Abstract
Lung cancer is a malignant disease with increasing mortality rates. Cytokines play a role in normal cell growth regulation and differentiation and are also implicated in malignant disease. Among these cytokines, Transforming Growth Factor β type 1 (TGF‐β1) acts as a tumor promoter in malignant cells. Several clinical studies have found high levels of TGF‐β1 in various cancer types. The aim of this study was to establish a TGF‐β1 cut‐off point as a complementary diagnostic tool in lung cancer detection. Therefore, 72 clinically well‐characterized individuals were studied, 41 lung cancer patients and 31 healthy subjects. Serum TGF‐β1 concentration was measured by an enzyme‐linked immunosorbent assay (ELISA). We compared statistically the serum TGF‐β1 concentration between both groups with analysis of variance, linear regression and receiver operating curve analysis. We observed that lung cancer patients produced higher TGF‐β1 levels than healthy individuals (37,225±9,436 vs. 28,416±9,324 pg/ml, P<0.001). The cut‐point diagnostic value was 30,500 pg/ml with 80.5% sensitivity, 64.5% specificity and odds ratio: 7.5, 95% CI: 2.6–21.8. Conclusions: We found significantly higher TGF‐β1 levels in lung cancer patients than in healthy individuals. We propose the measurement of serum TGF‐β1 levels as a complementary diagnostic test in lung cancer detection. J. Clin. Lab. Anal. 25:238–243, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: TGF‐β1 serum concentration, lung cancer, diagnosis, biomarker
INTRODUCTION
Worldwide lung cancer is a disease with high mortality rates 1. Risk factors like cigarette smoking, wood smoke exposure, and lung cancer family history, among others, have been reported for lung cancer 2. The detection of lung relies on both clinical history and other diagnostic test such as chest radiography and transthoracic needle aspiration 1. Lung cancer is histologically classified as nonsmall cell lung cancer (including adeno‐, squamous cell‐, and large cell‐carcinoma) and Small Cell Lung Cancer. Each histological type requires specific treatment and has different prognosis 1. The biological changes that occur in carcinogenesis, depends on alterations in oncogene activation, tumor gene suppressor inactivation, as well as alterations in signaling pathways that affect growth factors. Included in these, cytokines play a key role in normal cell growth regulation and differentiation and are involved in many types of malignant disease 3. Transforming Growth Factor β type 1 (TGF‐β1) is a cytokine member of a super‐family that includes TGF‐β1 through 5, bone morphogenic proteins, activins, and inhibins. Human TGF‐β1 is a 25 kDa, disulfide‐linked, nonglycosylated homodimer 4. This cytokine acts as tumor suppressor and arrests cell growth in epithelial normal cells and in cells with an early malignant state 5. In contrast, in an advanced tumoral phase, TGF‐β1 produces a positive effect for survival, progression, and tumor metastasis, promoting epithelium–mesenchimal transition and angiogenesis as well as avoiding immuno‐surveillance 5.
Several clinical studies have found high levels of this cytokine in patients with colorectal carcinoma, esophagus carcinoma, gastric carcinoma, glioblastoma, and lung cancer 6, 7, 8, 9, 10, 11. Given the increasing incidence of lung cancer and the low effectiveness in the treatment for advanced lung cancer cases, it is important to investigate biomarkers that could complement and improve the diagnostic stage of lung cancer. Therefore, the aim of this study was to quantify TGF‐β1 serum concentration in lung cancer patients and clinically healthy individuals. A positive correlation between high TGF‐β1 serum levels and lung cancer disease was demonstrated. In addition, we found a TGF‐β1 concentration cut‐point value with acceptable sensibility and specificity to use as a complementary biomarker in the lung cancer diagnosis.
MATERIAL AND METHODS
Subjects
Eligible cases were identified at the Department of Neumology and Pulmonary Physiology, Centro Médico Nacional de Occidente, Instituto Mexicano del Seguro Social (IMSS), from August 2006 to December 2007. We analyzed clinically and histologically 102 patients suspicious for lung cancer; however, only patients with histopathological confirmation of lung cancer (gold standard diagnosis) were considered in the study (n=41). Patients diagnosed with lung cancer did not receive any treatment, drug, and/or chemotherapy before a blood sample was obtained. Pathological diagnosis was classified according to WHO classification of lung tumors 1, and a staging (TNM classification groups) was performed by an expert pathologist in lung diseases. Also, we studied 157 volunteers, from these we excluded individuals with the following conditions (as these conditions could alter the TGF‐β1 concentration 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22): body mass index up to 30 kg/m2, alcoholism history, cigarette smoking exposure, wood smoke exposure, chronic hepatic disease, chronic renal disease, acute or chronic respiratory diseases, diabetes mellitus, hypertension, immunodeficiency, patients previously transplanted, patients treated with anti‐inflammatory drugs or having any malignant disease. Only individuals without these criteria were considered as the control group (n=31). All participants were age matched.
Ethic Considerations
The internal Ethics Committee of Centro Médico Nacional de Occidente approved this study. A standardized, structured questionnaire was used to collect clinical data. All participants (patients and controls) gave informed consent.
Sample Collection and TGF‐β1 Measurement
Blood samples were obtained by vein puncture (5 ml), and serum was separated by centrifugation at 3,500 rpm for 15 min. Serum samples were stored at −20°C until TGF‐β1 analysis. TGF‐β1 serum concentration was quantified by enzyme‐linked immunosorbent assay (ELISA TGF‐β1 kit, R&D Systems, Minneapolis, MN) according to manufacturer's instructions. Briefly, to activate latent TGF‐β1 to the immunoreactive form, serum samples were acidified and lately neutralized with 1N HCI and 1.2N NaOH/0.5 M HEPES, respectively. 50 μl of assay diluent was added to each well and subsequently, standard, control, or activated serum samples were added and incubated for 2 hr at room temperature. Each well was aspirated and washed four times with wash buffer. Later, 100 μl conjugate was added to each well and incubated for 2 hr, and the last washing step was repeated. Subsequently, substrate solution was added to each well and incubated 30 min at room temperature. The reaction was stopped and the plate was read at 450 nm with a λ correction of 540 nm. To calculate TGF‐β1 concentration, the duplicate readings for each standard, control, or activated serum sample were averaged and subtracted to the average zero standard optical density. A standard curve was constructed and the best fit line was determined by regression analysis.
Statistical Analysis
An analysis of variance was used to compare TGF‐β1 serum concentration by several clinical data (age groups, gender, cigarette smoking, wood smoke exposure, signs and symptoms related to poor prognosis). Also, we performed a receiver operating curve (ROC) analysis to estimate a TGF‐β1 level cut‐point value and assess its sensibility and specificity as a diagnostic test. These analyses were processed by the statistical software SPSS version 15.0 for WindowsTM (SPSS Inc., Chicago, IL). In addition, we analyzed positive predictive value (PPV) and negative predictive value (NPV) to establish the proportion of patients correctly or incorrectly diagnosed. We also calculated an accuracy determination to analyze the probability of the test to classify correctly the patients.
Finally, we calculated odds ratio (OR) and a likelihood ratio in order to estimate the risk and to provide a direct estimation of how much this test result would change the probability of having lung cancer. These analyses were processed with the following software: Diagnostic test calculator (version 2006032401) 2002–2007 by Alan Schwartz (alansz@uic.edu), and Graph Pad PRISM version 5.0 for Windows™ (GraphPad Software Inc., La Jolla, CA).
RESULTS
The association between serum TGF‐β1 levels and age, gender, cigarette smoking, wood smoke exposure, and lung cancer family history signs and symptoms related to poor prognosis status was evaluated in healthy individuals and lung cancer patients. The clinical features of both groups are shown in Table 1.
Table 1.
Relevant Clinical Features of Lung Cancer Patients and Healthy Individuals
| Lung cancer n=41 (%) | Control group n=31 (%) | |
|---|---|---|
| Age (years, mean age±SD) | 64.3±10.6 | 61.5±19.9 |
| Gender | ||
| Male | 29 (71) | 17 (55) |
| Female | 12 (29) | 14 (45) |
| Wood smoke exposure | 8 (20) | 0 (0) |
| Tobacco smoking | 30 (73) | 0 (0) |
| Cancer familial history | 15 (37) | 0 (0) |
| Cancer Stagea | ||
| IIIb | 11 (30) | – |
| IV | 26 (70) | – |
| Signs and symptoms related to poor prognosis | – | |
| Weight loss | 4 (9.8) | 0 (0) |
| Anorexia | 2 (4.8) | 0 (0) |
| Fatigue | 3 (7.3) | 0 (0) |
| Histological type | ||
| Adenocarcinoma | 16 (39.0) | – |
| Squamous cell carcinoma | 11 (26.9) | – |
| Small cell carcinoma | 4 (9.7) | – |
| Large cell carcinoma | 2 (4.9) | – |
| Other typesb | 8 (19.5) | – |
aFour patients could not be classified in any stage (TNM staging of lung cancer, 1).
bTumors which cytological or in a small biopsy specimen do not allow specific differentiation according to the WHO lung tumors classification (1).
The mean age±SD was 64.3±10.6 and 61.5±19.9 years old for the lung cancer and healthy individuals groups, respectively. Other clinical symptoms present at the moment of the diagnostic included cough (16.9%), dyspnea (14.6%), chest pain (11.8%), weight loss (10.6%), haemoptysis (9.6%), weakness (6.5%), anorexia (5.2%), and others (24.8%) (data not shown). Similarly, 73% of lung cancer patients were cigarette smokers, 20% were exposed to wood smoke (indoor contamination), and 37% had cancer family history (first‐degree relatives). Furthermore, at the time of the diagnosis, 70% of patients with lung cancer presented metastatic disease (stage IV), and 30% had locally advanced disease (stage IIIb). We also studied the anatomical localization of the tumors, because this information is critical to establish a prognosis and treatment. We found that left hemithorax (46.3%) was the most frequent tumor location, followed by right hemithorax (39.0%), mediastinum (12.2%), and diffuse micronodular pattern (2.5 %). In regards to TGF‐β1 serum concentration, we observed that lung cancer patients produced a higher concentration than healthy individuals (37,225±9,436 vs. 28,416±9,324 pg/ml, P<0.001, Fig. 1). We also analyzed the association of TGF‐β1 serum concentration with age, gender, cigarette smoking, wood smoke exposure, lung cancer family history, histological lung cancer types, and signs and symptoms related to poor prognosis of lung cancer status, but no significant differences were found (P>0.05, data not shown).
Figure 1.
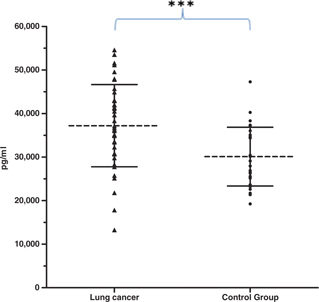
Distribution of TGF‐β1 serum concentration values (mean±SD) in patients with lung cancer (37,225±9,436 pg/ml) and control group (28,416±9,324 pg/ml). *** P<0.001.
In addition, to determine if the TGF‐β1 serum concentration could be useful in the lung cancer diagnosis, we performed a ROC analysis. We found that the data distribution generate a cut‐point value of TGF‐β1 concentration: 30,500 pg/ml, with 80.5% sensitivity, 64.5% specificity, and OR 7.5 (CI 95%: 2.6–21.8) (Table 2 and Fig. 2).
Table 2.
Statistical Parameters for the Use of TGF‐β1 Serum Levels as Diagnostic Test
| Value | 95% CI | |
|---|---|---|
| Sensitivity | 80.5% | 66.0–89.8% |
| Specificity | 64.5% | 46.9–78.9% |
| Positive predictive value | 75.0% | 60.6–85.4% |
| Negative predictive value | 71.4% | 52.9–84.7% |
| Accuracy | 73.6% | 62.4–82.4% |
| Odds ratio | 7.5 | 2.6–21.8 |
| Likelihood ratio | 2.27 | 1.38–3.73 |
CI=Confidence interval.
Figure 2.
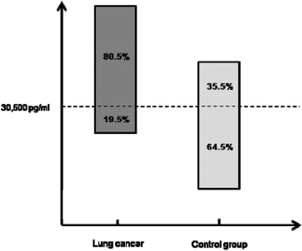
Sensitivity and specificity of the determination of TGF‐β1 levels in lung cancer patients and control subjects, according to ROC analysis.
After ROC curve evaluation, we also evaluated data with PTP that indicates the proportion of patients with positive test results who are correctly diagnosed. In addition, we tested NPV that is the proportion of patients with negative test results who are correctly diagnosed. Serum TGF‐β1 concentration had PTP values of 75.0% and a NPV of 71.4%.The accuracy of the test was calculated (probability to correctly classify patients) with a value of 73.6%. We calculated a positive likelihood ratio of 2.27 (95% CI: 1.38–3.73) (Table 2).
DISCUSSION
TGF‐β1 has been related to cancer promotion in later cancer stages. In our study, the majority of lung cancer patients (70%) exhibited advanced disease (presence of metastasis) and the rest (30%) locally advanced disease. Accordingly, we found that these patients had a higher TGF‐β1 serum concentration than healthy individuals (control group). Our data is in agreement with data previously reported 10, 11. The increase in TGF‐β1 serum concentration could be explained by alterations in several signaling pathways, including the TGF‐β1 signaling pathway 23 and/or gene expression of cell cycle proteins. Moreover, the microenvironmental changes of tumor cells can promote a high rate of TGF‐β1 production and its activation 5. On the other hand, it has been reported that this cytokine has a dual role in carcinogenesis exerting a tumor suppressor activity (normal cells) as well as promoting cancer progression (malignant cells) 5, 24. Its metastasic effect has been demonstrated in in vitro experiments where TGF‐β1 treatment of metastatic breast cancer cell lines enhanced metastases 25. Furthermore, Domagała‐Kulawik et al. 26 reported an association between high levels of TGF‐β1 in bronchioalveolar fluid and advanced lung cancer stage that could be related to metastasis. In summary, significantly higher TGF‐β1 production was found in several lung cancer cell lines as well as in lung cancer patients 10, 11, 27. Kong et al. reported a TGF‐β1 levels reduction after radiotherapy in lung cancer patients proposing this measurement as a marker to monitor disease persistence and recurrence after treatment 10. However, these authors did not find a correlation between TGF‐β1 plasma levels before therapy and histological lung cancer types 11. Similar results were found in this study (Data not shown, P value=0.846).
These results could be explained by the sample size and the heterogeneous distribution of the histological lung cancer types.
Otherwise, TGF‐β1 expression by tumor cells may inhibit immune response and enhance its tumorigenicity promoting angiogenesis and metastasis. To evaluate the role of TGF‐β1 in the disease progression, we compared the TGF‐β1 concentration with the symptoms related to poor prognosis (anorexia, weight loss, and fatigue) 28 but could not establish a correlation (P value=0.134).
The current diagnostic strategies have not impacted lung cancer mortality rates 29. As 70% of the lung cancer patients present locally advanced or metastatic disease at the time of diagnosis, new markers and diagnostic methods are needed to detect lung cancer at early stages 1. Currently, sputum cytology, transthoracic needle aspiration, bronchial biopsy, bronchial washing, chest radiography, computed tomography, 18F‐FDG Positron Emission Tomography, and/or Magnetic Resonance are the most common diagnostic tests (Table 3). All of these methods have different sensitivities and specificities, its selection depends on tumor localization (central, peripheral, and/or spread disease, Table 3), and its combinatorial use could increase the diagnostic accuracy 30, 31, 32, 33, 34, 35. In this study, the measurement of TGF‐β1 concentration yielded enough sensitivity to detect lung cancer (80.5%). The detection of high TGF‐β1 levels provided a risk of 7.5 fold (OR) associated with lung cancer. Besides, we found that if these levels are up to 30,500 pg/ml (cut‐point value) augment the lung cancer risk. Nevertheless, it is important also consider the clinical condition and lung cancer target symptoms. Actually, to improve the lung cancer algorithm diagnosis is necessary the combination of several clinical tests and markers.
Table 3.
Lung Cancer Diagnostic Methods
| Diagnostic method | Sensitivity (%) | Specificity (%) | Indication | Comments |
|---|---|---|---|---|
| Invasive diagnostic test | ||||
| Thoracocentesis | 80 | >90 | Pleural spillage | Pleural fluid cytology |
| Thoracotomy | – | – | Only clearly resectable tumors | Recommended for diagnosis and treatment of early nonsmall cell carcinoma |
| Excisional biopsy of an accessible node | – | – | Palpable lymphadenopathy | – |
| Flexible bronchoscopy with or without transbronchial needle aspiration | Central tumors: 88 Peripheral tumors: 60–70 | 90 | Central or peripheral tumors and mediastinal lymphadenopathy | Fluoroscopic or CT guidance; transbronchial needle aspiration improves sensitivity in peripheral tumors |
| Transthoracic needle aspiration | Peripheral tumors: 90 | 97 | Peripheral tumor in nonsurgical candidates or when transbronchial needle aspiration is inconclusive | Fluoroscopic or CT guidance; the assistance of a cytopathologist improves diagnostic yield |
| Video‐assisted thoracoscopy | – | – | Small peripheral tumors (<2 cm in diameter), pleural tumors, or pleural effusions | May prevent the need for thoracotomy |
| Noninvasive diagnostic test | ||||
| Sputum cytology (at least three specimens) | Central tumors: 71 Peripheral tumors <50 | 99 | Central tumor and haemoptysis | Noninvasive; further testing needed after negative result |
| Computed tomography | 80–90% | – | – | Positive predictive value less than 20% in lung cancer screening |
| Magnetic resonance imaging | 94% | 95% | To differentiate malignant SPNs from bening SPNs | To evaluate microvessel density, staging lung cancer and for a treatment followup |
| Chest radiography | 54–84% | 90–99% | To detect presymptomatic disease and initial diagnosis | 3–5% of lung lesions are identifiable only with lateral X‐rays, and 5–17% can be observed better laterally than frontally |
| 18F‐FDG positron emission tomography | 96% | – | Evaluating on indeterminate SPNs | Emerging as a pre‐operative assessment in NSCLC. Combined with CT Scan improves accuracy |
SPNs, solitary pulmonary nodules. –: Nonspecified.
aAdapted with permission from “Lung Cancer: Diagnosis and Management,” January 1, 2007, American Family Physician. Copyright © 2007 American Academy of Family Physicians. All rights reserved” Information added from 30, 32–35.
In conclusion, our results indicate that the quantification of TGF‐β1 serum concentration could be useful as complementary diagnostic test, because of the following reasons: easy perform, high sensitivity and specificity, and high cost‐effectiveness. In addition, the combination of an expanded number of biomarkers, clinical evaluation, and current diagnostic tests will allow more accurate detection of lung cancer.
Acknowledgements
The authors acknowledge the assistance of R. C. Zarate and the helpful comments of S. Garcia in the manuscript.
Conflict of interest statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
REFERENCES
- 1. Travis WD, Brambilla E, Mueller‐Hermelink HK, Harris CC. World Health Organization Classification Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. p 10–20. [Google Scholar]
- 2. Wakai K, Inoue M, Mizoue T, et al. Tobacco smoking and lung cancer risk: An evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 2006;36:309–324. [DOI] [PubMed] [Google Scholar]
- 3. Tang JH, Zhao JH, Lu JW, et al. Circulating levels of angiogenic cytokines in advanced breast cancer patients with system chemotherapy and their potential value in monitoring disease course. J Cancer Res Clin Oncol 2011;137:55–63. [DOI] [PubMed] [Google Scholar]
- 4. Lawrence DA. Transforming growth factor‐beta: A general review. Eur Cytokine Netw 1996;7:363–374. [PubMed] [Google Scholar]
- 5. Derinck R, Akhurst RS, Balmain A. TGF beta signaling in tumor suppression and cancer progression. Nat genet 2001;29:117–128. [DOI] [PubMed] [Google Scholar]
- 6. Shim KS, Kim KH, Han WS, Park EB. Elevated serum levels of transforming growth factor beta 1 in patients with colorectal carcinoma. Its association with tumor progression and its significant decrease after radiotherapy. Cancer 1999;85:544–561. [DOI] [PubMed] [Google Scholar]
- 7. Su‐Ping S, Ye‐Ning J, Hong‐Peng Y, Yi W, Zhao D. Serum transforming growth factor beta 1 levels reflects disease status in patients with esophageal carcinoma after radiotherapy. Word J Gastroenterol 2007;13:5267–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li X, Yue Z, Zhang YY, et al.Elevated serum level and gene polymorphisms of TGF‐beta 1 in gastric cancer. J Clin Lab Anal 2008;22:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider T, Sailer M, Ansorge S, Firsching R, Reinhold D. Increased concentrations of transforming growth factor beta1 and beta 2 in the plasma of patients with glioblastoma. J Neurooncol 2006;79:61–65. [DOI] [PubMed] [Google Scholar]
- 10. Kong FM, Washington MK, Jirtle RL, Anscher MS. Plasma transforming growth factor‐beta 1 reflects disease status in patients with lung cancer after radiotherapy: A possible tumor marker. Lung cancer 1996;16:47–59. [DOI] [PubMed] [Google Scholar]
- 11. Kong FM, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma transforming growth factor‐b1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer 1999;86:1712–1719. [PubMed] [Google Scholar]
- 12. Sepúlveda‐Flores RN, Vera‐Cabrera L, Flores‐Gutiérrez JP, et al. Obesity‐related non‐alcoholic steatohepatitis and TGF‐beta1 serum levels in relation to morbid obesity. Ann Hepatol 2002;1:36–39. [PubMed] [Google Scholar]
- 13. Yuang Z, Wi L, Yuliang Z, et al. Analysis of serum cytoquines in patients with severe acute respiratory syndrome. Infect Immun 2004;72:4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim YK, Lee BC, Ham BJ, et al. Increased transforming growth factor‐beta1 in alcohol dependence. J Korean Med Sci 2009;24:941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Churg A, Wang RD, Wright JL. Cigarette smoke causes small airway remodeling by direct growth factor induction and release. Proc Am Thorac Soc 2006;3:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo JC, Bao JF, et al. Level of serum and liver tissue TGF‐beta1 in patients with liver fibrosis due to chronic hepatitis. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2008;22:354–357. [PubMed] [Google Scholar]
- 17. Suthanthiran M, Gerber LM, Schwartz JE, et al. Circulating transforming growth factor‐beta1 levels and the risk for kidney disease in African Americans. Kidney Int 2009;76:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yener S, Comlekci A, Akinci B, et al. Serum transforming growth factor‐beta 1 levels in normoalbuminuric and normotensive patients with type 2 diabetes. Effect of metformin and rosiglitazone. Hormones 2008;7:70–76. [DOI] [PubMed] [Google Scholar]
- 19. Mak JC, Chan‐Yeung MM, Ho SP, et al. Elevated plasma TGF‐beta1 levels in patients with chronic obstructive pulmonary disease. Respir Med 2009;103:1083–1089. [DOI] [PubMed] [Google Scholar]
- 20. Zhu S, Liu Y, Wang L, Meng QH. Transforming growth factorbeta1 is associated with kidney damage in patients with essential hypertension: Renoprotective effect of ACE inhibitor and/or angiotensin II receptor blocker. Nephrol Dial Transplant 2008;23:2841–2846. [DOI] [PubMed] [Google Scholar]
- 21. Goulet S, Bihl MP, Gambazzi F, Tamm M, Roth M. Opposite effect of corticosteroids and long‐acting beta(2)‐agonists on serum‐ and TGF‐beta(1)‐induced extracellular matrix deposition by primary human lung fibroblasts. J Cell Physiol 2007;210:167–176. [DOI] [PubMed] [Google Scholar]
- 22. Johnson MD, Kim P, Tourtellotte W, Federspiel CF. Transforming growth factor beta and monocyte chemotactic protein‐1 are elevated in cerebrospinal fluid of immunocompromised patients with HIV‐1 infection. J NeuroAIDS 2004;2:33–43. [DOI] [PubMed] [Google Scholar]
- 23. Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor β transduction. J Leukoc Biol 2002;71:731–740. [PubMed] [Google Scholar]
- 24. Roberts AB, Wakefield LM. The two faces of transforming growth factor β in carcinogenesis. Proc Natl Acad Sci USA 2003;100:8621–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welch DR, Fabra A, Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci USA 1990;87:7678–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Domagała‐Kulawik J, Hoser G, Safianowska A, Grubek‐Jaworska H, Chazan R. Elevated TGF‐beta1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Arch Immunol Ther Exp 2006;54:143–147. [DOI] [PubMed] [Google Scholar]
- 27. Fukuyama T, Ichiki Y, Yamada S, et al. Cytokine production of lung cancer cell lines: Correlation between their production and the inflammatory/immunological responses both in vivo/in vitro. Cancer Sci 2007;98:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feinstein AR, Wells CK. A clinical‐severity staging system for patients with lung cancer. Medicine 1990;69:1–33. [DOI] [PubMed] [Google Scholar]
- 29. U.S. Preventive Services Task Force . Lung cancer screening: Recommendation statement. Ann Intern Med 2004;140:738–739. [DOI] [PubMed] [Google Scholar]
- 30. Rivera MP, Detterbeck F, Mehta AC, for the American College of Chest Physicians . Diagnosis of lung cancer: The guidelines. Chest 2003;123:129S–136S. [DOI] [PubMed] [Google Scholar]
- 31. Collins LG, Haines C, Perkel R, Enck RE. Lung Cancer: Diagnosis and Management. Am Fam Physician 2007;75:56–63. [PubMed] [Google Scholar]
- 32. Gavelli G, Giampalma E. Sensitivity and specificity of chest x‐ray screening for lung cancer.Cancer 2000;89:2453–2456. [DOI] [PubMed] [Google Scholar]
- 33. Joshi SC, Pant I, Hamzah F, Kumar G, Shukla AN. Integrated positron emission tomography/ computed tomography fusion imaging: An emerging gold standard in lung cancer. Indian J Cancer 2008;45:137–141. [DOI] [PubMed] [Google Scholar]
- 34. Fujimoto K. Usefulness of contrast‐enhanced magnetic resonance imaging for evaluating solitary pulmonary nodules. Cancer Imaging 2008;8:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Black C, de Verteuil R, Walker S, et al. Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature. Thorax 2007;62:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]


