Abstract
Background
Superficial wound infections after gastrointestinal surgery markedly impair the affected patients’ quality of life. As it is still unknown which method of skin closure is best for the reduction of wound infections in elective gastrointestinal surgery, we compared the frequency of wound infections after intracutaneous suturing versus skin stapling.
Methods
In a prospective, randomized, single-center study, patients undergoing elective gastrointestinal surgery were intraoperatively randomized to skin closure either with an intracutaneous suture or with staples. The primary endpoint—the occurrence of a grade A1 wound infection within 30 days of surgery—was evaluated according to the intention-to-treat principle.
Results
Out of a total of 280 patients, 141 were randomized to intracutaneous suturing and 139 to stapling. The groups did not differ significantly with respect to age, sex, or ASA classification. 19 of the 141 patients in the intracutaneous suturing group (13.5%) had a grade A1 wound infection, compared with 23 of 139 in the stapling group (16.6%) (odds ratio [OR]: 0.79; 95% confidence interval: [0.41; 1.52]; p = 0.47). A multiple regression analysis revealed that the type of surgery (colorectal vs. other), the approach, and the incision length were independent risk factors for a grade A1 wound infection. When wound dehiscences were additionally considered, wound complications were found to have arisen significantly more often in the stapling group than in the intracutaneous suturing group (16.3% [23/141] versus 30.2% [42/139], OR: 0.45 [0.25; 0.80]; p = 0.006).
Conclusion
In elective gastrointestinal surgery, intracutaneous suturing was not found to be associated with a lower rate of superficial wound infections than skin stapling, but fewer wound dehiscences occurred in the intracutaneous suturing group.
The prevention of wound infections after surgical procedures is of great interest for every surgical discipline, in order to improve the quality of patient care. Surgical site infections are among the most common nosocomial infections (1); they often result in prolonged hospital stays and increased costs (2– 4). Surgical site infections are the third most common nosocomial infection, with an incidence of 16% (5). In Germany, the estimated number of hospital acquired postoperative wound infections amounts to 225 000 cases per year (6).
In 1999, the US Centers for Disease Control and Prevention (CDC) in Atlanta, USA, developed an internationally recognized and accepted classification for surgical site infections and devised a guideline for preventing these (7) (table 1), which was updated in 2017 (8). In Germany, the CDC criteria were adopted and implemented by the Robert Koch-Institute (National Reference Center for the Surveillance of Nosocomial Infections) (9, 10). The World Health Organization in its Global Guidelines for the Prevention of Surgical Site Infection recommends 29 preoperative and intraoperative measures to prevent surgical site infections (11).
Table 1. Definitions for nosocomial infections: postoperative surgical site infections*.
| Category A1 | Infection at the skin incision site within 30 days after the operation, which affects only skin or subcutaneous tissue, and one of the following criteria applies: – Purulent secretion/discharge from the superficial incision – Confirmation of pathogen on culture grown from aseptically harvested wound secretion or tissue from the superficial incision – One of the following signs: pain or tenderness/sensitivity to touch, localized swelling, erythema, or overheating, and the surgeon opens up the superficial incision on purpose/intentionally. This criterion does, however, not apply if the microbiological cultures from the superficial incision yields a negative result. – Diagnosis of the treating physician |
| (Postoperative superficial surgical site infection) | |
| Category A2 | Infection within 30 days after the operation and infection seems to be linked to the operation and affects the fascia and muscle tissue and one of the following criteria applies: – Purulent secretion from deep inside the incision, … – Spontaneously opened or intentionally opened by the surgeon, … – Abscess or other signs of infection affecting the deeper tissues, … |
| (Postoperative deep surgical site infection) | |
| Category A3 | Infection within 30 days postoperatively and infection seems linked to the operation and affects organs or body cavities that were opened up or manipulated during the operation, and one of the following criteria applies: – Purulent discharge from a drain that accesses the organ or body cavity… – Pathogen confirmed on culture… – Abscess or other signs of an infection of the organ or body cavity … |
| (Infections of organs and body cavities in the operating field) |
*Abbreviated version of the classification of the Centers for Disease Control and Prevention (CDC) (7)
In spite of evidence based preoperative antiseptic measures, the patient’s own flora is considered to be the main source for surgical site infections and causes endogenous infections with a ratio of 90% to 10% compared with exogenous infections as a result of contamination with external substances (7). No difference was found regarding the preoperative preparation of the skin, (12). Flushing wounds before suturing leads to a significant reduction in surgical site infections, especially in colorectal surgery (13). Wound healing can be affected, among others, by (9, 14– 18):
The length of the preoperative inpatient stay
Intraoperative antibiotic prophylaxis
Surgical hand disinfection
The surgical site
The air condition technology in the operating theater
The patient’s body temperature
The protection of the surgical wound margin.
The treating surgeon can influence most of these factors only to a limited degree. A universally applicable option for optimizing surgical site healing in every patient may be the selection of the “correct” suturing material and the “correct” suturing technique for skin closure. To date, hardly any prospective data exist on the skin closure technique after gastrointestinal surgery. Six randomized trials since 1981 have studied the occurrence of wound infections after using staples or sutures in visceral surgery (19– 24). The study populations and designs were heterogeneous, and different suturing techniques and materials were used (25, 26), so no unequivocal results in favor of sutures or staples were collected. A Japanese study showed (19) in a subgroup analysis that using subcoreal sutures in the subgroup of patients who had surgery of the lower gastrointestinal tract resulted in a significantly lower rate of surgical site infections than staples. Another prospective randomized trial from 2016 of 401 patients showed no difference in the occurrence of surgical site infections between subcuticular sutures or staples used in abdominal surgery (20).
Ultimately, on the basis of existing data, no unequivocal recommendation can be made for the optimal skin closure technique after elective gastrointestinal procedures. We conducted a prospective randomized trial that compared the use of continuous intracutaneous sutures and staples with regard to the development of surgical site infections after elective gastrointestinal surgery (27).
Methods
Study design and patients
The study was conceived as a single center prospective randomized controlled trial with an intervention group and an active control group. In the intervention group the skin was closed by using continuous absorbable intracutaneous sutures (Mososyn 4–0 [glyconate] “suture group”), in the control group, staples were used (WECK Visistat 35W 6.5 × 4.7 mm, “staples group”).
The ethics committee of Philipps University of Marburg approved the study. The study was registered with the German Clinical Trials Register (DRKS 00004542). The study protocol was published (27).
We included patients who had elective abdominal surgery by means of midline or transverse laparotomy. The procedures undertaken included small bowel resection, colorectal procedures including laparoscopy-assisted surgery using a Pfannenstiel-Kerr incision measuring at least 6 cm, esophageal resection, stomach or duodenal interventions, pancreas resection, liver resection, open cholecystectomy with or without common bile duct revision, gastrointestinal bypass surgery, and biliodigestive anastomoses. The patients’ minimum life expectancy had to be 12 months.
Exclusion criteria were antibiotic treatment within the 14 days preceding the operation and inpatient admission more than 4 days before the procedure. Patients who had already undergone midline or transverse laparotomy were excluded if the same access was reopened for the current procedure.
The primary endpoint of the study was the occurrence of an A1 surgical site infection according to the CDC classification (7) within 30 days after the operation, diagnosed by the treating surgeon. According to the CDC criteria, A1 surgical site infection was defined as an infection of the skin incision within 30 days postoperatively, which affected only skin or subcutaneous tissue (7) (table 1).
Secondary endpoints were:
The duration of skin closure
Cosmetic result after 30 days
Length of inpatient stay
Duration of sickness absence/unfitness for work.
Randomization and blinding
Participating patients were randomized to the suture group or staples group intraoperatively after fascial closure by means of a telephone call 1:1 in the coordinating center for clinical studies of the Philipps University of Marburg. The randomization method was based on a randomization list generated in the center with permuted blocks of random lengths. The results of the randomization was communicated to the treating surgeons only during the phone call preceding fascial closure.
Course of the study
The published protocol described the course of the study in detail (27). The surgical procedure in both groups was unaffected by participation in the study, as the intervention related only to the skin closure. Surgeons were unblinded after the procedure had been completed and the fascia closed.
Intraoperative complications, such as unexpected contamination of the abdominal cavity, relevant blood loss of more than 500 mL, and intraoperative cardiovascular complications were documented.
Skin closure was undertaken either by the primary surgeon or the assistant, in accordance with daily clinical routine.
The follow-up examinations were done on the 2nd, 5th, 10th, and 30th postoperative day by the responsible study doctors, and a certified wound manager undertook their assessment in parallel. The signs of an A1 surgical site infection according to CDC criteria were documented, and the wounds were documented photographically at each follow-up examination. Skin staples were removed on the 10th postoperative day; sutures did not need removing. Any sign of A2 or A3 surgical site infections or any necessary re-operation resulted in exclusion from further follow-up examinations.
Statistical analysis
The sample size calculation was based on a retrospective evaluation of internal hospital data of 387 patients from 2009 and 2011. We assumed an A1 surgical site infection rate of 14% in the staples group and 4% in the sutures group. A sample size of 128 patients per group achieves 80% power in the chi-squared test, with a two-tailed significance level of 0.05. Under the assumption of a dropout rate of 10%, a total of 286 patients were randomized.
The primary endpoint was the occurrence of an A1 surgical site infection within 30 days postoperatively, defined as the number of patients with A1 surgical site infection relative to the total number of patients randomized into the relevant group. The primary analysis was undertaken in a modified intention to treat population. Patients in whom the main outcome measure was achieved earlier than 30 days postoperatively, without an A1 surgical site infection occurring, were categorized as without A1 surgical site infection in the sense of a “last observation carried forward” substitution. The rates of surgical site infections were compared by using a two-sided chi-squared test for the 0.05 significance level. For the sensitivity analysis, multiple logistical regression models with the type of operation and age were calculated as additional covariates.
Secondary endpoints and patient characteristics were exploratively analyzed by using appropriate statistical methods (median and interquartile range [IQR], Mann-Whitney U-test for continuous distributions, percentages and chi-squared tests for discrete distributions). We used the software package SAS 9.4 for our analyses.
Results
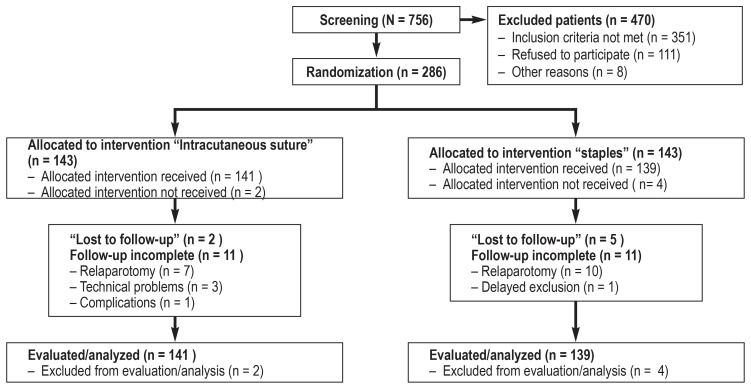
A total of 756 patients were screened in the period from March 2013 to December 2015, of whom 470 did not meet the inclusion criteria. 286 patients were randomized—143 into the sutures group and 143 into the staples group (figure). The evaluation of cases showed that 2 patients in the sutures group and 4 patients in the staples group did not meet the inclusion criteria (for example, a life expectancy of less than 12 months in a patient with intraoperatively diagnosed peritoneal carcinomatosis). The modified intention to treat population therefore comprised 141 patients in the sutures group and 139 patients in the staples group. Reoperation within 30 was required in 7 patients in the sutures group and 10 patients in the staples group. Five further patients (1 in the sutures group, 4 in the staples group) were excluded because of technical problems.
Figure.
Flow of participants through the study (CONSORT)
The demographic and clinical characteristics of patients in both groups were balanced, only the patients’ age was slightly higher in the sutures group (table 2). The type of surgery, operative access, length of skin incision, thickness of subcutaneous fatty tissue, intraoperative blood loss, and extraordinary contamination of the surgical site did not differ in the two groups (table 2).
A postoperative surgical site infection within 30 days occurred in 42 of 280 patients (15.0%) (table 3). 83% of surgical site infections developed after the 10th postoperative day. The difference between rates of A1 surgical site infections in both groups did not reach significance: 13.5% (19 out of 141 patients) in the sutures group, and 16.6% (23 out of 139 patients) in the staples group (odds ratio 0.79; 95% confidence interval [0.41; 1.54]; P=0.47).
Table 3. Rates of A1 surgical site infections within the subgroups defined according to the site of the surgery.
| Total | Lower GI tract | Upper GI tract | Hepatobiliary/pancreatic | |
| A1 SSI (total) |
42/280 (15.0%) |
32/165 (19.4%) |
2/44 (4.6%) |
8/71 (11.3%) |
| A1 SSI suture group |
19/141 (13.5%) |
16/84 (19.0%) |
0/22 (0.0%) |
3/35 (8.6%) |
| A1 SSI staples group |
23/139 (16.6%) |
16/81 (19.8%) |
2/22 (9.1%) |
5/36 (13.9%) |
| Odds ratio [95% CI] for A1 SSI |
0.79 [0.41; 1.52] |
0.96 [0.44; 2.07] |
Not defined | 0.58 [0.13; 2.64] |
GI tract, gastrointestinal tract; SSI, superficial site infection;
95% CI, 95% confidence interval
Univariate logistical regression showed an association between the type of operation and the rate of surgical site infections (P=0.04; likelihood ratio test). A1 surgical site infections manifested most commonly after operations of the lower gastrointestinal tract (colorectal procedures, 32 out of 165 patients, 19.4%), followed by hepatobiliary and pancreatic surgery (8 of 71 patients, 11.3%) and operations of the upper gastrointestinal tract (2 of 44 patients, 4.6%) (table 3). This association remained significant even if the type of skin closure in the multiple regression was added (data not shown).
Of note is the fact that in the staples group, cases of wound dehiscence increased once staples were removed on the 10th day (21 of 139 patients, 15.1%), which were not classed as surgical site infections according to the CDC classification, although they still represented a wound complication. Wound dehiscence was less common in the sutures group (4 of 141 patients, 2.8%; P=0.0002). This result was notable in the context of the study.
A2 and A3 wound infections were equally common in both groups: 4 (2.8%) and 5 (3.6%) of patients in the sutures group and 4 (2.9%) and 6 (4.3%) of patients in the staples group were affected.
The time required to close the skin was on average 6 minutes longer in the sutures group with a mean duration of 7.4 minutes versus 1.3 minutes in the staples group (P<0.0001). The average inpatient stay was 12 days for both groups (IQR 9–17). The cosmetic result and length of sickness absence were not evaluated because of incomplete data.
Discussion
None of the evidence-based recommendations issued by health organizations for the prevention and treatment of surgical site infections takes into account the skin closure technique (9– 11). Only two multicenter randomized controlled trials (RCTs) compared subcuticular sutures and staples for skin closure after gastrointestinal operations (19, 2). Tsujinaka et al. did not find a significant difference between the two techniques in 1080 patients in 24 hospitals; but a subgroup analysis showed a reduction in the rate of surgical site infections when subcuticular sutures were used after colorectal operations (19). A further multicenter study including 401 patients confirmed this result (20).
The current study is the first to compare prospectively the occurrence of A1 surgical site infections in elective gastrointestinal surgery after using intradermal sutures or staples. The two skin closure techniques were selected intentionally because of their differences with regard to application and tightness of the closure. Both skin closure techniques have advantages and disadvantages. Staples are applied quickly by using a one-way stapler. This reduced the time taken by the surgery (19, 20), as was also obvious in our study. After an appropriate healing period, the staples will have to be removed. The theoretical disadvantage of a stapled scar is the fact that skin closure is not tight/continuous, but point by point. Furthermore, staples often damage hair follicles, sweat glands, and sebaceous glands. With regard to the patient’s own flora as the main source of surgical site infections, tight skin closure without injury to dermal structures might possibly help prevent surgical site infections. For this reason we chose continuous intracutaneous sutures as the comparator group. Intracutaneous sutures are generally accepted for the closure of clean wounds, but are regarded with skepticism in gastrointestinal surgery. Intracutaneous sutures promise tight closure of the epidermis without compromising dermal structures. Dissolvable/absorbable sutures do not have to be removed, which makes them more comfortable for patients. Cosmetic results have been rated positively. The disadvantages of intracutaneous sutures are their low stability/robustness and possible skin perforation with local inflammation.
The difference between intracutaneous sutures and staples in terms of the occurrence of A1 surgical site infections did not reach significance, neither in the total study population nor in the subgroups. As expected, rates of A1 surgical site infections were highest in the subgroup of lower gastrointestinal tract operations (19.4%), followed by hepatobiliary and pancreas operations (11.3%). The literature describes surgical site infection rates of between 8.7% and 32.2% (28– 31). The total rate of A1 surgical site infections in the current study was 15.0%. Earlier studies of elective abdominal surgery documented A1 wound infection rates of between 9.8% and 13.4% (19, 20).
The relatively high rates of surgical site infections in our study can be explained as follows. The additional assessment of the wounds by an independent wound expert classified surgical site infections that in routine clinical practice would probably not have been classified as such. Firstly, if, for example, a surgical site infection was treated very briefly by using local antiseptic measures, this might well not have been documented outside the study. Secondly, the follow-up period of 30 days means a greater number of documented surgical site infections that would not have been documented if patients had already been discharged from hospital. More than 80% of the A1 surgical site infections were diagnosed only after Day 10.
Of note were the many occurrences of wound dehiscence (15.1%) after removal of staples on the 10th postoperative day, which did not represent a surgical site infection but did represent a surgical site complication. Almost no wound dehiscence was seen in the sutures group (2.8%). When considering any kind of wound complications—infection and dehiscence—then intracutaneous sutures emerge as more beneficial: 23/141 patients (16.3%) in the sutures group versus 42/139 patients (30.2%) in the staples group (OR 0.45; [0.25; 0.80]; P=0.006). Although our study was not a non-inferiority study, the results show that intracutaneous sutures in gastrointestinal surgery are non-inferior to staples with regard to the rate of surgical site infections, and are more beneficial with regard to wound dehiscence.
Multiple regression analysis showed that surgical access and the length of the skin incision were surgical risk factors for an increased rate of surgical site infections. For midline laparotomy, a higher incidence of A1 surgical site infections was found than for transverse laparotomy (OR=3.5). In the multiple regression model, the length of the skin incision was also associated with higher rates of A1 surgical site infections. Intraoperative blood loss and contamination did not affect the incidence of surgical site infections. In the univariate analysis, the thickness of the subcutaneous fat layer was more often associated with A1 surgical site infections. The study thus confirmed the results of earlier data collections (32, 33).
The limitations of this study include the fact that it is a single center study. Study participants had to meet stringent inclusion criteria; more than 750 patients were screened in order to randomize 286 patients. The study was characterized by a standardized patient selection undertaking of the intervention. Furthermore, the follow-up at four time points, with photographic documentation of the wound findings, was very precise.
Conclusion
Intracutaneous sutures do not confer any advantage over staples as far as superficial A1 surgical site infections after elective gastrointestinal surgery are concerned. However, wound dehiscence is rarer after intracutaneous sutures. The authors have adapted their clinical practice accordingly and use intracutaneous sutures to close the skin after elective surgery of the gastrointestinal tract.
Table 2. Demographic and clinical patient characteristics.
|
Intracutaneous suture (n = 141; 50.4 %) |
Surgical staples (n = 139; 49.6 %) |
P value | |
| Demographic data | |||
| Age (years) | 66 (54–74) | 61 (53–72) | 0.0496 |
| Sex – Male – female |
96 (68.1 %) 45 (31.9 %) |
91 (65.5 %) 48 (34.5 %) |
0.64 |
| ASA 1 2 3 4 |
29 (20.6 %) 54 (38.3 %) 56 (39.7 %) 2 (1.4 %) |
31 (22.3 %) 70 (50.4 %) 38 (27.3 %) 0 (0.0 %) |
0.06 |
| BMI | 26.6 (23.8–30.8) | 25.8 (23.5–30.3) | 0.19 |
| Diabetes | 22 (15.6 %) | 25 (18.0 %) | 0.59 |
| Smoker | 21 (14.9 %) | 24 (17.3 %) | 0.29 |
| On steroid medication | 2 (1.4 %) | 5 (3.6 %) | 0.24 |
| Clinical data | |||
| Duration of surgical procedure (min) | 201 (139–283) | 190 (140–265) | 0.81 |
| Time taken to close skin (min) | 7.4 (5.3–9.7) | 1.3 (0.9–1.8) | <0.001 |
| Access – Midline laparotomy – Transverse laparotomy |
70 (49.6 %) 71 (50.4 %) |
67 (48.2 %) 72 (51.8 %) |
0.81 |
| Length of skin incision (cm) (interquartile range) | 25 (19– 30) | 23 (18– 29) | 0.29 |
| Surgical site – Lower GI tract – Upper GI tract – Hepatobiliary and pancreatic |
84 (59.6 %) 22 (15.6 %) 35(24.8 %) |
81 (58.3 %) 22 (15.8 %) 36 (25.9 %) |
0.97 |
| Thickness of subcutaneous fat layer (cm) | 3.0 (2.0–3.5) | 2.5 (1.6–3.5) | 0.25 |
| Intraoperative blood loss >500 mL | 6 (4.3 %) | 3 (2.2 %) | 0.32 |
| Relevant contamination intraoperatively | 2 (1.4 %) | 1 (0.7 %) | 0.57 |
Data as n (%) or median (IQR).
ASA, American Society of Anesthesiologists (ASA 1: normal, otherwise healthy patient, ASA 2: mild general illness, ASA 3: severe general illness, ASA 4: severe general illness that poses a continuous threat to life); BMI, body mass index; GI tract, gastrointestinal tract.
Differences between baseline characteristics of the two groups did not reach significance except in older patients (P = 0.0496).
Differences between times, types of surgery, and intraoperatively collected data did not reach significance except for the time taken to close the skin (p <0.0001, Mann-Whitney-U-Test).
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Acknowledgements
Our sincerest thanks go to the patients who participated in our study. Further, we wish to express our thanks for financial funding of our study in the context of the cooperation agreement between the University Hospital of Giessen and Marburg GmbH and the Philipps University of Marburg.
Registration
Study registration number DRKS 00004542.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Behnke M, Aghdassi SJ, Hansen S, Diaz LAP, Gastmeier P, Piening B. The prevalence of nosocomial infection and antibiotic use in German hospitals. Dtsch Arztebl Int. 2017;114:851–857. doi: 10.3238/arztebl.2017.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcome: a systematic review in six European countries. J Hosp Infect. 2017;96:1–15. doi: 10.1016/j.jhin.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broex EC, van Asselt AD, Bruggeman CA, van Tiel FH. Surgical site infections: how high are the costs? J Hosp Infect. 2009;72 doi: 10.1016/j.jhin.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Rüden H, Daschner F, Schuhmacher M. Nosokomiale Infektionen in Deutschland: Erfassung und Prävention; (NIDEP-Studie); Teil 1 Das Bundesministerium für Gesundheit (eds.): Nosokomiale Infektionen in Deutschland: Erfassung und Prävention. Baden-Baden: Nomos Verlagsgesellschaft, Band. 1995;56 [PubMed] [Google Scholar]
- 6.Robert Koch-Institut. Basisdaten der stationären Krankenhausversorgung in Deutschland - nosokomiale Infektionen. Epidemiologisches Bulletin. www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2010/Ausgaben/36_10.pdf?__blob=publicationFile (last accessed on 16 April 2019) 2010;36 [Google Scholar]
- 7.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. The Hospital Infection Control Practices Advisory Committee: Guideline for prevention of surgical site infection. Am J Infect Contro. 1999;27:97–132. [PubMed] [Google Scholar]
- 8.Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection. JAMA Surg. 2017;152(8):784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 9.Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen. www.nrz-hygiene.de/surveillance/kiss/cdc-definitionen (last accessed on September 2016) [Google Scholar]
- 10.Prävention postoperativer Wundinfektionen - Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut. Bundesgesundheitsbl. 2018;61:448–473. doi: 10.1007/s00103-018-2706-2. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organisation. Global guidelines for prevention of surgical site infection. www.who.int/infection-prevention/publications/ssi-guidelines/en/ (last accessed on 15 April 2019) [Google Scholar]
- 12.Park HM, Han SS, Lee EC, et al. Randomized clinical trial of preoperative skin antisepsis with chlorhexidine gluconate or povidone-iodine. Br J Surg. 2017;104:e145–e150. doi: 10.1002/bjs.10395. [DOI] [PubMed] [Google Scholar]
- 13.Mueller TC, Loos M, Haller B, et al. Intra-operative wound irrigation to reduce surgical site infections after abdominal surgery: a systematic review and meta-analysis. Langenbecks Arch Surg. 2015;400:167–181. doi: 10.1007/s00423-015-1279-x. [DOI] [PubMed] [Google Scholar]
- 14.Smyth ET, Emmerson AM. Surgical site infection surveillance. J Hosp Infect. 2000;45:173–184. doi: 10.1053/jhin.2000.0736. [DOI] [PubMed] [Google Scholar]
- 15.Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38:1706–1715. doi: 10.1086/421095. [DOI] [PubMed] [Google Scholar]
- 16.Leaper D, Burman-Roy S, Palanca A, et al. Prevention and treatment of surgical site infection: summary of NICE guidance. BJM. 2008;337 doi: 10.1136/bmj.a1924. [DOI] [PubMed] [Google Scholar]
- 17.Reid K, Pockney P, Draganic B, Smith SR. Barrier wound protection decreases surgical site infection in open elective colorectal surgery: a randomized clinical trial. Dis Colon Rectum. 2010;53:197–204. doi: 10.1007/DCR.0b013e3181ed3f7e. [DOI] [PubMed] [Google Scholar]
- 18.Hawn MT, Vick CC, Richman J, et al. Surgical site infection prevention: time to move beyond the surgical care improvement program. Ann Surg. 2011;254:494–499. doi: 10.1097/SLA.0b013e31822c6929. [DOI] [PubMed] [Google Scholar]
- 19.Tsujinaka T, Yamamoto K, Fujita J, et al. Subcuticular sutures versus staples for skin closure after open gastroinstestinal surgery: a phase 3, multicentre, open-label, randomized controlled trial. Lancet. 2013;382:1105–1112. doi: 10.1016/S0140-6736(13)61780-8. [DOI] [PubMed] [Google Scholar]
- 20.Imamura K, Adachi K, Sasaki R, et al. Randomized comparison of subcuticular sutures versus staples for skin closure after open abdominal surgery: a multicenter open-label randomized controlled trial. J Gastrointest Surg. 2016;20:2083–2092. doi: 10.1007/s11605-016-3283-z. [DOI] [PubMed] [Google Scholar]
- 21.Eldrup J, Wied U, Andersen B. Randomized trail comparing proximate stapler with conventional skin closure. Acta Chirurgica Skandinavia. 1981;147:501–502. [PubMed] [Google Scholar]
- 22.Gatt D, Quick CR, Owen-Smith MS. Staples for wound closure: a controlled trial. Ann R Coll Surg Engl. 1985;67:318–320. [PMC free article] [PubMed] [Google Scholar]
- 23.Pickford IR, Brennan SS, Evans M, Pollock AV. Two methods of skin closure in abdominal operations: a controlled clinical trial. Br J Surg. 1983;70:226–228. doi: 10.1002/bjs.1800700414. [DOI] [PubMed] [Google Scholar]
- 24.Ranaboldo IR, Rowe-Jones DC. Closure of laparotomy wounds: skin staples versus sutures. Br J Surg. 1992;79:1172–1174. doi: 10.1002/bjs.1800791122. [DOI] [PubMed] [Google Scholar]
- 25.Zwart HJ, De Ruiter P. Subcuticular, continuous and mechanical skin closure: cosmetic results of a prospective randomized trial. Neth J Surg. 1989;41:57–60. [PubMed] [Google Scholar]
- 26.Wang ZX, Jiang CP, Cao Y, Ding YT. Systematic review and meta-analysis of triclosan-coated sutures for prevention of surgical-site infection. Br J Surg. 2013;100:465–473. doi: 10.1002/bjs.9062. [DOI] [PubMed] [Google Scholar]
- 27.Maschuw K, Heinz C, Maurer E, Reuss A, Schade-Brittinger C, Bartsch DK. Intracutaneous suture versus transcutaneous skin stapling for closure of midline or horizontalskin incision in elective abdominal surgery and their outcome on superficial surgical site infections - INTRANS: study protocol for a randomized controlled trial. Trials. 2014;15 doi: 10.1186/1745-6215-15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann Surg. 2004;239:599–605. doi: 10.1097/01.sla.0000124292.21605.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi S, Ito M, Yamamoto S, et al. Randomized clinical trial of skin closure by subcuticular suture or skin stapling after elective colorectal cancer surgery. Br J Surg. 2015;102:495–500. doi: 10.1002/bjs.9786. [DOI] [PubMed] [Google Scholar]
- 30.Connolly TM, Foppa C, Kazi E, Denoya PI, Bergamaschi R. Impact of a surgical site infection reduction strategy after colorectal resection. Colorectal Dis. 2016;18:910–918. doi: 10.1111/codi.13145. [DOI] [PubMed] [Google Scholar]
- 31.Segal C, Waller DK, Tilley B, Piller L, Bilimoria K. An evaluation of differences in risk factors for individual types of surgical site infection after colon surgery. Surgery. 2014;156:1253–1260. doi: 10.1016/j.surg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Fujii T, Tsutsumi S, Matsumoto A, et al. Thickness of subcutaneous fat as a strong risk factor for wound infections in elective colorectal surgery: impact of prediction using preoperative CT. Dig Surg. 2010;27:331–335. doi: 10.1159/000297521. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa H, Ohno K, Ikeda S, Muto M. The effect of preoperative subcutaneous fat thickness on surgical site infection risk in patients undergoing colorectal surgery: Results of a multisite, prospective cohort study. Ostomy Wound Manager. 2016;62:14–20. [PubMed] [Google Scholar]