND METHODS
Reagents
Immunoturbidimetric assays for 12 of the 14 proteins were developed using monospecific goat antihuman serum from Midland BioProducts Corporation (Boone, IA). For α1AT and α2M, goat antihuman serum was obtained from International Immunology Corporation (Murrieta, CA) and DiaSorin (Stillwater, MN), respectively. Each antiserum was diluted off‐line in a Tris buffer containing 24.2 g/l Tris‐HCl base, 29.1 g/l sodium chloride, and 0.5 g/l sodium azide, adjusted to pH 7.5. Sample diluent was phosphate‐buffered saline (PBS), consisting of 8 g/l sodium chloride, 1.39 g/l sodium dibasic anhydrous, and 0.24 g/l sodium monobasic, adjusted to pH 7.4. For apo AI and B, samples were diluted in DiaSorin's Diluent A. Reaction buffer was PBS containing 48 g/l polyethylene glycol (MW 8,000). For apo AI, 1 g of Tween 20 was added to 1 l of reaction buffer. All chemicals were reagent grade quality, and all buffers and diluents were filtered through a 0.45 µm filter before use. The reaction buffer and diluted antisera were dispensed into positions A and B of individual cobas c pack MULTI cassettes, respectively.
Calibrator 1 (a 3× concentrate of pooled human serum) and SPQ™ controls (low, normal, and high) for serum proteins were obtained from DiaSorin. Protein values assigned to Calibrator 1 were revised following direct measurement of the Certified Reference Material ERM‐DA470 from the Institute for Reference Materials and Measurements (IRMM, Geel, Belgium) according to the method of Blirup‐Jensen et al. 14. From Calibrator 1, a series of six stock calibrants (10, 20, 30, 50, 75, and 100%) were prepared that were further diluted to 1:21 in PBS (40 µl calibrant+800 µl PBS) before assay. For apos AI and B, standard and control sera were obtained from Siemens Inc. (Newark, DE). Values ascribed to these materials were based on the International Federation of Clinical Chemists (IFCC) Reference Preparation SP1‐01 for apo AI 15 and the IFCC Reference Preparation SP3‐07 for apo B 16. After reconstituting the standard with 0.5 ml of deionized water, a series of six calibrants (1:91, 1:41, 1:31, 1:21, 1:14, and 1:11) were prepared in Diluent A. All dilutions were made with the programmable Microlab® ML500 BP dilutor (Hamilton Company, Reno, NV).
Samples
Deidentified, surplus serum samples stored at −20°C (<2 months) were used for the method comparison studies. Samples were selected to encompass a broad range of analyte concentrations.
Instrumentation
The c 501 of the cobas® 6000 analyzer series (Roche Diagnostics, Indianapolis, IN) is a fully automated, user‐programmable analyzer designed to perform potentiometric and photometric assays in serum, plasma, urine, and cerebrospinal fluid, as well as various supernatant sample types.
Laboratory Analysis
The selected methodology was based on timed endpoint measurement analysis (approximating 95% of endpoint) with sample blanking. Microvolumes of diluted calibrator, control or patient serum, and reaction buffer were automatically pipetted into the reaction cell, mixed by ultrasonic action, and incubated for approximately 1.5 min. Next, specific antiserum was added to each reaction cell, mixed and incubated for 4–8 additional minutes, depending on the assay. Throughout the incubation period, bichromatic photometric measurements were taken at 700 and 340 nm for each reaction cell every 8.5 sec. The change in absorbance of the sample‐blanked portion of the reaction was subtracted from the final change in absorbance of the antigen and antibody reaction. Roche's nonlinear reaction calculation models (RCM or RCM2T1) were used to create a calibration curve from which controls and patient samples were interpolated. Once the individual assays were optimized their performance was verified as described below.
Precision
Precision studies were performed in accordance with CLSI document EP5‐A 17. Control materials with low, normal, and high levels of the specific protein were assayed in duplicate on a daily basis for 20 days. The within‐run precision component and the total precision were calculated from all results for each of the controls.
Linearity
Linearity studies were performed using blends of samples with specific protein concentrations that fell within the lower and upper limits of the calibration range. The resultant pools (consisting of 12 points) were assayed in duplicate, using the assay's normal sample volume settings. The observed mean concentration was plotted vs. the expected concentration and the data were examined by linear regression and visual inspection of the bias plots (expected values on the x‐axis vs. the ratio of the observed to expected values on the y‐axis).
To evaluate the accuracy of the instrument's automatic rerun capabilities serum samples with concentrations near the lower and upper limits of the calibration range were diluted off‐line to force them outside the calibration range (1:42 for evaluation of the lower limit and 1:10 for evaluation of the upper limit). The dilutions were run in triplicate using each assay's programmed setting for either an increase or decrease in the volume of sample delivered to the reaction cell (Table 1).
Table 1.
Assay Conditions and Instrument Parameters for Serum Protein Measurement on the cobas c 501
| α1AT | α2M | Alb | Apo AI | Apo B | C3 | C4 | Hpt | IgA | IgG | IgM | Oro | Tf | Ttr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiserum dilutiona | 1:7 | 1:10 | 1:7 | 1:6 | 1:5 | 1:15 | 1:10 | 1:10 | 1:10 | 1:15 | 1:10 | 1:7 | 1:15 | 1:8 |
| Number of tests/cassette | 178 | 200 | 178 | 178 | 190 | 190 | 190 | 172 | 190 | 190 | 190 | 190 | 200 | 198 |
| Fill volume (ml) | ||||||||||||||
| R1, position A | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 | 25.65 |
| R2, position B | 7.7 | 7.3 | 7.7 | 6.8 | 6.7 | 6.7 | 6.7 | 6.2 | 7.7 | 6.5 | 7.7 | 7.7 | 6.4 | 7.7 |
| Instrument settings | ||||||||||||||
| Assay type | 2 point end | |||||||||||||
| Wavelength (2nd/1st) | 700 nm/340 nm | |||||||||||||
| Reaction time (min) | 10 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 10 | 6 | 10 |
| Assay points (initial) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| (final) | 70 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 70 | 42 | 70 |
| Reaction direction | Increasing | |||||||||||||
| Sample dilutionb | 1:21 | |||||||||||||
| Sample volume (µl) | ||||||||||||||
| Normal | 3.0 | 9.3 |
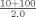
|
3.0 | 12.6 | 10.5 | 21.0 | 4.0 | 4.6 | 2.1 | 10.5 | 5.5 | 3.7 | 20.2 |
| Decrease |
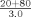 c
c
|
5.6 |
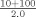
|
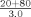
|

|
6.3 | 8.4 |
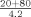
|
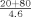
|
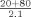
|
2.1 | 2.9 | 3.7 | 11.2 |
| Increase | 14.9 | 18.7 |
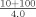
|
6.0 | 21.0 | 21.0 | 35.0 | 33.6 | 32.2 | 21.6 | 31.5 | 10.5 | 7.5 | 35.0 |
| Reagent bottle setting | ||||||||||||||
| Reagent volume (µl)d | ||||||||||||||
| R1, position A | 112 | 100 | 112 | 112 | 105 | 105 | 105 | 116 | 105 | 105 | 105 | 105 | 100 | 101 |
| R2, position B | 28 | 23 | 28 | 23 | 21 | 21 | 21 | 21 | 26 | 20 | 26 | 26 | 20 | 25 |
| Calibration mode | RCM | RCM2T1 | RCM | RCM | RCM | RCM2T1 | RCM2T1 | RCM2T1 | RCM | RCM | RCM | RCM | RCM2T1 | RCM2T1 |
| Point | 6 | 6 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Span point | 6 | 6 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Calibration curve | Concentrations are expressed in g/l (actual mass values are lot specific) | |||||||||||||
| Standard 1 | 0.36 | 0.56 | 10.88 | 0.38 | 0.22 | 0.38 | 0.08 | 0.32 | 0.49 | 2.62 | 0.25 | 0.42 | 0.74 | 0.06 |
| Standard 2 | 0.71 | 1.13 | 21.76 | 0.85 | 0.49 | 0.76 | 0.16 | 0.65 | 0.98 | 5.24 | 0.49 | 0.64 | 1.48 | 0.13 |
| Standard 3 | 1.07 | 1.69 | 32.64 | 1.12 | 0.64 | 1.14 | 0.23 | 0.97 | 1.47 | 7.86 | 0.74 | 1.06 | 2.22 | 0.19 |
| Standard 4 | 1.79 | 2.82 | 54.40 | 1.66 | 0.95 | 1.91 | 0.39 | 1.62 | 2.45 | 13.11 | 1.24 | 1.59 | 3.71 | 0.32 |
| Standard 5 | 2.68 | 4.22 | 81.60 | 2.58 | 1.48 | 2.86 | 0.58 | 2.42 | 3.68 | 19.66 | 1.85 | 2.12 | 5.56 | 0.48 |
| Standard 6 | 3.57 | 5.63 | − | 3.17 | 1.82 | 3.81 | 0.78 | 3.23 | 4.90 | 26.21 | 2.47 | 3.48e | 7.41 | 0.64 |
aAntiserum is diluted in 0.2 M Tris HCl (see text for details).
bAssay controls and specimens are diluted 1:21 in PBS with the exception of apos AI and B which are diluted 1:21 in Diluent A.cIn this example, the analyzer delivers 3 µl of sample from a 1:5 dilution (20+80 µl).
dR1 is reaction buffer; R2 is diluted antiserum.
eStandard 6 for Oro is a 1:13 dilution of the neat calibrator 1.
Detection Limit
The lower limit of detection was defined as the lowest protein concentration corresponding to the mean absorbance value plus 3 SDs of 10 repeated measurements of the sample diluent 7.
Interferences
The effectiveness of these assays for hemolyzed and icteric sera was evaluated by preparing a series of sera supplemented with fresh hemolysate (hemoglobin range: 0–8.9 g/l) or bilirubin (range: 1.5–414 mg/l) from Sigma Chemicals (St. Louis, MO), according to the procedure of Glick et al. 18. The turbidity of lipemia was simulated by adding dilutions of Intralipid™ −20% to serum, such that the measured triglyceride concentration ranged from 0.15 to 28 g/l. Last, the influence of rheumatoid factor (RF) on these assays was evaluated by blending different amounts of a normal serum sample with a serum containing an RF concentration of 3,930 IU/ml.
Carryover
Specimen‐dependent carryover was evaluated, based on the recommendations described by the International Union of Pure and Applied Chemistry 19. Two pools were prepared containing low and high levels of each specific protein. Replicates of the low pool (n=11) and the high pool (n=10) were assayed in varying sequence for each protein. Paired values were compared, to determine whether a carryover effect was present.
Antigen Excess
All assays were evaluated for antigen excess by studying the entire dose–response curve and ensuring that concentration values in the antigen excess zone were incompatible with the pathophysiological concentration for the protein in question. The immunoglobulin assays were further evaluated by assaying stored sera (−20°C) from 58 subjects with monoclonal gammopathies, as established by the presence of a monoclonal protein (M‐protein) on serum protein electrophoresis and immunofixation. Of these sera, 18 M‐proteins were IgA (range: 6.9–47.8 g/l), 20 were IgG (range: 13.8–80.5 g/l), and 20 were IgM (range: 11.8–76.0 g/l).
Method Comparison
Method comparison studies were performed with Tina‐quant® assays from Roche Diagnostics for α1AT, apo AI, apo B, C3, C4, Hpt, IgA, IgG, IgM, Oro, Tf, and Ttr, on the c 501. Measurements of α2M and Alb were performed on the Behring Nephelometer II™ (BN II) from Siemens. Each assay was run in accordance with the manufacturers' package inserts. Protein values for the standards and controls from both companies are traceable to ERM‐DA470, whereas the apolipoprotein values are traceable to the respective IFCC reference preparations described above.
Method comparison studies were performed on the three instruments in a side‐by‐side analysis to minimize potential variability owing to specimen handling. Regression analysis was calculated using the Deming method 20 and Analyse‐it software, version 2.21 (Analyse‐it, Leeds, UK) for Microsoft Excel® (Microsoft Corporation, Redmond, WA).
RESULTS
Antiserum avidity and titer were evaluated to ensure adequate sensitivity, a broad measuring range, and antigen excess security, according to the method of Hudson et al. 21, 22. Through experimentation, a common, off‐line sample dilution (1:21) was established for the 14 proteins. With this approach, all 14 proteins could be measured with 60 µl of sample. Owing to the high concentration of Alb in serum, a further 1:11 on‐board dilution was necessary. For Apo AI, the immunoreactivity of the assay was enhanced with the addition of Tween 20 to the reaction buffer. Attempts to incorporate Tween 20 into the reaction buffer for other proteins proved unsatisfactory, as it tended to suppress immunoprecipitin formation. The optimized antiserum dilutions and the assay parameters for the 14 proteins on the c 501 are presented in Tables 1, 2, where they list each assay's dynamic range and the corresponding lower limit of detection.
Table 2.
Assay Characteristics
| Protein | Dynamic rangea (g/l) | Low detection limit (g/l) |
|---|---|---|
| α1AT | 0.07–18.50 | 0.02 |
| α2M | 0.32–8.80 | 0.01 |
| Alb | 5.67–85.00 | 0.06 |
| Apo A1 | 0.18–15.00 | 0.02 |
| Apo B | 0.12–5.07 | 0.01 |
| C3 | 0.18–5.83 | 0.02 |
| C4 | 0.05–2.00 | <0.01 |
| Hpt | 0.05–17.00 | 0.03 |
| IgA | 0.07–26.80 | 0.03 |
| IgG | 0.43–127.8 | 0.05 |
| IgM | 0.08–13.00 | 0.03 |
| Oro | 0.24–7.09 | 0.03 |
| Tf | 0.40–8.14 | 0.03 |
| Ttr | 0.04–1.26 | 0.01 |
aThe dynamic range is based on the programmed rerun sample volumes.
As noted above, the dose–response curves were optimized to ensure adequate antibody excess and to avoid erroneous results owing to antigen excess in the sample. For the immunoglobulin assays, all 58 samples from the subjects with an M‐protein were properly flagged at the routine 1:21 dilution.
The within‐run imprecision (coefficient of variation, CV) for the 14 assays ranged between 0.4 and 2.4%, whereas the total imprecision CVs ranged from 1.4 to 3.6% (Table 3). The assays were linear over the calibration ranges with no more than 5% deviation from the expected value. Samples that fell outside of the assay's calibration range were automatically detected and rerun by the instrument. We observed recoveries that were within 5% of the expected value and CVs for the replicates that were less than 3%. We observed no carryover effect for any of the proteins (Wilcoxon signed rank test, two‐tailed P>0.1). Calibration for the 14 proteins was stable for at least 14 days, with no more than 5% variation in values for controls.
Table 3.
Precision Studies for the 14 Proteins
| Low | Mid | High | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (g/l) | Within (CV%) | Total (CV%) | Mean (g/l) | Within (CV%) | Total (CV%) | Mean (g/l) | Within (CV%) | Total (CV%) | |
| α1AT | 0.53 | 2.5 | 3.6 | 1.21 | 1.1 | 1.5 | 2.38 | 0.8 | 1.8 |
| α2M | 0.91 | 1.0 | 2.0 | 1.82 | 0.7 | 1.8 | 3.59 | 1.2 | 2.0 |
| Alb | 24.4 | 2.0 | 2.8 | 40.6 | 1.1 | 1.9 | 88.3 | 1.9 | 3.2 |
| Apo A1 | 1.21 | 0.5 | 1.4 | 1.49 | 0.6 | 2.1 | 1.63 | 0.4 | 1.6 |
| Apo B | 0.88 | 0.4 | 2.0 | 1.12 | 0.8 | 1.9 | 1.61 | 0.4 | 1.5 |
| C3 | 0.69 | 0.8 | 2.2 | 1.15 | 0.9 | 2.0 | 2.44 | 1.2 | 2.1 |
| C4 | 0.15 | 1.2 | 2.2 | 0.26 | 0.7 | 1.5 | 0.48 | 0.7 | 1.8 |
| Hpt | 0.65 | 1.4 | 2.6 | 1.25 | 1.1 | 1.9 | 2.59 | 0.9 | 2.1 |
| IgA | 1.17 | 0.8 | 2.4 | 1.98 | 0.9 | 1.7 | 4.04 | 0.7 | 1.6 |
| IgG | 5.34 | 1.1 | 2.3 | 9.29 | 0.9 | 2.0 | 18.73 | 1.0 | 1.4 |
| IgM | 0.47 | 2.2 | 2.8 | 0.88 | 1.0 | 2.4 | 1.56 | 0.8 | 1.6 |
| Oro | 0.52 | 1.3 | 3.4 | 0.75 | 1.4 | 2.1 | 1.67 | 0.7 | 1.6 |
| Trf | 1.61 | 0.8 | 1.9 | 2.67 | 0.6 | 1.7 | 5.77 | 1.3 | 2.4 |
| Ttr | 0.11 | 1.1 | 2.7 | 0.23 | 0.8 | 1.4 | 0.51 | 0.9 | 2.0 |
Interference was defined as significant if the concentration of the interferent resulted in a consistent bias of more than ±10% from the expected value. We observed no interference from bilirubin (up to 414 mg/l), triglyceride (up to 28 g/l), or RF (up to 3,930 IU/ml) for the 14 proteins. Similarly, no interference was detected from hemoglobin (up to 8.9 g/l) with the exception of the Hpt assay, in which a maximum –25% bias in the measured Hpt concentration was observed at ≥2.0 g/l of added hemoglobin.
Method comparison plots between the 14 developed and the existing commercial assays are displayed in Figure 1; data from the Deming regression analysis are presented in Table 4. The Pearson correlation coefficient (r) exceeded 0.975 for all methods. The accuracy of each method's calibration was assessed by measuring ERM‐DA470 in triplicate for 12 of the 14 proteins (excluding the apos) on the three test systems (Table 5). Recovery of the assigned protein values for ERM‐DA470 was within 3% of the target value for 11 of the 12 analytes tested by the newly developed methods compared with only 2 of the 10 Roche Tina‐quant® assays and 1 of the 2 Siemens assays, respectively.
Figure 1.
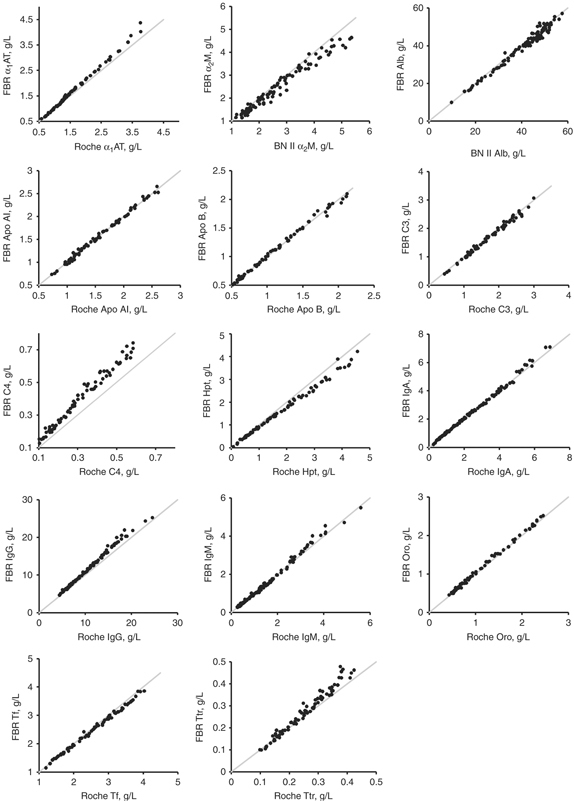
Correlation plots for the method comparison studies between the test method (Foundation for Blood Research, FBR) and the reference method (either the Roche Tina‐quant® or the Siemens BN II). The solid line indicates the line of identity (x=y). Refer to Table 4 for the Deming regression statistics.
Table 4.
Summary Statistics From Deming Regression Analysis of the Method Comparison Studies
| Protein | Comparative method (instrument, reagents) | Range (g/l) | n | Slope (SE) | Intercept (SE) | Sy/x | r |
|---|---|---|---|---|---|---|---|
| α1AT | c 501, Roche | 0.58–3.77 | 67 | 1.12 (0.019) | −0.09 (0.026) | 0.052 | 0.998 |
| α2M | BN II, Siemens | 1.16–5.35 | 97 | 0.90 (0.019) | 0.06 (0.045) | 0.176 | 0.985 |
| Alb | BN II, Siemens | 9.72–57.50 | 100 | 0.99 (0.013) | −0.70 (0.449) | 1.677 | 0.988 |
| Apo AI | c 501, Roche | 0.73–2.61 | 72 | 1.01 (0.010) | −0.01 (0.016) | 0.037 | 0.998 |
| Apo B | c 501, Roche | 0.52–2.12 | 66 | 0.99 (0.012) | 0.01 (0.011) | 0.034 | 0.997 |
| C3 | c 501, Roche | 0.44–3.00 | 64 | 1.00 (0.015) | −0.03 (0.022) | 0.064 | 0.995 |
| C4 | c 501, Roche | 0.10–0.58 | 72 | 1.20 (0.020) | 0.02 (0.005) | 0.023 | 0.991 |
| Hpt | c 501, Roche | 0.07–4.55 | 72 | 0.88 (0.013) | 0.02 (0.016) | 0.086 | 0.997 |
| IgA | c 501, Roche | 0.25–6.88 | 100 | 1.02 (0.010) | 0.00 (0.017) | 0.105 | 0.998 |
| IgG | c 501, Roche | 4.44–24.54 | 100 | 1.11 (0.019) | −0.17 (0.172) | 0.408 | 0.996 |
| IgM | c 501, Roche | 0.26–5.60 | 99 | 1.04 (0.018) | −0.05 (0.021) | 0.104 | 0.996 |
| Oro | c 501, Roche | 0.43–2.46 | 65 | 1.01 (0.006) | 0.02 (0.007) | 0.033 | 0.999 |
| Tf | c 501, Roche | 1.20–4.03 | 72 | 0.94 (0.008) | 0.07 (0.019) | 0.052 | 0.997 |
| Ttr | c 501, Roche | 0.10–0.42 | 72 | 1.18 (0.029) | −0.03 (0.006) | 0.018 | 0.986 |
Table 5.
Recovery of ERM‐DA470 by Methoda
| Roche c 501 | Siemens BN II | FBR | ||
|---|---|---|---|---|
| Protein | ERM‐DA470 assigned value (g/l) | Observed bias, % | ||
| α1AT | 1.206 | −1.1 | − | −0.3 |
| α2M | 1.641 | − | −3.3 | −3.0 |
| Alb | 39.76 | − | +1.3 | −1.4 |
| C3 | 1.091 | +3.9 | − | −2.6 |
| C4 | 0.151 | −5.5 | − | +4.4 |
| Hpt | 0.893 | +12 | − | −1.6 |
| IgA | 1.955 | −0.6 | − | −2.1 |
| IgG | 9.67 | −8.2 | − | −1.5 |
| IgM | 0.797 | +6.6 | − | −0.2 |
| Oro | 0.656 | +4.2 | − | −1.4 |
| Tf | 2.449 | +3.3 | − | −2.1 |
| Ttr | 0.243 | −4.1 | − | −2.2 |
aMean value of three replicates.
DISCUSSION
Over the past several decades, numerous commercial platforms for the measurement of serum proteins have been introduced. Presently, 40% of laboratories that participate in the College of American Pathologists (CAP) proficiency program use a dedicated nephelometer (source: CAP's 2009 Diagnostic Immunology Survey C). There are, however, a growing number of laboratories who see the adaptation of chemistry analyzers for protein measurement based on immunoturbidimetry as a logical extension. We selected the c 501 because of its wide applicability in clinical chemistry, the availability of open channels for user‐defined applications, and its high sample throughput capabilities.
Our evaluation revealed within‐run and total precision performance that was equivalent or superior to the existing commercial nephelometric and turbidimetric immunoassays 23, 24. We found the assays to be linear over the calibration range and the analytical sensitivity consistent with previously described findings for serum proteins 7.
Interference from serum that contains immune complexes, immunoglobulin aggregates, or lipoproteins is a concern with all light‐scattering systems. Moreover, because apos exist in complex associations with lipids, this can pose unique problems in method development 25, 26. In order to minimize interference from triglyceride‐rich lipoproteins, we pretreated samples with DiaSorin's Diluent A. This pretreatment step effectively eliminated the influence of lipemia in samples spiked with up to 28 g/l of triglyceride. In addition, because some of the antigenic sites on apo AI are masked by its lipid component, we found that the addition of Tween 20 to the reaction buffer enhanced the reactivity of apo AI, consistent with the findings of Macjieko et al. 27.
Potential interferents, including bilirubin or free hemoglobin, may adversely impact absorbance readings and result in erroneous reporting. These effects were minimized by using the analyzer's bichromatic wavelength feature, a large sample dilution, and measurement of a sample blank. We found no interference from bilirubin or hemoglobin (with the exception of Hpt) at concentrations up to 414 mg/l and 8.9 g/l, respectively. That the Hpt concentration was influenced by the addition of hemoglobin is not surprising, because one function of this protein is to form complexes with free hemoglobin. The reduction we observed is very similar to data from Bossuyt et al. 28 and is of no relevance for patient samples because in vivo the Hpt‐hemoglobin complex is rapidly removed from the circulation. Last, although overestimation of protein values in sera containing RF has been previously reported 29, we found no significant difference in protein levels after the addition of up to 3,930 IU/ml of RF.
Monoclonal immunoglobulins have restricted populations of epitopes and can reach antigen excess at concentrations well below levels seen for normal polyclonal immunoglobulins. Commercial assays have used different approaches to detect antigen excess, including the addition of additional antibody or antigen to the reaction, and by studying the antigen–antibody reaction velocity as a function of time using kinetic or rate measurements 30, 31. In this system, all the samples containing an M‐protein were properly identified at the routine 1:21 dilution. Consistent with previous publications 21, 32, we found that accurate quantification of M‐proteins required samples to be diluted and assayed until linearity with dilution was established. As a matter of practice, if an M‐protein was known to be present based on clinical or laboratory findings (e.g., protein electrophoresis), the sample was assayed at two or more dilutions, preferably near the lower range of the calibration curve, until the results were within ±10% of each other.
Method comparison studies between the developed assays and either the Roche or Siemens tests showed excellent agreement. Differences in slope between some assays may be related to methodology, antiserum specificity, or differences in calibration. By assaying ERM‐DA470 on each system, we were able to confirm that method calibration problems exist. For several proteins (C4, Hpt, IgG, IgM, and Tf), the observed bias in the recovered values for ERM‐DA470 was consistent with the slopes seen in the method comparison studies. Persisting bias among manufacturers whose calibrators are traceable to ERM‐DA470 has been previously documented 12, 13. For some proteins, the bias has been shown to worsen progressively over time. Suboptimal value transfer from ERM‐DA470 to a manufacturer's internal calibrants was cited as the most likely explanation for the differences. The use of either an imprecise method of value transfer or curve fitting that only approximates the obtained curve can also result in bias. In the former case, use of a single point value transfer in particular is likely to result in bias. These observations have lead the IFCC committee on plasma proteins to create written guidelines for all manufacturers to follow when conducting serum protein value assignment 14, 33. It should be noted that ERM‐DA470 has been recently replaced by ERM‐DA470 k, which is available from the IRMM (Geel, Belgium).
On‐board sample dilution is available on the c 501; however, additional conservation of sample and improved throughput was achieved by preparing these dilutions off‐line with a benchtop programmable dilutor. With this approach, we were able to perform more than 300 analyses in 1 hr compared with roughly 130 for the Roche assay on the same platform. Such high throughput is unattainable with the existing nephelometric systems. Moreover, some of the dedicated nephelometers require a rather large sample volume, which may be problematic when dealing with pediatric samples, research samples of limited volume, or samples that must be split for processing of other tests. All 14 proteins could be measured using the developed protocols, with as little as 60 µl of serum.
In conclusion, we found these 14 serum protein assays well suited for routine clinical use. The reduced size of the reaction cell enables the use of relatively small sample and reagent volumes, and has lead to cost savings in our laboratory. Importantly, the c 501 offers research laboratories a flexible, automated, high‐throughput analyzer for homogeneous immunoassays without the purchase of a dedicated platform.
Acknowledgements
This work was supported by funds from the Foundation for Blood Research Development Program.
REFERENCES
- 1. Ritchie RF. A simple, direct, and sensitive technique for measurement of specific protein in dilute solution. J Lab Clin Med 1967;70:512–517. [PubMed] [Google Scholar]
- 2. Ritchie RF, Alper CA, Graves J, Pearson N, Larson C. Automated quantitation of proteins in serum and other biologic fluids. Am J Clin Pathol 1973;59:151–159. [DOI] [PubMed] [Google Scholar]
- 3. Killingsworth LM, Savory J. Automated immunochemical procedures for measurement of immunoglobulins IgG, IgA, and IgM in human serum. Clin Chem 1971;17:936–940. [PubMed] [Google Scholar]
- 4. Sternberg JC. A rate nephelometer for measuring specific proteins by immunoprecipitin reactions. Clin Chem 1977;23:1456–1464. [PubMed] [Google Scholar]
- 5. Buffone GJ, Savory J, Cross RE, Hammond JE. Evaluation of kinetic light scattering as an approach to the measurement of specific proteins with the centrifugal analyzer. I. Methodology. Clin Chem 1975;21:1731–1734. [PubMed] [Google Scholar]
- 6. Hills LP, Tiffany TO. Comparison of turbidimetric and light‐scattering measurements of immunoglobulins by use of a centrifugal analyzer with absorbance and fluorescence/light‐scattering optics. Clin Chem 1980;26:1459–1466. [PubMed] [Google Scholar]
- 7. Blirup‐Jensen S. Protein standardization III: Method optimization basic principles for quantitative determination of human serum proteins on automated instruments based on turbidimetry or nephelometry. Clin Chem Lab Med 2001;39:1098–1109. [DOI] [PubMed] [Google Scholar]
- 8. Whicher J, Johnson M. The present state of protein analysis and interpretation. Clin Chem Lab Med 2001;39:1017–1018. [DOI] [PubMed] [Google Scholar]
- 9. Whicher JT. BCR/IFCC reference material for plasma proteins (CRM 470). Community Bureau of Reference. International Federation of Clinical Chemistry. Clin Biochem 1998;31:459–465. [DOI] [PubMed] [Google Scholar]
- 10. Whicher JT, Ritchie RF, Johnson AM, et al. New international reference preparation for proteins in human serum (RPPHS). Clin Chem 1994;40:934–938. [PubMed] [Google Scholar]
- 11. Goodall SR. Advances in plasma protein standardization. Ann Clin Biochem 1997;34:582–587. [DOI] [PubMed] [Google Scholar]
- 12. Johnson AM, Whicher JT, Ledue TB, Carlstrom A, Itoh Y, Petersen PH. Effect of a new international reference preparation for proteins in human serum (certified reference material 470) on results of the College of American Pathologists Surveys for plasma proteins. Arch Pathol Lab Med 2000;124:1496–1501. [DOI] [PubMed] [Google Scholar]
- 13. Ledue TB, Johnson AM. Commutability of serum protein values: Persisting bias among manufacturers using values assigned from the certified reference material 470 (CRM 470) in the United States. Clin Chem Lab Med 2001;39:1129–1133. [DOI] [PubMed] [Google Scholar]
- 14. Blirup‐Jensen S, Johnson AM, Larsen M. Protein standardization V: Value transfer. A practical protocol for the assignment of serum protein values from a reference material to a target material. Clin Chem Lab Med 2008;46:1470–1479. [DOI] [PubMed] [Google Scholar]
- 15. WHO . 1993. Technical report series 840, 9. Geneva.
- 16. WHO . 1994. Technical report series 848, 17. Geneva.
- 17. NCCLS . Evaluation of precision performance of clinical chemistry devices (EP5‐A). Villanova, PA: NCCLS, 1999.
- 18. Glick MR, Ryder KW, Jackson SA. Graphical comparisons of interferences in clinical chemistry instrumentation. Clin Chem 1986;32:470–475. [PubMed] [Google Scholar]
- 19. Haeckel R. Proposals for the description and measurement of carry‐over effects in clinical chemistry. Pure Appl Chem 1991;63:301–306. [Google Scholar]
- 20. Cornbleet PJ, Gochman N. Incorrect least‐squares regression coefficients in method‐comparison analysis. Clin Chem 1979;25:432–438. [PubMed] [Google Scholar]
- 21. Hudson GA, Poulin SE, Ritchie RF. Twelve‐protein immunoassay profile on the COBAS FARA. J Clin Lab Anal 1987;1:191–197. [Google Scholar]
- 22. Hudson GA, Ritchie RF, Haddow JE. Method for testing antiserum titer and avidity in nephelometric systems. Clin Chem 1981;27:1838–1844. [PubMed] [Google Scholar]
- 23. Salden HJ, Bas BM, Hermans IT, Janson PC. Analytical performance of three commercially available nephelometers compared for quantifying proteins in serum and cerebrospinal fluid. Clin Chem 1988;34:1594–1596. [PubMed] [Google Scholar]
- 24. Schotters SB, McBride JH, Rodgerson DO, Higgins S, Pisa M. Determination of five protein analytes using the Beckman ArrayTM and the Behring nephelometer system. J Clin Lab Anal 1988;2:108–111. [Google Scholar]
- 25. Rifai N, King ME. Immunoturbidimetric assays of apolipoproteins A, AI, AII, and B in serum. Clin Chem 1986;32:957–961. [PubMed] [Google Scholar]
- 26. Siedel J, Schiefer S, Rosseneu M, et al. Immunoturbidimetric method for routine determinations of apolipoproteins A‐I, A‐II, and B in normo‐ and hyperlipemic sera compared with immunonephelometry. Clin Chem 1988;34:1821–1825. [PubMed] [Google Scholar]
- 27. Maciejko JJ, Levinson SS, Markyvech L, Smith MP, Blevins RD. New assay of apolipoproteins A‐I and B by rate nephelometry evaluated. Clin Chem 1987;33:2065–2069. [PubMed] [Google Scholar]
- 28. Bossuyt X, Blanckaert N. Evaluation of interferences in rate and fixed‐time nephelometric assays of specific serum proteins. Clin Chem 1999;45:62–67. [PubMed] [Google Scholar]
- 29. Chambers RE, Whicher JT, Perry DE, Milford‐Ward A, White PA, Fifield R. Overestimation of immunoglobulins in the presence of rheumatoid factor by kinetic immunonephelometry and rapid immunoturbidimetry. Ann Clin Biochem 1987;24:520–524. [DOI] [PubMed] [Google Scholar]
- 30. Skoug JW, Pardue HL. Kinetic turbidimetric method for the immunochemical quantification of immunoglobulins, including samples with excess antigen. Clin Chem 1988;34:309–315. [PubMed] [Google Scholar]
- 31. Skoug JW, Pardue HL. Effects of reaction variables on nephelometric and turbidimetric responses for the immunochemical reaction of immunoglobulin G. Clin Chem 1988;34:300–308. [PubMed] [Google Scholar]
- 32. Van Lente F. Light‐scattering immunoassays In: Rose NR, de Macario EC, Fold JD, Lane HC, Nakamura RM, editors. Manual of Clinical Laboratory Immunology. Washington, DC: American Society for Microbiology, 1997;13–19. [Google Scholar]
- 33. Blirup‐Jensen S, Johnson AM, Larsen M. Protein standardization IV: Value transfer procedure for the assignment of serum protein values from a reference preparation to a target material. Clin Chem Lab Med 2001;39:1110–1122. [DOI] [PubMed] [Google Scholar]


