ABSTRACT
The clinical scenario of heart failure (HF) in older hospitalized patients is complex and influenced by acute and chronic comorbidities, coexistent geriatric syndromes, the patient's ability for self‐care after discharge, and degree of social support. The impact of all these factors on clinical outcomes or disability evolution is not sufficiently known. FRAIL‐HF is a prospective observational cohort study designed to evaluate clinical outcomes (mortality and readmission), functional evolution, quality of life, and use of social resources at 1, 3, 6, and 12 months after admission in nondependent elderly patients hospitalized for HF. Clinical features, medical treatment, self‐care ability, and health literacy were prospectively evaluated and a comprehensive geriatric assessment with special focus on frailty was systematically performed in hospital to assess interactions and relationships with postdischarge outcomes. Between May 2009 and May 2011, 450 consecutive patients with a mean age of 80 ± 6 years were enrolled. Comorbidity was high (mean Charlson index, 3.4 ± 2.9). Despite being nondependent, 118 (26%) had minor disability for basic activities of daily living, only 76 (16.2%) had no difficulty in walking 400 meters, and 340 (75.5%) were living alone or with another elderly person. In addition, 316 patients (70.2%) fulfilled frailty criteria. Even nondependent older patients hospitalized for HF show a high prevalence of clinical and nonclinical factors that may influence prognosis and are usually not considered in routine clinical practice. The results of FRAIL‐HF will provide important information about the relationship between these factors and different postdischarge clinical, functional, and quality‐of‐life outcomes.
Introduction
Heart failure (HF) is the leading cause of hospitalization in the elderly,1, 2 is associated with a significant mortality during and after admission, and is associated with a high readmission rate.3, 4 Hospitalization is, in addition, the main cause of new disability in the older population.5
The clinical picture of HF in the older hospitalized patient is complex. In addition to the direct causes and consequences of HF and its treatment, a constellation of other conditions or situations influences the development, clinical evolution, and prognosis of the disease. These include comorbidities, acute concomitant diseases, functional consequences of hospitalization, and polypharmacy. A number of cardiac and noncardiac factors have been identified as prognostic markers in patients with HF,6, 7, 8, 9, 10 but other factors frequent in the elderly, such as geriatric syndromes (eg, frailty, cognitive impairment, depression, mobility impairment), seem to be gaining importance in explaining the prognosis of these patients.11, 12, 13, 14, 15 Moreover, the quality of postdischarge care in the elderly (ie, ability of the patients for self‐care and social support), something that has not been formally evaluated in patients with HF, may play an important role in the course of the disease.
We hypothesize that some factors not routinely evaluated in the elderly, such as the presence of geriatric syndromes (frailty more prominently), the ability for self‐care related to the treatment of HF, and the presence of social support after discharge, may be of key importance in the clinical (ie, readmissions) and functional (ie, new disability) outcomes of older patients with HF. Therefore, a comprehensive clinical and geriatric analysis of the hospital and postdischarge phases in older patients with HF is needed to untangle these multiple and complex relationships.
The FRAIL‐HF study was designed to assess the additive influence of coexisting diseases and geriatric conditions on short‐term and long‐term clinical and functional outcomes in elderly patients hospitalized for HF, and to determine whether the influence of these factors is mediated by the manifestation of the phenotype of frailty, the underuse of indicated treatments, or an impairment in the ability of patients for self‐care secondary to the cumulative deficits. This article outlines the rationale, methods, and baseline results of the study.
Methods
Study Design and Objectives
FRAIL‐HF is a prospective observational cohort study comprising consecutive elderly patients hospitalized for HF in a large‐volume academic center with a 1‐year follow‐up after index admission. The main objectives of the study are (1) to describe the characteristics of elderly patients hospitalized for HF, including a comprehensive geriatric assessment; (2) to determine the role of some factors not usually evaluated in routine clinical practice, such as frailty and other geriatric conditions, or the coexistence of acute diseases, on HF prognosis; (3) to evaluate the real ability for HF self‐care using a new specific scale of observed performance in essential care tasks; and (4) to explore the interaction between frailty and treatment prescription or frailty and ability for self‐care as determinants of prognosis and potential goals for intervention after HF hospitalization.
Patient Enrollment
All consecutive patients age ≥70 years admitted to the department of cardiology, department of geriatric medicine, and one of the 3 services of the department of internal medicine of Gregorio Marañón General Hospital, a large university hospital in Madrid, with an admission diagnosis of HF during the study period were evaluated.
Inclusion and Exclusion Criteria
Heart failure diagnosis was confirmed by the presence of ≥1 of the following symptoms: shortness of breath, orthopnea, paroxysmal nocturnal dyspnea, or confusion, and one of the following signs: pulmonary rales, leg edemas, gallop rhythm, respiratory frequency >24, hypotension, radiologic signs of HF, and the prescription of new treatment for HF or the increase in the dosing of previous medications used for treating HF as documented in the emergency room report.
Patients were not eligible to participate in the study if any of the following criteria were present: (1) inability to perform independently ≥3 basic activities of daily living (ADLs) among the 6 tested (bathing, dressing, transferring from a chair, using the toilet, feeding, and grooming) before index admission; (2) transfer in from a nursing home or from another hospital; and (3) presence of moderate to severe dementia (defined as Mini Mental State examination [MMS] score ≤15).16 Patients with severe dependence or severe dementia were excluded because these are irreversible conditions and independently associated with very poor prognosis.
All patients with inclusion criteria underwent a mental evaluation with the Confusion Assessment Method.17 If the patient had confusion or cognitive impairment that limited communication, a proxy was interrogated to check exclusion criteria. Patients who finally met eligibility criteria were approached by a physician or research nurse, who provided them with a verbal explanation of the study. If the patient desired to proceed, the researcher obtained written consent from the patient. When needed, a proxy was asked to sign the informed consent.
Data Collection
Baseline data were collected by trained physicians or research nurses during index admission.
Sociodemographic Variables
Recorded were patient age, place of residence, educational and economic level, health literacy (using the Rapid Estimate of Adult Literacy in Medicine [REALM] test, reduced version),18 and social and emotional support (using the Duke‐UNC Functional Social Support Questionnaire).19 The Duke‐UNC questionnaire measures the individual's perception of the amount and type of personal social support. It includes 11 items, with responses ranging from 1 (“much less than I would like”) to 5 (“as much as I would like”). The addition of points is the total score.
Clinical Variables
These included chronic comorbidities (a list of predefined comorbidities and the Charlson Comorbidity Index20), HF characteristics, New York Heart Association functional class prior to admission, laboratory and echocardiography parameters, and medical treatment at discharge.
Geriatric Conditions
Functional status was evaluated as the independence to perform 6 basic ADLs—bathing, dressing, transferring, toileting, continence, and feeding21—2 weeks before index admission. These were obtained by interviewing the patient or a proxy if needed. Each item is scored “1” for complete independence and “0” when personal assistance is needed. Mobility was examined using a scale that includes 4 components: ability to walk inside, ability to walk one‐quarter mile, ability to walk up a flight of stairs, and average of time (in hours) walked per day. Possible scores range from 0 to 8, with 8 being the maximum mobility disability.22 To evaluate balance, we used the balance item of the Short Physical Performance Battery.23 Cognitive impairment was evaluated using the validated Spanish version of the MMS examination, a test that scores from 0 (worst) to 35 (normal).16 We also used the clock test that adds information about executive function, with a score from 0 (worse) to 10 (best).24 The presence of depression was evaluated by the Yesavage geriatric depression scale, a 15‐item scale with 15 points as the maximum score and a score of >9 as an indication of the presence of established depression.25 Sensorial impairments were evaluated using the “whispering test” for hearing26 and the Snellen test for visual acuity.27
Frailty
Frailty was assessed using the Cardiovascular Health Study frailty definition.27 Patients were considered frail if they met ≥3 of the following criteria: physical exhaustion, slowness, low physical activity, unintentional weight loss, and weak grip strength. Physical exhaustion was assessed according to self‐report using the question “How often in the last week did you feel that everything you did was an effort or you could not get going?” The answer of >3 days or most of the time was considered positive. Slow walking speed was considered if the time to walk 4.6 m was in the lowest 10% of the sex‐ and height‐adjusted time in the population28 and measured after clinical stabilization. Unintentional weight loss was considered if there was an affirmative answer to the question “In the past year did you lose more than 5% of your regular weight or more than 5 kilograms unintentionally?” Low physical activity was evaluated with the short version of the Minnesota Leisure Time Physical Activity Questionnaire,29 where <2.5 hours per week of any of described activities is considered poor. Grip strength was measured using a hand dynamometer in kilograms of force (Jamar; Patterson Medical, Bolingbrook, IL) and was considered weak if the average of 3 measures was in the lowest 20% of the sex‐adjusted and body mass index–adjusted community‐dwelling older adults.28
Concomitant Acute Diseases
The medical record of the entire admission was reviewed, searching for a predefined list of acute diseases. Concomitant acute or chronic exacerbated noncardiac diseases present at any moment during hospitalization were considered. The list included acute renal failure or chronic renal failure exacerbation (serum creatinine >1.5 mg/dL previously unknown or an increase of 0.3 mg/dL over the usual creatinine level); pneumonia (defined by clinical symptoms and radiological imaging); respiratory infection (respiratory symptoms with signs of infection without radiological image of pneumonia); exacerbation of chronic obstructive pulmonary disease (increase in bronchodilator treatment during hospitalization in patients with previous diagnosis of COPD); urinary tract infection (urinary symptoms plus abnormal urine analysis or positive urine culture); and other infections (any other infection that required antibiotics).
Heart Failure Self‐Care Ability
We evaluated the ability to perform 6 essential tasks for correct self‐care in HF with the following tests: (1) to stand up on a scale without help for the time needed to have a stable measure of weight; (2) to read and write correctly one's own weight as measured in the previous test; (3) to identify the prescribed diuretic drug pills from the drug boxes of the patient's regular treatments for HF; (4) to identify a number of highly salted foods that should be avoided from a short list, which included cheese, cured ham, snacks, olives, boiled rice, apples, and canned food; (5) to explore one's own ankles and identify the presence or not of edemas; and (6) to adjust the prescribed dose of diuretic treatment according to a simple rule based on weight changes. Each one of the tasks correctly performed was scored as “1,” and if the patient was unable or needed help to perform it, the score was “0.” Questions are simple, have theoretical consistence, and can be easily reproduced.
The European Heart Failure Self‐Care Behaviour Scale (EHFScBS) was also recorded.30 This scale is a questionnaire with 12 questions in these areas: weight control, HF symptoms and signs identification, low‐salt diet, correct treatment, exercise, and influenza vaccination. The range of the scale is from 12 to 60, where lower scores indicate better self‐care. All the patient assessments were done after clinical stabilization on a day close to discharge by a physician involved in the study.
Outcomes
Clinical and functional outcomes including mortality, readmission, functional decline, the need for new social help, and quality of life were evaluated. The primary outcome of the study is the occurrence of death or readmission at 6 months of follow‐up. Secondary outcomes are functional decline, quality of life, and use of social resources at different stages of follow‐up.
All patients underwent telephone interviews at 1, 3, 6, and 12 months after discharge. The patient, or a caregiver if needed, was asked about vital status, the occurrence of any readmissions and their causes, the ability to perform independently the 6 ADLs previously described, mobility ability, and the need for new personal help for daily living. Medical records were reviewed to check causes of mortality and readmission during follow‐up.
Readmission was defined as any unplanned hospitalization during follow‐up after index discharge. Recorded were the mode of readmission (urgent or not) and the primary reason for hospitalization, grouped in the following conditions: HF, infection, anemia or bleeding, renal failure or electrolyte imbalance, and procedures and surgery. Visits to the emergency room during follow‐up were also registered. Only the first readmission was considered for analyses.
Functional decline was defined as the loss of ≥1 point in the ADL total score at any point during follow‐up with respect to preadmission status (baseline ADL).
Need for new social help was defined as the need for institutionalization or increase in personal support for the performance of ADLs as compared with the situation before admission.
Quality of life was measured at each stage of follow‐up using the Short Form (SF)‐12 questionnaire.31 SF‐12 physical and mental summaries were calculated.
Statistical Analysis
For descriptive analysis, baseline characteristics are presented as frequency (percent) for categorical variables, mean ± SD for normally distributed continuous variables, and median (interquartile range) for continuous variables with skewed distribution. The rate of patients with acute diseases coexisting with HF, the rate of different geriatric syndromes, and the description of self‐care ability will be presented.
For the primary endpoint analysis, relative risks and odds ratios with their 95% confidence intervals will be calculated using generalized linear models that include frailty, other geriatric conditions, presence of acute diseases, and self‐care ability as predictors. The secondary endpoints will be analyzed in the same way.
Additionally, covariate adjusted analysis by logistic regression controlling for age, sex, different factors of HF severity (N‐terminal pro‐brain natriuretic peptide [NT‐proBNP], left ventricular ejection fraction [LVEF]), and comorbidity will be performed to test the independent influence of frailty and other geriatric conditions on main outcomes.
The χ2 test and Mann‐Whitney U test will be used for comparative purposes. Interaction between frailty and use of disease‐modifying treatments, frailty and self‐care ability, and frailty and social support will be formally tested.
Sample size was calculated upon the assumption that the prevalence of frailty would be about 60% to 65% of patients and the rate for the primary endpoint of death or hospitalization 45%. To find a significant difference between frail and nonfrail groups and assuming a 2‐sided α error of 0.05 and 80% power (β error 20%), a sample size of 354 patients will be necessary. Dropouts and losses were estimated to be 15% over the duration of the trial. An addition of 10% was done to allow for other analyses, so a total of 450 patients were included.
Including 450 patients, we estimate 180 events to occur. According to the rule of 10, multivariate regression models involving more than 15 variables may be used for the analysis.
Results
Between May 2009 and May 2011, 1187 admissions for HF in the services of cardiology, internal medicine, and geriatrics were evaluated. We used administrative data searching for HF as the primary diagnosis in the referred services and including only 1 hospitalization per patient. During the study period, 952 patients were approached; 85 of them did not fulfill HF inclusion criteria and 417 were excluded, as shown in Figure 1. The main reason for exclusion was the coexistence of severe dependency for basic ADLs.
Figure 1.
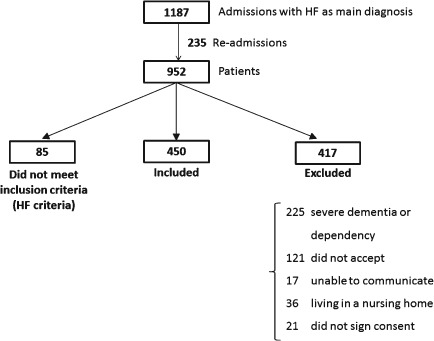
FRAIL‐HF recruitment profile.
Finally, 450 patients were enrolled, 311 in the department of cardiology, 78 in internal medicine, and 61 in geriatrics. The mean age of enrolled patients was 80 ± 6 years; 49.6% of them were female. The comorbidity burden was high; 276 (58.4%) of the patients had a Charlson index ≥3, 284 (63.1%) had other coexistent acute disease during admission, and 274 (61%) had previous hospitalizations for HF. Patient demographics, risk factors, chronic conditions, and HF characteristics are summarized in Table 1.
Table 1.
Baseline and HF Characteristics by Admission Department
| Total | Cardiology | Internal Medicine | Geriatrics | |
|---|---|---|---|---|
| No. of patients (%) | 450 (100) | 311 (69.1) | 78 (17.3) | 61 (13.6) |
| Age, y, mean ± SD | 80.1 ± 6.1 | 78.6 ± 5.2 | 80.2 ± 5.4 | 87.3 ± 5.7 |
| Female sex, n (%) | 223 (49.6) | 152 (48.7) | 34 (44.2) | 37 (60.7) |
| Risk factors, n (%) | ||||
| DM | 152 (34) | 111 (35.4) | 22 (28.1) | 19 (31.1) |
| Hypertension | 392 (87.7) | 262 (84.8) | 73 (93.6) | 57 (95) |
| Dyslipidemia | 238 (53.6) | 179 (58.5) | 35 (49.4) | 24 (40) |
| Smoking | 96 (21.5) | 66 (21.3) | 21 (26.9) | 9 (15.3) |
| Charlson index, mean ± SD | 3.4 ± 2.9 | 3.4 ± 2.4 | 3.3 ± 1.9 | 3.00 ± 1.9 |
| COPD, n (%) | 101 (22.4) | 58 (18.7) | 26 (33.3) | 17 (27.9) |
| AF, n (%) | 240 (53.3) | 165 (53.1) | 44 (56.4) | 31 (50.8) |
| Chronic renal failure, n (%) | 135 (30) | 92 (29.6) | 24 (30.7) | 17 (27.9) |
| Anemia, n (%) | 233 (51.8) | 156 (50.2) | 45 (57.7) | 32 (52.5) |
| Regular use of NSAID, n (%) | 78 (17.3) | 53 (17.2) | 17 (23) | 8 (14) |
| Previous cardiovascular diseases, n (%) | ||||
| MI | 166 (37.1) | 128 (41.3) | 27 (34.6) | 11 (18.6) |
| PAD | 180 (40.4) | 129 (41.7) | 25 (32.1) | 26 (44.1) |
| Stroke | 44 (9.8) | 33 (10.6) | 7 (9) | 4 (6.7) |
| Previous diagnosis of HF, n (%) | 331 (73.9) | 225 (72.6) | 61 (78.2) | 45 (75) |
| HF etiology, n (%) | ||||
| Hypertensive | 118 (26.2) | 66 (21.1) | 25 (32.0) | 27 (44.2) |
| Ischemic | 155 (34.4) | 114 (36.7) | 29 (37.2) | 12 (19.7) |
| Valvular | 106 (23.7) | 82 (26.4) | 12 (15.4) | 12 (19.7) |
| Unknown | 71 (15.7) | 49 (15.8) | 12 (15.4) | 10 (16.4) |
| Preadmission NYHA class, n (%) | ||||
| I | 88 (19.6) | 69 (22.3) | 13 (16.6) | 6 (9.8) |
| II | 241 (53.6) | 158 (50.8) | 42 (54.5) | 42 (62.7) |
| III | 113 (25.1) | 80 (25.8) | 3 (24.7) | 13 (21.3) |
| IV | 2 (0.4) | 2 (0.64) | 0 | 0 |
| SBP, mm Hg, mean ± SD | 140 ± 29 | 139 ± 30 | 144 ± 28 | 138 ± 28 |
| DBP, mm Hg, mean ± SD | 75.1 ± 17 | 75.1 ± 17 | 75.3 ± 15 | 74.3 ± 18 |
| LVEF, n (%) | ||||
| <30% | 116 (27.6) | 96 (32.7) | 12 (17.1) | 8 (14.3) |
| 30%–50% | 90 (21.4) | 65 (22.1) | 17 (24.3) | 8 (14.3) |
| >50% | 214 (51) | 133 (45.2) | 41 (58.6) | 40 (71.8) |
Abbreviations: AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSAID, nonsteroidal anti‐inflammatory drug; NYHA, New York Heart Association; PAD, peripheral arterial disease; SBP, systolic blood pressure; SD, standard deviation.
The description of the geriatric assessment are shown in Table 2. Most patients (75.5%) were living alone or with another elderly person, even though they reported, on average, a good social‐emotional support level. The cultural level was not high; 68% abandoned school at 12 years of age or younger, and only 5% had university studies. In addition, 57.8% of patients had a low level of health literacy.
Table 2.
Results of Geriatric Assessment by Admission Department
| Total | Cardiology | Internal Medicine | Geriatrics | |
|---|---|---|---|---|
| Married, n (%) | 293 (65.1) | 209 (67.2) | 54 (69.2) | 30 (49.2) |
| Living status, n (%) | ||||
| Alone | 96 (21.3) | 65 (20.9) | 15 (19.2) | 16 (26.2) |
| With an elderly person | 244 (54.2) | 179 (57.6) | 42 (53.8) | 23 (37.7) |
| With young family | 80 (17.8) | 52 (16.7) | 18 (23.1) | 10 (16.4) |
| With a caregiver | 19 (4.2) | 11 (3.5) | 2 (2.6) | 6 (9.8) |
| DUKE‐UNC Scale, mean ± SDa | 42.2 ± 8.4 | 42.4 ± 8.3 | 42.2 ± 8.3 | 41.5 ± 9 |
| Independence for 6 ADL, n (%) | 332 (73.8) | 241 (77.5) | 56 (71.8) | 35 (57.4) |
| MMS <24, n (%) | 89 (19.8) | 57 (18.3) | 14 (18.2) | 18 (29.5) |
| Mobility scale, mean ± SDb | 4.3 ± 2.4 | 4.07 ± 2.4 | 4.6 ± 2.3 | 5.1 ± 2.6 |
| No difficulty walking at home, n (%) | 191 (42.4) | 141 (45.3) | 28 (35.9) | 22 (36.1) |
| No difficulty walking 400 m, n (%) | 73 (16.2) | 57 (18.3) | 8 (10.3) | 8 (13.1) |
| Depression, n (%) | 61 (13.6) | 38 (12.2) | 13 (16.7) | 10 (16.4) |
| REALM‐Rc | 5.59 ± 2.2 | 5.62 ± 2.2 | 5.56 ± 2.3 | 5.45 ± 2.3 |
| Frailty, n (%) | 316 (70.2) | 210 (67.4) | 57 (73.1) | 49 (80.3) |
| EHFScBS, mean ± SDd | 29.35 ± 5.5 | 29.54 ± 5.2 | 29.31 ± 5.3 | 28.47 ± 5.2 |
Abbreviations: EHFScBS, European Heart Failure Self‐Care Behaviour Scale; MMS, Mini Mental State examination; REALM, Rapid Estimate of Adult Literacy in Medicine.
Scoring from 11 to 55; higher score reflects higher perceived social support.
Scoring from 0 (better mobility) to 8 (worse mobility); the scale includes mobility at home, ability to walk one‐quarter mile and to walk up stairs, and time walked daily.
REALM‐reduced scale, scoring from 0 (worst) to 8 (best) health literacy.
EHFScBS possible scores range from 12 to 60; lower scores indicate better self‐care.
An important number of patients (70.2%) showed a frailty phenotype associated with HF, but frailty was not associated with poorer left ventricular function; among frail patients, 51% had preserved LVEF, and 46.9% among the nonfrail had preserved LVEF. Nor was frailty associated with higher levels of NT‐proBNP at admission.
Gait speed at discharge was available in 284 patients; 140 were unable to walk the 4.5 m required, without help. Mean gait speed was 0.61 ± 0.25 m/sec.
As major disability was an exclusion criterion, most patients were fully independent; only 26% of included patients had a minor disability at baseline. Both conditions, frailty and disability, were more frequently found among patients admitted to the geriatrics department.
Discussion
FRAIL‐HF is one of the first and most comprehensive studies to evaluate the heterogeneity of elderly patients hospitalized for HF with a significant follow‐up after discharge with sequential evaluation of several clinical and nonclinical outcomes. The study will assess not only classic clinical outcomes, such as mortality and readmission, but also the evolution of functional outcomes (ie, disability), quality of life, and use of social resources in such difficult patients.
Older patients hospitalized for HF present complex clinical pictures that challenge the delivery of simple general recommendations for diagnosis, early treatment, and chronic management. Given its complexity, HF has been considered a geriatric syndrome per se.32 Patients not only present with the typical symptoms and signs of HF, but frequently they show other clinical conditions, chronic and acute, that interact with or modify the course of HF. Acutely decompensated chronic illnesses as well as acute de novo diseases may trigger HF, or may be triggered by HF, complicating diagnosis and the decision for early treatment. Chronic geriatric syndromes (eg, cognitive impairment, lack of mobility, falls) may add more complexity to the clinical course. Heart failure in the elderly is characterized by a clinically challenging course during hospitalization, with frequent cardiac and noncardiac complications.33 In addition, specific geriatric complications, such as delirium or loss of functionality, are frequent and have an important impact on patient well‐being, use of resources, and potentially on outcomes. Moreover, syndromes such as frailty, cognitive impairment, depression, and mobility impairment seem to be gaining importance in explaining the prognosis of these patients.11, 12, 13 However, although the individual effect of these conditions has been evaluated separately, the interaction that may be occurring between cardiac conditions, noncardiac diseases, and geriatric syndromes, and their effects on outcomes and prognosis after HF hospitalization, has not been studied. The importance of quality of postdischarge care in elderly patients with HF also needs to be better understood. This means considering not only evidence‐based treatments and medical care (ie, continuity of care) but also other important aspects, such as the ability of patients for self‐care related to HF treatment or the impact that social support may have to help patients overcome these potential limitations. These factors may play an important role in the course of the disease but have not been formally evaluated in patients with HF (Figure 2).
Figure 2.
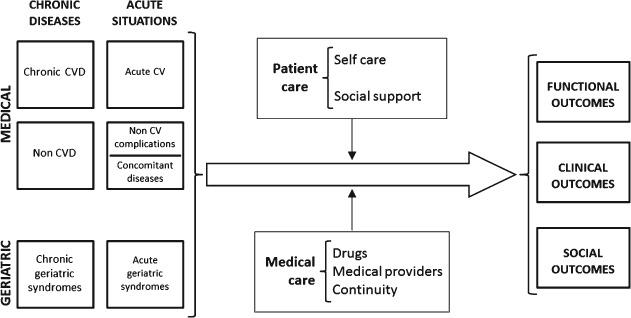
Conceptual framework used by FRAIL‐HF to describe the complex relationship between cardiovascular diseases, other medical conditions and geriatric syndromes (chronic and acute) in the acute phase, the different components of postdischarge care, and potential outcomes in the study of older patients with heart failure. Abbreviations: CV, cardiovascular; CVD, cardiovascular diseases.
Even after establishing a relatively low age cutoff for enrollment and having excluded patients with dementia and important dependence, our study groups comprise a population of very advanced age with a high comorbidity index and a high prevalence of geriatric syndromes, especially frailty. Despite these features, many patients were living at home, either alone or with another elderly person. This seems to be a good representation of the majority of patients currently admitted to hospitals for HF. Interestingly, these characteristics have been presumed to be responsible for the known gap between the proven benefits of different drugs in clinical trials and the still‐poor prognosis after hospitalization in the usual clinical practice of HF patients.34
Due to their poor prognosis and the frequent use of health resources after hospitalization, many interventions and disease‐management programs have been developed for HF patients. These interventions are viewed as means to increase the use of evidence‐based therapies and improve patient education on HF, ultimately to improve outcomes. Nevertheless, the association between HF management programs and improved outcomes is inconsistent among studies,35 and, although a number of them have shown significant reductions in readmission rates,36, 37, 38, 39, 40, 41, 42, 43 none of them improve survival, functional capacity, or quality of life.44 Most of these programs are probably not designed to understand and manage the complexity of patients with coexistent geriatric syndromes and who suffer the harms of hospitalization that the present study will describe.
Our initial results already point out some of the particularities of this population. Despite being a nondependent cohort of patients, a significant number showed limitations in walking or fulfilled criteria of frailty. In fact, the prevalence of frailty found in our cohort, 70.2%, is very high for such a selected population of older independent patients. Frailty is an age‐associated biological syndrome characterized by a decline in overall function or biological reserve and response to situations that require rapid adaptive responses, resulting from the deterioration of multiple physiological systems. Frailty frequently coexists with cardiovascular diseases.45, 46 Other studies have described that frailty is associated with increased mortality and health care utilization among community patients with HF,12, 47, 48 but its influence in prognosis after HF hospitalization is less well known.
Self‐care is essential in patients with HF, and different instruments have been used for its assessment, most of them by asking the patient about behaviors related to HF care. The FRAIL‐HF study, in contrast, will prospectively evaluate the real ability to perform the essential tasks for self‐care in these patients, an innovative approach in HF studies. Also, the study of potential interaction between frailty (more related to physical function) and self‐care ability (more related to education and health literacy) will be prospectively evaluated, as this may have implications for the design of suitable interventions for these patients.
The large proportion of patients with HF approached but not enrolled in the study deserves a comment. Most of these patients were admitted in the wards of internal medicine and geriatrics services and were excluded due to the presence of dementia or severe dependency. Although this might be considered a limitation, we decided to restrict the inclusion of these patients because severe dementia or dependency are irreversible factors that are associated with poor prognosis, independently of the underlying disease. Lastly, our aim is to provide useful information for future potential interventions to improve the care of complex older patients with HF.
Conclusion
The results of FRAIL‐HF will provide important prospective information about elderly patients admitted to hospital for HF, their clinical outcomes, and functionality and quality of life evolution after discharge. Designing and assessing the effectiveness of interventions in older patients with HF is challenging and requires the knowledge of multiple factors besides the cardiovascular signs and symptoms traditionally studied. More global and integrated care for these patients is indeed needed.
Acknowledgments
The authors thank Cristina González, MD; Raquel Barrera, MD; Fabricio Flores, MD; Guillermo Ferreira, MD; and Guadalupe Mendieta, MD, for their collaboration in data collection for this study.
This study was funded with a grant from the Spanish Ministry of Economy and Competitiveness (Carlos III Health Institute, Fund for Health Research [Fondo de Investigación Sanitaria {FIS}] 08/1461).
Dr. Vidán, Dr. Fernández‐Avilés, and Dr. Ortiz have no financial disclosures. Dr. Sánchez has received a Spanish government research grant through the Fund for Health Research (Fondo de Investigación Sanitaria [FIS]), Contrato Río Hortega, CM07/00202, Carlos III Health Institute. Dr. Serra has received lecture fees from Lilly, Abbott and Nestlé. Dr. Bueno has received advisory/consulting fees from AstraZeneca, Bayer, Bristol‐Myers Squibb, Daiichi‐Sankyo, Eli Lilly, Novartis, Servier, and Pfizer, and a research grant from AstraZeneca.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Rodríguez‐Artalejo F, Guallar‐Castillón P, Banegas Banegas JR, et al. Trends in hospitalization and mortality for heart failure in Spain, 1980–1993. Eur Heart J. 1997;18:1771–1779. [DOI] [PubMed] [Google Scholar]
- 2. Fang J, Mensah JA, Croft JB, et al. Heart failure‐related hospitalization in the US, 1979–2004. J Am Coll Cardiol. 2008;52:428–434. [DOI] [PubMed] [Google Scholar]
- 3. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gill TM, Allore HG, Holford TR, et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. [DOI] [PubMed] [Google Scholar]
- 6. Fonarow GC, Abraham WT, Albert NM, et al; OPTIMIZE‐HF Investigators and Hospitals . Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE‐HF. Arch Intern Med. 2008;168:847–854. [DOI] [PubMed] [Google Scholar]
- 7. Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. [DOI] [PubMed] [Google Scholar]
- 8. Kerzner R, Gage BF, Freedland KE, et al. Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction. Am Heart J. 2003;146:286–290. [DOI] [PubMed] [Google Scholar]
- 9. Kosiborod M, Smith GL, Radford MJ, et al. The prognostic importance of anemia in patients with heart failure. Am J Med. 2003;114:112–119. [DOI] [PubMed] [Google Scholar]
- 10. Hernández MB, Schwartz RS, Asher CR, et al. Predictors of 30‐day readmission in patients hospitalized with decompensated heart failure. Clin Cardiol. 2013;36:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sánchez E, Vidán MT, Serra JA, et al. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart. 2011;97:1602–1606. [DOI] [PubMed] [Google Scholar]
- 12. Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. [DOI] [PubMed] [Google Scholar]
- 13. Chaudhry SI, Wang Y, Gill TM, et al. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pilotto A, Addante F, Franceschi M, et al. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short‐term mortality in older patients with heart failure. Circ Heart Fail. 2010;3:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodríguez‐Pascual C, Vilches‐Moraga A, Paredes‐Galán E. Comprehensive geriatric assessment and hospital mortality among older adults with decompensated heart failure. Am Heart J. 2012;164:756–762. [DOI] [PubMed] [Google Scholar]
- 16. Lobo A, Saz P, Marcos G, et al. Revalidation and standardization of the cognition mini‐exam (first Spanish version of the Mini‐Mental Status Examination) in the general geriatric population [article in Spanish; published correction appears in Med Clin (Barc). 1999;113:197]. Med Clin (Barc). 1999;112:767–774. [PubMed] [Google Scholar]
- 17. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. [DOI] [PubMed] [Google Scholar]
- 18. Bass PF 3rd, Wilson JF, Griffith CH. A shortened instrument for literacy screening. J Gen Intern Med. 2003;18:1036–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Broadhead WE, Gehlbach SH, de Gruy FV, et al. The Duke‐UNC Functional Social Support Questionnaire: measurement of social support in family medicine patients. Med Care. 1988;26:709–723. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 21. Katz S, Ford AB, Moskowitz RW, et al. Studies of Illness in the Aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 22. Gill TM, Guo Z, Allore HG. Subtypes of disability in older persons over the course of nearly 8 years. J Am Geriatr Soc. 2008;56:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 24. Manos PJ, Wu R. The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients. Int J Psychiatry Med. 1994;24:229–244. [DOI] [PubMed] [Google Scholar]
- 25. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17:37–49. [DOI] [PubMed] [Google Scholar]
- 26. Macphee GJ, Crowther JA, McAlpine CH. A simple screening test for hearing impairment in elderly patients. Age Ageing. 1988;17:347–351. [DOI] [PubMed] [Google Scholar]
- 27. Sue S. Test distance vision using a Snellen chart. Community Eye Health. 2007;20:52. [PMC free article] [PubMed] [Google Scholar]
- 28. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 29. Taylor HL, Jacobs DR, Schuker B, et al. A questionnaire for the assessment of leisure‐time physical activities. J Chronic Dis. 1978;31:745–755. [DOI] [PubMed] [Google Scholar]
- 30. Jaarsma T, Strömberg A, Mårtensson J, et al. Development and testing of the European Heart Failure Self‐Care Behaviour Scale. Eur J Heart Fail. 2003;5:363–370. [DOI] [PubMed] [Google Scholar]
- 31. Ware J, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 32. Rich MW. Heart failure in the 21st century: a cardiogeriatric syndrome. J Gerontol A Biol Sci Med Sci. 2001;56:M88–M96. [DOI] [PubMed] [Google Scholar]
- 33. Stein GY, Kremer A, Shochat T, et al. The diversity of heart failure in a hospitalized population: the role of age. J Cardiac Fail. 2012;16:645–653. [DOI] [PubMed] [Google Scholar]
- 34. Masoudi FA, Havranek EP, Wolfe P, et al. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J. 2003;146:250–257. [DOI] [PubMed] [Google Scholar]
- 35. Gonseth J, Guallar‐Castillón P, Banegas JR, et al. The effectiveness of disease management programs in reducing hospital re‐admission in older patients with heart failure: a systematic review and meta‐analysis of published reports. Eur Heart J. 2004;25:1570–1595. [DOI] [PubMed] [Google Scholar]
- 36. Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. [DOI] [PubMed] [Google Scholar]
- 37. Stewart S, Vandenbroek AJ, Pearson S, et al. Prolonged beneficial effects of a home‐based intervention on unplanned readmissions and mortality among patients with congestive heart failure. Arch Intern Med. 1999;159:257–261. [DOI] [PubMed] [Google Scholar]
- 38. Jaarsma T, Halfens R, Huijer Abu‐Saad H, et al. Effects of education and support on self‐care and resource utilization in patients with heart failure. Eur Heart J. 1999;20:673–682. [DOI] [PubMed] [Google Scholar]
- 39. Hughes SL, Weaver FM, Giobbie‐Hurder A, et al; for the Department of Veterans Affairs Cooperative Study Group on Home‐Based Primary Care . Effectiveness of team‐managed home‐based primary care: a randomized multicenter trial. JAMA. 2000;284:2877–2885. [DOI] [PubMed] [Google Scholar]
- 40. Blue L, Lang E, McMurray JJ, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riegel B, Carlson B, Kopp Z, et al. Effect of a standardized nurse case‐management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162:705–712. [DOI] [PubMed] [Google Scholar]
- 42. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83–89. [DOI] [PubMed] [Google Scholar]
- 43. Whellan DJ, Hasselblad V, Peterson E, et al. Meta‐analysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149:722–729. [DOI] [PubMed] [Google Scholar]
- 44. Jovicic A, Holroyd‐Leduc JM, Straus SE. Effects of self‐management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fried L, Kronmal RA, Newman AB, et al. Risk factors for 5‐year mortality in older adults. The Cardiovascular Health Study. JAMA. 1998;279:585–592. [DOI] [PubMed] [Google Scholar]
- 46. Ariza‐Solé A, Formiga F, Vidán MT, et al. Impact of frailty and functional status on outcomes in elderly patients with ST‐segment elevation myocardial infarction undergoing primary angioplasty: rationale and design of the IFFANIAM study. Clin Cardiol. 2013;36:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long‐term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. [DOI] [PubMed] [Google Scholar]
- 48. McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]


