Abstract
Background
Myeloperoxidase (MPO) plasma values predict major adverse cardiac events (MACE) in cases of acute coronary syndrome. The effect of serial testing in patients who are suspected for acute coronary ischemia is unclear.
Hypothesis
We hypothesize that sequential MPO measurement may improve prediction of MACE in patients with suspected acute coronary ischemia.
Methods
The present prospective observational study examined the prognostic significance of MPO in 917 patients with suspicion of acute coronary syndrome. Blood samples were taken at cardiac catheter laboratory admission and the day after coronary angiography. We recorded patients' mortality, the occurrence of cardiac ischemia, and repeated percutaneous coronary intervention through the next 6 months.
Results
Mortality among patients with increased MPO plasma levels the day after coronary angiography was increased significantly (P < 0.01). Patients with MPO values above 306.3 pmol/L had a significantly higher incidence of 6‐month MACE (P < 0.0001) than patients with lower plasma values. Cox proportional hazards multivariate regression analyses revealed that MPO was an independent marker for MACE after suspected acute coronary ischemia (P = 0.048). However, MPO plasma levels at cardiac catheter laboratory admission showed no prognostic significance.
Conclusions
In patients with suspected myocardial infarction, MPO levels above 306.3 pmol/L measured 24 hours after onset of symptoms were independent predictors of 6‐month mortality and MACE.
Introduction
Atherosclerosis is known as a chronic inflammatory disease leading to acute coronary syndromes (ACS).1, 2 Today, early risk stratification strategies use clinical markers and highly sensitive assays of myocardial necrosis to predict major adverse cardiac events (MACE). The ability to detect patients at increased risk for MACE among individuals with existing atherosclerotic heart disease by using markers of progression and rupture of atherosclerotic plaques is of considerable interest for the treatment of patients at high risk. Despite actual and aggressive treatments, the incidence of MACE after myocardial infarction (MI) still remains high.3
Myeloperoxidase (MPO), an enzyme derived from activated neutrophils and monocytes, possesses proinflammatory and pro‐oxidative properties that has been shown as being involved in the development and rupture of atherosclerotic plaques.4 MPO catalyzes oxidative modifications of lipoproteins,5 enhances bioavailability of nitric oxide,6, 7 and promotes plaque instability.8 MPO is suggested as a solid marker to predict mortality in patients at risk for MACE.9, 10, 11, 12 MPO concentrations reliably correlate with the degree of endothelial dysfunction.7 Systemic MPO concentrations have been shown to provide prognostic information among patients with chest pain,9, 10 asymptomatic patients,13 or patients with present ACS.9, 14, 15 However, additional studies found that MPO was not able to predict the risk of short‐ or long‐term mortality,16, 17 and is not a reliable marker for detecting ACS.18
Several investigations reported the influence of preanalytic handling on MPO plasma concentration. Increased plasma MPO levels have been shown after the admission of heparin in vivo and in vitro.19, 20 Furthermore, the use of different blood collecting tubes influences the measurement of MPO plasma levels. MPO concentrations appeared to be reliably stable in ethylenediaminetetraacetic acid (EDTA) plasma.21 Time dependent changes of MPO serum concentration were also shown after primary percutaneous coronary intervention (PCI) in patients with MI presenting anterior ST‐segment elevation.22 However, so far there exists no standardized laboratory MPO test pattern for routine clinical use. Risk scores and clinically used biomarkers were proven to predict mortality among patients with suspected ACS.23, 24
In the current study, in patients with suspected myocardial ischemia, we examined the predictive value of a sequential MPO measurement at 2 different time points for the prediction of mortality and the occurrence of myocardial ischemia within the next 6‐months.
Methods
Study Population
From October 2004 until December 2006, a 2‐center, prospective cohort study was performed. The study group consisted of 917 consecutive patients with suspected ACS who were admitted to the cardiac catheter laboratory at the Department of Cardiology at the University of Giessen or to the Department of Cardiology at the Kerkhoff Klinik, Bad Nauheim, Germany. All included patients (273 women and 644 men, age 65 ± 13 years) met clinical criteria for ACS. ACS was defined according to the criteria of the American College of Cardiology/American Heart Association (ACC/AHA) guideline for the management of patients with unstable angina and non–ST‐segment elevation MI in 2002, the ACC/AHA guidelines for the management of patients with acute MI (AMI) in 1999, and the ACC/AHA/American College of Physicians–American Society of Internal Medicine guidelines for the management of patients with chronic stable angina from 1999.25, 26
Baseline data were recorded for standard cardiac risk factors including hypertension, diabetes, hypercholesterolemia, cigarette smoking, and familial disposition. Biometric data of patients, such as weight and height, were also recorded for calculation of body mass index. Additionally, we recorded the presence of acute cardiogenic shock and the need for surgical intervention. Coronary angiography was performed according to standard practice, and the examination results were reported. All study subjects gave informed consent, and the local ethics committee approved the study protocol in accordance with the guidelines in the Declaration of Helsinki 2000.
End Points
MACE were defined as death by any cause or nonfatal MI during the observation period after enrollment into the study. The end point MI was defined as reported MI during the 6 months after hospital admission. The end point information was collected by prospective follow‐up examination and direct contact by study staff or standardized interviews by phone. We used information given from patients, their relatives, or from medical data provided by the families' physicians. We ascertained patients' outcome over the next 6 months after enrollment.
Plasma MPO Assay
Venous blood samples were collected at cardiac catheter laboratory admission and 24 hours after admission in EDTA tubes, processed, and frozen at −80°C until further analysis. After completion of the patient recruitment phase, blood samples were analyzed centrally to avoid multiple freeze and thaw cycles. MPO concentrations were determined by using the Abbott Architect system (Abbott Diagnostics, Abbott Park, IL). The Architect MPO assay is an automated chemiluminescent microparticle immunoassay using MPO‐specific monoclonal antibodies in a 2‐step sandwich format. The assay has a limit of detection <20.0 pmol/L, functional sensitivity of 125 pmol/L (total coefficient of variation [CV] = 10%), and a range of 277 to 3457 pmol/L (CV = 2.6%–6.8%).21, 27
Statistical Analysis
The study end points were defined as 6‐month composite occurrence of MACE including reinfarction, repeated PCI, or 6‐month mortality. Continuous variables are presented as mean (±standard deviation) or median (with interquartile range [IQR]), and categorical variables as number and percentage. The relationship between mortality and MPO concentration was analyzed by using the Mann‐Whitney test. Receiver operating characteristic (ROC) curves were generated to identify the threshold level for MPO that provided the highest predictive value to stratify patients according the risk of adverse cardiac events. The area under the curve (AUC) was calculated with estimated risk. The optimal cutoff was chosen to maximize AUC values. The rates of overall survival and the quartile analysis were determined by using Kaplan‐Meier analyses. Youden index was used for the detection of the correct MPO cutoff level.28
The corresponding P value of the Kaplan‐Meier curves was calculated by using the log‐rank test. The Wilcoxon rank sum test was used to compare between the different patient groups for nonparametric data. Comparisons between groups were analyzed by t test. Multivariate Cox proportional hazards model analysis was performed to determine associations between MPO and established risk factors (gender male, age, hypertension, dyslipidemia, diabetes mellitus, cigarette smoking, ST‐segment elevation MI [STEMI], cardiogenic shock, need for coronary artery bypass graft [CABG], PCI, elevated creatinine) and survival.
Comparisons of categorical variables were generated by the Pearson χ22 test or Fisher exact test. Probability P values ≤0.05 were considered statistically significant. The entire statistical analysis were performed using SPSS version 19.0.0 (IBM, Armonk, NY).
Results
Baseline Characteristics
A total number of 917 consecutive patients with suspected cardiac ischemia were enrolled in this study. Baseline characteristics of the study population are shown in Table 1. The mean age of these patients was 65 years, and 70% were male. The median time from onset of symptoms until the first collected blood sample was 6.1 hours (IQR = 2.6–14.5). The median time from onset of symptoms until the second blood sample collection was 26.1 hours (IQR = 20.5–34.5) (Figure 1). Percentage of patients representing 6‐month MACE was 8.5%. Six‐month mortality was 7% in the observation group.
Table 1.
Baseline Characteristics
| Characteristic | Value |
|---|---|
| No. of patients | 917 |
| Gender, female, no. (%) | 273 (30%) |
| Age, y (SD) | 65 (±13) |
| Hypertension, no. (%) | 628 (69%) |
| Dyslipidemia, no. (%) | 386 (42%) |
| Diabetes mellitus, no. (%) | 196 (21%) |
| Current smoking, no. (%) | 305 (33%) |
| ACS family history, no. (%) | 182 (20%) |
| STEMI, no. (%) | 393 (43%) |
| No cardiac ischemia | 108 (12%) |
| Time from onset of chest pain to first blood sample, h, median (IQR) | 6.1 (2.6–14.5) |
| Time from onset of chest pain to second blood sample, h, median (IQR) | 26.1 (20.5–34.5) |
| MACE, no. (%) | 78 (8.5%) |
| 6‐month mortality, no. (%) | 65 (7%) |
Abbreviations: ACS, acute coronary syndrome; IQR, interquartile range; MACE, major adverse cardiac event; SD, standard deviation; STEMI, ST‐elevation myocardial infarction;
Data are mean, median and IQR, or number (%) of patients.
Figure 1.
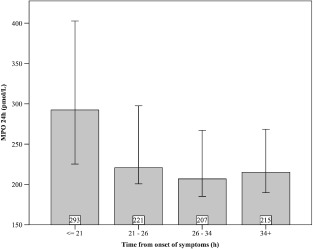
Bar chart demonstrating the time‐dependent changes in myeloperoxidase (MPO) levels at day 1 after suspected acute coronary syndrome. MPO values shown are mean with standard deviation (pmol/L) and time from onset of the symptoms (hours).
Plasma MPO and MACE
In the baseline group (n = 917), median MPO concentration was 893.5 pmol/L compared to a median concentration of 234.4 pmol/L in the 24‐hour group (n = 663). The baseline median MPO plasma levels were not predictive for 6‐month mortality (P = 0.35). However, in blood samples taken 24 hours following admission, MPO levels were significantly elevated in patients who died during the next 6 months (P = 0.016).
ROC curve analyses were generated to identify the optimal MPO cutoff value as described. Kaplan‐Maier‐analysis revealed that MPO plasma level ≥306.3 pmol/L was the most powerful predictive factor for 6‐month MACE. (log‐rank test P < 0.0001) (Figure 2). In this regard, patients representing MPO plasma level ≥306.3pmol/L in 24‐hour blood samples were categorized into the high MPO level group (group 1). Patients representing MPO plasma levels <306.3 pmol/L were assigned to the low MPO level group (group 2). Patients of these 2 groups were similar in age, gender, vascular risk factors, blood sampling time, and history of PCI and CABG. The incidence of STEMI, cardiogenic shock, chest pain, and PCI did also not differ between the 2 groups (Table 2). However, the groups showed significant differences in the number of troponin I‐positive (>0.05 ng/mL) patients (72.5% vs 81.3; P = 0.009) and in the need for CABG (2% vs 7.1%; P = 0.001). The 2 groups also differed in mortality (3.5% vs 9.1%; P = 0.003) and MACE (3.8% vs 10.9%; P = 0.001) during the next 6 months.
Figure 2.
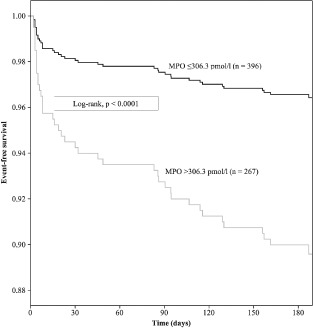
Kaplan‐Meier analysis for 6‐month major adverse cardiac events after suspected cardiac ischemia. Myeloperoxidase (MPO) values at 24 hours after hospital admission are shown. Cutoff MPO values were ≥306.3 pmol/L. Log‐rank P < 0.0001.
Table 2.
Comparison of Baseline Characteristics: Laboratory Findings and 6‐Month Clinical Outcomes Between Patients With High and Low MPO Levels of the Second Blood Sample
| MPO ≤306.3 pmol/L, n = 396 | MPO >306.3 pmol/L, n = 267 | P Value | |
|---|---|---|---|
| Age, y, mean ± SD | 63.7 ± 13.0 | 65 ± 12 | 0.199 |
| Male gender | 69.7% (276) | 68.9% (184) | 0.83 |
| Body mass index | 28 ± 4 | 27 ± 4 | 0.483 |
| Hypertension | 70.9% (280) | 67.8% (181) | 0.722 |
| Hypercholesterolemia | 44.8% (177) | 37.5% (100) | 0.06 |
| Diabetes mellitus | 19.2% (76) | 23.6% (63) | 0.177 |
| Currently smoking | 32.9% (130) | 33% (88) | 0.99 |
| ACS family history | 20.5% (81) | 17.2% (46) | 0.293 |
| Previous MI | 11.6% (46) | 11.3% (30) | 0.885 |
| Previous PCI | 12.2% (48) | 11.7% (31) | 0.847 |
| Previous CABG | 5.8% (23) | 6.8% (18) | 0.622 |
| Angina pectoris | 92.9% (367) | 87.6% (233) | 0.21 |
| STEMI | 42.5% (168) | 42.5% (113) | 0.99 |
| Cardiogenic shock | 0.3% (1) | 3.8% (10) | 0.001 |
| Blood sampling time, h | 31.8 ± 20.4 | 30.4 ± 19.6 | 0.633 |
| ACS negative | 11.6% (46) | 9.8% (26) | 0.449 |
| PCI | 78.7% (311) | 76.7% (204) | 0.535 |
| CABG | 2% (8) | 7.1% (19) | 0.001 |
| 6‐Month mortality | 3.5% (14) | 9.1% (24) | 0.003 |
| Reinfarction | 0.8% (3) | 1.9% (5) | 0.195 |
| MACE | 3.8% (16) | 10.9% (29) | 0.001 |
| LVEF, % | 50 ± 12 | 47 ± 12 | 0.274 |
| Troponin I, >0.05 ng/mL | 72.5% (287) | 81.3% (217) | 0.009 |
| MPO, pmol/L | 162.7 ± 67.0 | 1775.1 ± 2627.9 | <0.0001 |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MPO, myeloperoxidase; PCI, percutaneous coronary intervention; SD, standard deviation; STEMI, ST‐elevation myocardial infarction.
Data are mean ± SD or % (no.) of patients. Continuous variables were analyzed using the Wilcoxon rank sum test. Categorical variables were analyzed by using the χ2 test or Fisher exact test.
MPO Plasma Level as an Independent Predictor of Risk
Cox proportional hazards multivariate regression model for death and nonfatal myocardial infarction during the 6 months of follow‐up was further explored to assess the predictive value of MPO. MPO plasma levels ranging above 306.3 pmol/L at day 1 after admission (hazard ratio [HR] = 1.946, 95% confidence interval [CI]: 1.008‐3.756; P = 0.048) and serum creatinine baseline level above 1.1 mg/dL (HR = 2.585, 95% CI: 1.334‐5.012; P = 0.005) were identified as independent predictive markers of 6‐month MACE in patients who were suspected for cardiac ischemia (Table 3).
Table 3.
Multivariate Cox Proportional Hazards Regression Model for Death and Nonfatal Myocardial Infarction During 6 Months of Follow‐Up
| HR | 95% CI | P | |
|---|---|---|---|
| Gender male | 0.724 | 0.334‐1.569 | 0.414 |
| Age >65 years | 1.270 | 0.602‐2.677 | 0.530 |
| Hypertension | 0.879 | 0.424‐1.826 | 0.730 |
| Dyslipidemia | 0.565 | 0.278‐1.151 | 0.116 |
| Diabetes mellitus | 1.582 | 0.795‐3.149 | 0.192 |
| Cigarette smoking | 1.051 | 0.477‐2.316 | 0.902 |
| STEMI | 0.849 | 0.447‐1.611 | 0.616 |
| Cardiogenic shock | 2.762 | 0.753‐10.127 | 0.125 |
| CABG | 2.151 | 0.511‐9.055 | 0.297 |
| PCI | 1.77 | 0.609‐5.141 | 0.294 |
| Creatinine, >1.1 mg/dL | 2.585 | 1.334‐5.012 | 0.005 |
| MPO, 24 hours, >306.3 pmol/L | 1.946 | 1.008‐3.756 | 0.048 |
Abbreviations: CABG, coronary artery bypass graft; CI, confidence interval; HR, hazard ratio; MPO, myeloperoxidase; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
Discussion
Clinical criteria and established laboratory tests do not predict the exact risk of fatal cardiovascular events and mortality in patients with suspected cardiac ischemia. Previous studies found that inflammation is highly related to endothelial dysfunction, progression, and formation of atherosclerotic plaque, which leads to ischemic coronary disease.1, 2, 29 MPO and its oxidants are believed to be involved in the formation and propagation of atherosclerotic disease.4 MPO is also associated with plaque instability and rupture.30 Previous studies have identified the prognostic value of MPO after AMI,9, 10 acute heart failure,31 and chronic stable heart failure.32 Following ACS and stenting, patients are at high risk for cardiovascular events during the next month.33 MPO could help to identify patients presented with chest pain but without evidence of myocardial necrosis who are at risk for further myocardial ischemia.10 The present study investigated the predictive value of MPO on mortality, PCI, and MI during the next 6 months. Therefore, we used a 2‐times sequential MPO measurement at admission to the cardiac catheter laboratory (6.1 hours, IQR = 2.6–14.5) and the day after (26.1 hours, IQR = 20.5–34.5). The relative risk for MACE by 6 months was significantly higher when the MPO value was above the established cutoff value 24 hours after cardiac catheter laboratory admission. Overall, our findings for a heterogeneous group of patients, who are suspected for cardiac ischemia at hospital admission, suggest that MPO might serve as a predictor of further cardiovascular risk.
The impact of preanalytic conditions and collection tubes on MPO measurement is a very important fact, as it may cause falsely increased results. We used an automated US Food and Drug Administration‐cleared MPO assay (Abbott Architect research assay; Abbott Diagnostics) for the MPO measurement and EDTA plasma sample tubes, in which MPO concentrations have been shown to be stable.21 Previous studies reported that MPO concentrations were elevated by using serum or heparinized plasma samples 21
Our findings also revealed that in addition to the prognostic information generated by MPO for 6‐month MACE, we found that elevated serum creatinine was an independent predictor of risk.
The fact that MPO plasma levels at admission to the cardiac catheter laboratory did not correlate with 6‐month mortality or MACE differs from previous studies. MPO values at admission were significant higher than 24 hours after admission. Nevertheless, we do not see our findings as in contrast to previous MPO outcome studies. Table 1 shows that the median times from onset of symptoms to MPO measurements were 6.1 hours vs 26.1 hours. Mocatta et al reported that they collected study blood samples 24 to 96 hours after admission.34 They also identified high MPO level as a risk factor for long‐term mortality. Stankovic et al demonstrated that in sequential MPO measurements during a period of 168 hours after hospital admission, only MPO levels at 24 hours were independent predictors of in‐hospital mortality.22 Morrow et al reported in their MPO outcome study that plasma samples were obtained in patients within 24 hours after onset of clinical symptoms but did not provide the mean time to MPO measurement.15
All patients were treated according to the ESC/AHA guidelines for management of AMI.35, 36, 37 In the present study, all patients of the cohort undergoing coronary angiography received high‐dose unfractionated heparin. Heparin was identified to increase endothelial nitric oxide bioavailability by liberating vessel‐immobilized MPO.19, 20 This effect leads to a dose‐dependent increase of MPO plasma values in patients with or without symptoms of AMI.20, 38 We suggest that high‐dose heparin application before MPO measurement caused elevated MPO plasma values at hospital admission and led to the lack of predictive significance. Until now, there were no studies that examined the time‐ and dose‐dependent dynamic of MPO after heparin treatment. Further investigations are necessary to generate these data, but it seems that preclinical blood sample collections before the administration of heparin could be another option for generating valid data.
Nevertheless, we did not include MPO in established cardiac risk score systems (Global Registry of Acute Coronary Events, Thrombolysis in Myocardial Infarction). Therefore, further prospective trials are needed to investigate the predictive value of MPO levels 24 hours after onset of symptoms in addition to these proven risk scores.
Conclusion
In patients with suspected myocardial infarction, MPO levels above 306.3 pmol/L measured 24 hours after onset of symptoms were independent predictors of 6‐month mortality and MACE. These results highlight the use of plasma MPO for risk stratification of patients with suspected AMI undergoing primary coronary angiography.
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 2. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 3. Campo G, Saia F, Guastaroba P, et al. Prognostic impact of hospital readmissions after primary percutaneous coronary intervention. Arch Intern Med. 2011;171:1948–1949. [DOI] [PubMed] [Google Scholar]
- 4. Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. [DOI] [PubMed] [Google Scholar]
- 5. Hazen SL, Zhang R, Shen Z, et al. Formation of nitric oxide‐derived oxidants by myeloperoxidase in monocytes: pathways for monocyte‐mediated protein nitration and lipid peroxidation In vivo. Circ Res. 1999;85:950–958. [DOI] [PubMed] [Google Scholar]
- 6. Baldus S, Heitzer T, Eiserich JP, et al. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic Biol Med. 2004;37:902–911. [DOI] [PubMed] [Google Scholar]
- 7. Vita JA, Brennan ML, Gokce N, et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hazen SL. Myeloperoxidase and plaque vulnerability. Arterioscler Thromb Vasc Biol. 2004;24:1143–1146. [DOI] [PubMed] [Google Scholar]
- 9. Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. [DOI] [PubMed] [Google Scholar]
- 10. Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. [DOI] [PubMed] [Google Scholar]
- 11. Apple FS, Smith SW, Pearce LA, et al. Myeloperoxidase improves risk stratification in patients with ischemia and normal cardiac troponin I concentrations. Clin Chem. 2011;57:603–608. [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Zhang F, Dong L, et al. Long‐term prognostic value of myeloperoxidase on acute coronary syndrome: a meta‐analysis. Arch Med Res. 2011;42:368–374. [DOI] [PubMed] [Google Scholar]
- 13. Meuwese MC, Stroes ES, Hazen SL, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC‐Norfolk Prospective Population Study. J Am Coll Cardiol . 2007;50:159–165. [DOI] [PubMed] [Google Scholar]
- 14. Cavusoglu E, Ruwende C, Eng C, et al. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol. 2007;99:1364–1368. [DOI] [PubMed] [Google Scholar]
- 15. Morrow DA, Sabatine MS, Brennan ML, et al. Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS‐TIMI 18. Eur Heart J. 2008;29:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eggers KM, Dellborg M, Johnston N, et al. Myeloperoxidase is not useful for the early assessment of patients with chest pain. Clin Biochem. 2010;43:240–245. [DOI] [PubMed] [Google Scholar]
- 17. Apple FS, Pearce LA, Chung A, et al. Multiple biomarker use for detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2007;53:874–881. [DOI] [PubMed] [Google Scholar]
- 18. Peacock WF, Nagurney J, Birkhahn R, et al. Myeloperoxidase in the diagnosis of acute coronary syndromes: the importance of spectrum. Am Heart J. 2011;162:893–899. [DOI] [PubMed] [Google Scholar]
- 19. Baldus S, Rudolph V, Roiss M, et al. Heparins increase endothelial nitric oxide bioavailability by liberating vessel‐immobilized myeloperoxidase. Circulation. 2006;113:1871–1878. [DOI] [PubMed] [Google Scholar]
- 20. Li G, Keenan AC, Young JC, et al. Effects of unfractionated heparin and glycoprotein IIb/IIIa antagonists versus bivalirdin on myeloperoxidase release from neutrophils. Arterioscler Thromb Vasc Biol. 2007;27:1850–1856. [DOI] [PubMed] [Google Scholar]
- 21. Shih J, Datwyler SA, Hsu SC, et al. Effect of collection tube type and preanalytical handling on myeloperoxidase concentrations. Clin Chem. 2008;54:1076–1079. [DOI] [PubMed] [Google Scholar]
- 22. Stankovic S, Asanin M, Trifunovic D, et al. Time‐dependent changes of myeloperoxidase in relation to in‐hospital mortality in patients with the first anterior ST‐segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Clin Biochem. 2012;45:547–551. [DOI] [PubMed] [Google Scholar]
- 23. Bawamia B, Mehran R, Qiu W, et al. Risk scores in acute coronary syndrome and percutaneous coronary intervention: a review. Am Heart J. 2013;165:441–450. [DOI] [PubMed] [Google Scholar]
- 24. Chatterjee S, Kim J, Dahhan A, et al. Use of high‐sensitivity troponin assays predicts mortality in patients with normal conventional troponin assays on admission‐insights from a meta‐analysis. Clin Cardiol. 2013;36:649–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol. 2000;36:970–1062. [DOI] [PubMed] [Google Scholar]
- 26. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guideline update for the management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction—2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). Circulation. 2002;106:1893–1900. [DOI] [PubMed] [Google Scholar]
- 27. Datwyler SA, Hsu SC, Matias MA, et al. Evaluation of the ARCHITECT myeloperoxidase (MPO) assay in development. Clin Chem Lab Med. 2007;45(Suppl):T051. [Google Scholar]
- 28. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 29. Nicholls SJ, Zheng L, Hazen SL. Formation of dysfunctional high‐density lipoprotein by myeloperoxidase. Trends Cardiovasc Med. 2005;15:212–219. [DOI] [PubMed] [Google Scholar]
- 30. Apple FS, Wu AH, Mair J, et al. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51:810–824. [DOI] [PubMed] [Google Scholar]
- 31. Tang WH, Tong W, Troughton RW, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–2370. [DOI] [PubMed] [Google Scholar]
- 32. Tang WH, Brennan ML, Philip K, et al. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol. 2006;98:796–799. [DOI] [PubMed] [Google Scholar]
- 33. Bonaca MP, Murphy SA, Miller D, et al. Patterns of long‐term thienopyridine therapy and outcomes in patients with acute coronary syndrome treated with coronary stenting: observations from the TIMI‐38 Coronary Stent Registry. Clin Cardiol. 2014;37:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mocatta TJ, Pilbrow AP, Cameron VA, et al. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007;49:1993–2000. [DOI] [PubMed] [Google Scholar]
- 35. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention‐Summary Article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006;47:216–235. [DOI] [PubMed] [Google Scholar]
- 36. Hamm CW. Guidelines: acute coronary syndrome (ACS). 1: ACS without persistent ST segment elevations [in German]. Z Kardiol. 2004;93:72–90. [DOI] [PubMed] [Google Scholar]
- 37. Weber M, Hamm C. Myocardial infarct and unstable angina pectoris: diagnostics and therapy [in German]. Internist (Berl). 2007;48:399–410; quiz 411–412. [DOI] [PubMed] [Google Scholar]
- 38. Marshall CJ, Nallaratnam M, Mocatta T, et al. Factors influencing local and systemic levels of plasma myeloperoxidase in ST‐segment elevation acute myocardial infarction. Am J Cardiol. 2010;106:316–322. [DOI] [PubMed] [Google Scholar]


