Abstract
Background:
Many patients with coronary artery disease who are not candidates for revascularization suffer from refractory angina despite standard medical therapy. The coronary sinus Reducer, a balloon-expandable stainless steel hourglass-shaped device, creates a focal narrowing and increases pressure in the coronary sinus, thus redistributing blood into ischemic myocardium.
Methods:
Patients with Canadian Cardiovascular Society (CCS) class 3 to 4 angina (n=104) and myocardial ischemia, who were not candidates for revascularization, were randomized to implantation of a Reducer or a sham procedure. The primary end point was the proportion of patients with an improvement of at least 2 CCS angina classes at 6 months.
Results:
The primary end point was achieved in 35% (18/52) of the Reducer-treated patients versus 15% (8/52) of the sham-control patients (p=0.02). The Reducer was also associated with improvement of at least 1 CCS angina class in 71% (37/52) vs. 42% (22/52) in the sham-control group (p=0.003). Quality of life using the Seattle Angina Questionnaire was significantly improved in the Reducer group compared to the sham-control group (improvement of 17.6 vs. 7.6 points, respectively, p=0.03). There were no significant differences in improvement in exercise time or in mean change in wall motion score index by dobutamine echocardiography. At 6 months, one participant experienced a myocardial infarction in the Reducer group, and one patient died and three experienced myocardial infarctions in the control group.
Conclusions:
In this small clinical trial, Reducer implantation significantly improved symptoms and quality of life in patients with refractory angina unsuitable for revascularization.
Trial Registration:
ClinicalTrials.gov identifier -
INTRODUCTION
A growing number of patients who suffer from severe and diffuse obstructive coronary artery disease not amenable to revascularization continue to experience debilitating angina despite medical therapy.1,2,3 The worldwide prevalence of patients with refractory angina is increasing, and new therapeutic options are needed.4
The Reducer (Neovasc Inc, Richmond, Canada) is an endoluminal, hourglassshaped, balloon-expandable stainless steel device designed for percutaneous implantation in the coronary sinus. The Reducer creates a focal narrowing that leads to increased coronary sinus pressure, which theoretically may relieve angina (Figure 1). A non-randomized first-in-man study of 15 patients with refractory angina who were treated with the Reducer demonstrated significant improvement in angina class.5 This clinical benefit was maintained at 3-year follow-up with documented patency of all Reducers by computed tomographic angiography and no evidence of device migration.6 Recently, the outcomes for 21 patients treated with the Reducer were reported, demonstrating improvement in anginal symptoms and in objective parameters of ischemia.7
Figure 1.
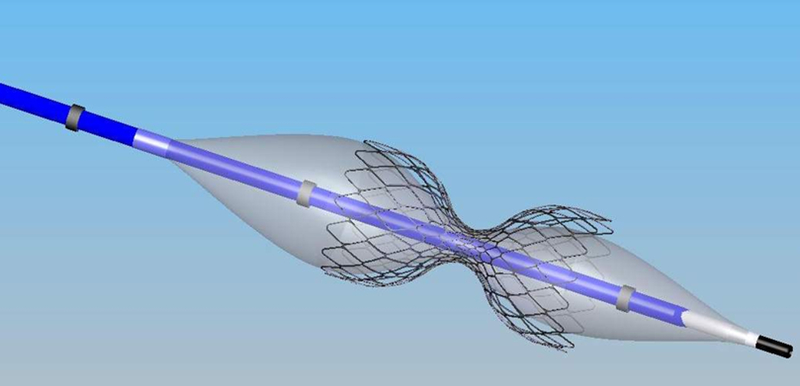
The Coronary Sinus Reducer System is comprised of the Reducer pre-mounted on the hour-glass shaped balloon catheter. After the Reducer is implanted in the coronary sinus, local flow disruption and vascular reaction leads to a hyperplastic response in the vessel wall, with occlusion of the fenestrations in the metal mesh. The central orifice of the Reducer remains patent and becomes the sole path for coronary sinus blood flow, resulting in a pressure gradient.
Future therapies in patients with refractory angina should focus not only on the reduction of risk for death and myocardial infarction, but also on angina relief and improved quality of life.8 The Coronary Sinus Reducer for Treatment of Refractory Angina (COSIRA) trial examined whether implantation of the Reducer could effectively improve angina symptoms in patients with obstructive coronary artery disease having concomitant evidence of reversible myocardial ischemia and who were considered unsuitable for revascularization.
METHODS
Study design
COSIRA was a phase 2, randomized, double-blind, sham-controlled, multi-center clinical trial to test the safety and efficacy of the Reducer. The trial was conducted at 11 clinical centers and was sponsored by Neovasc. The trial protocol, which is available with the full text of this article at NEJM.org, was designed by the academic authors with input from the sponsor. The data were collected, managed and analyzed by a contract research organization paid by the sponsor. The academic authors had full access to the data and take full responsibility for the accuracy and completeness of the data and the analyses reported, as well as for the fidelity of this report to the trial protocol.
The trial was overseen by an independent coordinating center, steering committee, clinical events committee and data and safety monitoring board, (see the Supplementary Appendix). The study protocol and amendments as well as the consent form were reviewed and approved by the national competent authority and the independent ethics committee at each participating center. The study was conducted in compliance with the provisions of the Declaration of Helsinki. All patients provided written informed consent before enrollment.
Study patients
The inclusion and exclusion criteria for COSIRA have been reported in detail elsewhere.9 Patients were considered for participation in the trial if they were over 18 years of age and suffered from Canadian Cardiovascular Society (CCS) class 3 to 4 angina, despite efforts to control symptoms with medical therapy for at least 30 days prior to screening. Medical therapy included beta-blockers, calcium-channel blockers, nicorandil, ivabradine and/or short- and long-acting nitrates used at maximally tolerable doses.10 All participants were required to have evidence of reversible myocardial ischemia and a left ventricular ejection fraction of greater than 25%. Only patients deemed unsuitable for coronary revascularization were eligible to participate in the study as decided by each institution’s heart team upon reviewing the recent coronary angiography, as detailed elsewhere.11 Patients were excluded if they had had a recent revascularization procedure (within 6 months), a recent acute coronary syndrome (within 3 months), or permanent pacemaker or defibrillator leads in the right heart. Detailed inclusion and exclusion criteria are presented in the Supplementary Appendix.
Randomization and intervention
Candidates meeting the above criteria underwent right heart catheterization with coronary sinus angiography prior to the planned intervention. Only patients with coronary sinus anatomy suitable for implantation of the Reducer (as outlined in the Supplementary Appendix exclusion criteria) were eligible for randomization.
Participants were randomized in a 1:1 ratio using a computer-generated random allocation sequence to undergo implantation of a Reducer (treatment group) or sham procedure (control group). Treatment assignments were concealed in numbered sealed envelopes. The allocation sequence remained concealed until the study arm was assigned.9
All participants remained blinded throughout the 6-month study period. While the implanting physician was not blinded, the investigator responsible for assessing the angina class at follow-up, all core laboratories, the biostatisticians performing the analysis and the members of the clinical events committee were blinded to treatment assignment.
Device design and implantation
The Reducer is a stainless steel device, available in one single model designed to fit a range of anatomies. Its expanded diameter is dependent on the inflation pressure of the semi-compliant balloon, which has an hour-glass shape, and it conforms to the tapering anatomy of the coronary sinus (Figure 1).
Dual antiplatelet therapy was given for at least one week prior to the procedure and for 6 months after the procedure in both groups. Those randomized to the Reducer were treated with intravenous heparin at the time of implantation. Participants were offered either headsets playing music or conscious sedation to mask the conversation in the room regarding the randomization and the procedure. The implanting physicians were instructed to behave similarly during both Reducer and sham implantations, including spending a comparable amount of procedure time in the two groups.
A 6F diagnostic catheter was introduced into the right atrium. Right atrial pressure was measured and recorded. The catheter was then introduced into the coronary sinus and an angiogram was performed with 30° left anterior oblique angulation. Implantation site was determined according to the vessel diameter and to avoid side branch bifurcation.
Participants assigned to the sham-control group had no additional invasive manipulation. In participants assigned to the treatment group, a preshaped 9F guiding catheter was introduced into the coronary sinus and a Reducer was implanted at the desired site using a 1.1:1.0 device-to-coronary sinus diameter ratio. Post-implantation angiography was performed to ensure appropriate implantation.
Study end points
The pre-specified primary end point was the proportion of patients with an improvement of 2 or more CCS classes from baseline to 6 months post procedure. Secondary end points included the proportion of patients with an improvement of 1 or more CCS classes from baseline to 6 months and exercise tolerance assessed on a symptom-limited stress test.12,13
Cardiac regional wall motion during stress and at rest was assessed by dobutamine echocardiography at baseline and 6 months. Motion of each of 16 wall segments at rest and during peak dobutamine infusion was quantified (1-normal, 2-hypokinetic, 3-akinetic, 4-dyskinetic, 5-aneurysmal),14 and the sum of the wall motion scores of the myocardial segments was divided by the number of segments to provide a wall-motion score index. A modified left coronary artery wall-motion score index was also computed using 11 segments attributed to the left coronary artery territory.
Angina-related quality of life was assessed using the Seattle Angina Questionnaire (SAQ), which is a 19-item questionnaire that measures five domains of health status related to coronary artery disease: angina stability, angina frequency, physical limitation, treatment satisfaction, and quality of life. Scores range from 0 to 100, with higher scores indicating fewer symptoms and better health status.15 Technical and procedural success as well as periprocedural and nonprocedural adverse events were also recorded. Details of the study outcome assessments are provided in the Supplementary Appendix.
Statistical analysis
An independent data and safety monitoring board was chartered to monitor and evaluate patient safety to identify any clinically relevant trends, and to advise the steering committee accordingly. Interim analyses, provided to the DSMB by the contract research organization, took place after 30 patients had completed 30-day follow-up, and after 50% of the originally planned cohort had completed their 6-month follow-up.
The study was designed to have 80% power to test the two-sided hypothesis at a Type I error level of 0.05 that 40% of participants assigned to the Reducer group would improve by 2 or more CCS angina classes compared to 15% of participants assigned to the sham-control group. A drop out/loss to follow-up rate of 10% was assumed because of uncertainties about deliverability of the device. Based on these assumptions, we originally calculated that 124 participants would need to be randomized in the study. Due to the longer than expected time to complete full enrollment, and the better than expected drop out/loss to follow-up rate, the sponsor elected to stop enrollment after 104 patients were randomized. The sponsor had no knowledge of the unblinded end point data when the decision to stop enrollment was taken; the randomization code was held by the study contract research organization.
Continuous variables are described as means and standard deviations and/or medians and interquartile ranges, as appropriate. Between-group means were compared via the use of paired Student’s t-tests. Categorical variables are expressed as proportions and compared with Pearson chi-square tests or Fisher’s exact tests, as appropriate. For continuous variables in secondary end points, the analysis of covariance (ANCOVA) was used to compare the variation of the change from baseline to six months between the Reducer and the sham-implanted patients after adjusting for baseline differences. All the efficacy analyses were performed following the intent-to-treat principle. The safety population included all randomized patients according to the actual treatment received. P values <0.05 were considered statistically significant. No type I error adjustment for multiple comparisons was planned. All analyses were performed using IBM SPSS version 21.
RESULTS
Baseline clinical characteristics
Between April 2010 and April 2013, 104 patients were enrolled in the trial. Fifty- two patients were assigned to the Reducer group and 52 to the sham-control group. Mean age was 67.8±9.4 (range: 35 to 87), and 81% were male. The cohort was characterized by high rates of risk factors and co-morbidities (Table 1).
Table 1.
Characteristics of the Patients.*
| Characteristic | Control Group (N = 52) |
Treatment Group (N = 52) |
|---|---|---|
| Age — yr | 66.0±9.8 | 69.6±8.7 |
| Male sex — no. (%) | 40 (77) | 44 (85) |
| Previous myocardial infarction — no. (%) | 30 (58) | 27 (52) |
| Previous CABG — no. (%) | 38 (73) | 42 (81) |
| Previous PCI — no. (%) | 40 (77) | 36 (69) |
| Hypercholesterolemia — no. (%) | 46 (88) | 50 (96) |
| Diabetes mellitus — no. (%) | 25 (48) | 21 (40) |
| Hypertension — no. (%) | 41 (79) | 42 (81) |
| Current or previous smoking — no. (%) | 31 (60) | 27 (52) |
| CCS angina class — no. (%)† | ||
| I | 0 | 0 |
| II | 0 | 0 |
| III | 45 (87) | 42 (81) |
| IV | 7(13) | 10 (19) |
| Left ventricular ejection fraction | 54.8±11.9 | 53.5±10.2 |
| Antianginal medication — no. (%) | ||
| Beta-blocker | 40 (77) | 40 (77) |
| Calcium-channel blocker | 26 (50) | 29 (56) |
| Nitrates | 32 (62) | 29 (56) |
| Nicorandil | 6(12) | 7(13) |
| Ivabradine | 5(10) | 4(8) |
| No. of antianginal medications — no. (%) | ||
| 0 | 4(8) | 3(6) |
| 1 | 10 (19) | 10 (19) |
| 2 | 18 (35) | 23 (44) |
| 3 | 18 (35) | 12 (23) |
| >3 | 2(4) | 4(8) |
Plus-minus values are means ±SD. There were no significant differences between the study groups at baseline. CABG denotes coronary-artery bypass grafting, and PCI percutaneous coronary intervention.
Canadian Cardiovascular Society (CCS) angina classes range from 1 to IV, with higher classes indicating greater limitations on physical activity owing to angina.
The Reducer was successfully implanted in 50 (96%) of the 52 patients randomized to the Reducer group. In 2 patients, the implantation failed due to a venous valve in the CS that could not be crossed with the device.
Efficacy end points
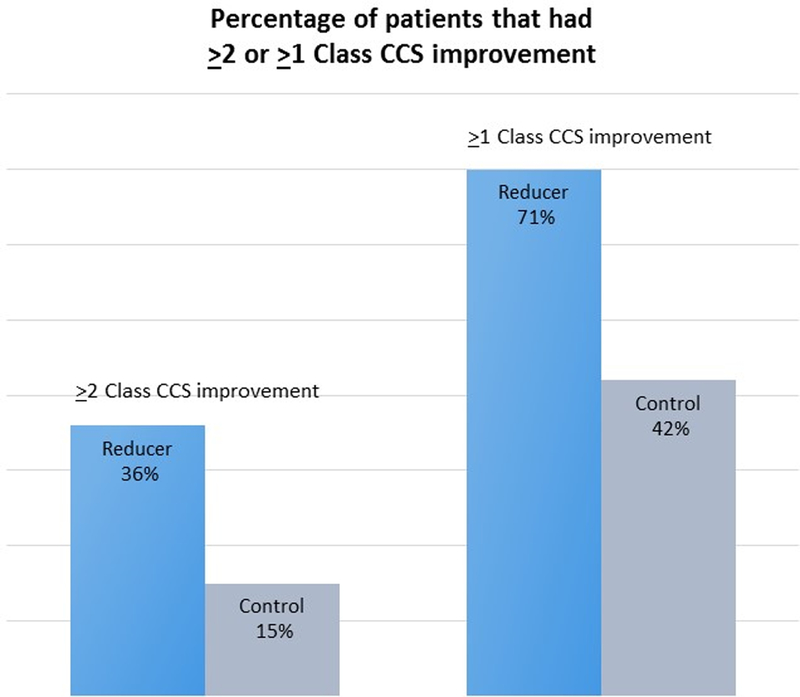
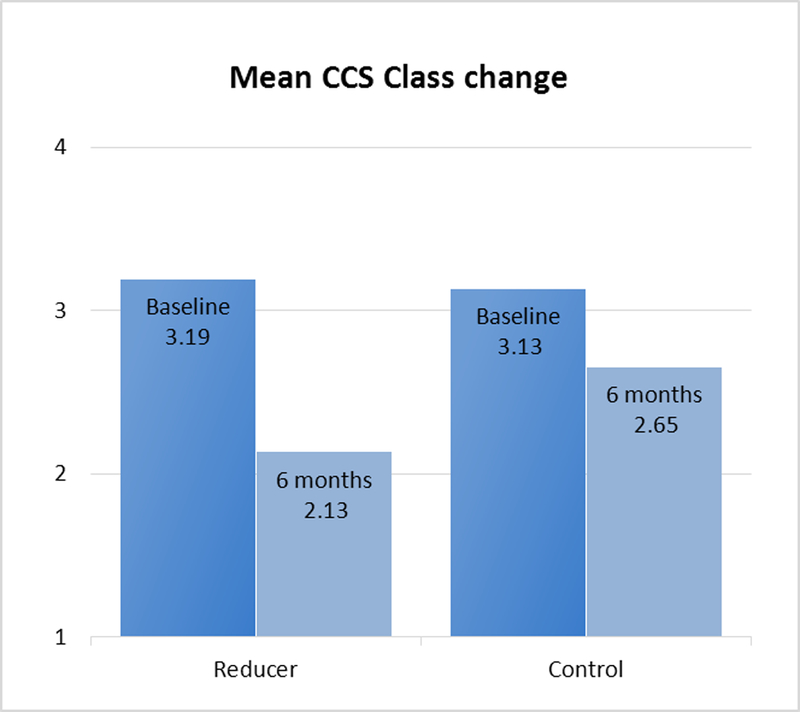
Baseline and follow-up information regarding CCS class was available for all 104 patients. A total of 18 of 52 patients in the Reducer group and 8 of 52 in the sham-control group had an improvement of at least two CCS angina classes (34.6% vs. 15.3%, respectively, p=0.02; Figure 2A). Mean CCS class was reduced from 3.2±0.4 at baseline to 2.1±1.0 at 6-month follow up in the Reducer group compared to 3.1±0.3 to 2.6±0.9 in the control group, p=0.001; Figure 2B). In the Reducer group, 71.1% (37 of 52) had an improvement of at least one CCS angina class, compared to 42.3% (22 of 52) in the sham-control group. (p=0.003, Figure 2A and Figure 3).
Figure 2.
A: The proportion of patients improving by at least 2 CCS angina grades, and by at least 1 CCS angina grade, was significantly higher among Reducer patients compared to control (34.6% vs. 15.3% for at least 2 CCS angina grades, p=0.02; and 71.1% vs 42.3% for at least 1 CCS angina grade, p = 0.003).
B: Mean CCS angina class was reduced from 3.19±0.4 at baseline to 2.13±0.97 at 6-month follow up in the Reducer group, and from 3.13±0.34 to 2.65±0.88 in the sham control group. The improvement was significantly better in the Reducer group (p=0.001).
Figure 3.
The proportion of patients in each CCS class, by randomized group, at baseline and 6 months after randomization.
Quality of life as measured by the Seattle Angina Questionnaire improved by 17.6 points in the Reducer group and by 7.6 points in the control group (p=0.03). There were no significant differences between groups with respect to improvement in angina stability (18.1 vs. 8.3 points, respectively; p=0.16) or angina frequency (15.3 vs. 11 points, respectively; p=0.4) (see Supplementary Appendix Table S1).
The baseline mean total exercise duration was 441±191 seconds in the Reducer group and 464±257 seconds in the control group. At 6 months follow-up, mean exercise duration improved by 60 seconds (13%) in the Reducer group, and by 4 seconds (1%) in the sham-control group (p=0.07). Mean time to 1 mm ST segment depression was prolonged by 49 seconds (13%) in the Reducer group and by 18 seconds (4%) in the control group (p=0.4) (see Supplementary Appendix Table S2).
The change from baseline to six months in dobutamine echocardiography stress wall-motion score index and the modified left coronary artery stress wall-motion score index did not differ significantly between the two groups. The stress wall- motion score index improved by 14% in the Reducer group and by 8.0% in the control group (p=0.20). The modified left coronary artery stress wall-motion score index improved by 13% in the Reducer group and by 3% in the control group (p=0.06) (see Supplementary Appendix Table S3).
Safety end points
A periprocedural myocardial infarction occurred in one patient in the Reducer group. Other periprocedural serious adverse events included a case of unstable angina, and a case of Crohn’s disease in the Reducer group and a case of unstable angina and a case of epigastric pain in the control group.
At least one adverse event was reported for 32 of 50 patients (64.0%) who received a Reducer implant and for 37 of 54 patients (68.5%) who did not receive the device (p=0.680). Overall there were 76 adverse events reported in the Reducer group and 93 in the control group.
There were three myocardial infarctions and one death (multi-organ failure at day 118) in the control group, and one periprocedural myocardial infarction and no deaths in the Reducer group. There were a total of 34 serious adverse events in the trial (10 in the Reducer group and 24 in the control group). The full lists of serious adverse events and of the periprocedural adverse events are shown in Supplementary Appendix Tables S4 and S5.
Computed tomographic (CT) angiography was performed in 36 of 50 patients treated with a Reducer, with no evidence of device migration or occlusion in any of the patients. Figure 4 demonstrates a longitudinal view and a series of cross sectional views from a CT angiogram of a Reducer, 6 months post-implantation.
Figure 4.
Cross sectional and longitudinal CT angiography views of the Reducer in the coronary sinus 6 months after implantation. The flow of contrast demonstrates continued patency of the device. The green and red bars in the longitudinal image, on the right, mark the positions of the cross-sectional images shown on the left. The red bars that appear in the third cross-sectional image and in the longitudinal image are in the same relative position in the two images and mark the positions of the opposing walls of the Reducer.
DISCUSSION
The COSIRA trial evaluated the Reducer as a new therapy for patients with refractory angina. Reducer implantation was significantly better than a sham intervention to improve angina symptoms in patients with advanced coronary artery disease unsuitable for revascularization and refractory to standard medical therapy. A reduction of at least 2 CCS angina classes at six months (the primary end point) occurred 2.4 times more frequently in the Reducer group than in the control group. Although underpowered for the pre-specified secondary outcomes, the trial nonetheless demonstrated more frequent improvements of at least 1 CCS angina class and greater improvement in in quality of life in the Reducer group than in the control group. Significant differences were not seen between the groups with respect to change in other secondary end points such as exercise duration, change in time to 1 mm ST segment depression, or change in wall-motion score index.
The prevalence of refractory angina continues to increase, associated with aging of the population and the increase in life expectancy of patients with ischemic heart disease. Therapeutic options for this group of patients should focus on angina relief and improved quality of life.4,8,11,16
The physiologic rationale for a beneficial effect of increased coronary sinus pressure in angina pectoris remains unclear. In 1954, Beck and Leighninger described a surgical procedure for partial occlusion of the coronary sinus, which was associated with relief of angina, improved functional class, and a reduction in mortality.17–21 The most commonly proposed mechanism of benefit is recruitment of coronary collateral flow with redistribution from the less ischemic epicardium to the ischemic endocardium.22–26
Our sham-controlled clinical trial was designed to control for both patient and investigator biases in the interpretation of outcome end points. Placebo treatment alone can lead to a substantial improvement in angina symptoms and in exercise duration. 27–32 In a previous study of transmyocardial laser revascularization for the treatment of angina, a pronounced placebo effect was noted, resulting in a 30% improvement in exercise duration and angina symptoms in the control group of patients.33 Consequently, in our trial, extensive precautions were taken to blind the patients and the investigators performing the follow up.
Our study was not statistically powered to detect improvement in ischemia by objective measures such as stress testing or wall-motion score index. A larger trial would be necessary to demonstrate such a benefit. Since the planning of this study, tools with better fidelity to detect improvement in myocardial ischemia such as magnetic resonance imaging (MRI) and positron-emission tomography (PET) have become more commonplace and would be attractive modalities to employ in the phase III studies of this device.
Among patients suffering from disabling angina pectoris, this small double-blind, randomized, sham-controlled trial demonstrated the efficacy of the Reducer in improving symptoms of angina and quality of life. These results require confirmation in larger and more definitive trials.
Supplementary Material
REFERENCES
- 1.Fihn SD, Gardin JM, Abrams J et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. J Am Coll Cardiol 2012. [Google Scholar]
- 2.Jolicoeur EM, Granger CB, Henry TD, Holmes DJ, Pepine CJ, Mark D, Chaitman BR, Gersh BJ, Ohman EM. Clinical and research issues regarding chronic advanced coronary artery disease: part I: Contemporary and emerging therapies. Am Heart J 2008;155(3):418–434. [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Menon M, Satran D, Hayward D, Hodges JS, Burke MN, Johnson RK, Poulose AK, Traverse JH, Henry TD. Patients with coronary artery disease not amenable to traditional revascularization: prevalence and 3-year mortality. Catheter Cardiovasc Interv 2010;75(6):886–891. [DOI] [PubMed] [Google Scholar]
- 4.Henry TD, Satran D, Jolicoeur EM. Treatment of refractory angina in patients not suitable for revascularization. Nat Rev Cardiol. 2014. February;11(2):78–95. 10.1038/nrcardio.2013.200 PMID: [DOI] [PubMed] [Google Scholar]
- 5.Banai S, Ben Muvhar S, Parikh KH, Medina A, Sievert H, Seth A, Tsehori J, Paz Y, Sheinfeld A, Keren G. Coronary sinus reducer stent for the treatment of chronic refractory angina pectoris: a prospective, open-label, multicenter, safety feasibility first-in-man study. J Am Coll Cardiol 2007;49(17):1783–1789. [DOI] [PubMed] [Google Scholar]
- 6.Banai S, Schwartz M, Sievert H, Seth A, Keren G, Parikh KH. Long Term Follow- Up to Evaluate the Safety of the Neovasc Reducer, A Device-Based Therapy for Chronic Refractory Angina. J Am Coll Cardiol 20l0; 55 (10- Supp A): 0919–07-A08. [Google Scholar]
- 7.Konigstein M, Meyten N, Verheye S, Schwartz M, Banai S: Transcatheter treatment for refractory angina with the Coronary Sinus Reducer. Eurointervention, 2014; 9(10): 1158–1164. [DOI] [PubMed] [Google Scholar]
- 8.Henry TD, Satran D, Hodges JS, Johnson RK, Poulose AK, Campbell AR, Garberich RF, Bart BA, Olson RE, Boisjolie CR, Harvey KL, Arndt TL, Traverse JH Long-term survival in patients with refractory angina. Eur Heart j. 2013. September;34(34):2683–8 [DOI] [PubMed] [Google Scholar]
- 9.Jolicoeur EM, Banai S, Henry TD, Schwartz M, Doucet S, White CJ, Edelman E, Verheye S: A phase II, sham-controlled, double-blinded study testing the safety and efficacy of the coronary sinus reducer in patients with refractory angina: study protocol for a randomized controlled trial. Trials. 2013. February 15;14:46. doi: 10.1186/1745-6215-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGillion M, Arthur HM, Cook A, Carroll SL, Victor JC, L’Allier PL, Jolicoeur EM, Svorkdal N, Niznick J, Teoh K, Cosman T, Sessle B, Watt-Watson J, Clark A, Taenzer P, Coyte P, Malysh L, Galte C, Stone J. Management of patients with refractory angina: canadian cardiovascular society/canadian pain society joint guidelines. Can J Cardiol 2012;28 Suppl A:S20–S41. [DOI] [PubMed] [Google Scholar]
- 11.Jolicoeur EM, Cartier R, Henry TD, Barsness GW, Bourassa MG, McGillion M, L’Allier PL. Patients with coronary artery disease unsuitable for revascularization: definition, general principles, and a classification. Can J Cardiol 2012;28 Suppl A:S50–S59. [DOI] [PubMed] [Google Scholar]
- 12.Chaitman BR, Stone PH, Knatterud GL, Forman SA, Sopko G, Bourassa MG, Pratt C, Rogers WJ, Pepine CJ, Conti CR. Asymptomatic Cardiac Ischemia Pilot (ACIP) study: impact of anti-ischemia therapy on 12-week rest electrocardiogram and exercise test outcomes. The ACIP Investigators. J Am Coll Cardiol 1995;26(3):585–593. [DOI] [PubMed] [Google Scholar]
- 13.Frolicher VF, Myers J. Manual of Exercise Testing. 2007. [Google Scholar]
- 14.Collins M, Hsieh A, Ohazama CJ, et al. Assessment of regional wall motion abnormalities with real-time 3-dimensional echocardiography. J Am Soc Echocardiogr 1999;12:7–14. [DOI] [PubMed] [Google Scholar]
- 15.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonnell M, Fihn SD. Development and evaluation of the Seattle Angina questionnaire: A new functional status measure for coronary artery disease. Journal of the American College of Cardiology 1995;25(2):333–341. [DOI] [PubMed] [Google Scholar]
- 16.Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Lu’scher T, Pasic M, Thelle D. The problem of chronic refractory angina: report from the European Society of Cardiology joint study group on the treatment of refractory angina. Eur Heart J 2002;23:355–370. [DOI] [PubMed] [Google Scholar]
- 17.Beck CS, Leighninger DS. Operations for coronary artery disease. J Am Med Assoc 1954;156(13):1226–1233. [DOI] [PubMed] [Google Scholar]
- 18.Beck CS, Leighninger DS. Scientific basis for the surgical treatment of coronary artery disease. J Am Med Assoc 1955;159(13):1264–1271. [DOI] [PubMed] [Google Scholar]
- 19.Sandler G, Slesser BV, Lawson CW. The Beck operation in the treatment of angina pectoris. Thorax 1967;22(1):34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wising PJ. The Beck-1 operation for angina perctoris: medical aspects. Acta Med Scand 1963;174:93–98. [DOI] [PubMed] [Google Scholar]
- 21.Brofman BL. Medical evaluation of the Beck operation for coronary artery disease. J Am Med Assoc 1956;162(18):1603–1606. [DOI] [PubMed] [Google Scholar]
- 22.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356(8):830–840. [DOI] [PubMed] [Google Scholar]
- 23.Ido A, Hasebe N, Matsuhashi H, Kikuchi K. Coronary sinus occlusion enhances coronary collateral flow and reduces subendocardial ischemia. Am J Physiol Heart Circ Physiol 2001;280(3):H1361–H1367. [DOI] [PubMed] [Google Scholar]
- 24.Paz Y, Shinfeld A. Mild increase in coronary sinus pressure with coronary sinus reducer stent for treatment of refractory angina. Nat Clin Pract Cardiovasc Med 2009;6(3):E3. [DOI] [PubMed] [Google Scholar]
- 25.Syeda Bonni, Schukro Christoph, Heinze Georg, Modaressi Kourosh, Glogar Dietmar, Maurer Gerald, Werner Mohl: The salvage potential of coronary sinus interventions: Meta-analysis and pathophysiologic consequences. The Journal of Thoracic and Cardiovascular Surgery, 2005,127(6);1703–1712 [DOI] [PubMed] [Google Scholar]
- 26.Mohl W, Kajgana I, Bergmeister H, Rattay F:Intermittent pressure elevation of the coronary venous system as a method to protect ischemic myocardium. Interactive CardioVascular and Thoracic Surgery 2005:4;66–69 [DOI] [PubMed] [Google Scholar]
- 27.Johnson AG. Surgery as a placebo. Lancet 1994;344:1140–2. [DOI] [PubMed] [Google Scholar]
- 28.Beecher H Surgery as placebo. JAMA 1961;176:1102–7. [DOI] [PubMed] [Google Scholar]
- 29.Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001;344:1594–1602. [DOI] [PubMed] [Google Scholar]
- 30.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol 2000;53:786–92. [DOI] [PubMed] [Google Scholar]
- 31.Bienenfeld L, Frishman W, Glasser SP. The placebo effect in cardiovascular disease. Am Heart J 1996;132:1207–21. [DOI] [PubMed] [Google Scholar]
- 32.Leon MB, Kornowski R, Downey WE, Weisz G, Baim DS, Bonow RO, Hendel RC, Cohen DJ, Gervino E, Laham R, Lembo NJ, Moses JW, Kuntz RE: A blinded, randomized, placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J Am Coll Cardiol 2005;46:1812–1819. [DOI] [PubMed] [Google Scholar]
- 33.Mohl W, Mina S, Milasinovic D, Kasahara H, Wei S, Maurer G. Is activation of coronary venous cells the key to cardiac regeneration? Nature Clin Pract Cardiovasc Med 2008;5:528–530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


