Abstract
Objective: Neonatal hypothyroidism is one of the most common endocrine disorders related to mental impairment and growth retardation in newborns. In many countries, the neonatal thyroid screening programs are performed for rapid diagnosis and treatment of hypothyroidism. The major aim of this investigation was to improve the thyroid screening program using primary blood TSH/back up TSH measurements as some patients are missed due to technical and human errors. Methods: A total of 9,118neonates were evaluated on the protocol. On top of that, the quality control procedures were applied to improve the sampling technique and the laboratory results. Results: Three missed neonates by current programs using the cutoff point more than 20 mU/l for blood TSH were found by our approach. Conclusion: Results showed that the programs based on the primary blood TSH/back up TSH measurements improve the thyroid screening results. J. Clin. Lab. Anal. 25:61–63, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: hypothyroidism, newborn screening, TSH, T4
INTRODUCTION
Neonatal hypothyroidism is one of the most common endocrine disorders in the world 1. Since rapid diagnosis and treatment of hypothyroidism during the first weeks of life prevent from mental impairment and growth retardation in neonates 2, 3, 4, 5 thus, the newborn thyroid screening programs are extensively developed in the world during the past 35 years 6, 7, 8. However, most of the current programs are switched on the TSH measurement but even in the more sensitive ones, 5–10% of neonates are missed due to technical and technician errors 9, 10, 11.
Three programs known as primary blood TSH/back up T4 measurements, primary blood T4/back up TSH measurements, and simultaneous measurements of T4 and TSH are currently used in newborn screening programs 12, 13, 14. The primary blood TSH/back up T4 measurements are used often in Europe, Japan, Mexico, and the United States. In this approach, cases with hypothyroxinemia, central hypothyroidism, and thyroid binding globulin deficiency together with the delayed TSH elevation are missed during the screening. Other approaches, such as the simultaneous measurements of T4 and TSH, however, are more sensitive for the thyroid screening but suffer from some problems such as high expenses. In almost all the programs, the dried blood TSH between 20 and 25 mU/l has been used as a cutoff point to recall neonates 15.
The aim of this investigation was to improve the previous protocol (16) by reducing the recall rate. In this study, we screened 9,118 neonates by the approach of primary blood TSH/back up TSH measurements.
NEONATAL SCREENING PROGRAM
The neonatal thyroid screening program, however, is performed by the government, but the expenses of laboratory tests are paid by families in Iran. At the beginning of the program, the cut off point for the dried blood TSH was 20 mU/l. Since several screened cases were found as hypothyroidism thus, the cutoff point was diminished to 5 mU/l. On this protocol, the neonates with the primary dried blood TSH more than 5 mU/l were recalled for measurements of the serum TSH, T4, and T3 and clinical examinations 16. However, the protocol improved the newborn screening program but the recall rate was high (3.6%).
In new approach, the newborns with primary dried blood TSH values between 5 and 25 mU/l were recalled for the secondary blood TSH between 10th and 15th days of age. Then, the ones with the secondary blood TSH above 5 mU/l and those with the primary blood TSH above 25 mU/l were recalled for the clinical examinations and serum tests (Fig. 1).
Figure 1.
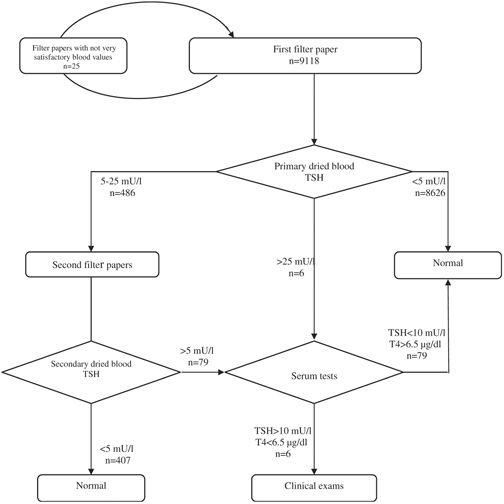
Newborn thyroid screening program's algorithm.
In addition, the neonates with birth weight more than 4,000 or less than 2,500 g and twins were screened between the third and fifth days and at 2 weeks of age.
DRIED BLOOD TSH ASSAY
The dried blood samples were collected between the third and seventh days of life on a filter paper (Schleicher and Schuell, NO 903) 17. The evaluation of spotted blood on circular areas of filter papers was under strict supervisions and those with not very satisfactory values were recalled for the second filter paper (2.8 by 1,000 filter papers). As the coefficient of within‐run variation (CVTSH) of disks was between 11.28 and 25.5% thus, all the discs were prepared from the center of circle and TSH was measured by ELISA technique (Stat‐Fax 3100; Awareness Technology, Inc., Palm city, Florida) 18.
Five standard samples (0, 5, 10, 30, and 60 mU/l used for standard graph), two calibrators (low and high values), and three standard replicates equal to the cutoff point (5 mU/l) were applied in each 96‐well plate.
The CV value of the three standard replicates was between 10.5 and 13%. The acceptable precision for each run was 5±1 mU/l and acceptable one‐way error (AOE) was calculated to be (CV×5)/100.
Actual cutoff point (ACP) for each run was also calculated by the linear regression equation (ACP=−0.0052A+5.847), as there was a correlation (R 2=0.999) between the replicates and absorbance (A) of standard sample (TSH=5 mU/l).
The ACP±AOE was the criteria for rechecking the filter papers. The AOE and ACP values were calculated to be 0.3–0.8 mU/l and 4.7–5.2 mU/l, respectively, and the repeat rate was 2.8 by 1,000 filter papers.
FINDINGS
On our screening protocol, six neonates with primary dried blood TSH above 25 mU/l were visited by the physician (Fig. 1). Three neonates had the serum TSH more than 50 mU/l and serum T4 less than 6.5 µg/dl. In addition, 486 neonates had the primary dried blood TSH values between 5 and 25 mU/l. The blood TSH values in seventy‐nine neonates were confirmed by the secondary dried blood TSH, two neonates were found as cases of hypothyroidism, and a neonate was under the clinical inquiries.
The recall rate for the measurements of serum tests was reduced to 0.85% in comparison with the previous report (3.6%). On the screening policy, however, some cases of hypothalamic–pituitary hypothyroidism with the normal TSH would be missed but the newborns with dyshormonogenesis or the thyroid gland dysgenesis characterized with the low T4 and the delayed TSH elevation can be found during an interval for the measurement of secondary dried blood TSH 19. In addition, we applied the quality control procedures covering almost all sectors in the program to improve false‐negative results 20.
In conclusion, the study showed that the thyroid screening approach based on the primary blood TSH/back up TSH measurements and strict supervisions on the sectors of program improve the neonatal screening results.
REFERENCES
- 1. Gruters A, Biebermann H, Krude H. Neonatal thyroid disorders. Horm Res 2003;59:24–29. [DOI] [PubMed] [Google Scholar]
- 2. Heindel JJ, Zoeller RT. Thyroid hormone and brain development: Translating molecular mechanisms to population risk. Thyroid 2003;13:1001–1004. [DOI] [PubMed] [Google Scholar]
- 3. Bunevicius R, Prange AJ Jr. Thyroid disease and mental disorders: Cause and effect or only comorbidity? Curr Opin Psychiatry 2010;23:363–368. [DOI] [PubMed] [Google Scholar]
- 4. Bongers‐Schokking JJ, Koot HM, Wiersma D, et al. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J Pediatr 2000;136:292–297. [DOI] [PubMed] [Google Scholar]
- 5. LaFranchi SH, Austin J. How should we be treating children with congenital hypothyroidism? J Pediatr Endocrinol Metab 2007;20:559–578. [DOI] [PubMed] [Google Scholar]
- 6. Bhatara V, Sankar R, Unutzer J, Peabody J. A review of the case for neonatal thyrotropin screening in developing countries: The example of India. Thyroid 2002;12:591–598. [DOI] [PubMed] [Google Scholar]
- 7. Honour JW, Torresani T. Evaluation of neonatal screening for congenital adrenal hyperplasia. Horm Res 2001;55:205–210. [DOI] [PubMed] [Google Scholar]
- 8. LaFranchi SH, Snyder DB, Sesser DE. Follow‐up of newborns with elevated screening T4 concentrations. J Pediatr 2003;143:296–301. [DOI] [PubMed] [Google Scholar]
- 9. American Academy of Pediatrics , Rose SR, Section on Endocrinology and Committee on Genetics , et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006;117:2290–2303. [DOI] [PubMed] [Google Scholar]
- 10. Yunis KA, Nasr MR, Lepejian G, et al. False‐negative primary neonatal thyroid screening: The need for clinical vigilance and secondary screening. J Med Screen 2003;10:2–4. [DOI] [PubMed] [Google Scholar]
- 11. Mengreli C, Kanaka‐Gantenbein C, Girginoudis P, et al. Screening for congenital hypothyroidism: The significance of threshold limit in false‐negative results. J Clin Endocrinol Metab 2010;95:4283–4290. [DOI] [PubMed] [Google Scholar]
- 12. Fisher DA. Disorders of the thyroid in the newborn and infant In: Sperling MA. (ed.). Clinical Pediatric and Adolescent Endocrinology. Philadelphia, PA: Saunders, 2002;164. [Google Scholar]
- 13. Foley T, Kaplowitz PB, Kaye CI, et al. Update of newborn screening and therapy for congenital hypothyroidism. American Academy of Pediatrics, Rose SR; Section on Endocrinology and Committee on Genetics, American Thyroid Association, Brown RS; Public Health Committee, Lawson Wilkins Pediatric Endocrine Society. Pediatrics 2006;117:2290–2303. [DOI] [PubMed] [Google Scholar]
- 14. Waller DK, Anderson JL, Lorey F, Cunningham GC. Risk factors for congenital hypothyroidism: An investigation of infant's birth weight, ethnicity, and gender in California, 1990–1998. Teratology 2000;62:36–41. [DOI] [PubMed] [Google Scholar]
- 15. American Academy of Pediatrics AAP Section on Endocrinology and Committee on Genetics, and American Thyroid Association Committee on Public Health . Newborn screening for congenital hypothyroidism: Recommended guidelines. Pediatrics 1993;91:1203–1209. [PubMed] [Google Scholar]
- 16. Najafi M, Khodaee GH, Bahari M, et al. Neonatal thyroid screening in a mild iodine deficiency endemic area in Iran. Indian J Med Sci 2008;62:113–116. [PubMed] [Google Scholar]
- 17. Elvers LH, Loeber JG, Dhondt JL, et al. First ISNS reference preparation for neonatal screening for thyrotropin, phenylalanine and 17alpha‐hydroxyprogesterone in blood spots. J Inherit Metab Dis 2007;30:609. [DOI] [PubMed] [Google Scholar]
- 18. Silva SA, Chagas AJ, Goulart EM, et al. Screening for congenital hypothyroidism in extreme premature and/or very low birth weight newborns: The importance of a specific protocol. J Pediatr Endocrinol Metab 2010;23:45–52. [DOI] [PubMed] [Google Scholar]
- 19. Bernal J, Guadano‐Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid 2003;13:1005–1012. [DOI] [PubMed] [Google Scholar]
- 20. Yordam N, Ozon A. Neonatal thyroid screening: Methods‐efficiency‐failures. Pediatr Endocrinol Rev 2003;2:177–184. [PubMed] [Google Scholar]


