Abstract
Background: Microalbuminuria is an indicator of kidney damage and a risk factor for the progression kidney disease, cardiovascular disease, and so on. Therefore, accurate and precise measurement of urinary albumin is critical. However, there are no reference measurement procedures and reference materials for urinary albumin. Methods: Nephelometry, turbidimetry, colloidal gold method, radioimmunoassay, and chemiluminescence immunoassay were performed for methodological evaluation, based on imprecision test, recovery rate, linearity, haemoglobin interference rate, and verified reference interval. Then we tested 40 urine samples from diabetic patients by each method, and compared the result between assays. Results: The results indicate that nephelometry is the method with best analytical performance among the five methods, with an average intraassay coefficient of variation (CV) of 2.6%, an average interassay CV of 1.7%, a mean recovery of 99.6%, a linearity of R=1.00 from 2 to 250 mg/l, and an interference rate of <10% at haemoglobin concentrations of <1.82 g/l. The correlation (r) between assays was from 0.701 to 0.982, and the Bland–Altman plots indicated each assay provided significantly different results from each other. Conclusion: Nephelometry is the clinical urinary albumin method with best analytical performance in our study. J. Clin. Lab. Anal. 25:324–329, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: urinary albumin, methodological evaluation, comparison, nephelometry
Microalbuminuria refers to amount of albumin in urine beyond the upper limit of the normal reference but clinical urinary protein measurement is negative. The reference range of a random urine sample is 20–200 mg/l 1. Urinary albumin is an important and independent predictor of diabetic nephropathy, cardiovascular, and renal outcomes in a diverse group of people, including the general population 2, 3, 4. Furthermore, urinary albumin reduction translates to reduced cardiovascular events among hypertensive subjects 5, 6, 7. When urinary protein is positive, kidney damage is not expected to recover 8. The accurate and precise measurement of urinary albumin is critical because even small increases in urinary albumin predict the progression of renal diseases and cardiovascular events 9, 10, 11. Traditionally, urinary albumin is measured using antibody‐based methods, including radioimmunoassay (RIA), enzyme‐linked immunosorbent assay (ELISA), nephelometry, and turbidimetry 12, 13. However, multicentric surveys revealed that the current urinary albumin measurement is composed of a number of different detection systems and lacks a generally accepted standard or reference 2, 3.
Based on the investigation of 50 hospitals in Tianjin, China 3, five common urinary albumin methods, including nephelometry, turbidimetry, colloidal gold method, RIA, and chemiluminescence immunoassay (CLIA), were selected for methodological evaluation and comparison. In the same investigation, most labs used urinary albumin concentration as an indicator for the evaluation of renal damage, so were the major labs in China.
MATERIALS
Patient Population
Forty patients with type 2 diabetes between 50 and 80 years old (half male and half female) were consecutively recruited from June to November in 2009 in Tianjin Union Medicine Centre in China. All patients tested were positive according to the WHO diagnostic criteria for diabetes, and urinary protein of these patients was negative. Twenty samples for verifying reference interval were from healthy people between 20 and 60 years old (half male and half female). The healthy people had no diabetes, cardiovascular disease, and without known renal disease or other diseases, not taking drugs may affect test results, and no history of alcohol abuse. Women in this study with diabetes or healthy were not menstruating or pregnant.
Fresh spot urine samples collected from midstream was obtained for urinary albumin measurement. The samples from patients were divided into aliquots and stored in plastic tubes at −80°C, samples were resumed to room temperature before testing, and the comparison test was completed in 3 days. Twenty samples from healthy people were tested within 2 hr since samples collected.
Instruments and Reagents
Urine samples were centrifuged at 650×g for 10 min before analysis. Before the test, a calibration curve was generated by assaying a series of calibrators supplied by the manufacturer. All methods were performed according to the instructions of each manufacturer (Table 1). The interfering substance was provided by SYSMEX (Japan SYSMEX Interference Check A Plus). The urinary albumin standard for recovery test was Dade Behring N protein standard SL, which could be traced back to the ERM‐DA470.
Table 1.
Instruments and Reagents for Five Urinary Albumin Measurements
| Instruments | Reagents | |
|---|---|---|
| Turbidimetry | Abbott Architect C8000 automatic biochemical analyzer | Randox Microalbumin kit |
| Nephelometry | Siemens BN ProSpec special protein analyzer | Manufacturer provided |
| Colloidal gold | Norway NycoCard Reader II multifunctional quantitative gold standard detector | Manufacturer provided |
| RIA | ShangHai Hefu optical instrument Corporation SN‐695 gamma RIA measurement system | Beijing Beifang Biotechnology Institute albumin RIA analysis kit |
| CLIA | Siemens Immulite 1000 analysator | Manufacturer provided |
RIA, radioimmunoassay; CLIA, chemiluminescence immunoassay.
Methodological Evaluation
Methodological evaluation study was performed on five urinary albumin measurements.
Intraassay imprecision
Two urinary samples from two patients (urinary albumin concentration <20 mg/l and 150 mg/l < albumin concentration <200 mg/l) were continuously measured for 20 runs, and intraassay coefficient of variation (CV) was determined.
Interassay imprecision
Twenty aliquots of two samples from two patients (urinary albumin concentration <20 mg/l and 150 mg/l < albumin concentration <200 mg/l) were used to measure urinary albumin in 1 day (five runs, four aliquots in each run). The interassay CV was then obtained.
Linearity studies
Two samples from two patients (urinary albumin concentration <20 mg/l and 150 mg/l < albumin concentration <200 mg/l) were mixed at different proportions, and five mixtures with serial urinary albumin concentrations were obtained. Urinary albumin was tested four times in each method and a linearity R was determined from 2 to 200 mg/l or from 2 to 60 mg/l (CLIA).
Recovery test
The recovery materials were prepared by the addition of standard albumin to a urinary sample with normal albumin concentration (<20 mg/l). Serial dilutions were performed to achieve concentrations of 5.5, 11.1, and 22.2 mg/l. The mixtures were added to a sample with high urinary albumin concentration (150 mg/l < albumin concentration <200 mg/l). The urinary albumin was measured and the recovery rate was determined.
Interference test
The basic sample was from a type 2 diabetic patient (50 mg/l < urinary albumin concentration <100 mg/l). According to the instructions of SYSMEX Interference Check A Plus, serial dilutions were performed to achieve haemoglobin concentrations of 0.11, 0.23, 0.46, 0.61, 0.91, 1.82, and 2.74 g/l, which were added to the basic samples, and then the albumin was measured to determine the interference rate.
Verify reference interval
Twenty healthy population samples measured urinary albumin by each method to verify whether or not the value was less than 20 mg/l.
Method Comparison
The urinary albumin of 40 samples was quantified using nephelometry, turbidimetry, colloidal gold method, RIA, and CLIA. Each system was compared with nephelometry.
Statistical Analysis
Statistical analysis was carried out with MedCalc statistics for biomedical research. Comparative analysis was performed between the various assays studied by scatter plots combined with correlation and regression analysis, as well as by difference plots (Bland–Altman bias plots) combined with bias calculations and 95% confidence intervals.
RESULTS
Methodological Evaluation
Results of the methodological evaluation are shown in Table 2, including the average intraassay and interassay imprecision CV, linearity (R), and average recovery. Nephelometry yielded better results compared with the other methods.
Table 2.
Result of Methodological Evaluation
| Intraassay and interassay imprecisiona (CV) (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Verify reference interval | |||||||
| Method | Mean** (mg/l) | Intra | Inter | Mean** (mg/l) | Intra | Inter | Linearity (R) | Recoverya (%) | (mg/l) (%)c |
| Nephelometry | 12.7 | 3.0 | 2.2 | 165 | 2.1 | 1.3 | 1.00 | 99.6 | 2.4–18.6 (100) |
| Turbidimetry | 15.8 | 5.7 | 4.9 | 187.4 | 4.3 | 3.2 | 0.95 | 97.8 | 4.8–32.7 (90) |
| Colloidal gold method | 18 | 2.9 | 2.0 | 186 | 3.3 | 1.5 | 0.99 | 96.3 | (<5)−15 (100) |
| RIA | 14.8 | 7.8 | 8.6 | 173.6 | 6.1 | 3.4 | 0.99 | 99.8 | 2.8–21.1 (95) |
| CLIA | 20.1 | 5.8 | 3.2 | 208.2 | 5.5 | 3.1 | 0.98 | 101.6 | 5.7–37.4 (80) |
RIA, radioimmunoassay; CLIA, chemiluminescence immunoassay.
aIntraassay and interassay imprecision CV, and recovery were represented as average.
bThese values represent the mean value of samples in intraassay imprecision by different methods.
c“%”means what the percentage of urinary albumin concentration from 20 healthy people were less than 20 mg /l.
Haemoglobin Interference Test
A haemoglobin interference test was performed on nephelometry, turbidimetry, colloidal gold method, and RIA. The average urinary albumin concentration in the basic sample was 46.5 mg/l by nephelometry, 87.8 mg/l by turbidimetry, 54.5 mg/l by colloidal gold method, and 70.0 mg/l by RIA. Haemoglobin had negative interference on the four methods (Table 3), and the interference rate increased as the haemoglobin concentration increased. However, haemoglobin produced a positive interference when haemoglobin concentration was ≥0.46 g/l using turbidimetry.
Table 3.
Interference Test of Four Methods
| Hemoglobin concentration (g/l) | Nephelometry (%) | Turbidimetry (%) | Immuno‐colloidal gold method (%) | RIA (%) |
|---|---|---|---|---|
| 0.11 | −6.9 | −1.8 | −10.1 | −2.2 |
| 0.23 | −7.7 | −2.6 | −10.1 | −4.3 |
| 0.46 | −7.7 | 1.7 | −11.9 | −10.0 |
| 0.61 | −8.4 | 6.7 | −17.4 | −8.7 |
| 0.91 | −8.8 | 14.1 | −13.8 | −10.4 |
| 1.82 | −9.5 | 26.3 | −17.8 | −11.8 |
| 2.74 | −11.4 | 27.8 | −21.1 | −12.5 |
Method Comparison
Each method was compared with nephelometry, which served as the reference method for this study because it performed best in methodological evaluation. Results of the correlation (Fig. 1) and Bland–Altman bias analyses (Fig. 2) are shown in Table 4.
Figure 1.
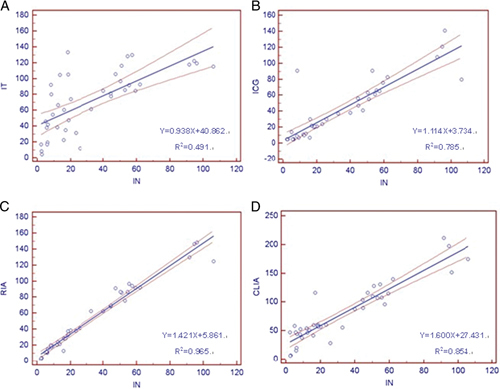
Regression lines between every two methods for quantitating urinary albumin concentration expressed as milligrams per liter. The dashed lines represent 95% confidence intervals for the regression line. This interval includes the true regression line with 95% probability. IN, nephelometry; IT, turbidimetry; ICG, immuo‐colloidal gold method; RIA, radioimmunoassay; CLIA, chemiluminescence immunoassay. (A) Nephelometry vs. turbidimetry, (B) nephelometry vs. immuo‐colloidal gold method, (C) nephelometry vs. radioimmunoassay, and (D) nephelometry vs. chemiluminescence immunoassay.
Figure 2.
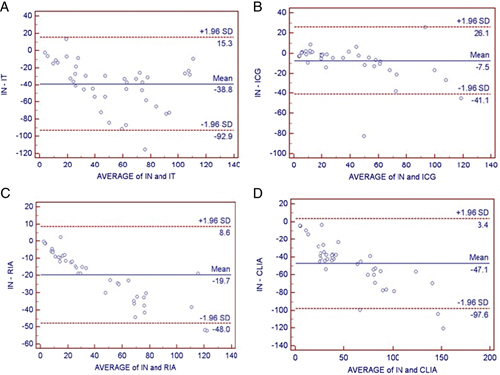
Bland–Altman bias plots between every two methods for quantitating urinary albumin concentration expressed as milligrams per liter. The solid lines represent the mean differences between the assays, and the dashed lines represent the bias±1.96 SD limits. IN, nephelometry; IT, turbidimetry; ICG, immuo‐colloidal gold method; RIA, radioimmunoassay; CLIA, chemiluminescence immunoassay. (A) Nephelometry vs. turbidimetry, (B) nephelometry vs. immuo‐colloidal gold method, (C) nephelometry vs. radioimmunoassay, and (D) nephelometry vs. chemiluminescence immunoassay.
Table 4.
Result of Method Comparison
| Correlation | 95% Limits of agreement | |||||||
|---|---|---|---|---|---|---|---|---|
| Assays | N | Mean (mg/l) | coefficient (r) | P value | Bias | SD of bias | From | To |
| IN vs. IT | 40 | IN 32.8; IT 71.7 | 0.701 | 0.000 | −38.8 | 27.6 | −92.9 | 15.3 |
| IN vs. IG | 40 | IN 32.8; IG 40.3 | 0.886 | 0.399 | −7.5 | 17.1 | −41.1 | 26.1 |
| IN vs. RIA | 40 | IN 32.8; RIA 52.5 | 0.982 | 0.027 | −19.7 | 14.4 | −48.0 | 8.6 |
| IN vs. CLIA | 40 | IN 32.8; CLIA 80.0 | 0.931 | 0.000 | −47.1 | 25.8 | −97.6 | 3.4 |
P value>0.05 indicates the significance between two methods. IN, nephelometry; IT, turbidimetry; IG, immuno‐colloidal gold method; RIA, radioimmunoassay; CLIA, chemiluminescence immunoassay.
Figure 1 shows the regression lines for nephelometry vs. turbidimetry (Fig. 1A), vs. colloidal gold method (Fig. 1B), vs. RIA (Fig. 1C), and vs. CLIA (Fig. 1D). The dashed lines in Figure 1 represent 95% confidence intervals for the regression line. This interval includes the true regression line with 95% probability.
Figure 2 shows the Bland–Altman plots. The solid lines represent the mean differences between the assays, and the dashed lines represent the bias±1.96 SD limits. The results of the bias±1.96 SD limits, including 95% confidence intervals are shown in Table 4. The limits of agreement appear to be high for each Bland–Altman plot, indicating that each assay gives significantly different results compared with nephelometry.
DISCUSSION
Urinary albumin is one of the earliest indicators of diabetic nephropathy. Early treatment with antihypertensive agents and improvements in glycemic control may slow or even prevent progression to persistent albuminuria and end‐stage renal failure 14. In the literature, method comparison of urinary albumin measurement mostly refers to one or two methods, such as turbidimetry, ELISA, and RIA 15, 16, 17, 18, 19. Currently, many investigations have focused on high‐performance liquid chromatography (HPLC) or liquid chromatography–tandem mass spectrometry (LC‐MS/MS), both of which quantify the intact urinary albumin 12, 14, 20, 21. In addition, Seegmiller demonstrated that LC‐MS/MS after trypsin digestion is a viable reference method for quantifying urinary albumin. This method improved analytical performance in the clinically relevant range compared with a commercially available turbidometric assay. Generally, HPLC and LC‐MS/MS are not very suitable for clinical laboratory, and immunoassays are more convenient. Therefore, five common urine albumin assays were selected for methodological evaluation and comparison.
In this study, the correlation and regression calculated for method comparison showed linear relationships (0.701–0.982) between nephelometry and the other methods. These results were confirmed by the Bland–Altman bias plots and the limits of agreement (Fig. 2 and Table 4). The Bland–Altman analysis for the comparison between assays shows that there are considerable differences between various assays. The average concentration of urinary albumin indicated by the other four methods is higher than that of nephelometry: 2.2‐fold higher for turbidimetry, 1.2‐fold higher for the colloidal gold method, 1.6‐fold higher for RIA, and 2.4‐fold higher for CLIA. Similar variations in immunoassays have been found by others: a 1.6‐fold increase was found using turbidimetry (ACA IV analyser using reagents from Dade) compared with nephelometry (Array 360 analyser using reagents from Beckman, Ireland), and calibrator cross‐over experiments demonstrated that some of the bias of methods could be accounted for by calibrator differences 22. However, contradictory results have also been observed: a threefold difference was found for urinary albumin concentrations measured using a Beckman nephelometry assay compared with Dade–Behring turbidimetry assay 14. Moreover, the result of verifying reference interval also demonstrates there are differences between assays. Urinary albumin has different antigenic site, antibody from different manufacturers could react with different urinary albumin or different antigenic sites of urinary albumin, and there has no reference material of urinary albumin, this maybe the reason can explain significantly different among assays.
Many factors influence urinary albumin measurement, including the methods used for sample acquisition, sample preservation, and so on. In general, haemoglobin produces a positive interference in majority of serum analyses 23, but there is no consistent conclusion regarding its effects during urine analysis. Haemoglobin interferes with the result during urinary albumin measurement when it is present in the urine of patients with renal damage 8, 24. Therefore, seven different haemoglobin concentrations were selected to perform an interference test. These concentrations covered three positive values of occult blood (OB) in urine: OB± (urinary haemoglobin concentration of 0.3 g/l), OB+ (0.75 g/l), and OB2+ (2.4 g/l). The results show that haemoglobin can interfere with urinary albumin determination using nephelometry, turbidimetry, colloidal gold method, RIA, even at OB± levels.
Five conventional methods for measuring urinary albumin concentration have been initially evaluated, and the results suggest that nephelometry performed best in methodological evaluation. Moreover, considerable differences were observed among the various assays, and the result of verifying reference interval confirmed this conclusion. These data indicate that the methodology chosen to quantify urinary albumin is important when assessing the presence of nephropathy in diabetic patients because inaccurate measurement could lead to incorrect therapeutic choices. Maybe different laboratories or different methods need to develop their own biological reference interval, and the technique employed for urinary albumin measurement must be carefully considered. Further studies of urinary albumin measurement should involve a larger sample size for the comparative test and include an interference test on CLIA. The results of the study hopefully provided some insights into the use of these methods in clinical practice.
References
- 1. Ning T. Clinical significance of microalbuminuria. Pract Med Tech 2008;15:4596–4597. [Google Scholar]
- 2. Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem 2009;55:24–38. [DOI] [PubMed] [Google Scholar]
- 3. Mu XY, Liu R, Cui XF, Yang QH. Current investigation of serum creatinine and urinary albumin measurement in 50 hospitals in Tianjin. Lab Med 2010;25:468–470. [Google Scholar]
- 4. Gansevoort RT, Nauta FL, Bakker SJ. Albuminuria: All you need to predict outcomes in chronic kidney disease? Curr Opin Nephrol Hypertens 2010;19:513–518. [DOI] [PubMed] [Google Scholar]
- 5. Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension 2005;45:198–202. [DOI] [PubMed] [Google Scholar]
- 6. Lee MY, Lin KD, Chang YH, Hsiao PJ, Shin SJ. Albuminuria is the stronger risk factor for peripheral arterial disease than eGFR decline in a type 2 diabetic Taiwanese population. Kidney Blood Press Res 2010;33:352–359. [DOI] [PubMed] [Google Scholar]
- 7. Aguilar MI, O'Meara ES, Seliger S, et al. Albuminuria and the risk of incident stroke and stroke types in older adults. Neurology. 2010;75:1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng SQ, Zhu MC. Microalbuminuria in early diagnosis of diabetic renal injury. J Lab Med 2006;28:740–741. [Google Scholar]
- 9. Lambers Heerspink HJ, Gansevoort RT, Brenner BM, et al. Comparison of different measures of urinary protein excretion for prediction of renal events. Clin J Am Soc Nephrol 2010;21:1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meigs JB, D'Agostino RB Sr, Nathan DM, Rifai N, Wilson PW. Longitudinal association of glycemia and microalbuminuria: The Framingham Offspring Study. Diabetes Care 2002;25:977–983. [DOI] [PubMed] [Google Scholar]
- 11. Cao JJ, Biggs ML, Barzilay J, et al. Cardiovascular and mortality risk prediction and stratification using urinary albumin excretion in older adults ages 68–102: The Cardiovascular Health Study. Atherosclerosis 2008;197:806–813. [DOI] [PubMed] [Google Scholar]
- 12. Shaikh A, Seegmiller JC, Borland TM, et al. Comparison between immunoturbidimetry, size‐exclusion chromatography, and LC‐MS to quantify urinary albumin. Clin Chem 2008;54:1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brinkman JW, Bakker SJ, Gansevoort RT, et al. Which method for quantifying urinary albumin excretion gives what outcome? A comparison of immunonephelometry with HPLC. Kidney Int 2004;92:S69–S75. [DOI] [PubMed] [Google Scholar]
- 14. Comper WD, Jerums G, Osicka TM. Osicka. Differences in urinary albumin detected by four immunoassays and high‐performance liquid chromato‐ graphy. Clin Biochem 2004;37:105–111. [DOI] [PubMed] [Google Scholar]
- 15. Gu XQ, Zhang XL, Xu JY, Yan MX, Guang L. Explore the immune turbidimetry assay urinary micro‐albumin. Mod Lab Med J 2004;19:36–37. [Google Scholar]
- 16. Wang YC, Chen RS, Tao SC. Comparison of microalbuminuria methods. J Clin Microbiol 1995;18:266. [Google Scholar]
- 17. Pan AP. Comparison and clinical application of microalbuminuria testing among Immunoturbidimetric assay and radioimmunoassay. Jingxi Med Lab Sci 2003;21:26. [Google Scholar]
- 18. Zhan BE, Lee WX, Liang GJ. Clinical evaluation of ELISA and chemiluminescent immunoassay method on urinary albumin measurement. China Trop Med 2008;8:2147. [Google Scholar]
- 19. Bao LiM, Qi RM, Gao X. Evaluation and clinical application of ELISA assay on urinary albumin measurement. Chin J Health Lab Technol 2006;16:227–228. [Google Scholar]
- 20. Seegmiller JC, Barnidge DR, Burns BE, Larson TS, Lieske JC, Kumar R. Quantification of urinary albumin by using protein cleavage and LC‐MS/MS. Clin Chem 2009;55:1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seegmiller JC, Sviridov D, Larson TS, Borland TM, Hortin GL, Lieske JC. Comparison of urinary albumin quantification by immunoturbidimetry, competitive immunoassay, and protein‐cleavage liquid chromatography–tandem mass spectrometry. Clin Chem 2009;55:1991–1994. [DOI] [PubMed] [Google Scholar]
- 22. Roberts WL, Calcote CB, Cook CB, et al. Comparison of four commercial urinary albumin (microalbumin) methods: Implications for detecting diabetic nephropathy using random urine specimens. Clin Chem Acta 1998;273:21–23. [DOI] [PubMed] [Google Scholar]
- 23. Liu L. The primary study on interference mechanism of hemoglobin, bilirubin and lipohemia on automatic biochemical analyzer. Shan Xi Clin Med J 2000;9:718–719. [Google Scholar]
- 24. Piao JZ, Nie MC. Investigation of urinary micro‐albumin measurement. J Chenzhou Med Coll 2003;5:31–32. [Google Scholar]


