Abstract
Introduction:
Intensive care medicine can contribute to population health in low-income countries by reducing premature mortality related to surgery, trauma, obstetrical and other medical emergencies. Quality improvement is guided by risk stratification models, which are developed primarily within high-income settings. Models validated for use in low-income countries are needed.
Methods:
This prospective cohort study consisted of 261 patients admitted to the intensive care unit (ICU) of K***** Central Hospital in Malawi, from September 2016 to March 2018. The primary outcome was in-hospital mortality. We performed univariable analyses on putative predictors and included those with a significance of 0.15 in the Malawi Intensive care Mortality risk Evaluation model (MIME). Model discrimination was evaluated using the area under the curve.
Results:
Males made up 37.9% of the study sample and the mean age was 34.4 years. A majority (73.9%) were admitted to the ICU after a recent surgical procedure, and 59% came directly from the operating theater. In-hospital mortality was 60.5%. The MIME based on age, sex, admitting service, systolic pressure, altered mental status, and fever during the ICU course had a fairly good discrimination, with an AUC of 0.70 (95% CI 0.63–0.76).
Conclusions:
The MIME has modest ability to predict in-hospital mortality in a Malawian ICU. Multicenter research is needed to validate the MIME and assess its clinical utility.
Keywords: global surgery, low-income, critical care, risk model
Introduction
The delivery of high-quality intensive care medicine may decrease mortality from trauma, infectious disease, obstetric conditions, and surgical complications[1–3]. Morbidity and mortality from these conditions are disproportionately high in low- and middle-income countries (LMICs), where intensive care unit (ICU) expertise and bed availability is lowest[4]. In order to effectively assess the provision and quality of intensive care medicine in LMICs, appropriate risk stratification models are needed. These models may allow for better interpretation of the severity of illness and corresponding mortality rates in regions where critical care services are still developing, facilitate risk-adjusted comparisons of critical care populations in disparate settings, and inform resource allocation in LMICs[5].
The majority of ICU risk stratification models developed to date have been based upon cohorts in high-income settings, which limits their generalizability to LMIC populations, where population demographics, environmental exposures, and critical care capabilities and practices are different [6–12]. Many ICU risk models are also not feasible for LMICs because they require too many assessments and/or laboratory measurements. The objective of this study was to utilize routinely collected data to develop an ICU mortality prediction model for use in Malawi and other LMICs.
Methods
This was prospective, observational cohort study of all patients admitted to the ICU of K***** Central Hospital (KCH) in Lilongwe, Malawi. Data collection occurred from September 2016 to March 2018 based on the funding period. Based on our previous work at this and nearby study sites[13, 14], we anticipated that this timeline would be adequate to achieve a sample size of approximately 250 patients, consistent with other similar studies in the field [15, 16]. The study protocol was developed a priori and approved by the National Health Sciences Research Council of Malawi and the Institutional Review Boards of both American universities with which the study was affiliated, and the requirement for written informed consent was waived by all. The study was registered at researchregistry.com under protocol 4330. Malawi is a country in southern Africa with a population of 18 million people, a life expectancy of 63.8 years, and a Human Development Index rank of 170 out of 187 countries[17]. It is the sixth poorest country in sub-Saharan Africa[18]. KCH is a central referral hospital in the central region of Malawi with a catchment area of approximately 5 million.
The data were collected prospectively by clerks specifically trained in ICU data abstraction. The clerks started data collection for each patient at the time of ICU admission by medical chart review and followed the patients to hospital discharge or death. Data collected included date of hospital admission, location before ICU admission (e.g. Emergency Room, Operating Theater, Ward), admitting service (e.g. Surgery, Obstetrics and Gynecology, Medicine, Pediatrics), vital signs and laboratory measurements at the time of ICU admission treatments utilized in ICU (e.g. mechanical ventilation, blood transfusion), location to which patients were discharged, and the hospital discharge date.
Vital signs collected included an assessment of mental status using the AVPU scale (Alert, Verbally-responsive, Painful-stimulus responsive, and Unresponsive), which was simpler and more acceptable to local clinicians than the Glasgow Coma Scale Because AVPU was frequently confounded by postoperative residual anesthesia, during data analysis we simplified it into an assessment of altered mental status (any value other than Alert). In addition to standard vital sign measurements, we also assessed for the clinical suspicion of infection at ICU admission and for the presence of fever (>38.4°C) at any time during the ICU course. Questions about data points were addressed by an author who is full-time ICU clinical officer at KCH (CK). All records were initially kept on paper and then maintained in a de-identified computer database.
Exclusion criteria for patients included age ≤15 years old, readmission to ICU (e.g. only the index admission was included), and ICU admission for a reported head injury; supplemental analyses included patients (1) with missing HIV serostatus managed via list-wise deletion, forced into the model, or imputed as negative and positive as per other studies in the literature[19], and (2) with a reported head injury to assess the validity of the predictive model in the larger cohort. The primary outcome was in-hospital mortality.
We first described the cohort, looking for differences between survivors and non-survivors. We performed a univariable analysis on all independent predictors for in-hospital mortality and included those that reached a significance level of ≤0.15 and had a low proportion of missing values (<10%) in the final model, the Malawi Intensive care Mortality risk Evaluation (MIME). A simplified Malawi Intensive care Mortality Evaluation (simple MIME) was explored and developed using a backward elimination procedure with a criterion of p < 0.10 from the full model, to provide an alternative that would be especially simple to implement in low-resourced environments. Model discrimination was evaluated using the area under the receiver operating characteristic curve (AUROC, or c-statistic) with 95% confidence intervals (CI). The area under the curve (AUC) summarizes how well a model is able to accurately delineate hospital survival after ICU admission. Model fit was assessed using Hosmer-Lemeshow Goodness-of-Fit, Akaike information criterion (AIC), and R-squared. Internal validation of model accuracy was performed using 10-fold cross validation. Statistical analyses were conducted using SAS Version 9.4 (SAS Institute, Inc., Cary, NC). The results are reported in line with the STROCSS criteria.[20]
Results
Between September 2016 and March 2018, 431 patients were admitted to the study ICU. After excluding readmissions (n=7), patients ≤15 years of age (n=84), and those with a head injury (n=88), 276 patients were eligible for analysis. Exclusion criteria were not mutually exclusive. Fifteen patients were missing outcome data and were also excluded, for a total cohort of 261 patients. Males accounted for 37.9% of the cohort, and the mean age was 34.4 ±15.1 years (range 16–84 years). A majority of the patients (73.9%) were admitted to the ICU after a recent surgical procedure, and 58.6% came directly from the operating theater while 24.5% were admitted from one of the hospital’s four High-Dependency Units. Overall in-hospital mortality in the cohort was 60.5%. (Table 1)
Table 1.
Univariable analysis of independent predictors for hospital mortality among ICU patients at a central referral hospital in Malawi.
| Variables | Hospital Mortality |
Crude OR (95% CI) | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Total | Survived | Died | ||||||
| N | Mean ± SD, Median (IQR) or No. of patients (%) | N | Mean ± SD, Median (IQR) or No. of patients (%) | N | Mean ± SD, Median (IQR) or No. of patients (%) | |||
| Age, years | 261 | 34.4 ± 15.1 | 103 | 32.6 ± 14.3 | 158 | 35.6 ± 15.6 | 1.01 (1.00, 1.03) | 0.12 |
| Male Sex | 261 | 99 (37.9) | 103 | 32 (31.1) | 158 | 67 (42.4) | 1.63 (0.97, 2.76) | 0.07 |
| ICU Admitting Service | 0.004 | |||||||
| General Surgery | 261 | 112 (42.9) | 103 | 33 (32) | 158 | 79 (50) | Reference | |
| Obstetrics & Gynecology | 261 | 78 (29.9) | 103 | 42 (40.8) | 158 | 36 (22.8) | 0.36 (0.20, 0.65) | |
| Medicine | 261 | 71 (27.2) | 103 | 28 (27.2) | 158 | 43 (27.2) | 0.64 (0.34, 1.20) | |
| Admitted to ICU Postoperatively | 260 | 192 (73.9) | 103 | 76 (73.8) | 157 | 116 (73.9) | 1.01 (0.57, 1.77) | 0.99 |
| Fever (> 38.4C) during ICU Course | 259 | 117 (45.2) | 102 | 34 (33.3) | 157 | 83 (52.9) | 2.24 (1.34, 3.76) | 0.002 |
| Measured at ICU Admission: | ||||||||
| Tachycardia (HR ≥ 100 bpm) | 257 | 198 (77) | 102 | 79 (77.5) | 155 | 119 (76.8) | 0.96 (0.53, 1.75) | 0.90 |
| Systolic Blood Pressure, mmHg | 256 | 115.4 ± 31 | 102 | 119.9 ± 30.9 | 154 | 112.4 ± 30.8 | 0.99 (0.98, 1.00) | 0.06 |
| Altered Mental Status | 255 | 212 (83.1) | 100 | 75 (75) | 155 | 137 (88.4) | 2.54 (1.30, 4.95) | 0.006 |
| Presence of Endotracheal Tube | 261 | 151 (57.9) | 103 | 58 (56.3) | 158 | 93 (58.9) | 1.11 (0.67, 1.83) | 0.68 |
| Provision of Tracheostomy | 259 | 12 (4.6) | 102 | 3 (2.9) | 157 | 9 (5.7) | 2.01 (0.53, 7.60) | 0.31 |
| Suspected Infection | 260 | 155 (59.6) | 103 | 59 (57.3) | 157 | 96 (61.2) | 1.17 (0.71, 1.95) | 0.53 |
| Hemoglobin, g/dL | 231 | 9.7 (7.3, 11.5) | 95 | 9.6 (7, 11.6) | 136 | 10 (7.5, 11.4) | 1.04 (0.95, 1.13) | 0.39 |
| HIV Status | 213 | 32 (15) | 86 | 8 (9.3) | 127 | 24 (18.9) | 2.28 (0.97, 5.33) | 0.06 |
| Malaria Status | 186 | 21 (11.3) | 74 | 6 (8.1) | 112 | 15 (13.4) | 1.75 (0.65, 4.75) | 0.27 |
HIV: Human immunodeficiency virus; HR: Heart Rate; ICU: Intensive Care Unit; IQR: interquartile range; No., N: Number; OR: odds ratio; SD: standard deviation.
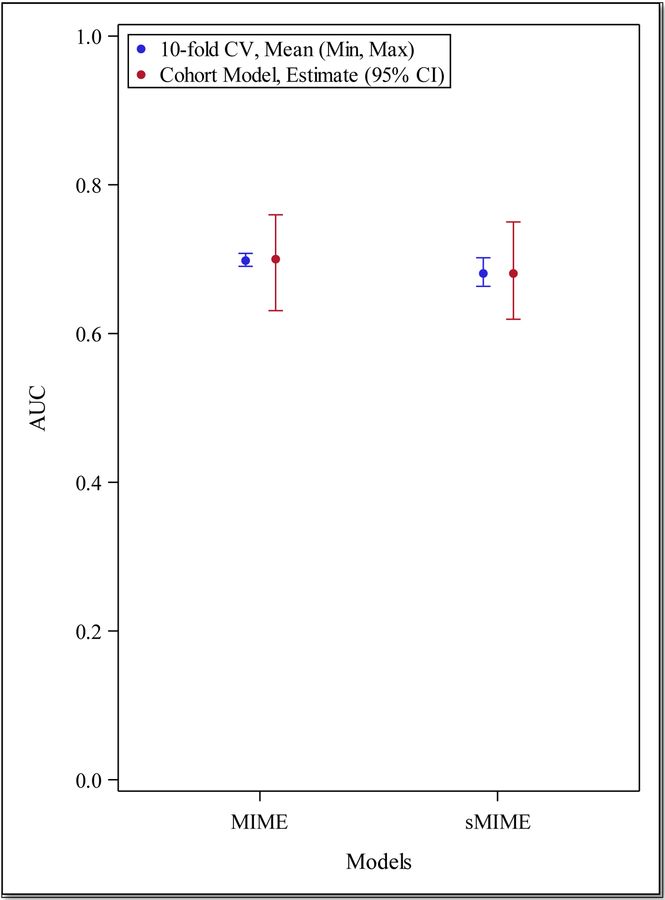
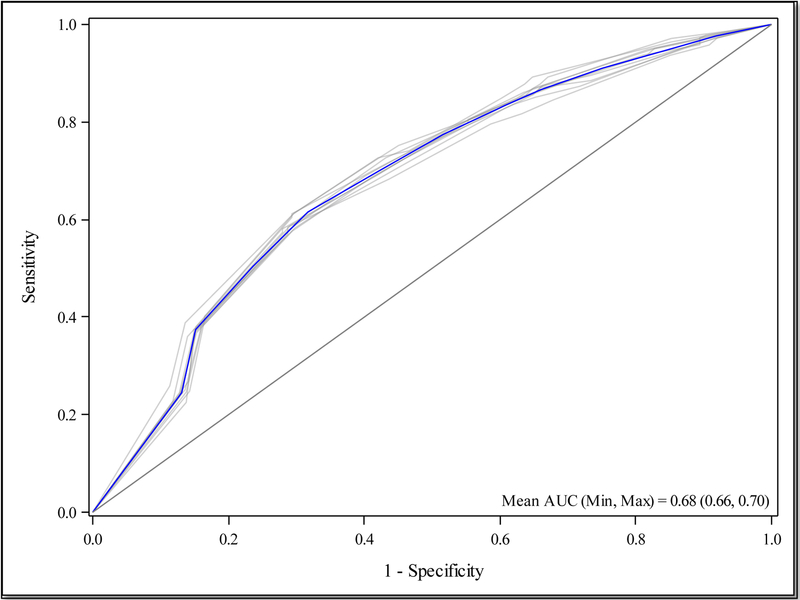
The full Malawi Intensive care Mortality risk Evaluation (MIME) model includes the following variables: age, sex, ICU admitting service, systolic blood pressure at ICU admission, altered mental status at ICU admission, and the presence of a fever during the ICU course. All variables included in the final model had <10% in missing responses. (Appendix, Table A1) The model demonstrated good discrimination, with an AUC of 0.70 (95% CI 0.63–0.76), an average sensitivity of 83.9% (±10.6) in the 10-fold cross-validation. (Tables 2 and 3, Figures 1 and 2)
Table 2.
Multivariable analysis of hospital mortality, for Malawi Intensive care Mortality risk Evaluation (MIME) Model and simplified MIME.
| Models | Hospital Mortality Yes vs No | P-value |
|---|---|---|
| MIME (N = 252) | Adjusted OR (95% CI) | |
| Age | 1.01 (0.99, 1.04) | 0.21 |
| Male Sex | 1.14 (0.57, 2.27) | 0.71 |
| ICU Admitting Service | 0.20 | |
| General Surgery | Reference | |
| Obstetrics & Gynecology | 0.55 (0.26, 1.19) | |
| Medicine | 1.01 (0.50, 2.08) | |
| Fever (> 38.4C) During ICU Course | 2.27 (1.30, 3.96) | 0.004 |
| Systolic Blood Pressure at ICU Admission | 0.99 (0.98, 1.00) | 0.11 |
| Altered Mental Status at ICU Admission | 2.13 (1.03, 4.38) | 0.04 |
| sMIME (N = 254) | Adjusted OR (95% CI) | |
| ICU Admitting Service | 0.01 | |
| General Surgery | Reference | |
| Obstetrics & Gynecology | 0.39 (0.21, 0.73) | |
| Medicine | 0.76 (0.39, 1.47) | |
| Fever During ICU Course | 2.08 (1.21, 3.58) | 0.008 |
| Altered Mental Status at ICU Admission | 2.07 (1.02, 4.20) | 0.04 |
HIV: Human immunodeficiency virus; HR: Heart Rate; ICU: Intensive Care Unit; IQR: interquartile range; No.: Number; OR: odds ratio; SD: standard deviation.
Table 3.
Model discrimination, fit, and internal validation for final scoring methods.
| MIME | sMIME | |
|---|---|---|
| Model Discrimination and Fit | ||
| AUC (0–1, higher indicates better prediction) | 0.70 (0.63, 0.76) | 0.68 (0.62, 0.75) |
| AIC (lower indicates better model fit) | 325.59 | 325.54 |
| Hosmer-Lemeshow chi-square statistic | 4.22 | 5.89 |
| Hosmer-Lemeshow p-value (lower indicates more evidence of a lack in model fit) | 0.84 | 0.44 |
| R2 (0–1, higher indicates better prediction) | 0.14 | 0.12 |
| Performance on 10-fold cross-validated testing cohort | ||
| Accuracy, %, Mean (SD) | 62.8 (7.9) | 66.3 (8.7) |
| Sensitivity, %, Mean (SD) | 83.9 (10.6) | 84.0 (10.1) |
| Specificity, %, Mean (SD) | 31.8 (12.7) | 39.7 (12.8) |
AIC: Akaike information criterion; AUC: Area Under the Receiver Operating Curve; SD: standard deviation.
Figure 1.
Area Under the Receiver Operating Curve (AUC) with 95% Confidence Intervals (CI) for Malawi Intensive care Mortality risk Evaluation (MIME) Model and simplified MIME (sMIME), and AUC for 10-fold cross validation (CV) results
Figure 2.
10-fold cross-validated Receiver Operating Curve for Malawi Intensive care Mortality risk Evaluation (MIME) Model
We examined an alternative model, the simplified Malawi Intensive care Mortality Evaluation (sMIME), which included ICU admitting service, altered mental status at ICU admission, and the presence of fever during the ICU course. (Table 2) This model included fewer variables and demonstrated similar discrimination as the full model with an AUC of 0.68 (95% CI 0.62–0.75). When internally validated, the sMIME model also performed with the same level of sensitivity as the full model. (Tables 2 and 3, Figure 3)
Figure 3.
10-fold cross-validated Receiver Operating Curve for simplified Malawi Intensive care Mortality risk Evaluation (sMIME) Model
Sensitivity analyses of the multivariable models were explored with the missing HIV serostatus cases managed via list wise deletion, when forced in the model, or imputed as negative and as positive. With the missing HIV serostatus cases imputed as negative or as positive, the model also included the HIV status. The model AUC for the MIME in both these analyses was 0.71 (95% CI 0.64–0.77). By excluding missing HIV status cases but forcing HIV status into the predictive model, the model AUC was 0.72 (95% CI 0.65–0.79). (Appendix Tables A2–3)
Supplemental analyses that included patients with a severe head injury at ICU admission yielded similar results. In these analyses, which included 325 patients, the MIME included the same variables as the primary analysis and demonstrated modest discrimination with an AUC of 0.69 (95% CI 0.63–0.75) and an average of 81% (±9.4) sensitivity in the 10-fold internal validation assessment. With the inclusion of patients with head injuries, the simplified MIME incorporated systolic blood pressure at ICU admission and demonstrated a model AUC of 0.68 (05% CI 0.62–0.74). (Supplement Tables S1–6, Supplement Figures S1–S3)
Discussion
The Malawi Intensive care Mortality Evaluation model (MIME) demonstrated modest ability to predict in-hospital mortality in a population of Malawian ICU patients. Its component variables were easily collected during the clinical care of an ICU patient in a low-resource setting without the need for invasive monitors or laboratory measurements. This score may be considered as a useful retrospective tool to evaluate expected versus observed in-hospital mortality for patients with critical illness in low-income settings but should be externally validated within other sub-Saharan African populations before broad application in future studies. We anticipate its utility to be derived in comparative retrospective studies of critical care services worldwide.
The World Health Organization recommends that any facility providing surgery should have critical care services[21]. This is still a challenge in many LMICs[4], including Malawi where ICU bed availability is only 1 bed per 1 million population[22]. The overall in-hospital mortality for ICU patients at this study site is high (60.5%). Although this is a relatively new area of research, the available prospective literature confirms high in-hospital mortality for ICU patients throughout the Eastern and Central African region, ranging from 46.6% to 50%[15, 16, 23]. An assessment of patients’ critical illness severity is imperative to contextualize this mortality rate. The MIME serves this purpose, and has discrimination within range of other newly developed models for sub-Saharan Africa[15, 16]. The development of all of these models suffers from small sample sizes, but it is nonetheless a first step towards framing critical care services in this region.
The past decade of research in global public health has demonstrated the importance of addressing non-communicable diseases [24, 25] and surgery[26] to improve population health in LMICs. Public health interventions are most successful when they are multimodal, incorporating both disease-specific interventions (e.g. medications) and systems-level improvements (e.g. protocols and infrastructural improvements). For example, the rollout of medications for human immunodeficiency virus (HIV) in Rwanda between 1996 and 2013 was supplemented by changes in the healthcare system (namely, decentralization of testing centers and changes in user fee structures), which led to improved overall success in slowing the HIV epidemic[27]. Improving access to safe surgical care should take these lessons into account; quality critical care services are essential to this mission. Scoring models feasible for use in low-resourced settings will contribute to better understanding of critically ill patients in this region and form one piece of this effort to improve access for safe surgery.
Critical care services must be tailored to the local disease epidemiology, practice patterns, and resources. Though there is scarce ICU research within LMICs to date, available data demonstrate that ICU patients in LMICs are younger and more likely to be admitted to ICU following trauma or surgery compared to cohorts in high-income settings[13, 14, 28–31]. Our findings are consistent with these observations. However, the effects of endemic tropical diseases (e.g. HIV, malaria) on critical illness are not yet well-defined. These conditions, including malaria, typhoid [32], schistosomiasis[33], and HIV[34] are common in hospitalized patients in sub-Saharan Africa. These infections affect endothelial and immune function[35, 36], which may have implications for the development, and severity of sepsis or other multiorgan dysfunction states. This may be a missing link in developing risk stratification models with better discrimination. We attempted to address this question in part by including sensitivity analyses on the HIV serostatus of our patients, but future research may aim to evaluate a broader spectrum of endemic tropical diseases.
There are several limitations to this study. First, the challenges of conducting prospective clinical research in a low-resource setting must be emphasized and underlies some of the missing values in our dataset. Our supplemental analyses were designed to remain consistent with other reports in the literature, but also to address this limitation. Second, we did not include laboratory or clinical values frequently measured in high-income settings. While we recognize that certain laboratories (e.g. white blood cell count, lactate) and clinical assessments such as the Systemic Inflammatory Response Syndrome (SIRS) have been used for critical illness risk stratification [37–40], these are not regularly available in the study ICU. Therefore, this was done as part of our commitment to working within the confines of the Malawi healthcare system, and to increase the generalizability of this work to other LMICs. Finally, since we developed the MIME and simple-MIME model from this dataset, the model AUC needs to be externally validated.
Conclusions
Risk stratification models are necessary to inform critical care systems worldwide. Models created within and for LMICs are critical to improving the quality of global surgery and treatments for non-communicable diseases. The MIME model provides moderate discrimination for ICU in-hospital mortality in Malawi. It may be considered as a measure of in-hospital mortality risk for patients with critical illness in low-resource settings and may facilitate comparisons with high-income regions. Further application will rely on external validation.
Supplementary Material
Appendix
Table A1.
Proportion of Missing Responses in Potential Variables for Malawi Intensive care Mortality risk Evaluation (MIME) Model
| Potential Variables in New Model | No. of Missing (%) |
| Age | 0 (0) |
| Male Sex | 0 (0) |
| ICU Admitting Service | 0 (0) |
| Post-Operative Status | 1 (0.4) |
| Fever (>38.4C) During ICU Course | 2 (0.8) |
| Measured at ICU Admission: | |
| Tachypnea (Heart Rate ≥100 bpm) | 4 (1.5) |
| Systolic Blood Pressure | 5 (1.9) |
| Altered Mental Status | 6 (2.3) |
| Presence of Breathing Tube | 0 (0) |
| Suspected Infection | 1 (0.4) |
| Plasma Hemoglobin | 30 (11.5) |
| HIV Status | 48 (18.4) |
| Malaria Status | 75 (28.7) |
Table A2.
Summary of hospital mortality and univariable analysis of independent predictors of hospital mortality, with HIV status excluded when missing versus imputation as positive or negative when missing
| Variables | Hospital Mortality | Hospital Mortality Yes vs No |
P-value | |||||
| Total | No | Yes | ||||||
| N | Mean ± SD, Median (IQR) or No. of patients (%) | N | Mean ± SD, Median (IQR) or No. of patients (%) | N | Mean ± SD, Median (IQR) or No. of patients (%) | Crude OR (95% CI) | ||
| HIV Status – exclude missing | 213 | 32 (15) | 86 | 8 (9.3) | 127 | 24 (18.9) | 2.28 (0.97, 5.33) | 0.06 |
| HIV Status – missing as negative | 261 | 32 (12.3) | 103 | 8 (7.8) | 158 | 24 (15.2) | 2.13 (0.92, 4.94) | 0.08 |
| HIV Status – missing as positive | 261 | 80 (30.7) | 103 | 25 (24.3) | 158 | 55 (34.8) | 1.67 (0.96, 2.91) | 0.07 |
CI: confidence interval; HIV: human immunodeficiency virus; IQR: interquartile range; No.: number; SD: standard deviation; OR: odds ratio
Table A3.
Sensitivity Analysis Tables for HIV in Primary Analysis of Model Performance (excluding head injury patients)
| MIME Model | sMIME Model | |||||||
| N | Adj. OR (95% CI) | Covariates in model | Model AUC (95% CI) | N | Adj. OR (95% CI) | Covariates in model | Model AUC (95% CI) | |
| HIV – missing as positive | 252 | 1.72 (0.94, 3.18) | Age, sex, service, systolic blood pressure, fever, altered mental status | 0.71 (0.64, 0.77) | 254 | 1.94 (1.07, 3.54) | Age, fever, and altered mental status | 0.68 (0.61, 0.74) |
| HIV – missing as negative | 252 | 2.19 (0.89, 5.38) | Age, sex, service, systolic blood pressure, fever, altered mental status | 0.71 (0.64, 0.77) | 254 | 2.21 (0.91, 5.35) | Service, fever, and altered mental status | 0.69 (0.63, 0.76) |
| HIV – exclude missing, force into model | 208 | 2.39 (0.96, 5.96) | Age, sex, service, systolic blood pressure, fever, altered mental status | 0.72 (0.65, 0.79) | 209 | 2.67 (1.09, 6.52) | Sex, fever, and altered mental status | 0.68 (0.61, 0.75) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Provenance and peer review
Not commissioned, externally peer-reviewed
References
- 1.Cubro H, Somun-Kapetanovic R, Thiery G, Talmor D, Gajic O: Cost effectiveness of intensive care in a low resource setting: A prospective cohort of medical critically ill patients. World J Crit Care Med, 5(2016)150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murthy S, Adhikari NK: Global health care of the critically ill in low-resource settings. Ann Am Thorac Soc, 10(2013)509–513. [DOI] [PubMed] [Google Scholar]
- 3.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD: Critical care and the global burden of critical illness in adults. Lancet, 376(2010)1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy S, Leligdowicz A, Adhikari NK: Intensive care unit capacity in low-income countries: a systematic review. PLoS One, 10(2015)e0116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prin M, Wunsch H: International comparisons of intensive care: informing outcomes and improving standards. Curr Opin Crit Care, 18(2012)700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE: APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med, 9(1981)591–597. [DOI] [PubMed] [Google Scholar]
- 7.Johnson AE, Kramer AA, Clifford GD: A new severity of illness scale using a subset of Acute Physiology And Chronic Health Evaluation data elements shows comparable predictive accuracy. Crit Care Med, 41(2013)1711–1718. [DOI] [PubMed] [Google Scholar]
- 8.Higgins TL, Kramer AA, Nathanson BH, Copes W, Stark M, Teres D: Prospective validation of the intensive care unit admission Mortality Probability Model (MPM0-III). Crit Care Med, 37(2009)1619–1623. [DOI] [PubMed] [Google Scholar]
- 9.Kajdacsy-Balla Amaral AC, Andrade FM, Moreno R, Artigas A, Cantraine F, Vincent JL: Use of the sequential organ failure assessment score as a severity score. Intensive Care Med, 31(2005)243–249. [DOI] [PubMed] [Google Scholar]
- 10.Harrison DA, Parry GJ, Carpenter JR, Short A, Rowan K: A new risk prediction model for critical care: the Intensive Care National Audit & Research Centre (ICNARC) model. Crit Care Med, 35(2007)1091–1098. [DOI] [PubMed] [Google Scholar]
- 11.Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR, Investigators S: SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med, 31(2005)1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul E, Bailey M, Pilcher D: Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand Risk of Death model. J Crit Care, 28(2013)935–941. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson J, Haac B, Kadyaudzu C, Samuel JC, Campbell EL, Lee CN, Charles AG: The burden of surgical diseases on critical care services at a tertiary hospital in sub-Saharan Africa. Trop Doct, 43(2013)27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prin M, Itaye T, Clark S, Fernando RJ, Namboya F, Pollach G, Mkandawire N, Sobol J: Critical Care in a Tertiary Hospital in Malawi. World J Surg, 40(2016)2635–2642. [DOI] [PubMed] [Google Scholar]
- 15.Riviello ED, Kiviri W, Fowler RA, Mueller A, Novack V, Banner-Goodspeed VM, Weinkauf JL, Talmor DS, Twagirumugabe T: Predicting Mortality in Low-Income Country ICUs: The Rwanda Mortality Probability Model (R-MPM). PLoS One, 11(2016)e0155858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sendagire C, Lipnick MS, Kizito S, Kruisselbrink R, Obua D, Ejoku J, Ssemogerere L, Nakibuuka J, Kwizera A: Feasibility of the modified sequential organ function assessment score in a resource-constrained setting: a prospective observational study. BMC Anesthesiol, 17(2017)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UN: Human Development Reports United Nations Development Programme; (2016)last accessed May 18, 2018. [Google Scholar]
- 18.Gregson J: The Richest Countries in the World: International Monetary Fund, World Economic Outlook Database. In Global Finance; 2017. [Google Scholar]
- 19.Moore CC, Hazard R, Saulters KJ, Ainsworth J, Adakun SA, Amir A, Andrews B, Auma M, Baker T, Banura P, et al. : Derivation and validation of a universal vital assessment (UVA) score: a tool for predicting mortality in adult hospitalised patients in sub-Saharan Africa. BMJ Glob Health, 2(2017)e000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agha RAB, MR; Vella-Baldacchino M; Thavayogan R; Orgill DP, STROCCS Group: The STROCSS Statement: Strengthening the Reporting of Cohort Studies in Surgery. International Journal of Surgery, (in press)(2017. [DOI] [PMC free article] [PubMed]
- 21.WHO: Surgical Care at the District Hospital Malta: Interprint Limited; 2003. [Google Scholar]
- 22.Manda-Taylor LM S; Baker T: Critical care in Malawi: the ethics of beneficence and justice. Malawi Medical Journal, 29(2017)268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker T, Blixt J, Lugazia E, Schell CO, Mulungu M, Milton A, Castegren M, Eriksen J, Konrad D: Single Deranged Physiologic Parameters Are Associated With Mortality in a Low-Income Country. Crit Care Med, 43(2015)2171–2179. [DOI] [PubMed] [Google Scholar]
- 24.Piot P, Caldwell A, Lamptey P, Nyrirenda M, Mehra S, Cahill K, Aerts A: Addressing the growing burden of non-communicable disease by leveraging lessons from infectious disease management. J Glob Health, 6(2016)010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alwan A, Maclean DR: A review of non-communicable disease in low-and middle-income countries. Int Health, 1(2009)3–9. [DOI] [PubMed] [Google Scholar]
- 26.Shrime MG, Bickler SW, Alkire BC, Mock C: Global burden of surgical disease: an estimation from the provider perspective. Lancet Glob Health, 3 Suppl 2(2015)S8–9. [DOI] [PubMed] [Google Scholar]
- 27.Nsanzimana S, Prabhu K, McDermott H, Karita E, Forrest JI, Drobac P, Farmer P, Mills EJ, Binagwaho A: Improving health outcomes through concurrent HIV program scale-up and health system development in Rwanda: 20 years of experience. BMC Med, 13(2015)216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olajumoke TO, Oyebamiji EO, Afolayan JM, Adekunle M: Trauma admissions into the intensive care unit and outcome of care in a tertiary health facility. Niger J Med, 23(2014)296–301. [PubMed] [Google Scholar]
- 29.Sawe HR, Mfinanga JA, Lidenge SJ, Mpondo BC, Msangi S, Lugazia E, Mwafongo V, Runyon MS, Reynolds TA: Disease patterns and clinical outcomes of patients admitted in intensive care units of tertiary referral hospitals of Tanzania. BMC Int Health Hum Rights, 14(2014)26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouedraogo N, Niakara A, Simpore A, Barro S, Ouedraogo H, Sanou J: [Intensive care in Africa: a report of the first two years of activity of the intensive care unit of Ouagadougou national hospital (Burkina Faso)]. Sante, 12(2002)375–382. [PubMed] [Google Scholar]
- 31.Kwizera A, Dunser M, Nakibuuka J: National intensive care unit bed capacity and ICU patient characteristics in a low income country. BMC Res Notes, 5(2012)475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitzer VE, Feasey NA, Msefula C, Mallewa J, Kennedy N, Dube Q, Denis B, Gordon MA, Heyderman RS: Mathematical Modeling to Assess the Drivers of the Recent Emergence of Typhoid Fever in Blantyre, Malawi. Clin Infect Dis, 61 Suppl 4(2015)S251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makaula P, Sadalaki JR, Muula AS, Kayuni S, Jemu S, Bloch P: Schistosomiasis in Malawi: a systematic review. Parasit Vectors, 7(2014)570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matoga MM, Rosenberg NE, Stanley CC, LaCourse S, Munthali CK, Nsona DP, Haac B, Hoffman I, Hosseinipour MC: Inpatient mortality rates during an era of increased access to HIV testing and ART: A prospective observational study in Lilongwe, Malawi. PLoS One, 13(2018)e0191944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gillrie MR, Ho M: Dynamic interactions of Plasmodium spp. with vascular endothelium. Tissue Barriers, 5(2017)e1268667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva CLM: Purinergic signaling in schistosomal infection. Biomed J, 39(2016)316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brun-Buisson C: The epidemiology of the systemic inflammatory response. Intensive Care Med, 26 Suppl 1(2000)S64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dulhunty JML, J.; Finfer S; Sepsis Study Investigators for the ANZICS Clinical Trials Group: Does severe non-infectious SIRS differ from severe sepsis? Results from a multi-centre Australian and New Zealand intensive care unit study. Intensive Care Med, 34(2008)1654–1661. [DOI] [PubMed] [Google Scholar]
- 39.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP: The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA, 273(1995)117–123. [PubMed] [Google Scholar]
- 40.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA, Emergency Medicine Shock Research Network I: Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA, 303(2010)739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.