Abstract
Hydroxyurea (HU) is the only drug approved for the induction of fetal hemoglobin. Besides this benefit, there are others such as the reduction of leukocyte and generation of nitric oxide (NO). Sickle cell anemia (SCA) is characterized by chronic hemolytic anemia and vaso‐occlusive phenomena. The aim of this study was to evaluate the correlation of parameters MDA and NO2 with the prognosis of patients with SCA as outpatients at Hospital Universitário Walter Cantídeo. In all, 65 patients with SCA—51 without the use of HU (group I) and 14 chronically treated with HU (group II)—were recruited. Nitrite and malonaldehyde were determined by biochemical methods. We found that in group II therewas a significant difference of serum MDA with clinical variables: two or more transfusions during the year (P<0.0469), the presence of malleolar ulcers (P<0.0400), and the occurrence of vaso‐occlusive episodes (P<0.0031), and Group I with the occurrence of three or more vaso‐occlusive episodes (P<0.0051). Correlating the malonaldehyde with clinical variables in groups I and II, we observed a statistically significant relationship with two or more transfusions during the year and the presence of malleolar ulcer. Our results demonstrate that MDA levels can be used as parameter for prognosis in SCA. J. Clin. Lab. Anal. 25:369–373, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: sickle cell disease, clinical profile, malonaldehyde, nitrite reductases, hydroxyurea
INTRODUCTION
Sickle cell anemia (SCA) is an inherited disorder of hemoglobin synthesis that is associated with significant morbidity and mortality due to vaso‐occlusive episodes 1. Recent studies have shown that the vasculopathy in SCA is associated with endothelial damage due to the accumulation of “sickled” red blood cells, leukocytes and platelets, activation of proinflammatory substances, reduction of anticoagulant factors (antithrombin III, Protein C and S coagulation), generation of lipid peroxidation products, and increased consumption of nitric oxide (NO) 2, 3.
Hydroxyurea (HU) reduces the incidence of vaso‐occlusive crises in SCA and has been used to treat SCA. It has the benefit of inducing fetal hemoglobin production with concomitant increase in total hemoglobin and reduced hemolysis. In addition, HU may also be beneficial by reducing the total count of leukocytes and the expression of adhesion molecules that contribute to vaso‐occlusive processes. Some studies have shown that HU is a donor of NO, which can be oxidized by hemoglobin and form nitrosylhemoglobin and NO 4. Moreover, HU can decompose to NO chemically or enzymatically by peroxidase, urease, and catalase. Other studies have shown that HU stimulates phosphorylation and activation of endothelial NO synthase, resulting in the production of NO 5, 6.
The occurrence of vaso‐occlusive episodes, especially in small vessels, is the decisive event in the pathophysiological origin of most of the signs and symptoms on clinical characteristics of patients with SCA, such as pain crises, hemolytic crises, lower limb ulcers, acute chest syndrome, splenic sequestration, priaprism, aseptic necrosis of femur, retinopathy, chronic renal failure, and stroke autosplenectomy 7.
Biological macromolecules can be damaged by oxidative insult, leading to oxidized products that act as biomarkers of oxidative stress status. Oxygen radicals may provoke oxidative stress that can induce lipid peroxidation. The malonaldehyde is an intermediate product of lipid peroxidation, which is detrimental to cellular integrity and function 8.
In this context, we studied the relationship between the levels of malonaldehyde and nitrite with prognostic parameters in SCA patients with and without HU treatment.
MATERIALS AND METHODS
Setting and Design
The study design was cross‐sectional and held in Fortaleza, Ceará, Brazil, in adults patients with SCA accompanied at the Hospital Universitário Walter Cantídeo outpatient clinic in the period from April 2008 to January 2009.
Material
In all, 65 patients were recruited and studied at random according to the criteria of inclusion and exclusion. Inclusion criteria were adults with a diagnosis of SCA confirmed by molecular biology with or without treatment with HU. The study excluded children and adults with SCA who have undergone transfusion therapy in the last 3 months, or who are in the use of chelating iron supplementation with vitamins C and E, or who refused to participate by not signing the informed consent.
The patients were stratified according to the use of HU, group I (n=51) of patients with SCA without the use of HU and group II (n=14) with the use of HU at a dosage of 30 mg/kg/day, in less than 6 months. Clinical variables were obtained from the patients' medical records.
Methodology
Samples were obtained from venous blood of each patient in a tube containing anticoagulant heparin and isolated for assay of plasma malonaldehyde, nitrite, and EDTA to confirm HbSS by PCR‐RFLP 9 in patients with SCA, and for confirmation of HbAA HPLC in individuals without hemoglobinopathies.
The method employed to determine the MDA was based on its reaction with thiobarbituric acid (TBARS) 10. The nitrite concentration was determined by Green's method 10.
Statistical Analysis
The data obtained were expressed as means±standard deviation using the statistical software SPSS®5.0. Student's t test was also used to compare clinical variables with the levels of MDA and nitrite. The criterion for significance was P<0.05 (confidence level 95%).
Ethical Aspects
The project was submitted to the Ethics Committee of the institution where the study was conducted, and approved according to Opinion 113.12.07.
RESULTS
The prevalence of women with a mean age of 31.37±11.29 years was observed in patients with SCA.
A correlation between the levels of MDA with clinical variables in patients with SCA without the use of HU, observed in group I showed a significant difference in the vaso‐occlusive episodes (P<0.0051) and in group II the difference was significant with two or more transfusions during the year (P<0.0469), the presence of malleolar ulcer (P<0.0400) and with the presence of three or more vaso‐occlusions (P<0.0031; Table 1). When comparing the levels of MDA with clinical variables between groups of patients with SCA with or without the use or no of HU (Groups I and II) found significant differences in two or more transfusions during the year (P<0.05) and with the presence of malleolar ulcer (P=0.045; Table 3).
Table 1.
Clinical Variables on the Level of MDA for Each Group
| MDA | ||||||
|---|---|---|---|---|---|---|
| Not on hydroxyurea | On hydroxyurea | |||||
| Clinical variables | Mean±SD | N | P value | Mean±SD | N | P value |
| Transfusions per year | ||||||
| 0–1 | 0.47±0.14 | 40 | >0.05 | 0.54±0.12 | 10 | <0.05* |
| ≥2 | 0.52±0.10 | 11 | 0.63±0.04 | 4 | ||
| Vaso‐occlusion | ||||||
| 1–2 crises | 0.43±0.12 | 24 | <0.05* | 0.43±0.14 | 4 | <0.05* |
| ≥3 | 0.53±0.13 | 27 | 0.6±0.05 | 10 | ||
| Leg ulcers/malleolar | ||||||
| No | 0.47±0.12 | 35 | >0.05 | 0.54±0.06 | 6 | <0.05* |
| Yes | 0.51±0.15 | 0.64±0.12 | ||||
| Recurrent infections | ||||||
| No | 0.46±0.15 | 16 | >0.05 | 0.54±0.11 | 8 | >0.05 |
| Yes | 0.50±0.11 | 26 | 0.64±0.02 | 10 | ||
* P<0.05, statistically significant.
Table 3.
Comparison of Clinical Variables on the Level of MDA Between the Groups I and II
| Observed values | |||||
|---|---|---|---|---|---|
| Not on hydroxyurea | On hydroxyurea | ||||
| MDA | MDA | ||||
| Clinical variables | Mean±SD | N | Mean±SD | N | P‐value |
| Transfusions per year | |||||
| 0–1 | 0.47±0.14 | 40 | 0.54±0.12 | 10 | >0.05 |
| ≥2 | 0.52±0.10 | 11 | 0.63±0.04 | 4 | <0.05* |
| Vaso‐occlusion | |||||
| 1–2 crises | 0.43±0.12 | 24 | 0.43±0.14 | 4 | >0.05 |
| ≥3 | 0.53±013 | 27 | 0.60±0.05 | 10 | >0.05 |
| Leg ulcers/malleolar | |||||
| No | 0.47±0.12 | 35 | 0.54±0.06 | 6 | >0.05 |
| Yes | 0.51±0.15 | 16 | 0.64±0.13 | 8 | <0.05* |
| Recurrent infections | |||||
| No | 0.46±0.15 | 26 | 0.53±0.12 | 10 | >0.05 |
| Yes | 0.50±0.11 | 24 | 0.64±0.02 | 3 | >0.05 |
* P<0.05, statistically significant.
Serum levels of nitrite in both groups showed no correlation with clinical variables (Table 2) when evaluated the groups alone or between groups I and II (Table 4).
Table 2.
Clinical Variables in Relation to Serum Levels of Nitrite (NO2 −) for Each Group
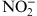
|
||||||
|---|---|---|---|---|---|---|
| Not on hydroxyurea | On hydroxyurea | |||||
| Clinical variables | Mean±SD | N | P value | Mean±SD | N | P value |
| Transfusions per year | ||||||
| 0–1 | 27.04±35.18 | 40 | >0.05 | 16.93±12.44 | 10 | >0.05 |
| ≥2 | 21.11±16.44 | 11 | 8.35±7.95 | 4 | ||
| Vaso‐occlusion | ||||||
| 1–2 crises | 28.78±40.76 | 24 | >0.05 | 21.86±10.59 | 4 | >0.05 |
| ≥3 | 23.10±22.07 | 27 | 21.86±11.31 | 10 | ||
| Leg ulcers/malleolar | ||||||
| No | 30.42±37.41 | 35 | >0.05 | 13.36±11.97 | 6 | >0.05 |
| Yes | 15.59±9.20 | 15.32±12.32 | ||||
| Recurrent infections | ||||||
| No | 30.82±39.29 | 16 | >0.05 | 13.63±11.75 | 8 | >0.05 |
| Yes | 20.51±21.72 | 26 | 19.60±14.61 | 12 | ||
Table 4.
Comparison of Clinical Variables in Relation to Serum Levels of Nitrite (NO2 −) Between the Groups I and II
| Observed values | |||||
|---|---|---|---|---|---|
| Not on hydroxyurea | On hydroxyurea | ||||
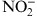
|
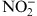
|
||||
| Clinical variables | Mean±SD | N | Mean±SD | N | P‐value |
| Transfusions per year | |||||
| 0–1 | 27.04±35.18 | 40 | 16.94±12.94 | 10 | >0.05 |
| ≥2 | 21.11±16.44 | 11 | 8.35±7.95 | 4 | >0.05 |
| Vaso‐occlusion | |||||
| 1–2 crises | 28.77±40.76 | 24 | 21.86±10.59 | 4 | >0.05 |
| ≥3 | 23.09±22.07 | 27 | 11.53±11.31 | 10 | >0.05 |
| Leg ulcers/malleolar | |||||
| No | 30.42±37.41 | 35 | 13.36±11.97 | 6 | >0.05 |
| Yes | 15.59±9.19 | 16 | 15.32±12.32 | 8 | >0.05 |
| Recurrent infections | |||||
| No | 30.82±39.29 | 26 | 13.63±11.75 | 12 | >0.05 |
| Yes | 20.77±22.15 | 24 | 16.15±11.93 | 3 | >0.05 |
DISCUSSION
SCA is the most common hereditary disease in Brazil and in the world 11. The phenotypic heterogeneity of the disease varies from chronic hemolytic anemia and vasculopathy with acute painful crises to multiple manifestations of specific organs with variable severity 12. Among the factors that may influence the disease process, we can highlight genetic variation, the proportion of sickle cells, endothelial activation, inflammation, oxidative stress, and hypoxia 13, 14.
Several studies have shown the involvement of malonaldehyde 15, 16, 17 and nitrite 18, 19, 20 in cases of SCA. The TBARS test is considered a key indicator of lipid peroxidation, which quantifies the malonaldehyde (MDA), a major breakdown product of hydroperoxides of polyunsaturated fatty acids, formed during the oxidation process 21.
When we evaluated the groups separately, we observed a significant increase in malonaldehyde in both groups of patients with SCA who had three or more vaso‐occlusive crises during the year. The same occurred in relation to the levels of malonaldehyde with the variables of two or more transfusions during the year and the presence of malleolar ulcer in patients with SCA using HU. These results are relevant since they show that the levels of malonaldehyde are directly proportional to the modulators of prognosis in SCA. In 2007, Fasola et al., evaluating the relationship between the frequency of vaso‐occlusive crises and total antioxidant status (TAS), found that the average BAC in 25 patients with SCA was lower in those who had suffered three or more attacks in the previous year 16.
Correlating the malonaldehyde with clinical variables in groups I and II, we observed a statistically significant relationship with two or more transfusions during the year and the presence of malleolar ulcer, suggesting that patients with SCA using HU have a higher lipid peroxidation, requiring more blood transfusions and showing more malleolar ulcer. This may be due to the fact that patients taking HU have a more severe clinical, if fitting the criteria for the use of HU, which is the presence of at least one clinical or laboratory criteria in the last 12 months. Among the clinical criteria are: three or more episodes of pain required medical hospital, acute chest syndrome, and chronic organ damage (priapism, bone necrosis, and proliferative retinopathy). Among the laboratorial criteria: concentration of Hb persistently lower than 7 g/dl and of the Hb F <8% after 2 years of age 22.
Recent studies 6, 23, 24 suggest that patients with SCA present a decrease in NO reserves, particularly during vaso‐occlusive crises, and NO levels are inversely proportional to the painful symptoms, so the decrease in the availability of NO may contribute to the pathophysiology of SCA 25.
The estimated levels of NO in this study were performed by measurement of serum nitrite, a breakdown product of NO that has good sensitivity and stability 17. The difficulties to measurement of nitrite result from oxidation of nitrite (within minutes) by heme proteins, such as hemoglobin. If nitrite is to be measured in plasma, blood needs to be centrifuged immediately to separate plasma from erythrocytes 26. There was no significant difference between the levels of nitrite with prognostic factors, probably due to the fact that they are not in crisis of acute hemolysis. This fact is demonstrated by researchers 4, 5, 18 who showed a transient increase in NO during vaso‐occlusive crises in sickle cell patients in use of HU. Morris et al. (2003) demonstrated increased levels of NO in patients with SCA in use of HU, this increase was potentiated when used in combination with arginine, but with a decline of between 3 and 4 hr 19. Lopez et al. (2003) found low levels of l‐arginine and NO in adult patients with SCD during vaso‐occlusive episodes when compared with SCD patients at baseline 20.
The results thus demonstrate that the levels of MDA can be used as prognostic marker in SCA.
Contributor Information
Romélia Pinheiro Gonçalves, Email: romeliapinheiro@ig.com.br.
Darcielle Bruna Dias Elias, Email: darcielle.bruna@gmail.com.
REFERENCES
- 1. Wood KC, Grander DN. Sickle cell disease: Role of reactive oxygen and nitrogen metabolites. Clin Exp Pharmacol 2007;34:926–932. [DOI] [PubMed] [Google Scholar]
- 2. Kaul DK, Liu XD, Xiaoqin Z, Ma L, Hsia CJC, Nagel RL. Inhibition of sickle red cell adhesion and vasooclusion in the microcirculation by antioxidants. Am J Physiol Heart Circ Physiol 2006;291:167–175. [DOI] [PubMed] [Google Scholar]
- 3. Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: A systematic review for efficacy and toxicity in children. Pediatrics 2008;122:1332–1342. [DOI] [PubMed] [Google Scholar]
- 4. Gladwin MT, Shelhamer JH, Ognibene FP, et al. Nitric oxide donor properties of hydroxyurea in patients with sickle cell disease. Br J Haematol 2002;116:436–444. [DOI] [PubMed] [Google Scholar]
- 5. Cokic VP, Andric SA, Stojikovic SS, Noguchi CT, Schechter AN. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood 2008;111:1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cannalli AA, Franco‐Penteado CF, Saad ST, Conran N, Costa FF. Increased adhesive properties of the neutrophils in sickle cell disease may be reversed by pharmacological nitric oxide donation. Hematol J 2008;93:605–609. [DOI] [PubMed] [Google Scholar]
- 7. Quinn CT, Shull EP, Ahmed N, Lee NJ, Rogers ZR, Buchanan GR. Prognostic significance of early vaso‐occlusive complications in children with sickle cell anemia. Blood 2007;109:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gizi A, Papassotiriou I, Apostolakou F, et al. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant–antioxidant status. Blood Cells Mol Dis 2011;46:220–225. [DOI] [PubMed] [Google Scholar]
- 9. Clark BE, Thein SL. Molecular diagnosis of haemoglobin disorders. Clin Lab Haematol 2004;26:159–176. [DOI] [PubMed] [Google Scholar]
- 10. Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990;186:421–431. [DOI] [PubMed] [Google Scholar]
- 11.Brasil. Ministério da Saúde—manual da anemia falciforme para a população; 2004. p 24.
- 12. Elagouz M, Jyothi S, Gupta, Sivaprasad S. Sickle cell disease and the eye: Old and new concepts. Surv Ophthalmol 2010;55:359–377. [DOI] [PubMed] [Google Scholar]
- 13. Vichinsky E. New therapies in sickle cell disease. Lancet 2002;360:629–631. [DOI] [PubMed] [Google Scholar]
- 14. Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: A state of nitric oxide resistance. Free Radic Biol Med 2008;44:1506–1528. [DOI] [PubMed] [Google Scholar]
- 15. Martins VD, Manfredini V, Peralba MCR, Benfato MS. Alpha‐lipoic acid modifies oxidative stress parameters in sickle cell trait subjects and sickle cell patients. Clin Nutr 2009;28:192–197. [DOI] [PubMed] [Google Scholar]
- 16. Fasola F, Adepapo K, Anetor J, Kuti M. Total antioxidants status and some hematological values in sickle cell disease patients in steady state. J Natl Méd Assoc 2007;99:891–894. [PMC free article] [PubMed] [Google Scholar]
- 17. Rusanova I, Escames G, Cossio G, et al. Oxidative stress status, clinical outcome, and b‐globin gene cluster haplotypes in pediatric patients with sickle cell disease. Eur J Haematol 2010;85:529–537. [DOI] [PubMed] [Google Scholar]
- 18. King SB. Nitric oxide production from hydroxyurea. Free Radic Biol Med 2004;37:737–744. [DOI] [PubMed] [Google Scholar]
- 19. Morris CR, Vichinsky EP, Van Warmendam J, et al. Hydroxyurea and arginine therapy: Impact n nitric oxide production in sickle cell disease. J Pediatric Hematol Oncol 2003;25:629–634. [DOI] [PubMed] [Google Scholar]
- 20. Lopez BL, Kreshak AA, Morris CR, Davis‐Moon L, Ballas SK, Ma XL. l‐arginine levels are diminished in adult acute vaso‐occlusive sickle cell crisis in the emergency department. Br J Haematol 2003;120:532–534. [DOI] [PubMed] [Google Scholar]
- 21. Apel K, Hir H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 2004;55:373–399. [DOI] [PubMed] [Google Scholar]
- 22. Cançado RD, Lobo C, Ângulo IL, Araújo PIC, Jesus JA. Protocolos clínico e diretrizes terapêuticas para uso de hidroxiureia na doença falciforme. Rev Bras Hematol Hemoter 2009;31:361–366. [Google Scholar]
- 23. Agil A, Sadrzadeh SMH. Hydroxyurea protects erythrocytes against oxidative damage. Redox Rep 2000;5:29–34. [DOI] [PubMed] [Google Scholar]
- 24. Vasconcelos SML, Goulart MOF, Moura JBF, Manfredini V, Benfato MS. Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: Principais métodos analíticos para sua determinação. Quim Nova 2007;30:1323–1338. [Google Scholar]
- 25. Mack AK, Kato GJ. Sickle cell disease and nitric oxide: A paradigm shift? Int J Biochem Cell Biol 2006;38:1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pelletier MM, Kleinbongard P, Ringwood L, et al. The measurement of blood and plasma nitrite by chemiluminescence: Pitfalls and solutions. Free Radic Biol Med 2006;41:541–548. [DOI] [PubMed] [Google Scholar]


