Abstract
Background:
Research on foot problems and frailty is sparse and could advance using wearable sensor-based measures of gait, balance, and physical activity (PA). This study examined the effect of foot problems on the likelihood of falls, frailty syndrome, motor performance, and PA in community-dwelling older adults.
Methods:
Arizona Frailty Cohort Study participants (community-dwelling adults aged ≥65 years without baseline cognitive deficit, severe movement disorders, or recent stroke) underwent Fried frailty and foot assessment. Gait, balance (bipedal eyes open and eyes closed), and spontaneous PA over 48 hours were measured using validated wearable sensor technologies.
Results:
Of 117 participants, 41 (35%) were nonfrail, 56 (48%) prefrail, and 20 (17%) frail. Prevalence of foot problems (pain, peripheral neuropathy, or deformity) increased significantly as frailty category worsened (any problem: 63% in nonfrail, 80% in prefrail [odds ratio (OR) =2.0], and 95% in frail [OR = 8.3]; P= .03 for trend) due to associations between foot problems and both weakness and exhaustion. Foot problems were associated with fear of falling but not with fall history or incident falls over 6 months. Foot pain and peripheral neuropathy were associated with lower gait speed and stride length; increased double support time; increased mediolateral sway of center of mass during walking, age adjusted; decreased eyes open sway of center of mass and ankle during quiet standing, age adjusted; and lower percentage walking, percentage standing, and total steps per day.
Conclusions:
Foot problems were associated with frailty level and decreased motor performance and PA. Wearable technology is a practical way to screen for deterioration in gait, balance, and PA that may be associated with foot problems. Routine assessment and management of foot problems could promote earlier intervention to retain motor performance and manage fear of falling in older adults, which may ultimately improve healthy aging and reduce risk of frailty.
Foot problems are common in older adults.1 Risk factors for foot problems include advancing age, sex, obesity, comorbidities, injudicious footwear, physical activity (PA) (high or low, depending on circumstances), and underlying geriatric syndromes and conditions.1 With age, feet widen and flatten, and the fat padding on the soles of the feet demonstrate greater stiffness, dissipate more energy when compressed, and are slower to recover after the load is removed.2 Frequent painful foot problems occur in an estimated 24% of older adults3 and have been shown to impair balance and foot function4 and more than double the risk of falling.5 This is especially relevant because one-third of adults older than 65 years fall each year and more than half of 80-year-olds fall annually, resulting in substantial health-care use and institutionalization (and associated costs) and deaths.6,7 In addition, 11% of community-dwelling elderly individuals have clinical frailty syndrome, increasing to nearly 26% by age 85 years.8 Despite numerous studies on the association between foot problems and falls, we believe that this is the first work to explore the relationship of foot problems to falls, frailty, and sensor-based measures of motor performance and PA.
Although foot problems are varied and the causes multifactorial, three major foot issues in older adults include pain, neuropathy, and deformities. Under certain circumstances, it can be difficult to tease apart these three major problems. Foot pain itself may be attributable to neuropathy, arthritis, foot deformity, plantar fasciitis, and poorly fitting shoes and is associated with falls, depression, and disability.9–11 Deformities of the feet, which can be congenital but are often caused by long-term use of ill-fitting footwear, can result in difficulties such as pain, ulceration, and risk of falling, among others.12 Foot problems are associated with an iterative cycle of reduced PA, deconditioning, impaired balance, increased fear of falling, and incident falls and have significant consequences when moderate or more in severity.10,13,14
Current research has emphasized the increased foot-related health risks, such as falls, for older adults.4,5,12,14 Foot and chronic pain are particularly strong risk factors for falls, and community-dwelling older adults with pain are more likely to have fallen in the past 12 months and to fall again in the future.15 The association between foot pathologies, decreased foot function, foot pain, and falls is established.5,16 The present study, however, evaluates the association of foot problems in relation to frailty syndrome; objectively measured gait, balance, and PA patterns; and incident falls. The use of wearable sensors provides an objective measure of free-living PA and allows for in-home gait and balance measures on individuals who cannot come to a laboratory or clinic.17,18 The objectives of this study, conducted as part of the Arizona Frailty Cohort, were to 1) characterize foot problems in older adults, particularly pain, deformities, and neuropathy; 2) evaluate the association of foot problems with frailty syndrome and prospective falls; and 3) examine the association between foot problems and objective wearable sensor-derived physical performance parameters (balance and gait) and PA.
Methods
Study Population
Reported data were abstracted from the National Institutes of Health-funded Arizona Frailty Cohort Study, an observational descriptive study of individuals 65 years or older conducted in Tucson, Arizona.17 Participants were recruited from primary, secondary, and tertiary health-care settings; community providers; assisted living facilities; retirement homes; and aging service organizations. The exclusion criteria included being nonambulatory (unable to walk 20 m with or without an assistive device), cognitive impairment as measured by a Mini-Mental State Examination19 score of 23 or less, movement disorders such as Parkinson’s disease or multiple sclerosis, or recent stroke. Eligible individuals signed a written informed consent form approved by the University of Arizona institutional review board. In-home assessments were completed between September 1, 2012, and July 31, 2014, by trained clinical research coordinators. The primary aim of this cohort study was to identify relevant objective wearable sensor data of physical function and everyday PA and to develop algorithms to be used for frailty and fall predictions.17
Measures
Participant Characteristics.
Demographic and health history data (age, sex, race/ethnicity, and daily medication count) were gathered through self-report. Height was taken on-site with a measuring tape, and weight was measured using a bathroom scale (Ozeri Touch II; Ozeri USA, San Diego, California) that provided weight, body fat percentage, and muscle percentage. Use of ambulatory assistive devices, such as canes and walkers, was noted, but participants were not asked whether such use was due to foot problems or whether foot problems were independent of needing an assistive device. In addition, participants answered questionnaires to assess tiredness when performing mobility-related tasks on the Mobility-Tiredness Scale (MOB-T),20 depression on the Center for Epidemiologic Studies Depression Scale, and independence as reflected by performance in activities of daily living from the Barthel Activities of Daily Living Index.21
Foot Pain, Deformity, and Neuropathy.
Participants were asked to rate their pain in each foot separately by marking their current pain level on a 10-cm visual analog scale that spanned from “no pain” to “worst pain ever.” This self-reported pain measurement was then given a numerical value between 0 and 10 based on the placement of the mark on the provided line. A single foot pain score on a scale from 0 to 10 was assigned to each participant as the greater self-reported score between the left and right feet. We categorized participants as having a foot pain problem if they reported moderate-to-severe pain (foot pain score >4) versus those with absent-to-mild pain (foot pain score ≤4), a cutoff point supported for general and various types of pain.22
Study coordinators, trained by podiatric medical specialists, performed a visual foot inspection using an in-color photograph card of general problems and deformities, including bunion, hammertoe, and flatfoot. We categorized participants as having a foot deformity if one of these conditions was observed on either foot.
Neuropathy is the result of nerve damage and can produce symptoms of numbness or stabbing or burning pain. According to the American Diabetes Association guidelines, peripheral neuropathy is confirmed if a patient does not sense a vibration perception threshold of 25 V or more under the big toes or does not respond to a 10-g monofilament test. To determine the existence of peripheral neuropathy, coordinators performed both a touch test and a vibration perception threshold test at the first metatarsal head and heel using a validated device (VibraTip; Henry Wellcome Laboratories, Bristol, England). The device vibrates at 25 V, the validated cutoff point.23 Participants who were unable to feel the touch or vibration in either of those locations on either foot were classified as having neuropathic symptoms.
We defined participants with any foot problem as having a self-reported foot pain problem, peripheral neuropathy, or deformity, allowing that some participants may have more than one problem.
Frailty Assessment.
Frailty was assessed using the five components specified by Fried ET AL24 of weight loss, weakness, walking speed, exhaustion, and low energy expenditure. Our methods, described in more detail elsewhere,17 included self-reported unintentional weight loss of 10 pounds or more in the past year; weakness based on the average of three measures of grip strength tested with a hydraulic hand dynamometer (Fabrication Enterprises Inc, Elmsford, New York), with stratified cutoff values24 by sex and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared); slow walking speed based on time to walk 15 ft, stratified by sex and height; self-reported exhaustion based on frequency that “everything I did was an effort” and “I could not get going”; and low energy expenditure as calories expended per week below sex-stratified cutoff points25 based on the short version of the Minnesota Leisure Time Physical Activity Questionnaire.26 Following this algorithm, participants were categorized as nonfrail if they met none of the criteria, prefrail if they met one or two criteria, and frail if they met three or more criteria. This scale has exhibited high validity and has become a gold standard for classifying frailty in adults older than 65 years.17
Fall Ascertainment.
We recorded separately self-reported history of recent falls (falls in the past 6 months) and self-reported prospective fall incidence (falls occurring in the 6 months after the initial baseline study visit). A fall was considered to be an unexpected event in which the participant comes to rest on the ground, floor, or lower level (Prevention of Falls Network Europe definition).27 At the baseline visit, participants were instructed to record all falls prospectively on a provided 6-month weekly fall diary log (date, time, activity before, injury symptoms, and need for medical attention) and additionally to report all falls by telephone to the study coordination office. A telephone interview after each reported call confirmed the details of the falls and injuries and resolved any missing data. Fall logs were collected in person at the 6-month follow-up visit. Individuals were also asked at baseline to subjectively assess their concerns about falling using the Falls Efficacy Scale-International (FES-I),28 which poses 16 questions regarding the level of fear of falling across various situations.
Gait, Balance, and PA Measures.
Gait and balance trials were performed using a validated wearable technology of five small inertial sensors (triaxial accelerometer and gyroscope) attached to the shins above ankles, thighs above knees, and lower back close to sacrum (LEGSys; BioSensics, Cambridge, Massachusetts).29,30 Balance was measured in two trials of 15 sec, one with eyes open with no visual target specified and one with eyes closed. For balance tests, participants were asked to stand silently and erectly with their arms crossed across their chest and their feet as close together as possible without touching. The balance software (BalanSens; BioSensics) analysis included sway of hip, ankle, and center of mass (COM) extracted from the sensors attached on the right shin and lower back.31 The ankle, hip, and COM sways are the product of mediolateral and anteroposterior sways for each parameter. In-home gait assessment was conducted as participants walked a distance of 15 feet at a self-selected speed using an unobstructed walking course that occasionally had to be created by moving furniture or using a smooth-surfaced outdoor patio. The gait software (LEGSys) analysis included gait speed, stride length, double support time, gait variability (coefficient of variation), and the COM sway in the mediolateral direction based on validated algorithms and data extracted from all five sensors.30,32 Participants who reported regular daily use of assistive devices (canes or walkers) used their device for the gait assessments.
Spontaneous daily PA, including postures (ie, sitting, standing, walking, lying, and transitions) and locomotion (walking bouts, number of steps, etc), was monitored over a 48-hour period using a validated triaxial accelerometer wearable technology device (PAMSys; BioSensics), which was inserted into a T-shirt with a device pocket located at the sternum. Participants were advised to wear this shirt at all times except while showering. Measures as specified in the study by Schwenk ET AL17 were chosen based on previously published Arizona Frailty Cohort analyses and were used to derive the variables of steps per day (24 hours), number of walking bouts per day, longest walking bout duration, and variability in walking bout duration.
Statistical Analysis
Statistical analyses were planned a priori by the epidemiologist and bioengineer co-principal inventigators in collaboration with an experienced biostatistician, and all of the analyses were performed with a statistical software program (Stata version 14; StataCorp LP, College Station, Texas). Participants with foot problems (any problem, pain score >4, peripheral neuropathy, or deformity) were compared with those with no problem. We compared demographic and clinical characteristics between participants with and without a foot problem using Student t tests for continuous variables, χ2 tests (or the Fisher exact test when a cell had fewer than five patients) for categorical variables, and the Cochran-Armitage test for ordinal variables. We present the frequencies of participants missing a categorical variable but excluded such individuals from the analysis of that variable. Associations between the presence of foot problems and categories of frailty, mobility tiredness (median MOB-T score ≤5), concern with falling (median FES-I score >25), and history of any fall in the past 6 months were evaluated with odds ratios and 95% confidence intervals, adjusted for age. Linear trends of odds ratios across increasing frailty were tested by the Wald test on frailty in logistic regression (age adjusted), treating the ordinal frailty variable as continuous, following a method described by Selvin.33 We evaluated the association between the presence of foot problems and incident falls with rate ratios of incidence per person-year and 95% confidence intervals. We performed two-sample t tests to compare mean sensor-based measures between each group with foot problems and the group with no foot problem, presenting means, standard deviations, and P values. We repeated these comparisons using multiple linear regression adjusted for age, and we noted in the text changes from crude comparisons in terms of qualitative patterns and statistical significance. Because certain PA measures (steps taken per day, number of walking bouts per day, longest walking bout duration, and walking bout duration variability) were markedly right-skewed in their distributions, we analyzed natural log-transformed measures but present the nontransformed mean and standard deviation.
Results
Demographic and Clinical Characteristics
The study included 128 individuals, of which 117 formed the analysis set, having nonmissing foot pain scores and ascertainment of peripheral neuropathy and foot deformities. These included 41 (35%) nonfrail, 56 (48%) prefrail, and 20 (17%) frail participants. Eight individuals dropped out after completing baseline measures and, thus, did not contribute to follow-up for incident falls. There were 22 participants (19%) with moderate-to-severe foot pain (score >4), 49 (42%) with peripheral neuropathy in the feet, 60 (51%) with at least one deformity, 90 (77%) with at least one of the three defined foot problems, and 31 (26%) with two or more of the three defined foot problems. The mean ± SD foot pain score was 1.7 ± 2.5, whereas the median (interquartile range) was 0.2 (0–2.5). There were 25 participants (21%) with a bunion, 26 (22%) with hammertoe, and 10 (8.5%) with flat feet.
Table 1 shows baseline demographic characteristics and results of clinical assessments. Participants with foot problems were significantly older (mean ± SD age, 80.0 ± 8.7 versus 76.1 ± 7.5 years; P = .037) and more likely to use a cane or walker on a daily basis (P = .068). The mean ± SD FES-I scores indicated that those with foot problems had significantly greater concerns about falling (29.9 ± 11.6 versus 23.3 ± 10.2; P = .009). Most of the participants were women (79.5%), but there was no significant difference in sex or race/ethnicity distribution by presence of a foot problem. There was no significant difference in the distribution of BMI categories, body fat percentage, and muscle percentage between those with any foot problem and those with no foot problem. However, when comparing those with moderate-to-severe foot pain (score >4) with those with no foot problem, we observed a significant trend of higher likelihood of having foot pain with increasing BMI category (P = .048), as well as a significantly higher mean body fat percentage in those with foot pain (30.9 ± 8.6 versus 24.6 ± 10.1; P = .024).
Table 1.
Demographic and Clinical Characteristics by Any Foot Problem versus No Foot Problem
| Characteristic | Any Foot Problem (n = 90) |
No Foot Problem (n = 27) |
Total (N = 117) |
P Valuea |
|---|---|---|---|---|
| Age (mean ± SD [years]) | 80.0 ± 8.7 | 76.1 ± 7.5 | 79.1 ± 8.5 | .037 |
| Sex (No. [%]) | .802 | |||
| Female | 72 (80.0) | 21 (77.8) | 93 (79.5) | |
| Male | 18 (20.0) | 6 (22.2) | 24 (20.5) | |
| Race/ethnicity (No. [%]) | .692 | |||
| Non-Hispanic white | 71 (78.9) | 20(74.1) | 91 (77.8) | |
| Hispanic white | 4 (4.4) | 1 (3.7) | 5 (4.3) | |
| African American | 3 (3.3) | 0 | 3 (2.6) | |
| Other/refused | 12 (13.3) | 6 (22.2) | 18 (15.4) | |
| Prescriptions (mean ± SD [No.]) | 4.1 ± 3.6 | 3.2 ± 2.3 | 3.9 ± 3.4 | .266 |
| Body mass index (No. [%]) | .533b | |||
| <25 | 32 (35.6) | 12 (44.4) | 44 (37.6) | |
| 25–29.9 | 24 (26.7) | 7 (25.9) | 31 (26.5) | |
| 30–34.9 | 23 (25.6) | 4 (14.8) | 27 (23.1) | |
| ≥35 | 11 (12.2) | 4 (14.8) | 15 (12.8) | |
| Fat (mean ± SD [%]) | 25.5 ± 10.2 | 25.6 ± 10.1 | 25.3 ± 10.1 | .680 |
| Muscle (mean ± SD [%]) | 33.4 ± 4.2 | 32.9 ± 4.6 | 33.3 ± 4.3 | .617 |
| Center for Epidemiologic Studies Depression Scale score, mean ± SD | 8.4 ± 7.4 | 6.5 ± 5.2 | 8.0 ± 7.0 | .212 |
| Barthel ADL Index, mean ± SD | 94.2 ± 7.7 | 93.7 ± 17.9 | 94.1 ± 10.8 | .817 |
| Mobility-Tiredness Scale score (mean ± SD) | 4.4 ± 1.9 | 4.9 ± 1.5 | 4.5 ± 1.8 | .257 |
| Falls Efficacy Scale-International score (mean ± SD) | 29.9 ± 11.6 | 23.3 ± 10.2 | 28.4 ± 11.6 | .009 |
| Social activities (No. [%]) | .061b | |||
| 0 | 6 (6.7) | 1 (3.7) | 7 (6.0) | |
| 1–3 | 43 (47.8) | 7 (25.9) | 50 (42.7) | |
| >3 | 37 (41.1) | 16 (59.3) | 53 (45.3) | |
| Missing | 4 (4.4) | 3 (11.1) | 7 (6.0) | |
| Use assistance devices (No. [%]) | .068 | |||
| Cane | 21 (23.3) | 2 (7.4) | 23 (19.7) | |
| Walker | 18 (20.0) | 3 (11.1) | 21 (18.0) | |
| None | 51 (56.7) | 22 (81.5) | 73 (62.4) | |
| Abnormal foot color (No. [%]) | .232 | |||
| Yes | 17 (18.9) | 2 (7.4) | 19 (16.2) | |
| No | 63 (70.0) | 23 (85.2) | 86 (73.5) | |
| Missing | 10 (11.1) | 2 (7.4) | 12 (10.3) | |
| Foot skin condition (No. [%]) | .411 | |||
| Yes | 40 (44.4) | 10 (37.0) | 50 (42.7) | |
| No | 41 (45.6) | 15 (55.6) | 56 (47.9) | |
| Missing | 9 (10.0) | 2 (7.4) | 11 (9.4) | |
| Nail condition (No. [%]) | .061 | |||
| Yes | 37 (41.1) | 6 (22.2) | 43 (36.8) | |
| No | 48 (53.3) | 20 (74.1) | 68 (58.1) | |
| Missing | 5 (5.6) | 1 (3.7) | 6 (5.1) |
Abbreviation: ADL, activity of daily living.
χ2, Fisher exact, and Cochran-Armitage tests do not include participants missing respective measures.
Cochran-Armitage test.
Foot Problems and Frailty
Table 2 presents frailty and fall measures compared by the presence of any foot problem, whereas Table 3 presents the same measures compared by the presence of specific foot problems or multiple (two or more) problems. With the nonfrail group as a reference, the odds of foot problems in the prefrail group was increased twofold or more and reached statistical significance for having two or more problems. For the frail group, the odds of foot problems was increased severalfold and reached statistical significance for foot pain, peripheral neuropathy, and having two or more problems. We observed significant linear trends for increasing odds ratios over increasing frailty categories for any foot problem and for each specific foot problem category.
Table 2.
Frailty and Falls by Any Foot Problem versus No Foot Problem
| Variable | Any Foot Problem (n = 90) |
No Foot Problem (n = 27) |
Odds Ratio (95% Cl)a |
|---|---|---|---|
| Frailty category (No. [%]) | |||
| Nonfrail | 26 (28.9) | 15 (55.6) | Reference |
| Prefrail | 45 (50.0) | 11 (40.7) | 2.0 (0.76–5.2) |
| Frail | 19(21.1) | 1 (3.7) | 8.3 (0.96–72)b |
| Weight loss (No. [%]) | |||
| Yes | 7 (7.8) | 0 | –c |
| No | 83 (92.2) | 27 (100.0) | |
| Weakness (No. [%]) | |||
| Yes | 35 (38.9) | 2 (7.4) | 6.6 (1.4–30)d |
| No | 55 (61.1) | 25 (92.6) | |
| Exhaustion (No. [%]) | |||
| Yes | 25 (27.8) | 2 (7.4) | 4.0 (0.85–18) |
| No | 65 (72.2) | 25 (92.6) | |
| Slowness (No. [%]) | |||
| Yes | 45 (50.0) | 10 (37.0) | 1.2 (0.45–3.2) |
| No | 45 (50.0) | 17 (63.0) | |
| Low activity (No. [%]) | |||
| Yes | 17 (18.9) | 4 (14.8) | 1.1 (0.33–3.7) |
| No | 73 (81.1) | 23 (85.2) | |
| Mobility-Tiredness Scale score ≤5 | |||
| Yes | 56 (62.2) | 15 (55.6) | 1.0 (0.41–2.6) |
| No | 34 (37.8) | 12 (44.4) | |
| Falls Efficacy Scale-International score >25 | |||
| Yes | 51 (56.7) | 5 (18.5) | 5.0 (1.7–15)d |
| No | 39 (43.3) | 22 (81.5) | |
| History of fall (No. [%])e | |||
| Yes | 37 (43.5) | 9 (37.5) | 1.3 (0.50–3.3) |
| No | 48 (56.5) | 15 (62.5) | |
| Fall incidence, rate per person-year (incidence rate ratio)f | 1.53 | 1.28 | 1.4 (0.74–2.5) |
Abbreviation: Cl, confidence interval.
Adjusted for age.
Linear trend over frailty categories: P= .03.
Not defined because none of the participants had weight loss and no foot problem.
Significantly (P < .05) increased odds of any foot problem compared with no foot problem.
History of at least one fall reported in the 6 months before baseline (eight individuals missing fall history).
Incidence of falls during 6-month follow-up (eight dropouts missing fall incidence).
Table 3.
Frailty and Falls by Foot Pain, Peripheral Neuropathy, and Foot Deformity versus No Foot Problem
| Variable | Foot Pain >4 (n = 22) |
Peripheral Neuropathy (n = 49) |
Foot Deformity (n = 60) |
≥2 Problems (n = 31) |
|---|---|---|---|---|
| Frailty category | ||||
| Nonfrail | Reference | Reference | Reference | Reference |
| Prefrail | 2.1 (0.52–8.6) | 3.2 (0.99–11) | 2.0 (0.72–5.4) | 4.6 (1.2–19) |
| Frail | 17.0 (1.6–181)a | 13.3 (1.4–132)a | 7.7 (0.86–68)b | 27.8 (2.6–298)a,c |
| Weaknessd | 17.9 (3.2–101)a | 6.5 (1.3–33)a | 6.2 (1.3–29)b | 11.4 (2.1–61 )a |
| Exhaustion | 9.7 (1.8–54)a | 5.7 (1.1–28)a | 3.9 (0.81–19) | 9.1 (1.7–48)a |
| Slowness | 1.2 (0.31–4.8) | 1.4 (0.45–4.2) | 1.0 (0.38–2.9) | 1.3 (.41–4.3) |
| Low activity | 1.5 (0.34–7.0) | 1.2 (0.30–4.6) | 1.4 (0.40–4.9) | 1.9 (.48–7.9) |
| Mobility-Tiredness Scale score ≤5 | 3.3 (0.84–13) | 1.3 (0.45–3.9) | 1.1 (0.45–2.9) | 2.6 (.79–8.4) |
| Falls Efficacy Scale-International score >25 | 13.3 (3.1–56)a | 5.9 (1.8–19)a | 5.0 (1.6–15)a | 7.7 (2.2–27)a |
| History of falle | 2.6 (0.76–9.0) | 2.4 (0.81–7.2) | 1.0 (0.39–2.8) | 2.1 (0.65–6.4) |
| Fall incidence (incidence rate ratio [95% Cl])f | 1.4(0.60–3.1) | 1.4 (0.73–2.8) | 1.3 (0.66–2.4) | 1.5 (0.76–3.1) |
Note: Participants with a foot problem can have more than one problem; the comparison group consisted of 27 participants with no foot problem. Odds ratios (95% Cls) are reported except where indicated otherwise, all adjusted for age.
Abbreviation: Cl, confidence interval.
Significantly increased (P < .05) odds versus those with no foot problem.
Linear trend over frailty categories: P < .05.
Linear trend over frailty categories: P < .01.
Fried criterion unintended weight loss odds ratio not defined due to no participants with weight lossandno foot problem.
History of at least one fall reported in the 6 months before baseline (eight participants missing fallhistory).
Incidence of falls during 6-month follow-up (eight dropouts missing fall incidence).
Among the individual Fried frailty criteria, odds ratios of 4 or more were observed for weakness and exhaustion across all foot problem categories, with a pattern of stronger and consistently significant associations for weakness. In contrast, the associations between foot problems and gait slowness and low activity were weak and did not reach statistical significance. However, comparison of the magnitude of the effect is limited by the wide confidence intervals observed for weakness and exhaustion and uncertainty about where the true odds ratio lies.
The association with weight loss could not be determined because no participants in the “no foot problem” cell had weight loss. Exhaustion was also assessed using the MOB-T, which assigns lower point values to higher levels of tiredness. The odds of scoring below the median (MOB-T score ≤5) for tiredness was not significantly elevated across foot problem categories.
Foot Problems and Falls
Foot problems were associated with increased concern about falling. The odds of high concern about falling (FES-I score >25) was significantly higher in those with any foot problem (Table 2), as well as those with foot pain, peripheral neuropathy, foot deformity, and two or more problems (Table 3). A history of at least one fall in the previous 6 months was reported by 46 participants (39.3%) (fall history was missing for eight participants [6.8%]). At least one incident fall during the 6-month follow-up was reported by 45 participants (38.5%) (information was unavailable for the seven individuals (6.0%) who dropped out). Neither history of a fall nor fall incidence was significantly associated with any foot problem (Table 2) or specific foot problems (Table 3).
Gait, Balance, and PA
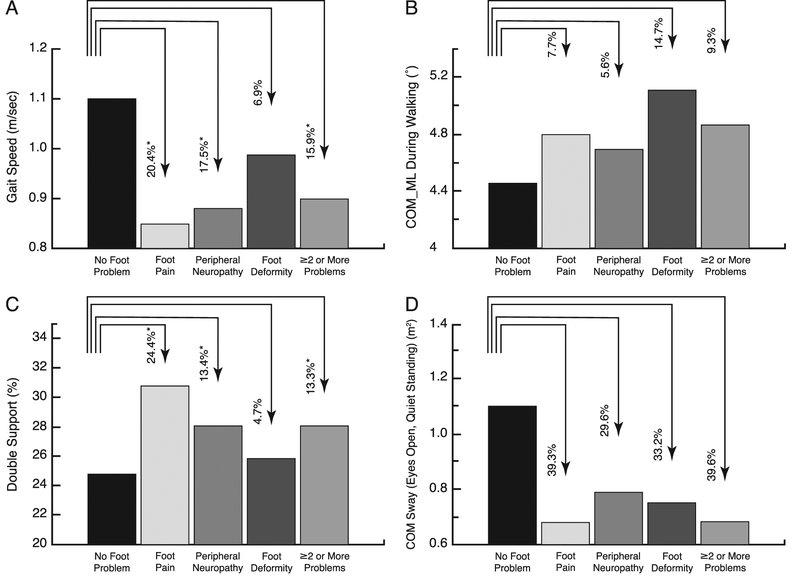
Table 4 shows sensor-based measures compared between subgroups with specific foot problems versus the group with no foot problem. Individuals with foot pain, peripheral neuropathy, or two or more foot problems had significantly lower gait speed (Fig. 1A) and stride length, as well as a higher proportion of time spent in double support, the portion of gait cycle with both feet simultaneously on the ground (Fig. 1C). Adjusting for age did not alter the patterns of contrasts for gait speed, stride length, and double support, but only the foot pain group means were significantly different than the group with no problem (P < .05). We observed consistently higher mediolateral sway of COM (for both gait initiation and steady state) for each foot problem type (Fig. 1B). After age adjustment, these differences were significant for peripheral neuropathy and deformity and suggested trends (P < .10) for foot pain and two or more problems. Among the balance measures tested during quiet standing, we observed across all foot problem types consistently lower ankle sway with eyes open and lower COM sway with eyes open (Fig. 1D), all of which showed significance after age adjustment with the exception of ankle sway (eyes open) for peripheral neuropathy (P = .095) and two or more problems (P = .052). Those with foot pain had lower body sway during quiet standing with eyes open on average by 39% unadjusted and 49% adjusted for age (P = .026).
Table 4.
Gait, Balance, and Physical Activity Sensor Assessment by Specific Foot Problems versus No Foot Problem
| Variable | No Foot Problem (n = 27) |
Foot Pain (n = 22) |
Peripheral Neuropathy (n = 49) |
Foot Deformity (n = 73) |
≥2 Problems (n = 31) |
|---|---|---|---|---|---|
| Gait | |||||
| Speed (m/sec) | 1.1 ± .22 | 0.85 ± 0.34 | 0.88 ± 0.28 | 0.99 ± 0.28 | 0.90 ± 0.29 |
| (P = .010)a | (P = .004)a | (P = .235) | (P = .017)a | ||
| Stride length (m) | 1.2 ± .15 | 1.0 ± 0.27 | 1.0 ± 0.23 | 1.1 ± 0.24 | 1.0 ± 0.24 |
| (P = .005)a | (P = .007)a | (P = .161) | (P = .013)a | ||
| Double support (%) | 24.8 ± 6.2 | 30.8 ± 7.7 | 28.1 ± 6.8 | 25.9 ± 6.6 | 28.1 ± 6.1 |
| (P = .004)a | (P = .042)a | (P = .442) | (P = .049)a | ||
| Speed variability during gait initiation (CV %) | 5.1 ± 4.3 | 6.0 ± 2.8 | 5.5 ± 3.2 | 4.8 ± 3.5 | 5.4 ± 4.3 |
| (P = .437) | (P = .663) | (P= .814) | (P = .776) | ||
| Speed variability during entire walk (CV %) | 6.8 ± 5.4 | 6.2 ± 2.6 | 5.8 ± 3.0 | 5.1 ± 3.0 | 5.7 ± 2.3 |
| (P = .648) | (P = .380) | (P = .098) | (P = .399) | ||
| Medial lateral sway of center of mass during gait initiation (deg) | 4.4 ± 1.8 | 4.7 ± 2.2 | 4.6 ± 2.4 | 5.0 ± 1.9 | 4.8 ± 2.1 |
| (P = .565) | (P = .587) | (P= .138) | (P = .272) | ||
| Medial lateral sway of center of mass during entire walk (deg) | 4.5 ± 1.8 | 4.8 ± 2.2 | 4.7 ± 2.4 | 5.1 ± 1.9 | 4.9 ± 2.2 |
| (P = .547) | (P = .637) | (P= .139) | (P = .450) | ||
| Balance | |||||
| Hip sway eyes closed (°2) | 10.2 ± 15.9 | 9.7 ±11.3 | 12.5 ± 14.4 | 8.9 ± 8.7 | 11.0 ± 11.0 |
| (P = .900) | (P = .543) | (P= .612) | (P = .838) | ||
| Hip sway eyes open, deg(°2) | 5.8 ± 6.1 | 4.7 ± 4.9 | 5.7 ± 7.1 | 4.6 ± 4.2 | 5.1 ± 4.6 |
| (P = .527) | (P = .951) | (P = .308) | (P = .645) | ||
| Ankle sway eyes closed, deg(°2) | 9.5 ±11.3 | 8.5 ± 6.5 | 9.4 ± 10.6 | 7.5 ± 6.3 | 8.3 ± 6.5 |
| (P = .707) | (P = .958) | (P = .286) | (P = .615) | ||
| Ankle sway eyes open (°2) | 5.7 ± 5.9 | 3.3 ± 2.2 | 4.3 ± 5.3 | 3.7 ± 2.8 | 3.6 ± 2.2 |
| (P = .091) | (P = .317) | (P = .038)a | (P = .085) | ||
| Center of mass, mean eyes closed (cm2) | 2.0 ± 2.8 | 1.6 ± 1.2 | 1.9 ± 1.6 | 1.5 ± 1.3 | 1.6 ± 1.4 |
| (P = .540) | (P = .800) | (P = .279) | (P = .451) | ||
| Center of mass, mean eyes open (cm2) | 1.1 ± 1.1 | .68 ± .49 | .79 ± .68 | .75 ± .61 | .68 ± .53 |
| (P = .109) | (P = .140) | (P = .057) | (P = .066) | ||
| Physical activity (48 hour) | |||||
| Walking duration (%) | 6.2 ± 2.2 | 4.2 ± 2.6 | 4.7 ± 2.9 | 5.8 ± 3.5 | 4.6 ± 3.1 |
| (P = .007)a | (P = .022)a | (P = .555) | (P = .032)a | ||
| Standing duration (%) | 15.9 ± 5.6 | 12.3 ± 4.5 | 12.8 ± 4.3 | 14.7 ± 5.5 | 11.9 ± 4.2 |
| (P = .022)a | (P= .010)a | (P = .344) | (P = .003)a | ||
| Sitting duration (%) | 34.8 ± 9.1 | 40.6 ± 10.3 | 42.1 ± 10.6 | 39.9 ± 9.1 | 42.2 ±11.6 |
| (P = .043)a | (P = .004)a | (P = .067) | (P = .009)a | ||
| Lying duration (%) | 43.1 ± 10.1 | 42.8 ±11.9 | 40.4 ± 10.5 | 39.6 ±11.7 | 41.2 ± 12.8 |
| (P = .926) | (P = .274) | (P = .183) | (P = .531) | ||
| Steps per daybc | 4772 ± 2323 | 3142 ± 2244 | 3487 ± 2749 | 4446 ± 3066 | 3538 ± 2671 |
| (P = .009)a | (P = .007)a | (P = .195) | (P = .021 )a | ||
| Number of walking bouts per dayb,c | 137 ± 55 | 106 ± 63 | 110 ± 59 | 132 ± 78 | 108 ± 58 |
| (P = .041 )a | (P = .036)a | (P = .273) | (P = .050) | ||
| Mean walking bout duration, secb | 42.1 ± 15.8 | 36.4 ± 16.6 | 37.5 ± 15.0 | 38.7 ± 14.9 | 38.0 ± 16.6 |
| (P = .1O5) | (P = .129) | (P = .241) | (P = .180) | ||
| Longest walking bout duration, secb | 747 ± 973 | 443 ± 489 | 491 ± 506 | 644 ± 629 | 505 ± 494 |
| (P = .106) | (P = .146) | (P = .651) | (P = .217) | ||
| Walking bout duration variability (SD) [sec])b | 74.1 ± 80.2 | 50.2 ± 38.7 | 54.0 ± 38.9 | 62.3 ± 45.2 | 55.3 ± 40.1 |
| (P = .137) | (P = .199) | (P = .700) | (P = .241) |
Note: Data are given as mean ± SD.
Abbreviation: CV, coefficient of variation.
Significantly different (P < .05) from those with no foot problem.
Test of difference performed on log-transformed physical activity data, but nontransformed mean ± SD presented.
Half of count over 48 hours.
Figure 1.
Changes in gait and balance parameters as a function of foot problems. Mean ± SE values are given for gait speed (A), center of mass in mediolateral (COM_ML) during walking (B), double support (C), and COM sway with eyes open during quiet standing (D). The percentage change from the no foot problem value is indicated above each bar, which is not the absolute change for those measures whose unit is percentage of time. *P < .05.
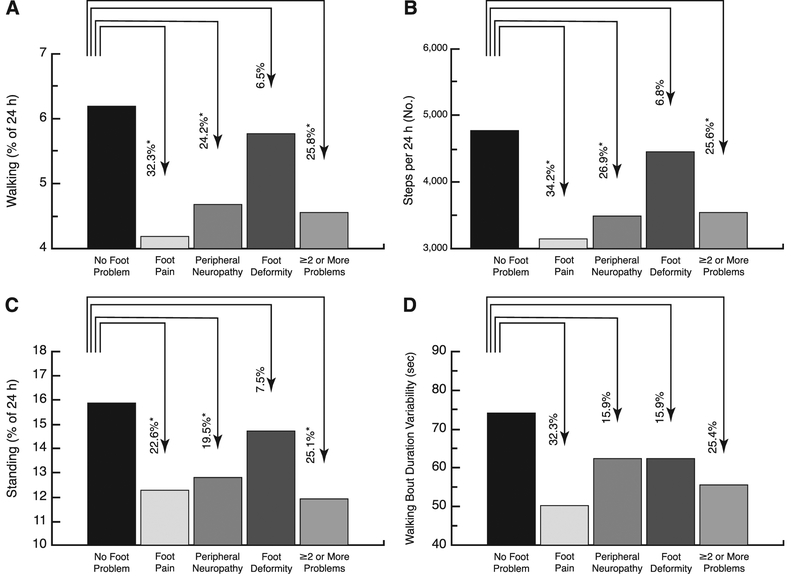
Participants with foot pain or peripheral neuropathy had significantly less total time walking and standing and more total time sitting (Table 4). Figure 2 illustrates some of the measured PA parameters across groups. The group with foot pain had on average 32.3% less total time walking (P = .007), 22.6% less total time standing (P = .02), 34.2% fewer steps per day (P = .009), and 32.3% less variability in walking bout duration (P = .14). Similarly, other foot problem types reduced PA, in particular duration of standing, steps per day, number of walking bouts, and walking bout duration variability. These patterns persisted after age adjustment, although only total time walking and number of steps per day in the group with foot pain remained statistically significant.
Figure 2.
Changes in spontaneous daily physical activities measured over 48 hours as a function of foot problems. Mean ± SE values per 24 hours are given for walking percent time (A), number of steps taken (B),standing percent time (C), and walking bout duration variability (D). The percentage change from the no foot problem value is indicated above each bar, which is not the absolute change for those measures whose unit is percentage of 24 hours. *P<.05.
Discussion
Although foot pain and problems have been recognized as important factors in the health and wellness of older adults,34 to our knowledge, no study has addressed the combination of relationships between foot problems, frailty, and wearable sensor-based measures of gait and PA. Thanks to the use of wearable technologies, we were able to complete all of the assessments at participants’ homes instead of at a clinic or gait laboratory. This allowed us to collect data from the frail population as well, who were often excluded in previous studies due to inability of frail people to commute to clinics or gait laboratories for the purpose of similar measurements. Accompanied by the use of objective wearable sensor-derived motor performance and ascertainment of self-reported incident falls, our approach aimed to address this knowledge gap by evaluating associations between foot problems and both stability and mobility in older adults.
This information may facilitate evaluation of individuals who are at increased risk for frailty, fall-related risks, and gait abnormalities due to their specific foot problem.
Our first aim was to examine foot problems and associated characteristics. The findings indicate that foot problems are associated with older age, as has been previously reported, but we did not observe an association with sex, contrary to previous reports13; however, some studies did not find associations between foot pain and either sex or age. The present sample was composed largely of women and, therefore, may have been underpowered for comparison by sex. We did not observe a significant difference in likelihood of foot problems by race/ethnicity, in contrast to reports suggesting that minorities are more likely to report higher levels of subjectively evaluated foot pain.35 Although BMI and body fat were not associated with combined foot problems, both were associated with foot pain, which aligns with other reports.36 The stronger association between foot pain and body fat percentage may stem from the inflammatory qualities of adipose tissue and the resulting systemic “inflammaging” response,37 defined as a global reduction in the capacity to cope with a variety of stressors and a concomitant progressive increase in proinflammatory status.38 Alternatively, foot pain could reduce PA, which, in turn, could lead to deconditioning and increased body fat.
Our second aim was to evaluate the association of foot problems with frailty syndrome and prospective falls. We observed a statistically significant linear trend of increasing odds of foot problems associated with increasing frailty level. Although foot problems, in general, have been reported in frail older people with diabetes, to our knowledge, it has not been previously reported in a frailty cohort. Interestingly, the present results suggest that the association of foot problems with weakness and exhaustion is much more pronounced (by a factor of >4) than the association of foot problems with gait slowness and low activity. This finding could be explained by the fact that foot problems mainly affect gait efficiency due to alteration in the biomechanics of walking, which, in turn, may increase the energy cost during locomotion and early fatigue. Another study is warranted to explore whether compensating foot problems via customized insoles may assist in reducing energy cost during locomotion, reducing the likelihood of weakness and exhaustion and, thus, delay in progression of frailty. Finding a link between the frailty criteria and foot problems is a challenge because it is difficult to identify a single plausible link between frailty and pain, neuropathy, or deformity. Although there may be a relation to the aforementioned variables of lower activity, sarcopenia, and other systemic characteristics, it is equally likely that chronic systemic inflammation frequently found in those in frail states may be culpable in part or in whole.37,39
We observed strong associations between fear of falling (as measured by the FES-I) and foot problems, along with each component of foot problems. This finding is consistent with recent findings on another sample suggesting that fear of falling is highly prevalent in older adults with neuropathy.40 Harada ET AL41 demonstrated that foot problems were correlated with fear of falling in 10,581 community-dwelling elderly people. The evidence shows that fear of falling often predicts future falls, while conversely, past falls often predict the development of a fear of falling.42 Both assistive device use and fear of falling are likely to influence fall risk and gait performance. However, because foot problems are likely a contributory cause of use of assistive devices and fear of falling, we did not adjust for them in the present analysis.43
We did not demonstrate significant increased risk in individuals with foot problems for either incident falls or a recent history of falls, associations that have been reported for combined problems,12,44 pain,11,14 and neuropathy.45 It is likely that the present study was underpowered to evaluate these associations because relatively small numbers had moderate-to-severe pain (n = 22) or no foot problem (n = 27). The power to assess falls was further eroded by eight participants (four nonfrail, three prefrail, and one frail) who did not report whether they had experienced a fall in the previous 6 months and seven different participants (one nonfrail, four prefrail, and two frail) who dropped out after baseline assessments and did not provide fall incidence. In addition, we cannot rule out the influence of recall bias in the analysis of fall history because the recall of falls, in particular the count, is inherently more biased than reporting incident falls. Furthermore, we used cross-sectional measurement for assessing foot problems, whereas some parameters, such as foot pain, could be developed after the initial assessments, which may have contributed to prospective falls. Further study is required to track changes in foot problems and the association with increased risk of falling.
In the third aim-an analysis of gait, balance, and PA-several gait measurements showed a significant relationship with foot problems, particularly foot pain and neuropathy. Participants with moderate-to-severe pain had significantly slower walking speed, a shorter stride length, and longer time spent in double support. This finding aligns with the literature on gait and pain, as pain in the lower extremities frequently alters gait patterns.46 Although walking speed as a continuous variable was significantly associated with foot pain, the dichotomous Fried slowness category of walking speed less than 1 ft/sec was not, an inconsistency likely due to statistical power issues. This distinction demonstrates the sensitivity provided by objective wearable sensors and their ability to augment traditional measures with those of “natural” daily activity.
Interestingly, the present results suggest that during walking, body sway (COM range of motion in mediolateral) is increased due to foot problems, whereas during quiet standing, COM sway and ankle sway are reduced. This observation suggests that individuals with problems (in particular with foot pain) may choose a rigidity postural control strategy while standing, when they have the chance to perceive body sway via sensory feedback, including intact visual and proprioception feedbacks.47 The eyes closed measures had high variation, which contributed to the lack of findings even in the presence of potentially important mean differences.
Finally, as expected, the present results suggest that foot problems, and in particular foot pain, reduce the level of PA as characterized by a lower percentage of walking and standing per day, as well as fewer total steps per day. In addition, foot problems alter the pattern of PAs as quantified by fewer unbroken walking bouts per day; a similar pattern was observed of shorter longest unbroken walking bouts per day and lower walking bout duration variability, but the mean differences were not statistically significant. In a previous study,17 we observed similar trends of reduced and altered patterns of PA in frail compared with nonfrail older adults. Thus, it could be speculated that foot problems may accelerate frailty in older adults via restriction of PA as well as alteration of the pattern of daily PA.
There are some limitations to consider in the interpretation of these findings. First, the parent study was designed to focus on frailty and falls; hypotheses involving foot problems were a secondary component of the study and may have been underpowered, particularly in subsets with specific types of foot problems. As a result, we observed large variability and wide confidence intervals on estimated odds ratios between foot problems and certain frailty categories and components. Although the point estimate is the most plausible estimate of the true association, wide confidence intervals indicate a lack of precision. Second, the study design is cross-sectional and descriptive, which limits our ability to infer cause and effect. However, it does provide information useful in developing future confirmatory research. Third, the study population consisted of individuals who were noninstitutionalized, community-dwelling, cognitively intact, and independent. Institutionalized and cognitively impaired older adults have been shown to have high levels of foot pain and problems in addition to a higher frequency of falls.48 Therefore, the present study results should be generalized to community-dwelling elderly people only and may underestimate the prevalence of foot pain and neuropathy in institutionalized older adults, along with the extent of its debilitating influence. Fourth, we were limited in the ability to infer causation of pain, particularly whether pain was secondary to neuropathy or deformity. Fifth, we classified participants as having foot problems if the condition existed on either foot. Therefore, we did not separately evaluate the effect of having problems on both feet. Finally, 48-hour PA assessment may not have accounted for day-to-day variability (such as weather-related differences or weekday versus weekend). However, 48-hour monitoring in the present study may have been sufficient to document habitual PA because PA is less variable in older compared with younger adults, and high day-to-day reliability of PA assessment has been reported in a sample older adults.49
The present study is not designed to differentiate between disease-related impairment and general aging-related (ie, frailty) symptoms. Furthermore, the cross-sectional design does not clarify the causal role of pain, which could be a result of disease, a contributory cause of frailty, or a result of frailty-related processes. We used the Fried frailty criteria to characterize those at increasingly high risk for poor outcomes related to hyperinflammatory, multisystem decline and accelerated aging and have tested whether foot problems differ by frailty category. The present findings suggest a need to assess for specific foot problems and intervene using targeted interventions to potentially reduce the likelihood of frailty and deterioration in functional status and, thus, promote healthy aging in older adults. Better understanding of the association of foot problems and frailty syndrome (hypothesized to reflect impairments in the regulation of multiple physiologic systems, embodying a lack of resilience to physiologic challenges, and elevated risk of poor outcomes) could lead to meaningful interventions to support strength, power, balance, and function in those with foot problems, who are at risk for further deconditioning and loss of independence. This information could also inform strategies to enhance adherence to interventions in frail elderly people, as worse physical health, greater fear of falling, and advanced age have been associated with worse adherence to foot-related fall interventions.50 Identification of older individuals who are frail or at risk for frailty with evaluation and intervention constitutes a cornerstone of geriatric medicine and quality care for our growing aging population.
In conclusion, this study presents compelling evidence of a relationship between foot problems and frailty that strengthens as the frailty level becomes more profound. In addition, we demonstrated several sensor-assessed gait decrements associated with foot problems. This information may prove useful for evaluating individuals who are at increased risk for falls and gait abnormalities due to specific foot problems. Assessment of frailty and gait characteristics could allow for earlier intervention to increase mobility and stability and to reduce the risk of falls, which may ultimately have an effect on improving not only mobility in those with foot problems but also quality of life for older adults.
Acknowledgment:
Arizona Frailty Cohort coordinator Marilyn Gilbert and student workers for the recruitment of participants and the collection of data and the participants for their kind participation.
Financial Disclosure: This project was supported in part by award 2R42AG032748 from the National Institute on Aging and by the Arizona Center on Aging. The sponsor had no role in the design or conduct of this study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: None reported.
References
- 1.Menz HB: Foot Problems in Older People: Assessment and Management, 1st Ed, Churchill Livingstone, New York, 2008. [Google Scholar]
- 2.Menz HB: Biomechanics of the ageing foot and ankle: a mini-review. Gerontology 61: 381, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Thomas MJ, Roddy E, Zhang W, et al. The population prevalence of foot and ankle pain in middle and old age: a systematic review. Pain 152: 2870, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Menz HB, Morris ME, Lord SR: Foot and ankle characteristics associated with impaired balance and functional ability in older people. J Gerontol A Biol Sei Med Sei 60: 1546, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Mickle KJ, Munro BJ, Lord SR, et al. Foot pain, plantar pressures, and falls in older people: a prospective study. J Am Geriatr Soc 58: 1936, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Chang JT, Morton SC, Rubenstein LZ, et al. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trials. BMJ 328: 680, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens JA, Corso PS, Finkelstein EA, et al. The costs of fatal and non-fatal falls among older adults. Inj Prev 12: 290, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 60: 1487, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Katon W, Sullivan MD: Depression and chronic medical illness. J Clin Psychiatry 51: 3, 1990. [PubMed] [Google Scholar]
- 10.Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA 302: 2214, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaiwanichsiri D, Janchai S, Tantisiriwat N: Foot disorders and falls in older persons. Gerontology 55: 296, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Menz HB, Lord SR: The contribution of foot problems to mobility impairment and falls in community-dwelling older people. J Am Geriatr Soc 49: 1651, 2001. [PubMed] [Google Scholar]
- 13.Leveille SG, Guralnik JM, Ferrucci L, et al. Foot pain and disability in older women. Am J Epidemiol 148: 657, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Menz HB, Morris ME, Lord SR: Foot and ankle risk factors for falls in older people: a prospective study. J Gerontol A Biol Sci Med Sei 61: 866, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Stubbs B, Binnekade T, Eggermont L, et al. Pain and the risk for falls in community-dwelling older adults: systematic review and meta-analysis. Arch Phys Med Rehabil 95: 175, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Menz HB, Dufour AB, Casey VA, et al. Foot pain and mobility limitations in older adults: the Framingham Foot Study. J Gerontol A Biol Sci Med Sei 68:1281, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwenk M, Mohler J, Wendel C, et al. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: baseline results of the Arizona frailty cohort study. Gerontology 61: 258, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Najafi B, Armstrong DG, Mohler J. Novel wearable technology for assessing spontaneous daily physical activity and risk of falling in older adults with diabetes. J Diabetes Sei Technol 7: 1147, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR: “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189, 1975. [DOI] [PubMed] [Google Scholar]
- 20.Fieo RA, Mortensen EL, Rantanen T, et al. Improving a measure of mobility-related fatigue (the Mobility-Tired-ness Scale) by establishing item intensity. J Am Geriatr Soc 61: 429, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahoney FI, Barthel DW: Functional evaluation: the Barthel index. Md State Med J 14: 61, 1965. [PubMed] [Google Scholar]
- 22.Palos GR, Mendoza TR, Mobley GM, et al. Asking the community about cutpoints used to describe mild, moderate, and severe pain. J Pain 7: 49, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds: a prospective study. Diabetes Care 17: 557, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sei Med Sei 56: M146, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sei 61: 262, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Taylor HL, Jacobs DR Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31: 741, 1978. [DOI] [PubMed] [Google Scholar]
- 27.Lamb SE, Jorstad-Stein EC, Hauer K, et al. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 53: 1618, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Yardley L, Beyer N, Hauer K, et al. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 34: 614, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Najafi B, Horn D, Marclay S, et al. Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J Diabetes Sei Technol 4: 780, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Najafi B, Helbostad JL, Moe-Nilssen R, et al. Does walking strategy in older people change as a function of walking distance? Gait Posture 29: 261, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Najafi B, Lee-Eng J, Wrobel JS, et al. Estimation of center of mass trajectory using wearable sensors during golf swing. J Sports Sci Med 14: 354, 2015. [PMC free article] [PubMed] [Google Scholar]
- 32.Najafi B, Khan T, Wrobel J: Laboratory in a box: wearable sensors and its advantages for gait analysis. Conf Proc IEEE Eng Med Biol Soc 2011: 6507, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Selvin S: Statistical Analysis of Epidemiologic Data, 3rd Ed, Oxford University Press, New York, 2004. [Google Scholar]
- 34.Plummer ES, Albert SG: Focused assessment of foot care in older adults. J Am Geriatr Soc 44: 310, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Dunn JE, Link CL, Felson DT, et al. Prevalence of foot and ankle conditions in a multiethnic community sample of older adults. Am J Epidemiol 159: 491, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Butterworth PA, Urquhart DM, Cicuttini FM, et al. Fat mass is a predictor of incident foot pain. Obesity 21: E495, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Morley JE, Baumgartner RN, Roubenoff R, et al. Sarcopenia. J Lab Clin Med 137: 231, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sei 908: 244, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 54: 991, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Kelly C, Fleischer A, Yalla S, et al. Fear of falling is prevalent in older adults with diabetes mellitus but is unrelated to level of neuropathy. JAPMA 103: 480, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harada K, Oka K, Shibata A, et al. Relationships between foot problems, fall experience and fear of falling among Japanese community-dwelling elderly [in Japanese]. Nihon Koshu Eisei Zasshi 57: 612, 2010. [PubMed] [Google Scholar]
- 42.Friedman SM, Munoz B, West SK, et al. Falls and fear of falling: which comes first? a longitudinal prediction model suggests strategies for primary and secondary prevention. J Am Geriatr Soc 50: 1329, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Greenland S, Pearl J, Robins JM: Causal diagrams for epidemiologic research. Epidemiology 10: 37, 1999. [PubMed] [Google Scholar]
- 44.Barr EL, Browning C, Lord SR, et al. Foot and leg problems are important determinants of functional status in community dwelling older people. DisabilityR-ehabilitation 27: 917, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Richardson JK, Hurvitz EA: Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sei 50: M211, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Benvenuti F,Ferrucci L, Guralnik JM, et al. Foot pain and disability in older persons: an epidemiologic survey. J Am Geriatr Soc 43: 479, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Toosizadeh N, Mohler J, Armstrong DG, et al. The influence of diabetic peripheral neuropathy on local postural muscle and central sensory feedback balance control. PLoS One 10: e0135255, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One 7: e40297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe DA, Kemble CD, Robinson TS, et al. Daily walking in older adults: day-to-day variability and criterion-referenced validity of total daily step counts. J Phys Act Health 4: 434, 2007. [PubMed] [Google Scholar]
- 50.Spink MJ, Fotoohabadi MR, Wee E, et al. Predictors of adherence to a multifaceted podiatry intervention for the prevention of falls in older people. BMC Geriatr 11: 51, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]