Abstract
Background: Nitrite and nitrate are exhaled in droplets of an aerosol during breathing and can be assayed in the exhaled breath condensate (EBC) as markers of nitrossative stress in the airways of patients with asthma, COPD, and idiopathic pulmonary fibrosis (IPF). Subjects and methods: Using HPLC with fluorescence detection, nitrite and nitrate were assayed in EBC of 14 atopic patients with mild‐to‐moderate stable asthma, 18 atopic asthmatics with exacerbation, 14 COPD patients without exacerbation, 18 patients with exacerbated COPD, 13 patients with active IPF, and in 29 healthy subjects. Results: The geometric mean [exp(mean±SD)] EBC concentrations of nitrite (micromol/l) in patients with asthma [5.1(2.1–12.3)], exacerbation of asthma [5.1(2.8–9.6)], exacerbation of COPD [5.3(3.2–8.7)], and with IPF [5.5(2.9–10.2)] were higher (P<0.05) compared with those of healthy subjects [2.9(1.6–5.3)] and patients with stable COPD [3.0(1.3–6.7)]. Nitrite concentration increased with decreased lung function of patients with asthma (r s=−0.31, P<0.02). Presumably owing to the contamination of the EBC sample with nitrate during collection, nitrate levels were highly variable among healthy subjects and higher compared with all groups of patients. Conclusion: EBC nitrite is a suitable marker of nitrossative stress in adult patients with lung diseases but cannot differentiate controlled and exacerbated asthma. Further improvements to the methods of EBC collection and sample handling are warranted. J. Clin. Lab. Anal. 24:317–322, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: nitrossative stress, breath condensate, nitrite, nitrate, pulmonary diseases
INTRODUCTION
Because the initial information that nitric oxide (NO) may be detected in exhaled air of humans 1 lots of studies have been published describing concentrations of NO and other markers in both healthy subjects and patients with inflammatory disease of airways 2, 3, 4, 5.
Endogenous NO is synthesized during the conversion of l‐arginine to l‐citruline, catalyzed by NO synthases (NOS). Three isoforms of NOS enzyme coded by different genes were described. Neuronal nNOS and endothelial eNOS produce relatively small amount of NO (picomols), which plays an important homeostatic role in many physiological and pathophysiological processes. Expression of the inducible NOS (iNOS) is controlled by proinflammatory stimuli and leads to a generation of much higher nanomolar level of NO in case of inflammatory processes of the respiratory tract 6. NO can be detected in the exhaled air. Nitrite and nitrate can be assayed in liquids of respiratory tract at the concentrations reflecting velocities of NO formation and oxidative metabolism.
Nitrite, nitrate, and other nonvolatile compounds are exhaled in droplets of an aerosol carried away from the respiratory tract during breathing. By cooling the expired gas during tidal breathing into an appropriate condenser it is possible to examine their concentrations noninvasively in exhaled breath condensate (EBC). Besides biomarkers of oxidative and nitrosative stress, a variety of other inflammatory mediators have been investigated in EBC, including adenosine, isoprostanes, leukotrienes, prostanoids, and cytokines. Noninvasive profiling of inflammatory biomarkers in EBC could help to classify the severity and type of inflammation in acute and chronic asthma, chronic obstructive pulmonary disease, and other lung diseases, and could be used for diagnosis and monitoring of therapy of these diseases 7.
This study aimed to measure the levels of nitrite and nitrate as biomarkers of nitrosative stress, in the EBC of adult patients with asthma, COPD, and idiopathic pulmonary fibrosis (IPF).
METHODS
This study was approved by the ethics committee of Charles University and informed consent was obtained from all subjects. The studied population included 106 subjects divided into the following subgroups: (a) 14 patients with mild persistent atopic asthma, (b) 18 asthmatics with an acute exacerbation, (c) 14 COPD patients without exacerbation, (d) 18 patients with exacerbated COPD, (e) 13 patients with active IPF, (f) 17 healthy nonsmokers, and (g) 12 smokers without any symptoms of pulmonary disease and with normal results of pulmonary function tests.
Patients with atopic asthma and COPD were recruited from the Department of Respiratory Diseases of the Regional Thomas Bata Hospital, Zlín, Czech Republic. The diagnosis was based on clinical history and examination and on measurement of pulmonary function parameters, according to international guidelines 8, 9. Patients had stable asthma and were taking neither inhaled nor oral corticosteroids during 2 months preceding the investigation or longer. Patients with asthma exacerbation were presenting to the Department of Respiratory Diseases with acute worsening of the disease and pharmacotherapy with corticosteroids was initiated after the EBC collection. Asthmatics used inhaled β2 agonists on demand. COPD patients were taking inhaled anticholinergics or β2 agonists.
Patients with IPF were selected among the patients of the Department of Pulmonary Medicine, 3rd Faculty of Medicine, Charles University, Prague, Czech Republic. They had the diagnosis confirmed by a surgical lung biopsy demonstrating presence of interstitial pneumonia coupled with appropriate clinical findings and signs of active disease proven by bronchoalveolar lavage and high‐resolution computer tomography 10.
Healthy subjects had no history of any chronic lung disease, allergy, or any other chronic illness including recurrent respiratory infections, and were taking no medication.
The collection of the EBC was done using EcoScreen II condenser (Jaeger Toennies, Germany). Patients breathed in rest condition for a period of 15 min using a nose clip. The EBC sample was frozen directly in the collecting cup to −70°C. It was then transported in dry ice to the laboratory and thawed only once before the analysis. The concentrations of nitrite and nitrate were determined using liquid chromatography with fluorescence detection validated in our earlier work 11. Briefly, nitrite concentration was assayed after its precolumn derivatization with 2,3‐diaminonaphthalene. The sum of nitrite and nitrate concentrations was determined after enzymatic conversion of nitrate into nitrite using bacterial nitrate reductase as originally described by Misko et al. 12. Nitrate concentration was calculated as the difference between the two results.
Statistical analyses were performed with Statistica (version 8.0, StatSoft, Inc., Tulsa, OK). Because the EBC nitrite and nitrate concentrations had a skewed distribution to the right, they were log‐transformed before statistical analysis with parametric tests. As descriptive characteristics, the geometric mean was used as a central value and antilogs of the mean±SD (log‐transformed data) were used as spread indicators. One‐way analysis of variance on the logarithmically transformed concentrations and Tukey's multiple comparison tests were used to compare EBC nitrite and nitrate between groups. Spearman's correlation was used to evaluate the relationship between lung function and nitrite concentration. Significance was defined as P<0.05.
RESULTS
Clinical and physiological characteristics of studied subjects are summarized in Table 1. The concentrations of nitrite and nitrate in EBC are given in Table 2.
Table 1.
Characteristics of Subjects
| Group | N | Sex (M/F) | Agea (years) | FEVl b(%) | TLC (%) |
|---|---|---|---|---|---|
| Asthma with exacerbation | 18 | 11/9 | 54.6±17.6 | 81.2±20.0 | 108±11.3 |
| Asthma without exacerbation | 14 | 9/6 | 51.3±18.8 | 94.4±14.4 | 102±11.2 |
| COPD with exacerbation | 18 | 16/6 | 68.3±12.0 | 52.5±22.3 | 108±12.2 |
| COPD without exacerbation | 14 | 20/5 | 67.4±7.00 | 72.2±21.9 | 101±13.1 |
| IPF | 13 | 9/9 | 57.3±15.1 | 87.6±22.2 | 84.5±19.3 |
| Healthy smokers | 12 | 7/4 | 41.2±14.8 | 115±8.8 | 107±9.8 |
| Healthy nonsmokers | 17 | 11/7 | 35.9±11.0 | 111±10.4 | 100±10.6 |
| All healthy subjects | 29 | 18/11 | 40.0±12.6 | 112±10.0 | 103±10.6 |
Table shows arithmetic means±SD.
aAll groups of patients were older compared with healthy subjects (P<0.05).
bAll groups of patients had a lower FEV1 compared with healthy subjects (P<0.01) and FEV1 of patients with COPD exacerbation was less compared with COPD patients without exacerbation (P<0.05).
Table 2.
Nitrite and Nitrate Concentrations in Exhaled Breath Condensate
| Nitritea (µmol/l) | Nitrateb (µmol/l) | |||||
|---|---|---|---|---|---|---|
| N | G. mean | G. mean±SD | N | G. mean | G. mean±SD | |
| Asthma with exacerbation | 18 | 5.09 | 2.11–12.3 | 18 | 30.5 | 16.1–58.0 |
| Asthma without exacerbation | 14 | 5.13 | 2.75–9.56 | 14 | 33.1 | 14.2–77.6 |
| COPD with exacerbation | 18 | 5.26 | 3.17–8.74 | 16 | 16.6 | 8.67–31.8 |
| COPD without exacerbation | 14 | 2.96 | 1.32–6.67 | 13 | 18.9 | 7.68–46.5 |
| IPF | 13 | 5.47 | 2.94–10.2 | 12 | 18.1 | 11.2–29.1 |
| Healthy smokers | 12 | 3.56 | 2.15–5.89 | 11 | 70.4 | 22.8–217 |
| Healthy nonsmokers | 17 | 2.49 | 1.31–4.72 | 16 | 52.0 | 19.5–139 |
| All healthy subjects | 29 | 2.89 | 1.58–5.28 | 27 | 58.9 | 20.9–165 |
Table shows geometric means and antilogs of the intervals (mean±SD of logarithmically transformed concentrations).
aAll groups of patients except of COPD without exacerbation had higher nitrite concentrations compared with healthy subjects (P<0.01), exacerbation increased the concentrations in COPD patients (P<0.05).
bThe concentrations of nitrates in the groups of healthy subjects were higher than results of patients (P<0.01).
Nitrite levels in EBC of healthy smokers were 43% higher than that of nonsmokers. The difference, however, did not reach statistical significance (P=0.12). Concentrations of EBC nitrite were higher in patients with asthma and exacerbated asthma compared with healthy individuals (P<0.05), but there was no difference between groups of patients with and without exacerbation of the disease (Fig. 1). Correlation analysis in both groups of asthmatic patients and healthy individuals showed that the concentration of nitrite increased with decreasing FEV1 (r s=−0.31, P<0.02) (Fig. 2). If asthma patients with and without exacerbation were analyzed, the correlation between nitrite levels and FEV1 was borderline significant (P=0.06). After exclusion of one smoker with asthma exacerbation and three smokers with stable asthma and comparison of their nitrite levels with those of healthy nonsmokers, the results of either between‐group comparison or correlation analysis remained unchanged (data not shown).
Figure 1.
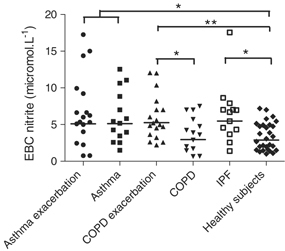
Nitrite concentration in the exhaled breath condensate (EBC) of healthy individuals, asthmatic patients, COPD patients, and patients with idiopathic pulmonary fibrosis (IPF). Horizontal lines are geometric means. * P<0.05, ** P<0.01.
Figure 2.
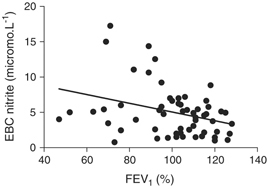
Correlation between lung function and nitrite concentration in exhaled breath condensate (EBC) in asthmatic patients and healthy individuals (r s=−0.31, P<0.02).
Nitrite concentration in EBC of patients with exacerbation of COPD was higher than in patients without exacerbation (P<0.05) and in healthy individuals (P<0.01), whereas patients without exacerbation had comparable results with healthy individuals (P=0.73) (Fig. 1). The correlation between the concentration of EBC nitrite and FEV1 did not reach statistical significance (P=0.12). Exclusion of eight smokers with COPD exacerbation and eight smokers with stable COPD did not change the results of statistical analysis (data not shown).
Patients with IPF (including two smokers) had higher levels of EBC nitrite compared with healthy controls (P<0.05) (Fig. 1). Nonsmokers with IPF had higher concentrations than healthy nonsmokers (P<0.05).
Surprisingly, the concentration of EBC nitrate in the groups of healthy nonsmokers and smokers showed a very high interindividual variability and were higher (P<0.01) than results of patients (Table 2). Differences between other groups were not found.
DISCUSSION
This study shows that the concentration of nitrite in EBC is increased in patients with asthma, IPF, and exacerbation of COPD indicating an increased nitrosative stress in the airways. In stable COPD, the level of EBC nitrite is comparable to healthy subjects.
Finding of the highest nitrate concentrations in healthy individuals, the average concentration in this group, and a very high interindividual variability are surprising. These results may, most probably, be explained by contamination of EBC with nitrate during collection. The contamination during sample processing and analysis is unlikely, as calibration standards and quality control samples were assayed in parallel in every analytical batch and no contamination was observed. Experience of other authors pointed to the fact that, in simulated conditions, EcoScreen condenser caused contamination of physiological solution and deionized water with up to 80 µmol/l nitrate 13. Other authors described three to four times worse short‐term repeatability (%CV) of nitrate concentration compared with nitrite of healthy subjects and asthmatic patients, which indirectly proves methodological problems of measuring nitrate in EBC collected using EcoScreen condenser 11.
Nitrite and nitrate are formed by oxidation of NO, which proceeds very quickly in human organism. The biological liquids of respiratory tract (liquid from bronchoalveolar lavage, condensate of expired air) contain mostly nitrate, which corresponds to the results of our study. Comparing our results with concentrations of nitrite and nitrate in studies of other authors is difficult owing to the differences in methods of EBC collection and analysis. If we focus on the results of healthy subjects, our results agree very well with findings obtained by other authors who used the EcoScreen condenser. Balint et al. reported similar concentrations of EBC nitrite using the same analytical method 14. Cruz et al. found very similar concentrations, but by using a commercial immunoassay 15. Other authors describe comparable values of nitrite in EBC collected into simple coolers of their own construction 16. Most studies, except that of Corradi et al., show lower concentrations of nitrate 15, 17, 18.
To our best knowledge, this is the first report on nitrite measurement in the EBC of patients with active IPF. Our finding of significantly elevated nitrite levels supports the evidence obtained with other experimental methods of increased nitrossative and oxidative stress associated with this lung disease. Saleh et al. used immunohistochemistry, histochemistry, and in situ hybridization, and found increased expression of nitrotyrosine and iNOS in macrophages, neutrophils, and alveolar epithelium in lungs of patients with active IPF compared with healthy controls 19. Patients with active IPF exhale higher concentrations of NO compared with healthy subjects 20 and have increased nitrite and nitrate concentrations in the bronchoalveolar lavage fluid 21, 22. An increased oxidative stress associated with IPF was repeatedly documented on the local as well as systemic level using exhaled ethane concentrations 23, EBC hydrogen peroxide and 8‐isoprostane concentrations 24 and other markers 25.
In the airways of patients with atopic asthma, formation of NO is augmented as a result of the induction of iNOS enzyme in the structural as well as inflammatory cells. Steroid‐naive patients with atopic asthma exhale high concentrations of NO 26. In accordance with our results, published articles 27, 28, 29 also reported higher concentration of nitrite in asthmatic patients. Practical usability of these findings, however, remains unclear as most of the studies did not prove relationship between the concentration of NO metabolites and the level of asthma control 29, 30. Only one study, so far, carried out on asthmatic children proved the importance of simultaneous examination of several markers in EBC together with the fractional concentration of NO in exhaled air (FENO) as indicators reflecting asthma control (FENO, 8‐isoprostane, γ‐interferon a IL‐4) and severity (8‐isoprostane, nitrite, nitrate, and FENO) 31. Negative results of some published studies may in part be owing to small numbers of patients in the groups and imprecision in evaluation of disease severity. The concentration of EBC nitrite in our study did not allow differentiating between controlled asthma and exacerbation, but it increased in patients with compromised lung function (decreased FEV1). Similarly, Ueno et al. found negative correlation between FEV1 and concentrations of nitrite and nitrate of asthmatic patients 29.
COPD patients exhale lower concentrations of NO in comparison to patients with asthma 32. According to the conclusions of most studies, the concentrations of exhaled NO of stable COPD patients are the same as those of healthy individuals 33, 34, 35. Other authors describe two to three‐fold elevated FENO as a maximum 17, 36, 37. COPD exacerbation is accompanied by an increase in FENO 36, which does not respond to inhaled corticosteroids as rapidly as in asthmatic patients. Unlike after asthma exacerbation, FENO concentrations decrease gradually during months after cessation of COPD exacerbation 38.
Relatively few articles focused on the concentrations of nitrate and nitrite of COPD patients, which might be owing to methodological problems of these assays 39. The EBC concentration of nitrite in our study was higher in patients with exacerbation of COPD than in stable disease and in healthy individuals. Corradi et al. found, compared with healthy smokers and nonsmokers, higher concentrations of nitrite of nonsmokers with COPD without exacerbation during one month preceding the investigation 40. Another study of Corradi et al. looked into EBC nitrate and described higher concentration in healthy smokers and asthmatic patients compared with healthy nonsmokers, whereas COPD patients selected using the same inclusion criteria, as in their earlier study, did not have higher values 18. Liu et al. focused on the impact of smoking and COPD on the sum of nitrite and nitrate (NOx). According to separate evaluations done with smokers and nonsmokers, COPD did not have any influence on the concentrations of NOx 17. The same study showed two‐fold elevated NOx concentrations in nonsmokers with more serious COPD, who were treated by inhaled corticosteroids, compared with nonsmokers with less severe disease without corticosteroid treatment. More severe COPD also causes higher concentrations of exhaled NOx 41.
Smoking complicates interpretation of concentrations of FENO and its metabolites in the EBC. It decreases FENO concentrations of healthy individuals 42. Moreover, the extent of the decrease correlates with the cigarette smoke exposure as measured by the concentration of cotinine in the serum 43. The study published by Balint et al. shows healthy smokers with similar EBC nitrite and nitrate concentrations as in nonsmokers 14. Corradi et al., on the contrary, reported a five‐fold increased values of smokers 18. Acute nitrosative and oxidative stress caused by cigarette smoke increased the EBC concentrations of NOx 30 min after smoking two cigarettes followed by a decrease to initial values after 90 min 14. Our study showed only a trend toward higher concentrations of nitrite in healthy smokers compared with nonsmokers. The same conclusion was reported by Liu et al, who examined the concentrations of NOx 17. However, the intersubject variability of EBC concentrations of nitrite/nitrate was high and the studies were carried out on small numbers of subjects. For the same reason, we could not examine the impact of smoking in detail and separate it from the influence of exacerbation. When the data from healthy nonsmokers were compared with those of patients after exclusion of smokers, the study findings remained unchanged.
Comparison of EBC nitrite levels between healthy controls and patients in our study could be confounded by the fact that the groups were not age‐matched. The main reason why healthy subjects were younger was the difficulty of recruiting older controls with no coexistent chronic diseases and no medication. However, we believe that this fact represents no serious limitation for our conclusions because a recent well‐conducted study found no relationship between age and nitrite or nitrate levels in the EBC in the healthy subjects between 18 and 80 years of age 15.
We conclude that EBC nitrites are increased in adult corticosteroid‐naive patients with asthma, active IPF, and exacerbation of COPD, whereas in stable COPD the concentration is comparable to healthy subjects. Considerable interindividual variability in each group and the overlap between EBC nitrite concentrations of healthy and diseased subjects may limit the value of this biomarker of nitrossative stress in the airways. Further improvements to the methods of EBC collection and sample handling are warranted as documented by the nitrate results in this study.
REFERENCES
- 1. Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 1991;181:852–857. [DOI] [PubMed] [Google Scholar]
- 2. Ojoo JC, Mulrennan SA, Kastelik JA, Morice AH, Redington AE. Exhaled breath condensate pH and exhaled nitric oxide in allergic astma and in cystic fibrosis. Thorax 2005;60:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kharitonov SA, Yates D, Robbins RA, Logan‐Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxid in exhaled air in asthmatic pacients. Lancet 1994;343:133–135. [DOI] [PubMed] [Google Scholar]
- 4. Persson MG, Zetterström O, Agrenius V, Ihre E, Gustafsson LE. Single breath nitric oxide measurements in asthmatic patients and smokers. Lancet 1994;343:146–147. [DOI] [PubMed] [Google Scholar]
- 5. Silkoff PE, Robbins RA, Gaston B, Lundberg JO, Townley RG. Endogenous nitric oxide in allergic airway disease. J Allergy Clin Immunol 2000;105:438–448. [DOI] [PubMed] [Google Scholar]
- 6. Moncada S, Higgs. The l‐arginine‐nitric oxide pathway. N Engl J Med 1993;329:2002–2012. [DOI] [PubMed] [Google Scholar]
- 7. Horváth I, Hunt J, Barnes PJ, et al. ATS/ERS task force on exhaled breath condensate exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 2005;26:523–548. [DOI] [PubMed] [Google Scholar]
- 8. Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2007. Available from: http://www.ginasthma.org
- 9. Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2007. Available from: www.goldcopd.com
- 10. American Thoracic Society/European Respiratory Society . International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 11. Chladkova J, Krcmova I, Chladek J, Cap P, Micuda S, Hanzalkova Y. Validation of nitrite and nitrate measurements in exhaled breath condensate. Respiration 2006;73:173–179. [DOI] [PubMed] [Google Scholar]
- 12. Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 1993;214:11–16. [DOI] [PubMed] [Google Scholar]
- 13. Jackson AS, Sandrini A, Campbell C, Chow S, Thomas PS, Yates DH. Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage. Am J Respir Crit Care Med 2007;175:222–227. [DOI] [PubMed] [Google Scholar]
- 14. Balint B, Donnelly LE, Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitric oxide metabolites in exhaled breath condensate after exposure to tobacco smoke. Thorax 2001;56:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cruz MJ, Sánchez‐Vidaurre S, Romero PV, Morell F, Muñoz X. Impact of age on pH, 8‐isoprostane, and nitrogen oxides in exhaled breath condensate. Chest 2009;135:462–467. [DOI] [PubMed] [Google Scholar]
- 16. Nightingale JA, Rogers DF, Barnes PJ. Effect of inhaled ozone on exhaled nitric oxide, pulmonary function, and induced sputum in normal and asthmatic subjects. Thorax 1999;54:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Sandrini A, Thurston MC, Yates DH, Thomas PS. Nitric oxide and exhaled breath nitrite/nitrate in chronic obstructive pulmonary disease patients. Respiration 2007;74:617–623. [DOI] [PubMed] [Google Scholar]
- 18. Corradi M, Pesci A, Casana R, et al. Nitrate in exhaled breath condensate of patients with different airway diseases. Nitric Oxide 2003;8:26–30. [DOI] [PubMed] [Google Scholar]
- 19. Saleh D, Barnes PJ, Giaid A. Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1997;155:1763–1769. [DOI] [PubMed] [Google Scholar]
- 20. Paredi P, Kharitonov SA, Loukides S, Pantelidis P, du Bois RM, Barnes PJ. Exhaled nitric oxide is increased in active fibrosing alveolitis. Chest 1999;115:1352–1356. [DOI] [PubMed] [Google Scholar]
- 21. Montaldo C, Cannas E, Ledda M, Rosetti L, Congiu L, Atzori L. Bronchoalveolar glutathione and nitrite/nitrate in idiopathic pulmonary fibrosis and sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2002;19:54–58. [PubMed] [Google Scholar]
- 22. Behera D, Kaur S, Sathyanarayana G, Bhatnagar A, Majumdar S. Nitric oxide derivative in bronchoalveolar lavage fluid from patients with idiopathic pulmonary fibrosis. Indian J Chest Dis Allied Sci 2002;44:21–24. [PubMed] [Google Scholar]
- 23. Kanoh S, Kobayashi H, Motoyoshi K. Exhaled ethane: An in vivo biomarker of lipid peroxidation in interstitial lung diseases. Chest 2005;128:2387–2392. [DOI] [PubMed] [Google Scholar]
- 24. Psathakis K, Mermigkis D, Papatheodorou G, et al. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest 2006;36:362–367. [DOI] [PubMed] [Google Scholar]
- 25. Bargagli E, Olivieri C, Bennett D, Prasse A, Muller‐Quernheim J, Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 2009;103:1245–1256. [DOI] [PubMed] [Google Scholar]
- 26. Taylor DR, Pijnenburg MW, Smith AD, De Jongste JC. Exhaled nitric oxide measurements: Clinical application and interpretation. Thorax 2006;61:817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Formanek W, Inci D, Lauener RP, Wildhaber JH, Frey U, Hall GL. Elevated nitrite in breath condensates of children with respiratory disease. Eur Respir J 2002;19:487–491. [DOI] [PubMed] [Google Scholar]
- 28. Hunt J, Byrns RE, Ignarro LJ, Gaston B. Condensed expirate nitrite as a home marker for acute asthma. Lancet 1995;346:1235–1236. [DOI] [PubMed] [Google Scholar]
- 29. Ueno T, Kataoka M, Hirano A, et al. Inflammatory markers in exhaled breath condensate from patients with asthma. Respirology 2008;13:654–663. [DOI] [PubMed] [Google Scholar]
- 30. Ratnawati, Morton J, Henry RL, Thomas PS. Exhaled breath condensate nitrite/nitrate and pH in relation to pediatric asthma control and exhaled nitric oxide. Pediatr Pulmonol 2006;41:929–936. [DOI] [PubMed] [Google Scholar]
- 31. Robroeks CM, van de Kant KD, Jöbsis Q, et al. Exhaled nitric oxide and biomarkers in exhaled breath condensate indicate the presence, severity and control of childhood asthma. Clin Exp Allergy 2007;37:1303–1311. [DOI] [PubMed] [Google Scholar]
- 32. Kharitonov SA, Barnes PJ. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers 2002;7:1–32. [DOI] [PubMed] [Google Scholar]
- 33. Delen FM, Sippel JM, Osborne ML, Law S, Thukkani N, Holden WE. Increased exhaled nitric oxide in chronic bronchitis: Comparison with asthma and COPD. Chest 2000;117:695–701. [DOI] [PubMed] [Google Scholar]
- 34. Ichinose M, Sugiura H, Yamagata S, Koarai A, Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am J Respir Crit Care Med 2000;162:701–706. [DOI] [PubMed] [Google Scholar]
- 35. Rutgers SR, van der Mark TW, Coers W, et al. Markers of nitric oxide metabolism in sputum and exhaled air are not increased in chronic obstructive pulmonary disease. Thorax 1999;54:576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maziak W, Loukides S, Culpitt S, Sullivan P, Kharitonov SA, Barnes PJ. Exhaled nitric oxide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:998–1002. [DOI] [PubMed] [Google Scholar]
- 37. Paredi P, Ward S, Cramer D, Barnes PJ, Kharitonov SA. Normal bronchial blood flow in COPD is unaffected by inhaled corticosteroids and correlates with exhaled nitric oxide. Chest 2007;131:1075–1081. [DOI] [PubMed] [Google Scholar]
- 38. Agustí AG, Villaverde JM, Togores B, Bosch M. Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary disease. Eur Respir J 1999;14:523–528. [DOI] [PubMed] [Google Scholar]
- 39. Borrill ZL, Roy K, Singh D. Exhaled breath condensate biomarkers in COPD. Eur Respir J 2008;32:472–486. [DOI] [PubMed] [Google Scholar]
- 40. Corradi M, Montuschi P, Donnelly LE, Pesci A, Kharitonov SA, Barnes PJ. Increased nitrosothiols in exhaled breath condensate in inflammatory airway diseases. Am J Respir Crit Care Med 2001;163:854–858. [DOI] [PubMed] [Google Scholar]
- 41. Vier C, Hecht B, Becher G, et al. Clinical significance of H2O2, nitrite/nitrate and pH in exhaled breath condensate during and after acute COPD exacerbation (abstract). Am J Respir Crit Care Med 2005;171:A932. [Google Scholar]
- 42. Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Torén K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest 2006;130:1319–1325. [DOI] [PubMed] [Google Scholar]
- 43. Sundy JS, Hauswirth DW, Mervin‐Blake S, et al. Smoking is associated with an age‐related decline in exhaled nitric oxide. Eur Respir J 2007;30:1074–1081. [DOI] [PubMed] [Google Scholar]


