Abstract
Maladaptive responses to pain-related distress, such as pain catastrophizing, amplify the impairments associated with chronic pain. Many of these aspects of chronic pain are similar to affective distress in clinical anxiety disorders. In light of the role of the amygdala in pain and affective distress, disruption of amygdalar functional connectivity in anxiety states, and its implication in the response to noxious stimuli, we investigated amygdala functional connectivity in 17 patients with chronic low back pain and 17 healthy comparison subjects, with respect to normal targets of amygdala subregions (basolateral vs centromedial nuclei), and connectivity to large-scale cognitive–emotional networks, including the default mode network, central executive network, and salience network. We found that patients with chronic pain had exaggerated and abnormal amygdala connectivity with central executive network, which was most exaggerated in patients with the greatest pain catastrophizing. We also found that the normally basolateral-predominant amygdala connectivity to the default mode network was blunted in patients with chronic pain. Our results therefore highlight the importance of the amygdala and its network-level interaction with large-scale cognitive/affective cortical networks in chronic pain, and help link the neurobiological mechanisms of cognitive theories for pain with other clinical states of affective distress.
Keywords: Amygdala, Chronic pain, Pain catastrophizing, Functional connectivity
1. Introduction
Chronic pain affects 100 million Americans with annual costs exceeding $500 million.49 Low back pain is the most common chronic pain condition31 with increasing prevalence, treatment and associated expenditures,48 and disability.40 Psychological distress is frequently associated with chronic pain18 and implicates an interaction between sensory perception, cognition, and emotion.13 Negative emotional states directly modulate pain experience,11,38 which in turn biases affective–motivational systems and elicits unpleasant feelings.42,45 Chronic pain additionally has a secondary emotional component, which is mediated by cognitive factors such as one’s beliefs, attitudes, and thoughts about the consequences of persistent pain on one’s work and life,44,63 an example of which is catastrophizing.60 Such responses predict poor outcomes54 and may maintain or worsen the illness.64
One brain region that may account for the emotional component of pain is the amygdala—a structure itself composed of several major subregions. Experimentally induced pain in patients with chronic pain increases activation in the basolateral amygdala (BLA),56 the major sensory input region of the amygdala. Additionally, the centromedial amygdala (CMA), which provides much of the descending output of the amygdala, also receives ascending nociceptive information.9 Recent resting-state functional magnetic resonance imaging (fMRI) studies have found that the BLA and CMA have dissociable patterns of functional connectivity in humans.24,50 Moreover, the amygdala may promote dysfunctional cognitive/emotional reactions such as pain catastrophizing through interactions with other networks implicated in a range of cognitive and emotional functions, which have been shown to be perturbed in chronic pain,7,14,41,61 namely, the frontoparietal “central executive network” (CEN), the dorsal anterior cingulate-anterior insula “salience network” (SN), and the medial prefrontal-medial parietal “default mode network” (DMN).
The CEN responds broadly to cognitive and emotional stimuli and is involved in attention selection16 and working memory modulation.39 The DMN activates when processing self-referential information and social and emotional inference of others.12 The SN tracks affective and pain-related sensations,17 maintains a stable cognitive set,20 and mediates interactions between emotion and cognitive control.37 Of note, amygdala–CEN connectivity is abnormally increased and amygdala–SN connectivity is decreased in patients with generalized anxiety disorder24,46—another clinical population featuring emotional distress mediated by dysfunctional cognitions.1
We therefore hypothesized that amygdala connectivity will be perturbed in patients with chronic pain to its subregion-selective targets and to the major cognitive/emotional networks (ie, CEN, SN, and DMN), and that this will relate to patients’ maladaptive pain-related cognitions (ie, pain catastrophizing).
2. Materials and methods
2.1. Subjects and clinical details
The study comprised 3 groups: (1) 17 patients (14 females and 3 males) with chronic low back pain (cLBP) recruited from the community (diagnosed by J.H.), (2) 17 healthy controls (14 females and 3 males) matched for age, gender, and education to the chronic pain group and recruited by advertisement, and (3) a healthy control group that was used only to determine region of interest targets for amygdala subregions (Table 1 for demographics). For the first 2 groups, all subjects gave their written informed consent. Stanford University’s institutional review board approved the study. The cLBP participants had experienced nonspecific cLBP for an average of 8.9 years (SE = 1.9, minimum = 0.5 years, maximum = 26 years). Their average pain severity was 6/10 (SE = 0.4, minimum = 3/10, maximum = 10/10) during the past month, and was 5.7/10 (SE = 0.5, minimum = 2/10, maximum = 10/10) for the past few days. They were without serious spinal pathology, radicular pain, comorbid pain syndromes, use of opioids, thyroid medication, antiepileptic or antidepressant medication, substance abuse, Diagnostic and Statistical Manual of Mental Disorders Axis I psychiatric disorders (determined through the MINI diagnostic interview),55 or ongoing legal or disability claims, and their first language was English. Thus, patients were a particularly homogenous sample, wherein conclusions would not be confounded by co-occurring psychiatric disorders, use of any psychotropic medications, or use of significant opioid or antineuropathic pain medication.
Table 1.
Demographics of subjects.
| Patients with cLBP (P) (N = 17) | Healthy controls (HC) (N = 17) | NKI sample (N = 36) | P vs HC comparisons | |
|---|---|---|---|---|
| Age, mean (SE) | 37.4 (2.5) | 33.9 (2.4) | 34.5 (1.7) | P = 0.33 |
| Female, n (%) | 14 (82) | 14 (82) | 19 (67) | P > 0.99 |
| Right handed, n (%) | 17 (100) | 17 (100) | 38 (100) | P > 0.99 |
| Education, mean (SE) (y) | 15.2 (0.6) | 16.2 (0.3) | NA | P = 0.11 |
| STAI_T, mean (SE) | 32.9 (2.3) | 30.1 (1.5) | NA | P = 0.30 |
| BAI, mean (SE) | 7.3 (1.8) | 2.4 (0.7) | NA | P < 0.05 |
| BDI-II, mean (SE) | 5.9 (1.3) | 4.5 (1.0) | NA | P = 0.41 |
| PCS_total, mean (SE) | 15.7(2.1) | NA | NA | NA |
| PCS_rumination, mean (SE) | 6.7 (1.0) | NA | NA | NA |
| PCS_helplessness, mean (SE) | 6.1 (1.1) | NA | NA | NA |
| PCS_magnification, mean (SE) | 2.8 (0.4) | NA | NA | NA |
BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory II; NKI, Nathan Kline Institute; PCS, Pain Catastrophizing Scale; SF-MPQ, Short-Form McGill Pain Questionnaire; STAI-T, Trait form of the State-Trait Anxiety Inventory.
Before their scan, all participants were given the Trait form of State-Trait Anxiety Inventory (STAI-T),58 Beck Depression Inventory (BDI-II),8 and the Beck Anxiety Inventory (BAI).4 Additionally, cLBP participants completed the Pain Catastrophizing Scale (PCS)60 to further characterize the psychological correlates of the functional connectivity abnormalities in this group. The PCS consists of 13 items that are rated for frequency on a 5-point Likert scale (0 = not at all, 4 = all the time). The PCS comprises 3 subscales: rumination (4 items; sample item: “I keep thinking about how badly I want the pain to stop”), helplessness (6 items; sample item: “It’s terrible and I think it’s never going to get any better”), and magnification (3 items; sample item: “I wonder whether something serious may happen”). The PCS is widely used in pain research and has good psychometric properties.60 For the latter control group, we used an independent age-matched sample group of 36 healthy subjects from the Nathan Kline Institute (NKI) data set made freely accessible online (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html) by the 1000 Connectome database.10 All individuals in the NKI sample have been given semistructured diagnostic psychiatric interviews.
2.2. Data acquisition
Imaging acquisition was performed on a GE 3T MRI system (GE Healthcare, Milwaukee, WI). Participants were told to keep their eyes closed, remain still, and try not to fall asleep during the resting-state scan. At the beginning of the scan, a magnetic fieldmap was acquired automatically by the pulse sequence. Six minutes of functional data were collected using a gradient echo, spiral-pulse sequence (repetition time, 2000 milliseconds; echo time, 30 milliseconds; flip angle, 77°; voxel size, 3.43 mm). Whole-brain coverage was obtained with 30 interleaved slices and 5-mm slice thickness. A T1-weighted spoiled grass gradient-recalled inverted recovery 3-dimensional MRI sequence (repetition time, 9.516 milliseconds; echo time, 2.896 milliseconds; flip angle, 15°; field of view, 22 cm; 124 axial slice; voxel size, 0.86 × 0.86 × 1.50 mm) was used for acquiring high-resolution structural images for preprocessing. Anatomical data were acquired in the same scan session with resting-state data. The NKI sample was acquired using SIEMENS 3T MR (MAGNETOM TrioTim, Siemens, Munich, Germany). The resting-state scan lasted for 10 minutes, and the scanning parameters were repetition time, 2500 milliseconds; echo time, 30 milliseconds; flip angle, 80°; interleaved; slices, 38; slice thickness, 3 mm; voxel size, 3.0 mm.
2.3. Data preprocessing
The first 8 volumes of resting-state data were discarded for all subjects to account for signal equilibration effects. A linear shim correction was used to reconstruct each slice using the acquired magnetic fieldmap.27 Preprocessing steps were implemented using FSL 5.0 (http://fsl.fmrib.ox.ac.uk/) as follows: (1) structural images were segmented and spatially transformed to standard stereotaxic space in the Montreal Neurologic Institute coordinate system25 with nonlinear normalization using standard settings for FNIRT tool; (2) functional images for each subject were registered to their structural images and corrected for motion with affine registration using MCFLIRT tool. All participants had movement within 3-mm translation and 3° of rotation; (3) functional images were spatially smoothed (6-mm full-width half-maximum gaussian kernel) and temporally band-pass filtered (0.008–0.1 Hz). The same preprocessing steps were applied to the NKI sample.
2.4. Functional connectivity analyses
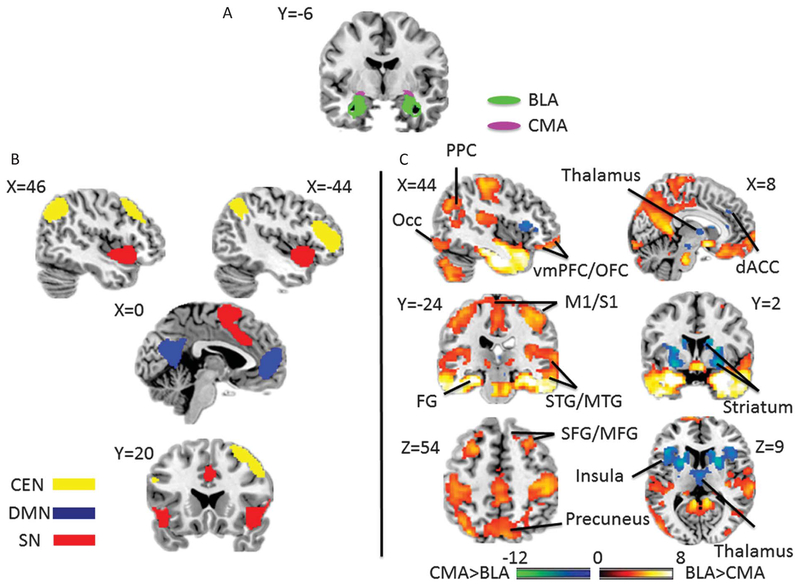
The amygdala subregional seed regions of interest (ROIs) used for connectivity analyses were the basolateral (BLA) and centromedial nulei (CMA). These were constructed from probabilistic cytoarchitectonic maps by including voxels whose probability to be assigned to BLA or CMA is no less than 40% compared with other amygdala subregions or surrounding medial temporal cortex21,22 and were identical to those used in our previous analyses24 (Fig. 1A). For each seed ROI, a BOLD time course was extracted from band-pass filtered resting-state data and then was correlated with the time courses from all other brain voxels using a first-level fixed-effect GLM model, regressing out signal from ventricular regions and white matter as well as 6 motion parameters. The correlation maps consisted of voxels whose values represented their degree of connectivity with the seed ROIs. These values were then converted to z scores by a Fisher z transform, producing z score maps with a normalized distribution.32 Average functional connectivity with the BLA or CMA seed for right and left hemispheres were extracted from individual z score maps for brain regions with high degrees of correlation with amygdala seeds and regions representing large-scale brain networks. The extracted functional connectivity measures were then analyzed using SPSS 19.0 (SPSS IBM, New York, NY). Normative target ROIs for amygdala subregions were derived from the independent control sample as described below in the results. Large-scale networks were obtained from thresholded ICA maps from an independent sample of our previous study, yielding ~1000 voxel ROIs.15 As shown in Figure 1B, the CEN was composed of clusters in the right lateral prefrontal cortex (Brodmann area [BA]: 6/8/9/46; number of voxels: 994), right lateral posterior parietal cortex (rLPPC; BA: 7/39/40; number of voxels: 1009), left lateral prefrontal cortex (lLPFC) (BA: 9/10/45/46/47; number of voxels: 1001), and left lateral posterior parietal cortex (lLPPC; BA: 7/19/39/40; number of voxels: 991); the SN consisted of clusters in the dorsal anterior cingulate cortex (BA: 24/32/6; number of voxels: 999) and frontoinsular cortices (FIC; BA: 13/44/45/47; number of voxels: 996); and the DMN included the medial prefrontal cortex (mPFC; BA: 9/10/11/32; number of voxels: 999) and posterior cingulate cortex (PCC) (BA: 23/29/30/31; number of voxels: 1009). Group-level voxel-wise analyses were performed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) using z score maps from the individual functional connectivity analyses, with a flexible factorial analysis of variance (ANOVA) model.
Figure 1.
(A) Seed regions of interest of amygdala subregions; BLA, basolateral amygdala; CMA, centromedial amygdala. (B) Regions of interest of large-scale networks; CEN, frontoparietal “central executive network”; DMN, medial prefrontal-medial parietal “default mode network”; SN, dorsal anterior cingulate–anterior insula “salience network.” (C) Regions of interest of expected targets for amygdala subregions resulting from a voxel-wise 1-way analysis of variance contrasting the BLA and the CMA for the independent NKI sample (false discovery rate (FDR) q < 0.05). Hot color indicates regions having greater connectivity with BLA than CMA, and cool color shows regions connected more with CMA than BLA. dACC, dorsal anterior cingulate cortex; FG, fusiform gyrus; M1/S1, primary somatosensory and motor cortices; Occ indicates occipital cortex; PPC, posterior parietal cortex; STG/MTG, superior temporal gyrus/middle temporal gyrus; SFG/MFG, superior frontal gyrus/middle frontal gyrus; vmPFC/OFC, ventromedial prefrontal cortex/orbitofrontal cortex; and VTA/SN, ventral tegmental area/substantia nigra.
2.5. Correlation analyses
For patients with cLBP, Pearson correlation was used for correlating individual extracted average amygdala functional connectivity with affect/pain scales, including STAI_T, BAI, BDI-II, and PCS, in SPSS.
2.6. Head motion analyses
Head motion was measured in terms of mean motion, number of movement, and mean rotation. Mean motion was the mean absolute displacement (displacement 5 square root (x2 + y2 + z2), where x, y, z represent translation parameters in the left/right, anterior/posterior, and superior/inferior directions, respectively) of each volume compared with its previous volume. The number of movement was calculated as the number of relative displacements larger than 0.1 mm. Mean rotation was estimated by averaging the absolute value of Euler angle (Euler angle = arccos[(cos(φ)cos(θ) + cos(φ)cos(ψ) + cos(φ)cos(θ) + sin(φ)sin(θ) sin(ψ) − 1)]/2), where φ, θ, and ψ are the rotation parameters along 3 axes.19 Mean motion, number of movement, and mean rotation were compared between patients with cLBP and healthy controls using t-tests and were correlated with functional connectivity estimates within each group using SPSS.
3. Results
3.1. Connectivity of amygdalar subregions to their expected targets
We first defined target mask ROIs for the subregion-specific connectivity maps of the BLA and CMA using the independent NKI sample by contrasting BLA- to CMA-seeded maps in an ANOVA. Target ROIs for the BLA (compared with CMA) and CMA (compared with BLA) were determined by thresholding the statistical maps at q < 0.05 false discovery rate (FDR)-corrected. Consistent with previous work, the BLA was more strongly connected with a number of cortical regions, encompassing primary and secondary sensory cortices, mPFC, ventromedial prefrontal cortex, precuneus, and PCC, as well as with the thalamus, pons, and cerebellum; the CMA was more associated with subcortical connectivity, including the striatum, midbrain, and cerebellum, as well as with the insula and dorsal anterior cingulate cortex (Fig. 1C; Ref. 24). We also did a 1-sample t test on the BLA and CMA connectivity, separately, to explore the source of the connectivity difference (see Supplemental Figure, available online at http://links.lww.com/PAIN/A272).
To test whether amygdalar subregional connectivity with their typical targets was altered in cLBP, average connectivity of both the BLA and CMA target ROIs were extracted from bilateral BLA- and CMA-seeded connectivity analyses (separated for amygdala subregions and hemisphere) for patients with cLBP and healthy controls. For healthy controls, a 2 × 2 × 2 ANOVA (target ROI × amygdala subregion × hemisphere of amygdala subregion) confirmed that the BLA and CMA connected differentially to their expected targets (determined from the independent NKI dataset maps; target ROI × amygdala subregion interaction, F(1,16) = 55.765, P < 0.001, η2 = 0.78), with no interaction by hemisphere (F(1,16) = 0.762, P = 0.396). Next, we conducted a between-group mixed 2 × 2 × 2 × 2 ANOVA (group × target ROI × amygdala subregion × hemisphere of amygdala subregion) to test for group differences in subregional connectivity, but found that subregional connectivity to amygdala targets was not significantly perturbed in patients with cLBP (group × target ROI × amygdala subregion interaction, F(1,32) = 1.915, P = 0.176).
3.2. Connectivity of the amygdala to large-scale cognitive–emotional networks
Next, we extracted bilateral average BLA- and CMA-seeded connectivity to core nodes of the CEN, SN, and DMN for patients with cLBP and healthy controls, and conducted an omnibus 3 × 2 × 2 × 2 ANOVA, with large-scale network, amygdala subregion, and hemisphere of amygdala subregion as within-subject factors and group as a between-subjects factor. We found a group effect for amygdala subregional connectivity (group × network × amygdala subregion interaction, F(2,64) = 3.850, P = 0.026, partial η2 = 0.107), with no interaction with amygdala seed hemisphere (amygdala hemisphere × group× network × amygdala subregion interaction, F(2,64) = 0.175, P = 0.840). We then decomposed this effect by performing 2 × 2 × 2 ANOVAs (group × amygdala subregion × amygdala hemisphere) separately for the CEN, SN, and DMN to examine group difference in amygdala subregional connectivity separately to each of the large-scale networks examined.
3.2.1. Central executive network
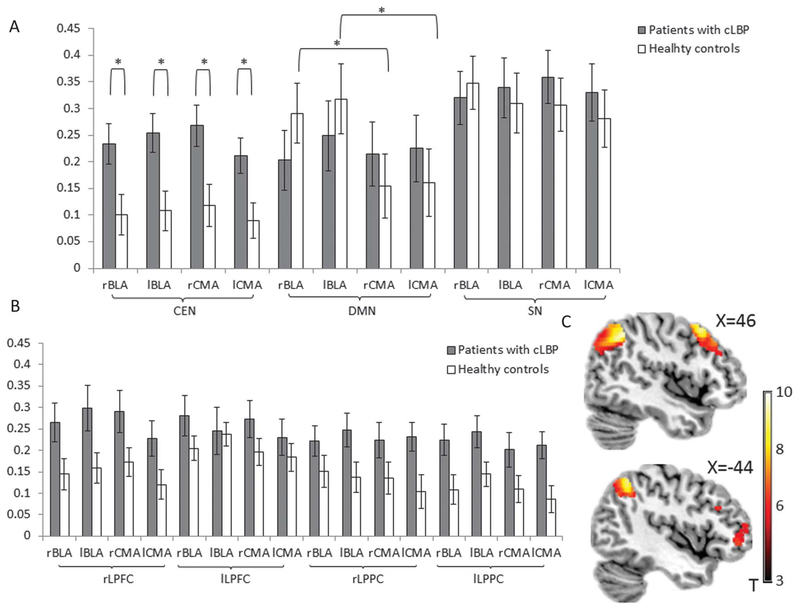
The 2 × 2 × 2 ANOVA (group × amygdala subregion × amygdala hemisphere) for amygdala connectivity to the CEN revealed a main effect of group (F(1,32) = 10.915, P = 0.003, partial η2 = 0.24), not moderated by interaction with amygdalar subregion (group × amygdala subregion interaction, F(1,32) = 0.008, P = 0.929) or amygdala seed hemisphere (group × amygdala hemisphere, F(1,32) = 0.040, P = 0.842). As shown in Figure 2A, this was driven by greater amygdala–CEN connectivity, across subregions, in patients with cLBP relative to controls. When breaking down the CEN into individual ROIs, we found no interaction of group with region of the CEN in a 2 × 2 × 2 × 2 ANOVA (CEN region × group × amygdala subregion × amygdala hemisphere; F(1,32) = 2.120, P = 0.103; Fig. 2B). To visualize the group main effect, we performed a voxel-wise group contrast collapsing across the amygdala subregion and amygdala seed hemisphere. The right LPPC, right lateral prefrontal cortex (LPFC), lLPFC, and left LPPC within the CEN showed especially strong amygdala connectivity in patients with cLBP compared with healthy controls (Fig. 2C, P < 0.05, voxel-wise small volume correction). This further supported our ROI analysis result that, in patients with cLBP, increased amygdala connectivity with CEN occurs across amygdalar subregions and to all regions of the CEN; in other words, there is a change in network-level connectivity between the amygdala and CEN in patients.
Figure 2.
(A) Connectivity of basolateral amygdala (BLA) or the centromedial amygdala (CMA), on the right or left hemisphere, with the large-scale networks in patients with cLBP and healthy controls. (B) Connectivity of BLA or the CMA, on the right or left hemisphere, with the core nodes within CEN in patients with cLBP and healthy controls. (C) A voxel-wise analysis of variance (group × amygdala seed × hemisphere of amygdala seed) showed the voxels within CEN with significant stronger amygdala connectivity in patients with cLBP, compared with healthy controls (P < 0.05, false discovery rate-corrected). Bars, mean values; error bar, SEM; lBLA, left basolateral amygdala; lCMA, left centromedial amygdala; rBLA, right basolateral amygdala; rCMA, right centromedial amygdala.
3.2.2. Default mode network
The patients with cLBP showed altered differential amygdala subregional connectivity to the DMN (group × amygdala subregion interaction, F(1,32) = 5.108, P = 0.031, partial η2 = 0.14), with no moderation by amygdala hemisphere (amygdala hemisphere × group × amygdala subregion interaction, F(1,32) = 0.042, P = 0.839) (Fig. 2A). This effect was driven by differential BLA/CMA connectivity with the DMN in healthy controls (F(1,16) = 10.365, P = 0.005, partial η2 = 0.39), such that DMN connectivity was stronger to the BLA than the CMA. This connectivity difference between amygdalar subregions was not found in patients with cLBP (F(1,16) = 0.018, P = 0.896). The lack of distinction between amygdala subregional connectivity in patients with cLBP was consistent for the 2 core nodes of the DMN, the mPFC, and the PCC, which suggests that differential amygdalar subregional connectivity is perturbed across the DMN (for patients with cLBP: FmPFC(1,16) = 0.033, P = 0.859; FPCC(1,16) = 0.005, P = 0.943; for healthy controls: FmPFC(1,16) = 9.430, P = 0.007, partial η2 = 0.37; FPCC(1,16) = 8.590, P = 0.01, partial η2 = 0.35). Thus, amygdalar connectivity to the DMN was perturbed in a different manner than for amygdala–CEN connectivity, such that the subregional specificity of amygdala–DMN connectivity was blunted in patients, rather than wholesale level of connectivity.
3.2.3. Salience network
There was no main effect of group (group main effect, F(1,32) = 0.171, P = 0.682) or group by amygdalar subregion interaction for amygdala–SN connectivity (group × amygdala subregion interaction, F(1,32) = 0.787, P = 0.381).
3.3. Relationship of perturbed amygdalar connectivity to head motion
No significant difference between healthy controls and patients with cLBP was found for the 3 head motion estimates: mean motion (t = −1.502, P = 0.143), number of movement (t = −0.758, P = 0.454), and mean rotation (t = −1.578, P = 0.124). Also, amygdala subregional connectivity with CEN and difference between BLA–DMN and CMA–DMN connectivity did not significantly correlate with these head motion estimates within and across both groups.
3.4. Relationship of perturbed amygdalar connectivity to pain catastrophizing
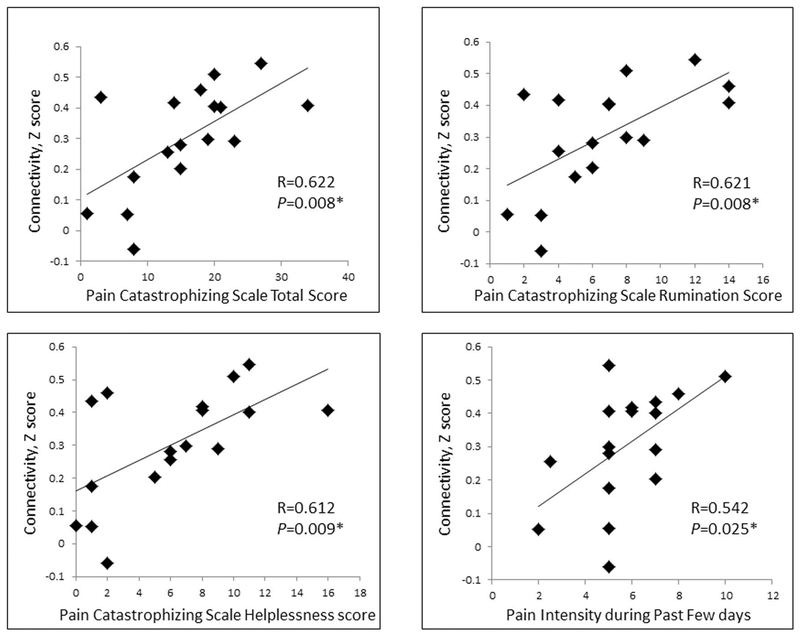
To further explore behavioral/symptom correlates of the cLBP patient abnormalities, we extracted amygdala–CEN connectivity values collapsing across amygdala subregions and hemisphere from patients and correlated these extracted average values with affect/pain scales, including STAI_T, BAI, BDI-II, and PCS. The results showed that exaggerated amygdala connectivity with the CEN in patients with cLBP was positively associated with total scores from the PCS (r = 0.622, P = 0.008). Breaking the PCS into subscales, we found positive correlations between increased amygdala–CEN connectivity and rumination (r = 0.621, P = 0.008) and also with helplessness (r = 0.612, P = 0.009). There was no significant correlation for magnification scores of PCS (r = 0.108, P = 0.681). We also found a positive relationship between increased amygdala–CEN connectivity and pain intensity during the past few days (r = 0.542, P = 0.025) (Fig. 3). After controlling for pain intensity during the past few days, amygdala connectivity remained significantly correlated with total (r = 0.603, P = 0.013), rumination subscale (r = 0.058, P = 0.025), and helplessness subscale (r = 0.604, P = 0.013) scores of the PCS. There was no significant association between reported pain intensity with the PCS or subscales. We also examined amygdalar connectivity relationships with anxiety and depression symptoms, to determine whether our findings in patients with chronic pain may secondarily reflect elevated anxiety symptoms (noting also that through our experimental design, no patient met criteria for a psychiatric disorder and that anxiety and depression levels were below clinical levels). Moreover, we found no relationship between amygdala–CEN connectivity and anxiety/depression symptoms (BDI: r = −0.310, P = 0.243; BAI: r = −0.002, P = 0.994). Finally, we explored the association between blunted subregional differentiation between BLA–DMN and CMA–DMN connectivity by correlating BLA–DMN connectivity, CMA–DMN connectivity and the subtraction of BLA–DMN, and CMA–DMN connectivity with the pain and affective scales above, and found no significant brain–symptom relationships.
Figure 3.
Significant correlations between amygdala connectivity strength (z score) and Pain Catastrophizing Scale and pain intensity during the past few days.
4. Discussion
In this study, we examined resting-state connectivity of the amygdala and its subregions in patients with chronic pain. Patients, compared with healthy controls, showed exaggerated amygdalar connectivity with the CEN. This network is believed to exert cognitive control through selective attention and working memory maintenance.20,39 Normally, the amygdala is only weakly associated with brain regions in the CEN,24,50,52 a finding we replicated in healthy controls in our study. The importance of this exaggerated connectivity is further amplified by the relationship we found between greater pain catastrophizing and greater amygdala–CEN connectivity. In addition, we found that the normal predominance of BLA connectivity with the DMN relative to the CMA that is observed in healthy participants was absent in patients with chronic pain. Thus, chronic pain is characterized by abnormalities in amygdalar connectivity with 2 large-scale networks implicated in cognitive/emotional processes.
Consistent with our amygdala–CEN finding, a meta-analysis of experimental pain studies showed that the prefrontal cortex is more activated in patients with chronic pain than in healthy subjects.2 Abnormal gray matter density in the amygdala and LPFC of people with cLBP has been shown to distinguish patients from healthy controls.62 Previous work in clinical anxiety has found increased amygdala–CEN connectivity in patients with generalized anxiety disorder and in individuals with elevated childhood anxiety.24,46 Because of our experimental design, however, the patients with chronic pain in this study were free of axis I anxiety or depressive disorders and had no clinically meaningful elevations in depressive (BDI-II) or anxiety (BAI) symptoms. Moreover, although patients with chronic pain had greater BAI scores compared with those of healthy controls, these subclinical anxiety scores were not associated with exaggerated amygdala–CEN connectivity. Thus, our findings reflect chronic pain-related amygdala–CEN abnormalities, rather than secondarily reflecting anxiety-related processes in these patients. In other words, our findings likely speak to a more generally relevant disruption in emotion/cognition circuit inter-actions observed separately across different clinical groups, and which is relevant for understanding chronic pain. Importantly, in the context of chronic pain, these are related to aspects of pain catastrophizing rather than more generally affective distress.
Previous work has found positive associations between pain catastrophizing and neural responses in bilateral lateral prefrontal and parietal cortices, as well as the extended amygdala, in patients with fibromyalgia in response to painful stimulation.28 In healthy subjects, activation of bilateral dorsolateral prefrontal and parietal cortices in response to mild pain has been found to correlate positively with catastrophizing scores.53 These results are therefore consistent with the relationship we observed in this study between greater abnormal amygdala–CEN connectivity and greater pain catastrophizing. Importantly, we found that helplessness and rumination drove the relationship between catastrophizing and exaggerated amygdala–CEN connectivity. A previous study on helplessness showed that perceived uncontrollability of pain stimuli in healthy subjects was associated with activation of the amygdala and the lateral prefrontal cortex.51 The amygdala and lateral prefrontal cortex are also more active during anticipation of pain in patients with depression than in healthy controls, and amygdala activation has also been associated with both the helplessness and rumination subscales of the PCS in patients with depression but not in healthy controls.59
Our finding extends this amygdalar–LPFC relationship to connectivity dynamics during the resting state, which suggests that catastrophizing responses may shape neural functioning and promote persistently abnormal cognitive–emotional interactions that occur even in the absence of noxious stimulus. Unlike acute pain, which is often only accompanied by an immediate and transient pain-induced state of negative affect, chronic pain has secondary effects on cognitive–emotional interactions. These include anticipating the consequences of persistent pain with regard to one’s long-term well-being.44Pain catastrophizing may be a specific manifestation of a negative cognitive bias in this appraisal process. Thus, the association we observed between chronic pain and exaggerated amygdala–CEN connectivity seems to be independent of anxiety, but parallels findings in anxious patients whose psychological distress is anxiety—instead of pain related. In other words, abnormally exaggerated amygdalar–CEN connectivity may represent a shared neural basis driving cognitive/emotional changes and distress symptoms (eg, catastrophizing) in anxiety and chronic pain, consistent with overlaps between the cognitive theories for these disorders.
We also found that the normative pattern of amygdala subregional connectivity with the DMN is disrupted in patients with chronic pain. In healthy participants, we found that the DMN is more strongly connected to the BLA than the CMA. The BLA is the major source of anatomical projections from the amygdala to the mPFC,3,33 and has a modulatory effect on the mPFC.26,29,30 Also, mPFC stimulation leads to activation of BLA neurons35 and indirectly inhibits CMA neurons through innervating inhibitory interneurons connecting the BLA and CMA.47 These amygdala–mPFC neural interactions, which are believed to be involved in the acquisition and extinction of learned fear,43 emotion preservation,57 and emotion appraisal,23 might underlie the normal pattern of amygdala subregional connectivity with the DMN. Our finding that this normal pattern is absent may thus reflect disturbed amygdala–mPFC interactions in chronic pain. Default mode network disruptions, especially in the mPFC, are consistently found in patients with chronic pain during rest or experimental tasks.5–7,61 Exaggerated mPFC–DMN connectivity has been associated with pain catastrophizing rumination in patients with chronic pain.34 Increased DMN–pgACC/mPFC connectivity is also related to self-initiated compensatory processing for the anticipated increased pain in patients with cLBP.36
In interpreting these results, it is also important to consider a key strength and limitation of this study. These patients were specifically selected to have low levels of anxiety and depression, and none met criteria for a psychiatric disorder. We did so to distinguish between pain-related abnormalities and those related to general affective distress. These patients were also free of psychotropic, opioids, antineuropathic pain medications, or nonopioid analgesic medications (eg, gabapentin and other anticonvulsants used for pain), and hence imaging data are not confounded by concurrent disorder or medication use. As such, these patients are not reflective of many of the patients with chronic pain seen in clinical practice, who often have comorbid anxiety or depressive disorders, and are frequently on a variety of medications. Nonetheless, these factors represent important strengths for understanding the neural circuitry of chronic pain from a more mechanistic perspective. In sum, although the amygdala has long been a research focus in affective disorders, its role in chronic pain is understudied—despite the important interplay between affect and pain and broad similarities between clinical anxiety and chronic pain conditions. We found changes in amygdalar connectivity with 2 large-scale networks that are important in cognitive and emotional operations, the CEN and DMN, and a relationship between abnormal amygdalar connectivity and pain-related affective distress (ie, pain catastrophizing). Together, these data argue for an important role for the amygdala and its network-level interactions in chronic pain, and help inform a broader understanding of the relationship between chronic pain and other states of affective distress.
Supplementary Material
Acknowledgments
This study was funded by an International Association for the Study of Pain International Collaborative Research Grant 2008–9 to Julia Hush, an NIH P01 AT006651, K24 DA029262, and the Redlich Pain Research Endowment to Sean Mackey, and the Sierra-Pacific Mental Illness Research Education and Clinical Center (MIRECC) for A. Etkin.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.painjournalonline.com).
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A272.
References
- [1].Aikins DE, Craske MG. Cognitive theories of generalized anxiety disorder. Psychiatr Clin North Am 2001;24:57–74. [DOI] [PubMed] [Google Scholar]
- [2].Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9:463–84. [DOI] [PubMed] [Google Scholar]
- [3].Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res 1996;720:211–9. [DOI] [PubMed] [Google Scholar]
- [4].BAI, Beck Anxiety Inventory: Manual Psychological Corporation, San Antonio, TX, USA, 1990. [Google Scholar]
- [5].Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci 2011;31:13981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006;26:12165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 2008;28:1398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: Manual. Psychological Corporation, 1996. [Google Scholar]
- [9].Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 1990;63:473–90. [DOI] [PubMed] [Google Scholar]
- [10].Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 2010;107:4734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bravo L, Rey-Brea R, Micό JA, P érez-Nievas B, Leza JC, Berrocoso E. Depressive-like states heighten the aversion to painful stimuli in a rat model of comorbid chronic pain and depression. Anesthesiology 2012;117:613–25. [DOI] [PubMed] [Google Scholar]
- [12].Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- [13].Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14: 502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DME. Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. Neuroimage Clin 2014;4:676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen AC, Oathes DJ, Chang C, Bradley T, Zhou Z-W, Williams LM, Glover GH, Deisseroth K, Etkin A. Causal interactions between frontoparietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A 2013;110:19944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002;3:201–15. [DOI] [PubMed] [Google Scholar]
- [17].Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655–66. [DOI] [PubMed] [Google Scholar]
- [18].Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, Levinson D, de Girolamo G, Nakane H, Mneimneh Z, Lara C, de Graaf R, Scott KM, Gureje O, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J, Von Korff M. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. PAIN 2007;129:332–42. [DOI] [PubMed] [Google Scholar]
- [19].Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012;59:431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A 2007;104:11073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 2006;32:570–82. [DOI] [PubMed] [Google Scholar]
- [22].Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005;25:1325–35. [DOI] [PubMed] [Google Scholar]
- [23].Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011;15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 2009;66: 1361–72. [DOI] [PubMed] [Google Scholar]
- [25].Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping 1995;2:165–89. [Google Scholar]
- [26].Garcia R, Vouimba R-M, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 1999;402:294–6. [DOI] [PubMed] [Google Scholar]
- [27].Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med 1998;39:361–8. [DOI] [PubMed] [Google Scholar]
- [28].Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004;127:835–43. [DOI] [PubMed] [Google Scholar]
- [29].Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature 2008;454:600–6. [DOI] [PubMed] [Google Scholar]
- [30].Herry C, Vouimba RM, Garcia R. Plasticity in the mediodorsal thalamoprefrontal cortical transmission in behaving mice. J Neurophysiol 1999;82: 2827–32. [DOI] [PubMed] [Google Scholar]
- [31].Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012;64:2028–37. [DOI] [PubMed] [Google Scholar]
- [32].Jenkins GM, Watts DG. Spectral analysis and its applications Holden-Day, San Francisco, CA, USA, 1968. [Google Scholar]
- [33].Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol 1990;298:40–9. [DOI] [PubMed] [Google Scholar]
- [34].Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 2014;34:3969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Likhtik E Prefrontal control of the amygdala. J Neurosci 2005;25: 7429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. PAIN 2013;154:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Luo Y, Qin S, Fernández G, Zhang Y, Klumpers F, Li H. Emotion perception and executive control interact in the salience network during emotionally charged working memory processing. Hum Brain Mapp 2014;35:5606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mackey SC, Maeda F. Functional imaging and the neural systems of chronic pain. Neurosurg Clin N Am 2004;15:269–88. [DOI] [PubMed] [Google Scholar]
- [39].Mitchell RLC. fMRI delineation of working memory for emotional prosody in the brain: commonalities with the lexico-semantic emotion network. Neuroimage 2007;36:1015–25. [DOI] [PubMed] [Google Scholar]
- [40].Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- [41].Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62:2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC. Neural correlates of individual differences in pain-related fear and anxiety. PAIN 2006;120:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol 2004;92:1–9. [DOI] [PubMed] [Google Scholar]
- [44].Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. [DOI] [PubMed] [Google Scholar]
- [45].Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. PAIN 1987; 28:297–307. [DOI] [PubMed] [Google Scholar]
- [46].Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry 2014;75:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 2003;23:8800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila. Pa. 1976) 2012;37:67–76. [DOI] [PubMed] [Google Scholar]
- [49].Research C on AP, Care, Education and, Policy B on HS, Medicine I of Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press, Washington, DC, USA, 2011. [PubMed] [Google Scholar]
- [50].Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 2009;45:614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Salomons TV, Johnstone T, Backonja M-M, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci 2004; 24:7199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27: 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. PAIN 2006;120:297–306. [DOI] [PubMed] [Google Scholar]
- [54].Severeijns R, Vlaeyen JWS, van den hout MA, Weber WEJ. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain 2001;17:165–72. [DOI] [PubMed] [Google Scholar]
- [55].Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- [56].Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 2014;35:527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sotres-Bayon F, Bush DEA, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem 2004;11:525–35. [DOI] [PubMed] [Google Scholar]
- [58].Spielberger CD, Gorsuch RL. STAI Manual for the State-trait Anxiety Inventory (“Self-evaluation Questionnaire”). Consulting Psychologists Press, Palo Alto, CA, USA, 1970. [Google Scholar]
- [59].Strigo IA, Simmons AN, Matthews SC, Craig ADB, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry 2008;65:1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment 1995;7:524–32. [Google Scholar]
- [61].Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett 2010; 485:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cereb. Cortex 2012;24:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wade JB, Dougherty LM, Archer RC, Price DD. Assessing the stages of pain processing: a multivariate analytical approach. PAIN 1996;68:157–67. [DOI] [PubMed] [Google Scholar]
- [64].Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: a systematic review. Spine (Phila. Pa. 1976) 2014;39:263–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.