Abstract
Background: Very few studies have investigated, in the elderly, the effect of rheumatic inflammatory states on phagocyte function and free radical production. The objective of this article is to evaluate phagocytosis by neutrophils and the production of nitric oxide (·NO) by monocytes in elderly women recruited among patients of the Brazilian Public Health System. Methods: Forty patients aged more than 60 years with rheumatic inflammatory diseases were studied. Phagocytosis was measured by flow cytometry. ·NO production was measured by the total nitrite assay and conventional inflammation markers were determined. Data were analyzed with the Mann–Whitney nonparametric test and P<0.05 was considered significant. Results: C‐reactive protein levels and white blood cell counts were significantly higher in inflammation than in the control group (P<0.05). The phagocytosis fluorescence intensity per neutrophil and the percentual of neutrophils expressing phagocytosis were significantly higher (P<0.05) in the test than in the control group. Furthermore, there was significant ·NO overproduction by monocytes, (P<0.05). Conclusion: Phagocytosis and ·NO production are affected by rheumatic states. This suggests that the increased ·NO levels may play a part in the increased oxidative stress in rheumatic diseases in elderly women. J. Clin. Lab. Anal. 25:47–51, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: nitric oxide, phagocytosis, elderly women, rheumatic diseases, oxidative stress
INTRODUCTION
A considerable amount of clinical research in geriatric medicine has been conducted in recent years; yet, there are few studies regarding the effect of rheumatic inflammatory diseases on the functions of phagocytes in elderly women.
Phagocytes, especially monocytes and neutrophils, play an essential part in the innate immune system via the oxidative burst, being involved in antimicrobial activity, the eradication of pathogens, and the production of free radicals and reactive oxygen and nitrogen species 1, 2. However, some studies have revealed that these free radicals, reactive oxygen species (ROS) and reactive nitrogen species (RNS) are also involved in deleterious effects in inflammation 1, 3. Furthermore, there are numerous discrepancies in the literature between in vivo and in vitro results, as well as between results for murine and human neutrophils 4.
The free radical nitric oxide (·NO) has an important role in the innate immune system, but it has also been found to play an important part in the pathophysiology of many human diseases. RNS derived from ·NO interact with biomolecules, such as proteins, carbohydrates, and lipids, modifying both their structure and function 5, 6. The generation of ·NO by human monocytes seems to be controversial, and it has not yet been explored in the elderly, especially those with rheumatic diseases.
Rheumatoid arthritis (RA) is a chronic joint disease leading to severe erosion of an adjacent bone, which is not observed in patients with osteoarthritis (OA) 7, and neutrophils and monocytes play an important role in overt inflammation. OA is the most common disease of the joints. It is a chronic condition that has periods of acute pain and affects the quality of life, owing to the impairment of normal functional activity 8.
Thus, the aim of this study was to investigate the phagocytosis in neutrophils and ·NO production by monocytes isolated from peripheral blood, in elderly women with OA and RA.
MATERIAL AND METHODS
Subjects
We performed a prospective controlled study of 40 elderly women (60–84 years old). They were recruited from patients attended by the Brazilian Public Health System in a geriatric department in Ribeirão Preto city. All subjects gave informed consent to participate in the study, and the procedures were approved by the Ethics Committee of Faculty of the Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, in keeping with the principles enunciated in the Declaration of Helsinki. Tobacco users and patients with malignancies, impaired kidney function, hemoglobinopathy, decompensated diabetes mellitus, bone marrow proliferative disorders, or infectious diseases were excluded from the study. Although the patients were under treatment with an antihypertensive (enalapril) and a hypocholesterolemiants (simvastatin) at enrollment, none had been treated with immunosuppressive drugs. Thus, these drugs had the same (homogeneous) distribution in both groups. All patients presenting hypertension and dyslipidemia had them controlled with medication. Under instructions from the leading cardiologist, blood pressure assessments were taken three times before venopunction at 15 min intervals. The control group of elderly women (n=20) had normal complete hemograms and no signs of inflammation. The inflammation group (n=20) was composed of elderly women with rheumatic disorders, specifically RA (77%) and OA (23%). They took Nonsteroidal Antiinflammatory Drugs, ibuprofen, diclofenac, and meloxican.
Materials
Lipopolysaccharides (LPS), fluorescein isothiocyanate (FITC), Ficoll‐Hypaque (Histopaque®, Sigma Aldrich, St. Louis, Missouri), RPMI 1640 medium, propidium iodide, trypan blue, fetal calf serum (FCS), neutral red solution, streptomycin, penicillin, and 2‐mercaptoethanol were purchased from Sigma‐Aldrich (St. Louis, MO).
Blood Samples
Human peripheral blood samples were drawn in the morning on a routine basis from volunteers who had fasted for 12 hr. Whole blood and serum samples were collected into tubes in the presence or in the absence of the anticoagulant, respectively. The following biochemical analyses were performed on the serum: creatinine, fasting glucose, gamma‐glutamyltransferase activity, and C‐reactive protein (CRP). Hematological tests were performed on the EDTA whole blood: white blood cell (WBC) counts and electrophoresis of hemoglobin (data not shown). Phagocytosis assay and isolation of mononuclear cells were analyzed on the heparinized whole blood.
Analytical Methods
The laboratory biochemical and hematological tests (Table 1) were performed in duplicate for each patient and reviewed by a physician to exclude subjects with hemoglobinopathy, kidney disorders, infection, liver disorders, or bone marrow proliferative disorders. WBC counts were obtained with an automated Micros 45‐ABX® counter (France), and differential counts were done by examining blood smears stained with Leishman. The high sensitivity CRP was measured by immunoturbidimetric assay (BioTécnica®, Brazil). The biochemical analyses were performed with colorimetric kits (Labtest®, Brazil) in a Beckman DU®‐70‐USA (Maryland) spectrophotometer.
Table 1.
Age, Biochemical and Hematological Parameters of Patients
| Parameters | Control group | Inflammation group |
|---|---|---|
| Age | 68 (65.5–75.0) | 69 (68.5–75.0) |
| Fasting glucose (mg/dl) | 85 (77.4–95.5) | 88 (81.7–110.8) |
| Serum creatinin (mg/dl) | 0.9 (0.8–1.2) | 0.92 (0.8–1.2) |
| GGT (U/l) | (21.6) (17.7–29.2) | (17.5) (8.5–24.4) |
| WBC/µl | 5,800 (4,750–6,450) | 6,100 (5,600–7,550) |
| CRP (mg/l) | 0.2 (0.2–0.9) | 4.3* (3.5–5.1) |
Data are reported as medians from 25th to 75th percentiles in brackets. *Significantly different from control, P<0.0001 (Mann–Whitney test).
Phagocytosis Function
Heparinized whole blood (100 µl) was collected from the peripheral blood of volunteers and incubated with 100 µl of FITC‐labeled bacteria (E. coli) 9 for 10 min at 37°C. After addition of 50 µl quenching solution, the leukocytes were washed twice with phosphate‐buffered saline (PBS) by centrifuging at 250×g, 4–8°C for 5 min. Red blood cells were lysed (NH4Cl lysis solution, 0.83%, pH 7.2). The leukocytes were washed once with PBS (centrifuged at 4–8°C, 250×g for 5 min) and resuspended in 500 µl of PBS‐D. The cell viability evaluations were analyzed and supplemented with propidium iodide (16.6 µg/ml). Phagocytosis was reported as percentual (%) of cells containing fluorescence and the median fluorescence intensity per cell. Tubes were kept at 4–8°C in the dark, until being read in a flow cytometer (FacScan®, Becton‐Dickinson, Bioscience, San José, CA). Data were taken from 10,000 cells per sample in the region for neutrophils, as well as monocytes.
Isolation of Cells
Heparinized peripheral blood was collected from volunteers. The mononuclear cells isolation was made by the difference of gradient density Ficoll‐Hypaque (Histopaque®) 1077. After centrifugation (400×g; 30 min at room temperature), the monocytes were found at the plasma/1077 interphase. Monocytes were collected carefully with a Pasteur pipette and washed in PBS twice (240×g for 10 min), and suspended in RPMI 1640 medium with 10% FCS.
Monocyte Cell Culture
Cells (1×105 monocytes/well) were counted in neutral red solution, plated in 24 well‐culture plates, and allowed to adhere at 37°C in a 5% CO2 atmosphere for 2 hr. The supernatant medium and nonadherent cells were washed off and the wells recharged with medium at 37°C in a 5% CO2 atmosphere. Cell preparations contained more than 98% viable monocytes, as assessed by the trypan blue exclusion method. The cells were cultured for 24 hr at 37°C in a 5% CO2 atmosphere, with (test) or without (control) 1 µg/ml LPS as stimulus, to analyze the ·NO production.
·NO Production
Concentration of nitrite (NO) was taken as an indirect measurement of ·NO produced by the monocytes. It was determined by the Total Nitric Oxide/Nitrite/Nitrate Assay kit (R&D Systems®, MN), a colorimetric method that determines nitric oxide concentrations by the enzymatic conversion of nitrate to nitrite by nitrate reductase. Absorbance at 540 nm was measured in duplicate with a Spectra Max Plus spectrophotometer (Molecular Devices®, Sunnyvale, CA), according to the manufacturer's instructions. Briefly, sodium nitrate (2,000 µmol/l) and sodium nitrite (2,000 µmol/l) solutions were provided as standards. The average duplicate readings for each standard and sample were subtracted from the average blank optical density. The measurement of the total endogenous nitrite concentration is the measurement of the total nitrite concentration after the conversion of nitrate to nitrite by the nitrate reductase.
Statistical Analysis
The Mann–Whitney nonparametric test was used to analyze the data and values are given as medians. The results were considered significant when P<0.05. All tests were analyzed by the program GraphPad Prism version 4.02.
RESULTS
Age, hematological, and biochemical parameters of patients are shown in Table 1. The CRP was significantly higher in the inflammation group than in the control group (P<0.05).
The percentage of neutrophils expressing phagocytosis and the phagocytosis fluorescence intensity per neutrophil were significantly higher in the inflammation group than in the control group (Figs. 1 and 2, respectively). The viability of cells in the phagocytosis assay was 96±3% (mean±SD).
Figure 1.
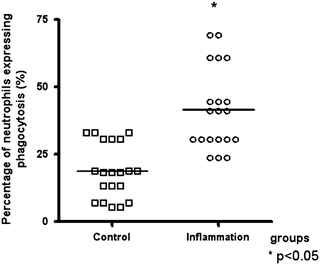
Percentage of neutrophils expressing phagocytosis in control and inflammation groups. Data are reported as medians. *Statistical difference vs. control group: P<0.05 (Mann–Whitney test).
Figure 2.
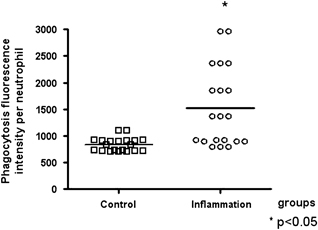
Phagocytosis fluorescence intensity per neutrophil in control and inflammation groups. Data are reported as medians. *Statistical difference vs. control group: P<0.05 (Mann–Whitney test).
The production of NO was significantly higher in the inflammation group than in the control group (Fig. 3).
Figure 3.
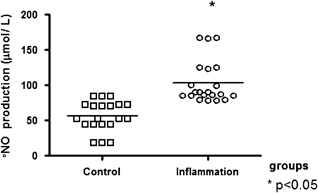
·NO production (µmol/l) by monocytes in control and inflammation groups. Monocytes were cultured at 1×105 cells/ml for 24 hr at 37°C in a 5% CO2 atmosphere. Total nitrite concentration in the samples was determined in the cell culture supernatant. Data are reported as medians. *Statistical difference vs. control group: P<0.05 (Mann–Whitney test).
DISCUSSION
There are very few studies assessing the effects of inflammatory states on phagocytosis in elderly women. In this investigation, this question was examined by studying the effects of rheumatic inflammatory conditions on phagocytosis by neutrophils and ·NO generation by monocytes, isolated from elderly women.
ROS and RNS are produced at high levels by phagocytes in the course of the inflammatory response. Free radicals are thought to contribute to the pathology of many inflammatory conditions. Leukocytes generate large amounts of ROS via activation of NADPH oxidase, neutrophils accumulate in large numbers, and are stimulated to produce superoxide and other reactive oxidants 10.
In this study, both the phagocytosis fluorescence intensity per neutrophil and the percentage of neutrophils expressing phagocytosis in the inflammation group were significantly higher than in the control group. The reason for this may involve the participation of phagocytes in host defense mechanisms, owing mainly to proinflammatory cytokines, such as TNF‐α and IL‐6, produced in large amounts during inflammation 11 and these proinflammatory cytokines are likely to be encountered in the circulation in peripheral blood neutrophils of RA patients as priming agents 12. Thus, in our study, it is probable that proinflammatory cytokines may be modulating and increasing phagocytosis 13.
The direct reaction of ·NO with most biological molecules does not occur at low concentrations (100–500 nmol/l) in vivo, but ·NO can be harmful at concentrations up to 1–3 µmol/l 6. There are reports that activated human neutrophils can catalyze the formation of nitrosated products 5, 14, an extensive event during oxidative stress. This shows that the NO production in our results (Fig. 3) represents overproduction of ·NO (above 50 µmol/l). It is believed to play a critical role in mediating inflammatory tissue injury. Several studies have reported increased endogenous ·NO synthesis. Overproduction of NO may be important in the pathogenesis of RA contributing to the inflamed joint and periarticular bone loss observed in RA 15. Recent evidence suggests that ·NO contributes to T‐cell dysfunction in autoimmune diseases, such as RA and systemic lupus erythematosus 16. Significantly, higher ·NO levels occur in acute appendicitis, which is strongly correlated with oxidative stress and conventional inflammation markers 17.
All the patients were under treatment with enalapril, an ACE inhibitor, and simvastatin at enrollment. These drugs have antiinflammatory effects and may interfere with the study. In fact, the use of the antiinflammatory drugs could characterize a limitation of the study. This finding should be investigated further, as this study was not designed to evaluate oral drugs action. However, the drugs have the same distribution in both groups, in order to minimize this effect.
Phagocytosis is an ancestral/innate component of the immune system that seems to be relatively preserved during aging. Taken together, these results suggest that ·NO is essential for the upregulation of the rheumatic inflammatory response in the target population. It suggests that the increased ·NO may play a role in the increased oxidative stress in rheumatic inflammation diseases in elderly women. This may reflect a characteristic of the inflammatory rheumatic condition and/or a current status in behavior and pathophysiology of the inflammatory rheumatic states.
REFERENCES
- 1. Winterbourn C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 2002;181–182:223–227. [DOI] [PubMed] [Google Scholar]
- 2. Sheppard RF, Kelher MR, Moore EE, Mclaughlin NJD, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol 2005;78:1025–1042. [DOI] [PubMed] [Google Scholar]
- 3. Ximenes VF, Paino IMM, De Faria‐Oliveira OMM, Da Fonseca LM, Brunetti IL. Indole ring oxidation by activated leukocytes prevents the production of hypochlorous acid. Braz J Med Biol Res 2005;38:1575–1583. [DOI] [PubMed] [Google Scholar]
- 4. Wessels I, Jansen J, Rink L, Uciechowski P. Immunosenescence of polymorphonuclear neutrophils. Scientific World Journal 2010;21;145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eiserich JP, Hristova M, Cross CE, et al. Formation of nitric oxide‐derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998;391:393–397. [DOI] [PubMed] [Google Scholar]
- 6. Eiserich JP, Patel RP, O'Donnell VB. Pathophysiology of nitric oxide and related species: Free radical reactions and modification of biomolecules. Mol Aspects Med 1998;19:221–357. [DOI] [PubMed] [Google Scholar]
- 7. Riepl B, Grässel S, Wiest R, Fleck M, Straub RH. Tumor necrosis factor and norepinephrine lower the levels of human neutrophil peptides 1–3 secretion by mixed synovial tissue cultures in osteoarthritis and rheumatoid arthritis. Arthritis Res Ther 2010;12:R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lanas A, Tornero J, Zamorano JL. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: The LOGICA study. Ann Rheum Dis 2010;69:1453–1458. [DOI] [PubMed] [Google Scholar]
- 9. Lopez‐Hurtado M, Flores‐Medina S, Diaz‐Garcia FJ, Guerra‐Infante FM. Partial characterization of phagocytic activity in neutrophils of the nine‐banded armadillo Dasypus novemcinctus. Vet Immunol Immunopathol 2005;106:269–275. [DOI] [PubMed] [Google Scholar]
- 10. Fialkow I, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 2007;42:153–164. [DOI] [PubMed] [Google Scholar]
- 11. Vondracek J. Effects of recombinant rat tumor necrosis factor‐α and interferon‐γ on respiratory burst of rat polymorphonuclear leukocytes in whole blood. Folia Biologica 1997;43:115–121. [PubMed] [Google Scholar]
- 12. Kowanko IC, Ferrante A, Clemente G, Youssef PP, Smith M. Tumor necrosis factor priming of peripheral blood neutrophils from rheumatoid arthritis patients. J Clin Immunol 1996;16:216–221. [DOI] [PubMed] [Google Scholar]
- 13. El‐Benna J, Dang PMC, Gougerot‐Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol 2008;30:279–289. [DOI] [PubMed] [Google Scholar]
- 14. Schopfer FJ, Baker PRS, Freeman BA. NO‐dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem Sci 2003;28:646–654. [DOI] [PubMed] [Google Scholar]
- 15. Van't Hof RJ, Ralston SH. Nitric oxide and bone. Immunology 2001;103:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagy G, Koncz A, Telarico T, et al. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther 2010;28:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yilmaz FM, Yilmaz G, Erol MF, Köklü S, Yücel D. Nitric oxide, lipid peroxidation and total thiol levels in acute appendicitis. J Clin Lab Anal 2010;24:63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


