Abstract
The diagnosis of American tegumentary leishmaniasis (ATL) is based on the visualization or isolation of the parasite, which is a time‐consuming and poorly sensitive method. In this study, we evaluated the accuracy and reliability of ELISA for the diagnosis of ATL using soluble (SF) and membrane‐enriched (MF) antigen fractions obtained from an infectious strain of Leishmania (Viannia) braziliensis. A total of 152 serum samples investigated at a referral center in Rio de Janeiro, Brazil, between 2005 and 2007 were studied. Each sample was tested twice with each fraction for the calculation of reliability (intraclass coefficient (ICC)). Cut‐off values of 0.22 (SF) and 0.33 (MF) were defined. The use of the fractions resulted in good discrimination between patients, with a large area under the curve (P<0.0001), but no difference was observed between the two fractions (P=0.45). Sensitivity was 89.5% for each fraction, specificity was 89.5% for SF and 93.4% for MF, and the positive likelihood ratio was 8.5 for SF and 13.6 for MF. The ICCs were excellent (SF: 0.96 and MF: 0.90). The antigens tested provided precision and accuracy for the diagnosis of ATL, with SF being recommended due to its lower cost and greater practicality. J. Clin. Lab. Anal. 24:289–294, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: ELISA, Leishmania (Viannia) braziliensis, reliability and validity, validation studies [type of studies], diagnostic techniques and procedures
INTRODUCTION
Leishmaniasis represents a global public health problem, with an estimated 12 million people being infected worldwide. American tegumentary leishmaniasis (ATL) occurs in the Americas from the southern region of the United States to the north of Argentina. In Brazil, ATL is present in all states and affects individuals of both genders and all ages. Different parasite species and vectors are involved in the disease, a fact resulting in a wide clinical and epidemiological diversity 1. In the southeastern region of Brazil, ATL is mainly caused by L. (Viannia) braziliensis, which is responsible for the cutaneous and mucocutaneous forms. Over the last decades, this species has spread into nonforest periurban and urban areas, infecting humans, dogs, and horses 1.
The diagnosis of ATL is based on the visualization or isolation of the parasite 2. However, the low sensitivity and long duration of these tests have resulted in the need for more sensitive, specific, and rapid methods. Serological tests, such as indirect immunofluorescence 3, 4, and enzyme immunoassays, such as ELISA, dot‐ELISA and immunoblotting 5, 6, have been used for the diagnosis of ATL. However, the accuracy of these tests may vary depending on the infectious parasite species and antigen used 7, 8.
ELISA has been employed successfully for the diagnosis of ATL because it is a rapid test that permits automation and the use of crude, purified, and recombinant antigens isolated from a noninfectious strain. A sensitivity ranging from 95% 9 to 100% 10 was reported when partially soluble antigen fractions from a noninfectious strain of L. (Viannia) braziliensis were used. There is increasing interest in the identification and characterization of molecules with immunogenic properties that can be used to increase the sensitivity and specificity of serological tests. Molecules with an immunogenic potential identified by different investigators include gp 63 11, GBP 12, HSP 60 13, and T26‐U2 and T26‐U4 14. During its life cycle, Leishmania sp. expresses various membrane glycoconjugates that can be employed as antigens for immunodiagnosis 15, 16. However, few studies have tested antigens obtained from infectious strains in immunoassays 17. Although the expression of certain proteins by the parasite is increased during the infectious phase 18, the performance of serological tests using antigens obtained from infectious promastigotes has not been evaluated.
The objective of this study was to compare the accuracy of ELISA for the diagnosis of ATL using soluble and membrane‐enriched antigen fractions obtained from an infectious strain of L. (Viannia) braziliensis.
MATERIALS AND METHODS
Samples
In this diagnostic accuracy study, 152 serum samples from patients with a suspicion of ATL seen for diagnostic investigation at the Evandro Chagas Clinical Research Institute for infectious diseases (IPEC), FIOCRUZ, between 2005 and 2007 were tested. The first serum sample obtained from these subjects before diagnostic confirmation, which was diluted in glycerin (1:2) and stored in a freezer (−20°C), was used. These samples were selected randomly from a consecutive series of patients. In 76 subjects, the diagnosis of ATL (ATL group) was established by demonstration of the parasite (imprint, culture, or histopathology). The control group consisted of 76 patients in whom the differential diagnosis confirmed another disease and imprint, culture, and histopathology were negative for ATL.
Eighty‐five (55.9%) of the samples analyzed were obtained from women. Thirty‐four of these samples were allocated to the ATL group and fifty‐one to the control group, with a mean age of the patients of 39.1 years (SD: 21.7) and 42.6 years (SD: 20.2), respectively. Among samples from patients living in the municipality of Rio de Janeiro, 48.7% were allocated to the ATL group and 45.9% to the control group. With respect to samples with a positive Montenegro skin test, 92.4% belonged to the ATL group and 7.9% to the control group.
The study was approved by the Ethics Committee of IPEC/FIOCRUZ (process 0049.0.009.000‐7). All subjects agreed to participate in the study and signed a free informed consent form.
Calculation of the Sample Size
On the basis of previous studies from our group, the minimum sample size necessary in each group was 76 subjects considering an ELISA sensitivity of 95% for partially soluble antigen 9 and of 80% for the membrane‐enriched fraction (MF) (unpublished data), in a two‐tailed test with an α error of 0.05 and power of 80%.
A minimum sample size of 137 sera was estimated for the analysis of reliability to obtain an intraclass coefficient (ICC) of 0.95, confidence level of 95%, and absolute error of 0.03. For the determination of repeatability, the assays were conducted on the same 152 patients in a blind fashion, i.e., the diagnostic status of the samples was unknown and the samples were therefore codified. A single observer (a health professional with a Master's degree and 3 years of training) tested the samples on two distinct occasions.
Parasites and Antigens
As we did not find any studies in the literature quantifying metacyclic forms of L. (Viannia) braziliensis in the infectious phase, a protocol developed for L. amazonensis 19 was adapted as follows. Infectious promastigote forms of L. (Viannia) braziliensis (MHOM/BR/02/R.787) in the stationary phase of growth were used. This strain was chosen because it was isolated from a patient from Rio de Janeiro who presented a standard clinical and therapeutic response. The strain was identified by isoenzyme electrophoresis and cryopreserved after the second passage. For construction of the growth curve and isolation of antigens, the strain was removed from liquid nitrogen, inoculated into culture medium, and only used until the fifth passage because of the large amount of metacyclic forms with a high degree of infectivity.
For establishment of the stationary phase, a growth curve was constructed by culture of the parasite in Schneider's Drosophila medium (Sigma‐Aldrich Co., St. Louis, MO) supplemented with 10% fetal bovine serum, 200 μg/ml penicillin, 200 μg/ml streptomycin, and 1% human urine. Growth was initiated by inoculation of 1×106 promastigotes into 1 ml culture medium in 15 ml Falcon tubes and incubation at 26–28°C. Growth was estimated as the number of cells/ml at intervals of 24 hr. Cells were counted in triplicate in a Neubauer chamber using 0.2% Trypan blue in PBS. The best time for parasite harvest was determined by analysis of the growth curve (beginning of the stationary phase on day 4), when the culture was transferred for antigen processing 20. For this purpose, the culture was quantified and transferred to about 500 ml culture medium under the same conditions as used for construction of the growth curve.
Collection of Antigens
The whole culture was centrifuged (Eppendorf 5810R) at 5,000 rpm for 10 min at 4°C and processed according to the protocol described by Genestra et al. 21. The sediment was resuspended in 0.25 M sucrose and 5 mM KCl (KCl/sucrose buffer). This procedure was repeated three times. The resulting sediment was resuspended in antiproteolytic buffer (0.1 mM PMSF, 0.01% (w/v) leupeptin, 0.2 mg/ml trypsin inhibitor, and 1 mM benzamidine) to a final volume of 5 ml and parasites were ruptured using a cavitation pump at a pressure of 1,500 psi for 10 min. Next, the antigen was centrifuged at 105,000g for 30 min at 4°C in a Beckman Coulter ultracentrifuge (2583 series, rotor type 35). The resulting supernatant was called the soluble fraction (SF) and the pellet was called the MF. The pellet was resuspended in KCl/sucrose buffer containing 1% Triton X‐100 and was solubilized in a Dounce homogenizer. After this step, the material was analyzed under a light microscope to determine the presence of clots. Protein content of the antigen fractions was determined by the Folin–Lowry method 22.
ELISA
The plates were sensitized with 100 μl of the antigen extract (SF and MF) obtained from promastigote forms of L. (Viannia) braziliensis at concentrations previously defined by protein titration and diluted in carbonate‐bicarbonate buffer, pH 9.6 (50 μg/ml SF and 100 μg/ml MF), and then incubated overnight (18 hr) at 8°C. After four washes in PBS, pH 7.2, containing 0.05% Tween 20 (PBS‐T) in an automatic washer (Tecan Columbus Washer®), 100 μl of the serum samples diluted 1:40 in PBS‐T plus 1% skimmed milk (Molico; PBS‐TM) was added to each well and the plates were incubated for 45 min at 37°C. After four new washes, 100 μl peroxidase‐conjugated anti‐IgG (Sigma) diluted in PBS‐TM and previously titrated (1:50,000 in the two fractions) was added. After incubation for 45 min at 37°C and four new washes, 100 μl 3,3′,5,5′‐tetramethylbenzidine (Sigma) was added and the plates were incubated for 30 min at room temperature in the dark. The reaction was stopped by the addition of 50 μl 2 N H2SO4 to each well. Absorbance was determined at 450 nm in an automatic plate reader (Tecan Spectra Classic®).
Statistical Analysis
Cut‐off values were defined using ROC curves and the respective 95% confidence intervals (CI), and areas under the curve (AUCs) were compared. The sensitivity, specificity, positive and negative predictive values, and likelihood ratio of ELISA employing the different antigen fractions (SF and MF) and their respective 95% CI were evaluated. The reliability of the tests was analyzed using the ICC (95% CI) of two optical density readings per sample. The level of significance was set at P<0.05 for all tests. Analysis was performed using the MedCalc 9.4 statistical software (Mariakerke, Belgium).
RESULTS
Under the conditions established, the beginning of the stationary phase was observed on day 4 of growth, when the parasites were harvested for antigen processing.
Table 1 shows the accuracy and reliability of the ELISA tests according to the type of fraction used. Expressive and statistically significant AUCs were observed for the two fractions. Both fractions showed good sensitivity and specificity. The likelihood ratios and predictive values were also satisfactory. Positive tests were more frequent among LTA patients than among controls for both fractions. The following cut‐off values were established.
Table 1.
Accuracy and Reliability of an Enzyme Immunoassay (ELISA) for the Diagnosis of American Tegumentary Leishmaniasis Using Soluble and Membrane‐Enriched Fractions Obtained From Infectious L. (Viannia) braziliensis (N=152)
| Parameter | Soluble fraction | Enriched membrane fraction |
|---|---|---|
| Cutoff | 0.22 | 0.33 |
| AUCa | 0.918 (80.3–95.5) | 0.934 (88.2–96.8) |
| Sensitivity | 89.5 (80.6–95.3) | 89.5 (80.3–95.3) |
| Specificity | 89.5 (80.3–95.3) | 93.4 (85.3–97.8) |
| Positive preditive value | 89.5 (81.7–94.2) | 93.2 (85.8–96.9) |
| Negative preditive Value | 89.5 (81.8–94.2) | 89.9 (82.4–94.4) |
| LRb positive | 8.5 (4.4–16.5) | 13.6 (5.8–31.8) |
| LR negative | 0.12 (0.06–0.23) | 0.11(0.06–0.22) |
| ICCc | 0.96 (94.1–96.8) | 0.90 (85.9–92.3) |
aArea under the curve.
bLikelihood ratio.
cIntraclass correlation coefficient.
Comparison of the performance of the two fractions showed no relevant (0.016, SD: 0.02) or significant difference (P=0.45). As can be seen in Figure 1, the two curves overlap.
Figure 1.
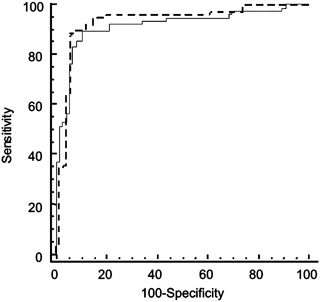
ROC curves of the soluble and membrane‐enriched fractions obtained from infectious L. (Viannia) braziliensis used for the diagnosis of American tegumentary leishmaniasis.
The ICCs obtained for the two fractions indicated excellent reliability of the tests, with values higher than 0.75 23. Then reliability (ICC) was excellent for both tests without an expressive variability between two readings of the same observer.
The distribution of optical density values obtained for sera of patients from the two groups tested by ELISA using the SF and MF antigen fractions is shown in Figures 2 and 3, respectively. Eight serum samples presented cross‐reactions when SF was used (three patients with lower limb ulcer, one with sporotrichosis, one with a neoplasm, one with pyodermitis, and two with other diagnoses). Five sera presented cross‐reactions when MF was used as antigen (three patients with lower limb ulcer, one with pyodermitis, and one with sporotrichosis).
Figure 2.
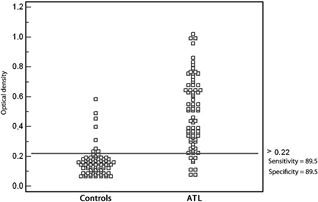
Distribution of optical density values, cut‐off value, sensitivity and specificity of the soluble fraction obtained from an infectious strain of L. (Viannia) braziliensis.
Figure 3.
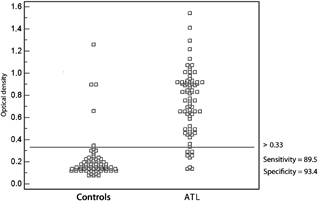
Distribution of optical density values, cut‐off value, sensitivity and specificity of the membrane‐enriched fraction obtained from an infectious strain of L. (Viannia) braziliensis.
DISCUSSION
In this study, ELISA employing antigen fractions (SF and MF) obtained from infectious L. (Viannia) braziliensis was used for the diagnosis of ATL. No difference was observed in the performance of the two tests. Similar results were obtained for ELISA employing partially SFs from a noninfectious strain. However, the performance of the tests was better than that reported by our research group for indirect immunofluorescence (sensitivity: 81.5%, specificity: 86.2%) 9.
Promising results have been reported in other studies using fractionated membrane components from noninfectious L. (Viannia) braziliensis as antigens in ELISA. However, no comparisons with the SF were made. Fraction 8 isolated by filtration chromatography (Sephadex G100) and ion‐exchange chromatography (DEAE‐Sepharose Fast‐Flow)presented 85.4% sensitivity and 91.2% specificity 24. Sensitivity ranged from 60 to 95% for antigen fractions isolated by chromatography (concanavalin‐A‐Sepharose and jacalin‐agarose columns) 8. In our laboratory, we obtained a sensitivity of 84.3% for the MF of noninfectious L. amazonensis in sera from patients with ATL (unpublished data).
Antigen expression may vary according to parasite species. The spectrum of disorders observed in ATL caused by L. (Viannia) braziliensis suggests a wide genetic variability between parasites of this species. However, most L. (Viannia) braziliensis strains isolated in the State of Rio de Janeiro present the same genotype (LbmtDNAGen1) 25.
Previous studies from our research group suggest that the parasite species involved is a relevant factor and might be even more important than infectivity of the strain. A sensitivity of 70% was observed for the partially SF obtained from noninfectious L. amazonensis and of 95% for the partially soluble antigen isolated from noninfectious L. (Viannia) braziliensis 9.
In this study, either fraction alone presented good sensitivity, specificity, predictive values, and likelihood ratios, a fact suggesting that ELISA may improve the diagnosis of ATL. Some investigators have demonstrated that ELISA employing crude L. (Viannia) braziliensis antigen was more sensitive than other methods used for the diagnosis of ATL such as PCR, biopsy, and indirect immunofluorescence 26. Although expression of membrane antigens is higher in infectious strains 18, the ELISA results obtained using MF and SF from an infectious strain were not better than those reported for antigens obtained from noninfectious strains.
This validation study was conducted according to the STARD guidelines 27. The sample of cases and controls was randomly selected from a cohort of subjects seen for diagnostic investigation of ATL, who were submitted to the same clinical protocol. Internal validity was evaluated and the diagnostic accuracy of the test should be sufficient to discriminate patients referred for the investigation of ATL and its differential diagnoses. Although serological cross‐reactions have been reported for patients with Chagas' disease, toxoplasmosis, and visceral leishmaniasis 28, 29, the control group did not include sera from those patients as these diseases are not part of the differential diagnosis in this context.
The present results suggest that the two fractions obtained from infectious L. (Viannia) braziliensis presented satisfactory accuracy for the diagnosis of ATL by ELISA. There was no evidence supporting the use of MF as antigen whose preparation is more time consuming and more expensive. The results obtained for ELISA using antigens from an L. (Viannia) braziliensis strain in the infectious phase are similar to those reported in previous studies from our research group using noninfectious strains, a finding suggesting that the species used is more important than the infectivity of the strain.
Acknowledgements
Jamyra Iglesias Cataldo was the recipient of a Master's fellowship from FIOCRUZ and Flávia Coelho Ribeiro was the recipient of a PhD fellowship from FAPERJ within the stricto sensu postgraduation course on infectious and parasitic diseases of Instituto de Pesquisa Clínica Evandro Chagas (IPEC/FIOCRUZ). Célia de Fátima Moreira Venâncio was the recipient of a technician fellowship from FAPERJ at the Laboratório de Vigilância em Leishmanioses. Armando de Oliveira Schubach and Fernanda Carvalho de Queiroz Mello were the recipients of productivity fellowships from CNPq.
REFERENCES
- 1. Ministério da Saúde . Manual de Vigilância da Leishmaniose Tegumentar Americana, second edition Brasília: Ministério da Saúde; 2007. [Google Scholar]
- 2. Furtado T. Critérios para o diagnóstico laboratorial da leishmaniose tegumentar americana. An Bras Dermatol 1980;55:81–86. [Google Scholar]
- 3. Marzochi MCA, Coutinho SG, Souza WTS, Amendoeira MMR. Leishmaniose visceral‐calazar. J Bras Med 1981;41:61–84. [Google Scholar]
- 4. Badaró R, Reed SG, Carvalho EM. Immunofluorescent antibody test in American visceral leishmaniasis: Sensitivity and specificity of different morphological forms of two Leishmania species. Am J Trop Med Hyg 1983;32:480–484. [DOI] [PubMed] [Google Scholar]
- 5. Hommel M. Enzymoimmunoassay in leishmaniasis. Trans R Soc Trop Med Hyg 1976;70:15–16. [Google Scholar]
- 6. Voller A, Bartlett A, Bidwell DE. Enzyme immunoassays for parasitic diseases. Trans R Soc Bras Med Hyg 1976;70:98–106. [DOI] [PubMed] [Google Scholar]
- 7. Ribeiro FC, Schubach AO, Confort EM, Schubach TMP, Madeira MF, Marzochi MCA. Use of ELISA employing Leishmania (Viannia) braziliensis and Leishmania (Leishmania) chagasi antigens for detection of IgG and IgG1 and IgG2 subclasses in the diagnosis of American Tegumentary Leishmaniasis in dogs. Vet Parasitol 2007;148:200–206. [DOI] [PubMed] [Google Scholar]
- 8. Gomes‐Silva A, Souza MA, Afonso‐Cardoso SR, et al. Serological reactivity of different antigenic preparations of Leishmania (Leishmania) amazonensis and the Leishmania braziliensis complex. Rev Soc Bras Med Trop 2008;41:135–141. [DOI] [PubMed] [Google Scholar]
- 9. Barroso‐Freitas APT, Passos SRL, Mouta‐Confort E, et al. Accuracy of an enzyme immunoassay (ELISA) and indirect immunofluorescence for the laboratory diagnosis of American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg 2009;103:383–389. [DOI] [PubMed] [Google Scholar]
- 10. Junqueira Pedras M, Orsini M, Castro VM, Rabello A. Antibody subclass profile against Leishmania braziliensis and Leishmania amazonensis in the diagnosis and follow‐up of mucosal leishmaniasis. Diagn Microbiol Dis 2003;47:477–485. [DOI] [PubMed] [Google Scholar]
- 11. Bouvier J, Etges RJ, Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem 1985;268:15504–15509. [PubMed] [Google Scholar]
- 12. Jensen ATR, Gaafar A, Ismail A, et al. Serodiagnosis of cutaneous leishmaniasis: Assessment of an enzyme‐linked immunosorbent assay using a peptide sequence from gene B protein. Am J Trop Med Hyg 1996;55:490–495. [DOI] [PubMed] [Google Scholar]
- 13. Rey‐Ladino JA, Joshi PB, Singh B, Gupta R, Reiner NE. Leishmania major molecular cloning, sequencing, and expression of the heat shock protein 60 gene reveals unique carboxy terminal peptide sequences. Exp Parasitol 1997;85:249–263. [DOI] [PubMed] [Google Scholar]
- 14. Montoya Y, Leon C, Talledo M, et al. Recombinant antigens for specific and sensitive serodiagnosis of Latin American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg 1997;91:674–676. [DOI] [PubMed] [Google Scholar]
- 15. Shreffler WG, Burns JM Jr, Badaró R, et al. Antibody responses of visceral leishmaniasis patients to gp63, a major surface glycoprotein of Leishmania species. J Infect Dis 1993;167:426–430. [DOI] [PubMed] [Google Scholar]
- 16. Cabrera GP, Silva VO, Costa RT, et al. The fucose‐mannose ligand‐ELISA in the diagnosis and prognosis of canine visceral leishmaniasis in Brazil. Am J Trop Med Hyg 1999;61:296–301. [DOI] [PubMed] [Google Scholar]
- 17. Oliveira LS, Julião FS, Souza VMM, et al. A utilização da imunofluorescência indireta no diagnóstico de rotina da leishmaniose visceral canina e suas implicações no controle da doença. C A B 2005;6:41–47. [Google Scholar]
- 18. Sacks DL. The structure and function of the surface lipophosphoglycan on different developmental stages of Leishmania promastigotes. Infect Agents Dis 1992;1:200–206. [PubMed] [Google Scholar]
- 19. Cysne‐Finkelstein L, Temporal RM, Alves AA, Leon LL. Leishmania amazonensis: Long‐term cultivation of axenic amastigotes is associated to metacyclogenesis of promastigotes. Parasitol Res 1998;89:58–62. [DOI] [PubMed] [Google Scholar]
- 20. Kweider M, Lemesre JL, Darcy F, Kusnierz JP, Capron A, Santoro F. Infectivity of Leishmania braziliensis promastigotes is dependent on the increasing expression of a 65,000‐dalton surface antigen. J Immunol 1987;138:299–305. [PubMed] [Google Scholar]
- 21. Genestra M, Cysne‐Finkelstein L, Leon L. Protein kinase A activity is associated with metacyclogenesis in Leishmania amazonensis . Cell Biochem Funct 2004;22:315–320. [DOI] [PubMed] [Google Scholar]
- 22. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275. [PubMed] [Google Scholar]
- 23. Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 1979;86:420–428. [DOI] [PubMed] [Google Scholar]
- 24. Vidigal CP, Marcussi VM, Marcussi LM, et al. Enzyme immunoassay using Leishmania (Viannia) braziliensis antigens for laboratorial diagnosis of American cutaneous leishmaniasis. Acta Trop 2008;107:208–212. [DOI] [PubMed] [Google Scholar]
- 25. Baptista C, Schubach AO, Madeira MF, et al. L. (Viannia) braziliensis genotypes identified in lesions of patients with atypical or typical manifestations of tegumentary leishmaniasis: Evaluation by two molecular markers. Exp Parasitol 2009;121:317–322. [DOI] [PubMed] [Google Scholar]
- 26. Ferreira MP, Roselino AMF, Nascimento MMP, Aires JM, Figueiredo JFC. Sensitivity of an immunoenzymatic test for the detection of anti‐L. braziliensis antibodies compared to other tests used for the diagnosis of American cutaneous leishmaniasis. Rev Inst Med Trop 2006;48:215–217. [DOI] [PubMed] [Google Scholar]
- 27. Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: Explanation and elaboration. Clin Chem 2003;49:7–18. [DOI] [PubMed] [Google Scholar]
- 28. Malchiodi EL, Chiaramonte MG, Taranto NJ, Zwirnwe NW, Margini RA. Cross‐reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp.; use of immunoblotting and ELISA with a purified antigen (Ag163 B6). Clin Exp Immunol 1994;97:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reed SG, Badaró R, Lloyd RM. Identification of specific and cross‐reactive antigens of Leishmania donovani chagasi by human infection sera. J Immunol 1987;138:1596–1601. [PubMed] [Google Scholar]


