Abstract
Organic anion transporter 3 (OAT3) plays a vital role in removing a broad variety of anionic drugs from kidney, thus avoiding their possible toxicity in the body. We earlier established that activation of protein kinase C (PKC) enhances OAT3 ubiquitination, which promotes OAT3 internalization from the cell plasma membrane to intracellular endosomes and consequent degradation. As a result, OAT3 expression and transport activity are reduced. In the current study, we discovered that protein kinase A (PKA) had an opposite effect to PKC on the regulation of OAT3. We showed that activation of PKA by Bt2-cAMP stimulated OAT3 transport activity, which was largely caused by an enhanced plasma membrane expression of the transporter, kinetically reflected as an augmented maximal transport velocity Vmax without notable alteration in substrate-binding affinity Km. Additionally, we showed that PKA activation accelerated the rate of OAT3 recycling from intracellular compartments to the plasma membrane and decelerated the rate of OAT3 degradation. We further showed that OAT3 is subjected to post-translational modification by SUMO-2 and SUMO-3 not by SUMO-1. PKA activation enhanced OAT3 SUMOylation, which was accompanied by a reduced OAT3 ubiquitination. Finally, insulin like growth factor 1 significantly stimulated OAT3 transport activity and SUMOylation through PKA signaling pathway. In conclusion, this is the first demonstration that PKA stimulated OAT3 expression and transport activity by altering the trafficking kinetics of OAT3 possibly through the crosstalk between SUMOylation and ubiquitination.
Keywords: Organic Anion Transporter, Drug Transport, Regulation, Protein Kinase A, SUMOylation
Introduction
Organic anion transporter 3 (OAT3) is a member of the organic anion transporter family, which play vital parts in the removal of many drugs from the kidney, such as anti-viral drugs, anti-tumor therapeutics, antibiotics, antihypertensive and anti-inflammatory drugs, and thereby avoiding their possible toxicity in the body (1–6).
The transport activity of OAT3 relies on its expression level at the plasma membrane. Our laboratory has earlier established that OATs naturally internalize from and recycle back to the plasma membrane. A crucial event prior to OAT internalization is the modification of lysine residues of cell surface OAT by ubiquitin conjugation. Ubiquitin is an 8-kDa polypeptide, and, when attached to cell surface OAT, can be identified by the elements of cell surface internalization machinery, and triggers OAT internalization from the plasma membrane to intracellular early endosomes partly through a clathrin-mediated pathway. As soon as in the endosomes, OAT either becomes deubiquitinated and recycles back to the plasma membrane or targets to a proteolytic system for degradation. We further demonstrated that activation of protein kinase C (PKC) down regulates OAT transport activity by enhancing OAT ubiquitination, which promotes an accelerated OAT internalization from the plasma membrane to intracellular early endosomes and subsequent degradation without affecting OAT recycling. Therefore, the expression level of OAT at the plasma membrane is significantly reduced, which results in a significant decrease in OAT transport activity. Clearly, post-translational modification of OAT by ubiquitination is a major mechanism that governs PKC-regulated OAT trafficking and transport activity (7–10).
Another important ubiquitin-like modifier is the small ubiquitin-related modifier SUMO (11, 12). SUMO family consists of three functional isoforms SUMO1–3. All three are polypeptides of ~12 kDa, and are broadly detected in many tissues, including brain, liver, and kidney. SUMO2 and SUMO3 are regularly mentioned as SUMO2/3 as they have 97% identity. In contrast, SUMO2/3 is only ~50% identical in the sequence with SUMO-1. Consistent with such sequence differences, SUMO1 and SUMO2/3 modify different substrates in vivo. The modification of substrate proteins by SUMOylation involves the consecutive steps of the E1 SUMO-activating enzymes and the E2 SUMO-conjugating enzyme 9 (Ubc9). This process leads to the formation of an isopeptide bond between the carboxy-terminal glycine of SUMO and lysine residues of specific target proteins. Although chemically stable, modification by SUMO is reversible by specific isopeptidases that break the bond between SUMO and the lysine residue(s) of target substrate. SUMOylation was initially identified as a major regulatory mechanism of protein function on nuclear proteins. Within the last decade, more and more membrane proteins such as channels and receptors were identified as SUMO substrates (13–19). And the roles of SUMOylation in the regulation of these membrane proteins have been investigated. Recently, it has become obvious that ubiquitin and SUMO, although performing different biological functions, frequently communicate, and cooperatively affect the properties of shared substrate proteins, in certain cases by modifying the same site in a competitive manner (20, 21). Whether OAT is subject to the regulation by SUMOylation is currently unknown.
Contrary to the inhibitory effect of PKC on OATs, protein kinase A (PKA) has been shown to stimulate OAT activity (22–24). However, its mechanism of action is not well understood. A thorough understanding of PKA-regulated OAT activity is of high significance because various physiological stimuli such as insulin-like growth factor exert its biological effect through the activation of PKA (25). In the present study, we examined the mechanism of PKA action on OAT3. We demonstrated that PKA stimulated OAT3 expression and transport activity by altering the trafficking kinetics of the transporter possibly through the crosstalk between ubiquitination and SUMOylation.
Materials and Methods
Materials –
COS-7 cells were obtained from ATCC (Manassas, VA). [3H]-labeled estrone sulfate (ES) was obtained from PerkinElmer (Waltham, MA). Membrane-impermeable biotinylation reagent NHS-SS-biotin, streptavidin-agarose beads and protein G-agarose beads were obtained from Pierce (Rockford, IL). cDNAs for HA-tagged SUMO-1, SUMO-2, SUMO-3, and Ubc9 were kindly provided by Dr. Jorge A Iñiguez-Lluhí from University of Michigan Medical School. Mouse anti-Myc antibody (9E10) was obtained from Roche (Indianapolis, IN). Rabbit anti-HA antibody and mouse anti-E-Cadherin antibody were obtained from abcam (Cambridge, MA). Mouse anti-ubiquitin antibody and mouse anti-β-actin were obtained from Santa Cruz (Santa Cruz, CA). Dibutyryl cyclic-AMP sodium salt (Bt2-cAMP), H-89 dihydrochloride hydrate (H-89), Insulin-like Growth Factor-I human (IGF-1) and all other reagents were from Sigma-Aldrich (St. Louis, MO).
Cell culture and Transfection –
Parental COS-7 cells were cultured in DMEM medium (Invitrogen, Carlsbad, CA) containing10% fetal bovine serum at 37 °C in 5% CO2. Cells stably expressing human OAT3 (hOAT3) were maintained in DMEM containing 0.2mg/ml G418 (Invitrogen, Carlsbad, CA), 10% fetal bovine serum.
Transfection with cDNA plasmids was done for 48 hrs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), following the manufacturer’s instructions.
Transport Measurements –
The uptake solution consisted of PBS/Ca2+/Mg2+ (pH 7.3) and [3H] estrone sulfate (ES) (0.3 μM). The uptake solution was added to the cells. At an indicated period of time, uptake was ended by aspirating the uptake solution and rapidly washing the cells with ice-cold PBS solution. The cells were then lyzed in 0.2 N NaOH, neutralized in 0.2 N HCl, and aliquoted for liquid scintillation counting.
Cell Surface Biotinylation –
The amount of hOAT3 at the cell surface was determined using the membrane-impermeable biotinylation reagent, NHS-SS-biotin as described in our previous publications (9, 26). hOAT3 in the pool of surface proteins was detected by SDS-PAGE and immunoblotting using an anti-Myc antibody 9E10.
Recycling Assay –
We followed the procedure previously established in our laboratory (7). hOAT3-expressing cells were biotinylated with sulfo-NHS-SS-biotin at 4 °C to label hOAT3 at the plasma membrane. Then, one set of cells was unceasingly biotinylated at 4 °C. The duplicate set of cells were warmed to 37 °C and unceasingly biotinylated at 37 °C. At the indicated time points, biotinylation was ended and biotin-labeled hOAT3 was examined by SDS-PAGE and immunoblotting as described above. Recycled hOAT3 was calculated as the difference between hOAT3 biotin-labeled at 37 °C and hOAT3 biotin-labeled at 4 °C.
Internalization Assay –
We followed the procedure established in our laboratory (7, 9) using a biotinylation approach in conjunction with immunoblotting. Relative OAT3 internalized was calculated as % of the total initial cell surface OAT3 pool.
Degradation Assay –
The procedure previously established in our laboratory was followed (9, 27). hOAT3-expressing cells were subjected to biotinylation with 0.5 mg/ml sulfo-NHS-SS-biotin at 4 °C. After biotinylation, followed by quenching of the unreacted NHS-SS-biotin with 2ml PBS containing 100mM glycine. The biotin-labeled cells were incubated with Bt2-cAMP at indicated time points and then lysed in lysis buffer with protease inhibitor cocktail, and centrifugated at 16,000 × g at 4 °C, 40 μl of streptavidin agarose beads were then added to the supernatant to isolate plasma membrane proteins, followed by immunoblotting with anti-Myc antibody.
Immunoprecipitation –
We followed the procedure previously established in our laboratory (27, 28). The cells were lysed with lysis buffer. Protein concentration for each sample was measured and same amount of proteins (1000μg) were incubated with 30μl protein G-agarose beads at 4 °C for 2hr to reduce the nonspecific binding. Meanwhile, appropriate primary antibody (1:100) was incubated with 30μl protein G-agarose beads (Pierce, Rockford, IL) at 4 °C for 2hr. After the 2hr-incubation, the precleared protein sample was mixed with antibody-bound protein G-agarose beads at 4 °C overnight. On day 2, the immunoprecipitated proteins were washed with lysis buffer three times, followed with elution and immunoblot with indicated antibodies.
Electrophoresis and Immunoblotting –
We followed the procedure previously established in our laboratory (27, 28). Protein samples were separated on SDS-PAGE minigels (Bio-Rad, Hercules, CA) and electroblotted on to PVDF membranes (Invitrogen, Carlsbad, CA). The blots were treated with 5% nonfat dry milk for 1hr in PBS-Tween 20 (PBST; 0.05% Tween-20 in PBS) at room temperature, washed, and incubated at 4 °C with appropriate primary antibodies. Then the blots were incubated with horseradish peroxidase-conjugated secondary antibodies for 1hr at room temperature, followed by detection with SuperSignal West Dura Extended Duration Substrate kit (Pierce, Rockford, IL). FluorChem 8000 imaging system (Alpha Innotech Corp., San Leandro, CA) was used to quantify the nonsaturating, immunoreactive protein bands.
Data Analysis –
Each experiment was repeated a minimum of three times. The statistical analysis was from multiple experiments. Between two groups, statistical analysis was performed using Student’s paired t-tests. Among multiple treatments, one-way ANOVA (Fig. 1, Fig. 3, Fig. 7d, Fig. 8 and Fig. 9a) or two-way ANOVA (Fig. 4, Fig. 5 and Fig. 6) Tukey’s test was applied by using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). A p-value of <0.05 was considered significant.
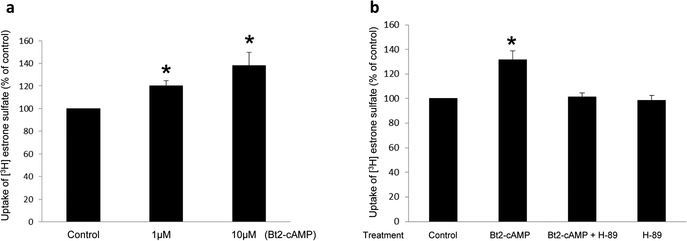
Fig. 1.
(a)Effect of PKA activator Bt2-cAMP on hOAT3 transport activity. hOAT3-expressing cells were treated with Bt2-cAMP at various doses for 30 min. 4-min uptake of [3H]-estrone sulfate (ES, 0.3 μM) was then determined. Transport activity was expressed as % of the uptake in control cells (mock cells). The data correspond to the uptake into hOAT3-expressing cells minus uptake into mock cells and was normalized to protein concentration. Values are mean ± S.D. (n = 3). *P<0.05. (b) Selectivity of PKA on hOAT3 transport activity. hOAT3-expressing cells were pretreated with or without a PKA inhibitor H-89 (4μM, 10min). After that, the cells were treated with PKA activator Bt2-cAMP (10μM, 30min) with or without PKA inhibitor H-89 (4μM, 30min), or H-89 alone, followed by measuring the uptake of [3H] estrone sulfate (ES, 4min, 0.3 μM). Transport activity was expressed as % of the uptake in control cells. The data correspond to the uptake into hOAT3-expressing cells minus uptake into mock cells and was normalized to protein concentration. Values are mean ± S.D. (n = 3). *P<0.05.
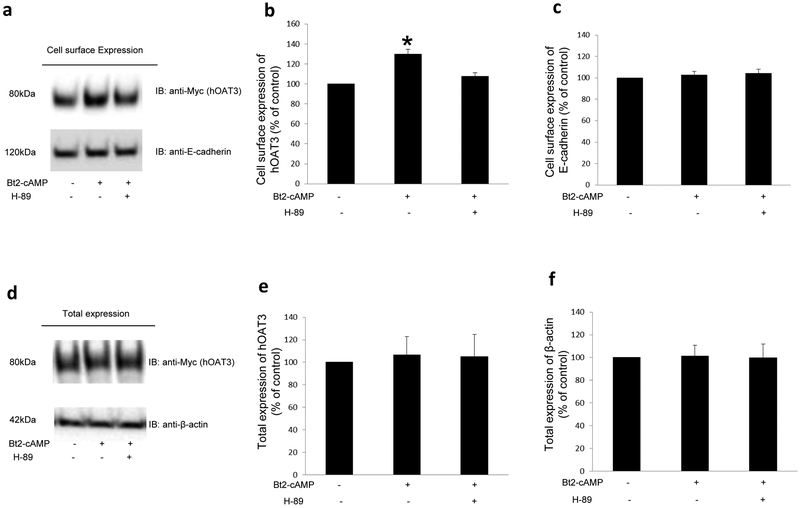
Fig. 3. Effect of PKA activator Bt2-cAMP on hOAT3 expression.
(a). Cell surface expression of hOAT3. Top panel: hOAT3-expressing cells were pretreated with or without H-89 (4μM, 10min). After that, cells were treated with Bt2-cAMP (10μM, 30min) in the presence and absence of PKA inhibitor H-89 (4μM, 30min). Biotinylation of treated cells was then performed, as described in the section of “Materials and Methods” followed by immunoblotting (IB) with an anti-Myc antibody (hOAT3 was tagged with the Myc epitope to facilitate the immunodetection). Bottom panel: The identical blot as the top panel was re-probed with anti-E-cadherin antibody. E-cadherin is a cell membrane marker protein. (b). Densitometry analyses of results from Fig. 3a, Top panel, along with other experiments. The values are mean ± S.D. (n = 3). *P<0.05. (c) Densitometry analyses of results from Fig. 3a, Bottom panel, along with other experiments. The values are mean ± S.D. (n = 3). (d). Total expression of hOAT3. hOAT3-expressing cells were pretreated with or without H-89 (4μM, 10min). After that, cells were treated with the Bt2-cAMP (10μM, 30min) in the presence and absence of PKA inhibitor H-89 (4μM, 30min). Cells were immunoblotted (IB) with an anti-Myc antibody. (e). Densitometry analyses of results from Fig. 3d, Top panel, along with other experiments. The values are mean ± S.D. (n = 3). (f) Densitometry analyses of results from Fig. 3d, Bottom panel, along with other experiments. The values are mean ± S.D. (n = 3).
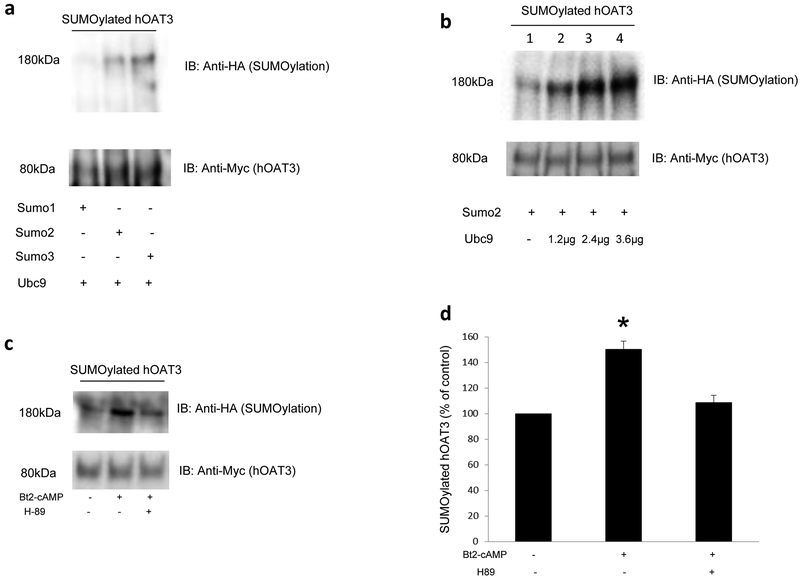
Fig. 7.
(a) Effects of SUMO1, SUMO2, SUMO3 and Ubc9 on hOAT3 SUMOylation. Top panel: cDNAs for HA-tagged SUMO1, SUMO2, or SUMO3 were transfected into COS-7 cells separately with 2.4μg of Ubc9, a SUMO-conjugating enzyme. 48 hrs after transfection, hOAT3 was pulled down by anti-Myc antibody (hOAT3 was tagged with the Myc epitope), with subsequent immunoblotting (IB) using anti-HA antibody. Bottom panel: The same blot from the top panel was re-probed with anti-Myc antibody to detect the amount of hOAT3 pulled down. (b) Effects of Ubc9 on hOAT3 SUMOylation. Top panel: cDNAs for HA-tagged SUMO2 was transfected into COS-7 cells with different amount of Ubc9 for 48 hrs. After transfection, hOAT3 was pulled down by anti-Myc antibody (hOAT3 was tagged with the Myc epitope), with subsequent immunoblotting (IB) using anti-HA antibody. Bottom panel: The same blot from the top panel was re-probed with anti-Myc antibody to detect the amount of hOAT3 pulled down. (c) PKA Specificity on hOAT3 SUMOylation. Top panel: hOAT3-expressing cells were transfected with HA-SUMO2 and 2.4μg of Ubc9 for 48h, then pretreated with or without H-89 (4μM, 10min). After that, cells were treated with the Bt2-cAMP (10μM, 30min) in the presence and absence of PKA inhibitor H-89 (4μM, 30min). hOAT3 was pulled down by anti-Myc antibody, with subsequent immunoblotting (IB) using anti-HA antibody. Bottom panel: The identical blot from the top panel was re-probed with anti-Myc antibody. (d) Densitometry analyses of results from Fig. 7c. Values are mean ± S.D. (n = 3). *P<0.05.
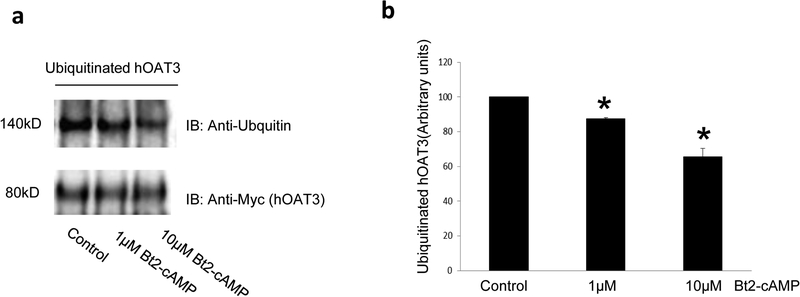
Fig. 8. The effect of PKA activator Bt2-cAMP on OAT3 ubiquitination.
(a). Top panel: hOAT3-expressing cells were treated with the Bt2-cAMP (1μM or 10μM, 30min). Cells were then treated with the PKC activator PMA (1 μM) for 30 min to enhance hOAT3 ubiquitination. hOAT3 was pulled down by anti-Myc antibody (hOAT3 was tagged with the Myc epitope), with subsequent immunoblotting (IB) using anti-ubiquitin antibody. Bottom panel: The identical immunoblot from Fig. 8a, Top panel was reprobed by anti-Myc antibody. (b). Densitometry analyses of results from Fig. 8a. The values are mean ± S.D. (n = 3). *P<0.05.
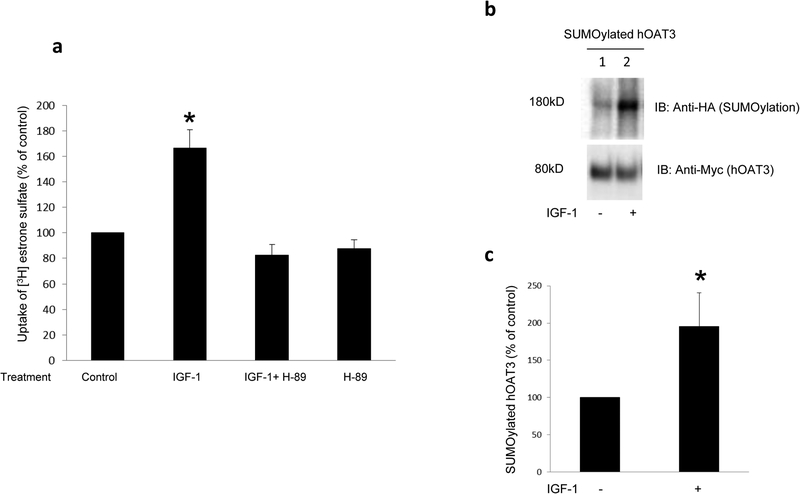
Fig. 9. The effect of IGF-1 on OAT3 transport activity and SUMOylation.
(a) The effect of IGF-1 on hOAT3 transport activity. hOAT3-expressing cells were pretreated with or without a PKA inhibitor H-89 (20μM, 10min). After that, the cells were treated with IGF-1 (100nM, 3hrs) in the presence and absence of PKA inhibitor H-89 (20μM, 3hrs), or H-89 alone, followed by [3H] estrone sulfate uptake (4min, 0.3 μM). Uptake activity was expressed as % of the uptake in control cells. The data correspond to the uptake into hOAT3-expressing cells minus uptake into mock cells and was normalized to protein concentration. Values are mean ± S.D. (n = 3). *P<0.05. (b) The effect of IGF-1 on OAT3 SUMOylation. hOAT3-expressing cells were transfected with HA-SUMO2 and 2.4μg of Ubc9 for 48hrs, then treated with the IGF-1 (100nM, 3hrs). hOAT3 was pulled down by anti-Myc antibody, in conjunction with immunoblotting (IB) using anti-HA antibody. (c) Densitometry analyses of results from Fig. 9b. Values are mean ± S.D. (n = 3). *P<0.05.
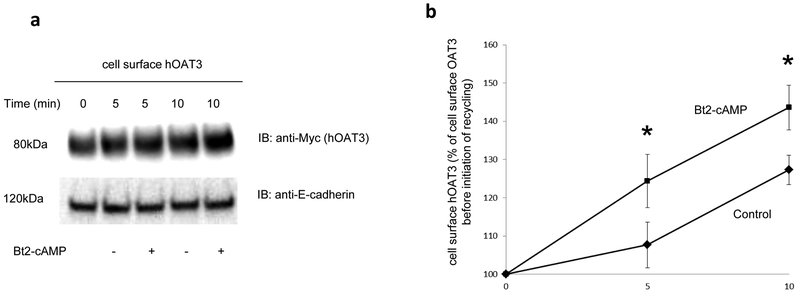
Fig. 4. Biotinylation analysis of Bt2-cAMP-modulated hOAT3 recycling.
(a). Top panel: hOAT3 recycling (5 min and 10 min) was analyzed as described in the section of “Materials and Methods” in the presence and the absence of Bt2-cAMP (10μM), in conjunction with immunoblotting (IB) using anti-Myc antibody (1:100). hOAT3 was tagged with the Myc epitope to facilitate the immunodecetion. Bottom panel: The identical blot as the top panel was re-probed with anti-E-cadherin antibody. E-cadherin is a cell membrane marker protein. (b) Densitometry analyses of results from Fig. 4a, Top panel along with other experiments. Total biotin-labeled hOAT3 was expressed as % of OAT3 biotinylated at 4 °C. Values are mean ± S.D. (n = 3). *P<0.05.
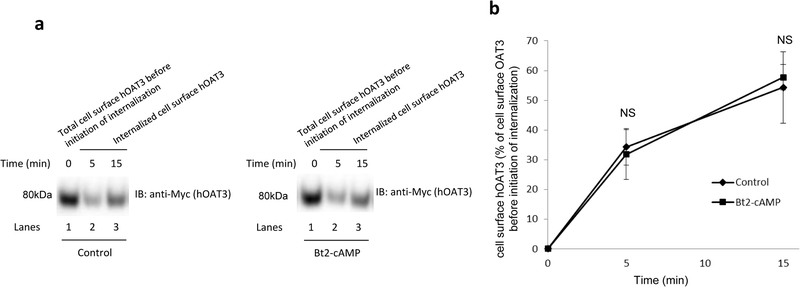
Fig. 5. Biotinylation analysis of hOAT3 internalization.
(a). hOAT3 internalization was examined as described in the section of “Materials and Methods”, in the presence and the absence of Bt2-cAMP (10μM), in conjunction with immunoblotting (IB) using anti-Myc antibody (1:100). (b). Densitometry analyses of results from Fig. 5a along with other experiments. Internalized hOAT3 was expressed as % of total initial cell surface hOAT3 pool. Values are mean ± S.D. (n = 3). NS: statistically not significant.
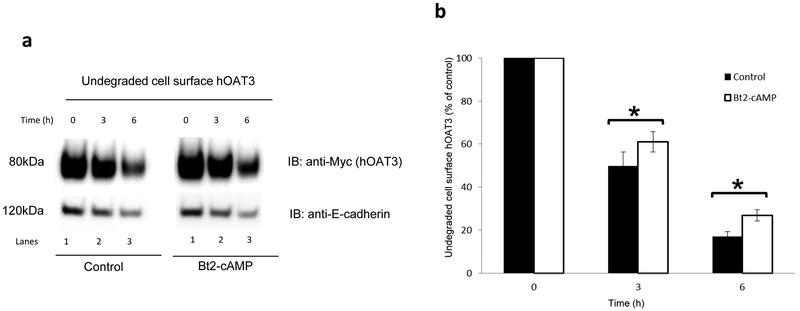
Fig. 6. Effect of Bt2-cAMP on the degradation of cell surface hOAT3.
(a) Top panel: COS-7 cells expressing hOAT3 were treated with the Bt2-cAMP (10μM). Cell surface hOAT3 degradation was then examined as described in the section of “Materials and Methods”, in conjunction with immunoblotting (IB) using anti-Myc antibody. Bottom panel: The identical blot as the top panel was re-probed with anti-E-cadherin antibody. E-cadherin is a cell membrane marker protein. (b) Densitometry analyses of results from Fig. 6a top panel along with other experiments. The amount of undegraded cell surface hOAT3 was expressed as % of total initial cell surface hOAT3 pool. Values are mean ± S.D. (n = 3). *P<0.05.
Results
Effect of PKA on hOAT3 transport activity –
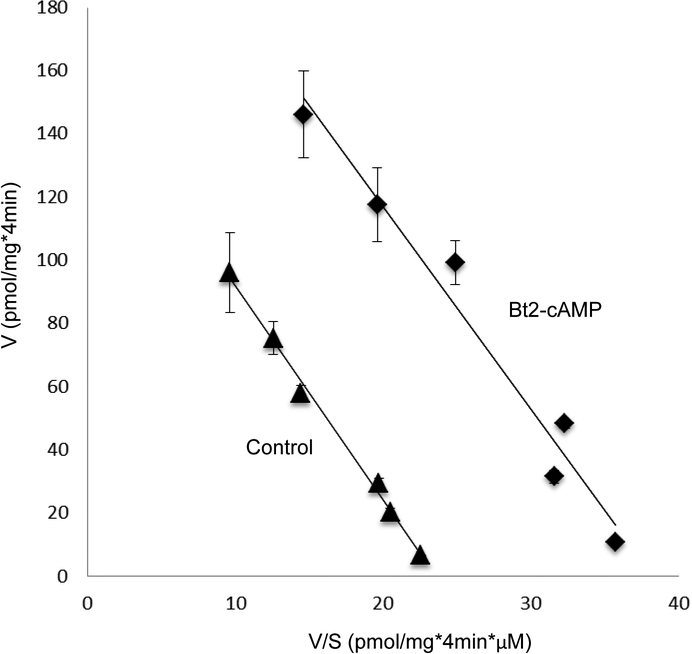
To examine the role of PKA in hOAT3 function, we treated hOAT3-expressing COS-7 cells with PKA activator Bt2-cAMP, followed by the measurement of hOAT3-mediated uptake of [3H] estrone sulfate (ES), a prototypical substrate for hOAT3. As shown in Fig. 1a, Bt2-cAMP induced a dose-dependent rise in the uptake in compared with that in control cells with a ~ 40% stimulation at Bt2-cAMP concentration of 10 μM. Such Bt2-cAMP-induced increase in hOAT3 transport activity was abrogated in the presence of a PKA-specific inhibitor H-89 (H-89 alone did not show non-specific cytotoxicity) (Fig. 1b), confirming the specific regulation of hOAT3 by PKA. To investigate the mechanism underlying PKA-induced increase in hOAT3 activity, we measured hOAT3-mediated uptake of [3H] ES at various substrate concentrations. An Eadie-Hofstee analysis (Fig. 2) revealed that activation of PKA by Bt2-cAMP caused an augmented maximal transport velocity Vmax of hOAT3 (147.33 ± 18.72 pmol·mg–1·4min–1 with control cells and 230.18 ± 16.46 pmol·mg–1·4min–1 with cells treated with Bt2-cAMP) without notable alteration in the substrate-binding affinity Km of the transporter (6.08 ± 0.65 μM with control cells and 5.84 ± 0.56 μM with cells treated with Bt2-cAMP).
Fig. 2. Effect of PKA activator Bt2-cAMP on the kinetics of hOAT3-mediated transport of estrone sulfate.
hOAT3-expressing cells were treated with the Bt2-cAMP (10μM, 30min), and initial uptake (4 min) of [3H] estrone sulfate was determined at the concentration of 0.1–10 μM. The data correspond to uptake into hOAT3-expressing cells minus uptake into mock cells. Values are means ± S.D. (n = 3). V, velocity; S, substrate concentration.
Effect of PKA on hOAT3 Expression –
Two possibilities could be responsible for a rise in the maximal transport velocity Vmax of hOAT3 shown in Fig. 2: either the quantity of the transporter at the plasma membrane could be increased or the transporter turnover rate could be enhanced. We conducted analyses that differentiate between these likelihoods by looking at the expression of the transporter both at the plasma membrane and in the total cell extracts. We revealed that PKA activation by Bt2-cAMP led to an increase of hOAT3 expression at the cell surface (Fig. 3a, top panel), without altering its total expression (Fig. 3d). Such a change in hOAT3 expression at the plasma membrane was not because of the overall disturbance of membrane proteins since the expression of plasma membrane protein marker E-cadherin was not affected under these situations (Fig. 3a, bottom panel & Fig. 3c).
Effect of PKA hOAT3 Trafficking –
We earlier demonstrated that the members of OAT family naturally internalize from and recycle back to the plasma membrane. In the current experiment, we assessed whether PKA-induced increase of OAT expression at the cell surface could result from altered trafficking kinetics. First, we examined whether PKA activation alters the rate of hOAT3 recycling. We observed that the quantity of surface-labeled hOAT3 recycled to the cell surface in the presence of PKA activator Bt2-cAMP was higher than that in the absence of Bt2-cAMP (Fig. 4a, Top panel), while the amounts of membrane protein marker E-cadherin were comparable between Bt2-cAMP treated group and non-treated group at indicated time points (Fig. 4a, Bottom panel), suggesting that PKA activation enhanced the rate of hOAT3 recycling. We next examined whether PKA activation alters the rate of hOAT3 internalization. We observed that the quantity of surface-labeled hOAT3 internalized with the PKA activator Bt2-cAMP present was similar as that in the absence of Bt2-cAMP (Fig. 5), indicating that PKA activation does not affect the rate of hOAT3 internalization.
Effect of Long-Term PKA Activation on hOAT3 Stability –
The above experiments were carried out with a short-term PKA activation (with 30-min Bt2-cAMP treatment). The effect of long-term activation of PKA (Bt2-cAMP treament for 3-hrs & 6-hrs) on cell surface hOAT3 was next evaluated using a biotinylation approach. Our results (Fig. 6) showed that there was significantly less amount of hOAT3 degraded after 3 hrs and 6 hrs of treatment with PKA activator Bt2-cAMP as compared to that of control (Fig. 6a, Top panel), while the amounts of membrane protein marker E-cadherin were comparable between Bt2-cAMP treated group and non-treated group at indicated time points (Fig. 6a, Bottom panel), indicating that PKA activation slowed down the degradation rate of hOAT3.
Effect of PKA on hOAT3 SUMOylation –
SUMO family consists of three functional isoforms SUMO1–3. To examine whether hOAT3 is a substrate for SUMOylation, we transfected hOAT3-expressing cells with epitope HA-tagged SUMO-1, SUMO-2, or SUMO-3 together with Ubc9, an enzyme catalyzing the conjugation of SUMO to its protein substrate. Transfected cells were then lysed and hOAT3 was immunoprecipitated with anti-Myc antibody (hOAT3 was tagged with the Myc epitope), followed by immunoblotting (IB) with anti-HA antibody to detect SUMOylated hOAT3. As shown in Fig. 7a, top panel, hOAT3 was SUMOylated by SUMO-2 and SUMO-3 but not by SUMO-1. hOAT3 SUMOylation depended on the amount of Ubc9 transfected with the maximum SUMOylation at 2.4 μg of Ubc9 (Fig. 7b, top panel). When cells were treated with PKA activator Bt2-cAMP, hOAT3 SUMOylation by SUMO-2 conjugation was significantly enhanced in comparison to that in control cells (Fig. 7c, top panel). Moreover, Bt2-cAMP-enhanced hOAT3 SUMOylation was abrogated in the presence of PKA-specific inhibitor H-89 (Fig. 7c, top panel), suggesting that hOAT3 SUMOylation is PKA-dependent. The difference in the hOAT3 SUMOylation was not due to the variance in the amount of hOAT3 immunoprecipitated because similar quantity of hOAT3 was pulled down in all samples (bottom panels of Fig. 7a, Fig. 7b, and Fig. 7c).
Effect of PKA on hOAT3 ubiquitination –
We earlier demonstrated that protein kinase C (PKC) down regulates hOAT3 activity by enhancing ubiquitin conjugation to the transporter. In contrast to the inhibitory effect of PKC on hOAT3, PKA activation stimulated hOAT3 activity. We, therefore, examined the effect of PKA on hOAT3 ubiquitination. hOAT3-expressing cells were incubated with PKA activator Bt2-cAMP. hOAT3 was then immunoprecipitated with anti-Myc antibody, followed by immunoblotting (IB) with anti-ubiquitin antibody to detect ubiquitinated hOAT3. As revealed in Fig. 8a, top panel, Bt2-cAMP suppressed hOAT3 ubiquitination in a dose-dependent manner as compared to that in control cells. The difference in hOAT3 ubiqitination was not due to the variance in the amount of hOAT3 pulled down because the same amount of hOAT3 was immunoprecipitated in all the samples (Fig. 8a, bottom panel).
Effect of Insulin like growth factor (IGF-1) on hOAT3 transport activity and SUMOylation –
cAMP signaling pathway has been shown to be an important mediator for many physiological hormones such as IGF-1. Therefore we investigated the effect of IGF-1 on hOAT3 transport activity and SUMOylation. As shown in Fig. 9a, IGF-1 (100nM and 3h treatment) significantly increased hOAT3-mediated uptake of estrone sulfate (ES). This stimulatory effect was blocked by the PKA-specific inhibitor H-89, indicating that IGF-1 regulates hOAT3 transport activity through PKA pathway. Moreover, IGF-1 greatly stimulated hOAT3 SUMOylation by SUMO2 conjugation (Fig. 9b).
Discussion
Active transport of organic anion carried out by organic anion transporters (OATs) is a major determining factor of the outcomes of therapeutical and toxic chemicals (1–6). Thus, it is of clinical and pharmacological importance to understand the mechanisms governing OAT regulation. Our current study investigated the regulatory mechanism of hOAT3 by PKA and revealed that PKA might modulate hOAT3 expression and transport activity through the cross-talk between SUMOylation and ubiquitination.
Our current work was conducted in COS-7 cells, an outstanding model system for examining the cloned organic anion transporter (7, 28–31). These cells are originated from the kidney, and many characteristics of OATs in these cells are consistent with those observed in other systems such as in animals (32). Therefore, our study in COS-7 cells will be an important step for future work focusing on assessing whether the comparable mechanisms are in play in native epithelia.
The transport activity of OAT is critically reliant on the expression level of the transporter at the cell surface. Our laboratory has previously demonstrated that OATs internalize naturally from and recycle back to the cell surface. OAT expression at the cell surface can be modulated by shifting the trafficking kinetics of the transporters. For instance, PKC inhibits OAT expression and transport activity by enhancing the ubiquitin conjugation to OAT, which then speeds up the rate of OAT internalization without affecting the rate of OAT recycling. Lengthy PKC activation leads to the targeting of the internalized OAT to proteolytic system for degradation (7, 9, 10). Our current study showed that PKA stimulated hOAT3 activity which is opposite to the action of PKC.
In contrast to the action of PKC, we showed that PKA stimulated hOAT3 expression and transport activity (Figs. 1–3) by accelerating the rate of hOAT3 recycling (Fig. 4) without affecting the internalization rate of the transporter (Fig. 5). Prolonged activation of PKA slowed down hOAT3 degradation (Fig. 6). We further showed that hOAT3 was SUMOylated by SUMO2 and SUMO3 but not by SUMO1 (Fig. 7a). SUMO2 and SUMO3 contain internal consensus motifs for SUMO conjugation, and therefore are capable of forming polySUMO chains. Yet, SUMO1 does not share such property. The molecular size for SUMOylated hOAT3 was ~ 180 kDa, 100 kDa larger than the size of hOAT3 (~ 80 kDa). Given that SUMO is a 12 kDa polypeptide, hOAT3 is most likely multi- or poly-SUMOylated. (Fig. 7a).
Our results also revealed that the activation of PKA promoted an enhancement in hOAT3 SUMOylation (Fig. 7b). Interestingly, the enhanced SUMOylation of hOAT3 by PKA activation is correlated with a reduced hOAT3 ubiquitination (Fig. 8a), indicating that there may be a communication between SUMOylation and ubiquitination. It has been suggested that SUMOylation and ubiquitination may crosstalk and mutually influence each other in a competitive manner (20, 21). In this context, SUMO and ubiquitin may modify the identical lysine residue(s). Alternatively, attachment of SUMO may potentially mask a nearby ubiquitination site. An increasing number of proteins have been reported to serve as substrates for both SUMOylation and ubiquitination. The two modifiers, often viewed as antagonists, enforce an opposite fate on their shared target and cooperatively exert regulatory control over a biological process. Therefore, it seems likely that the PKA-regulated and SUMOylation-dependent stimulation of hOAT3 expression and transport activity counters the PKC-regulated and ubiquitination-dependent inhibition of hOAT3 expression and transport activity. The work aiming at further identifying the relationship between SUMOylation and ubiquitination on hOAT3 is currently being pursued in our laboratory.
Most SUMO substrates bear the consensus motif, Ψ-K-x-D/E (where ψ is a hydrophobic residue, K is the lysine conjugated to SUMO, x is any amino acid, E is a glutamic acid and D is an aspartic acid) (11). Based on the computer modeling, there are ten intracellular lysine residues on hOAT3, two of which, K285 and K518, lie within the SUMO-modification consensus motif: indicating that these lysine residues could be the potential SUMO-conjugation sites. Additionally, several online programs are capable of predicting SUMOylation sites. A program called SUMOplot™ Analysis Program (http://www.abgent.com/sumoplot.html) predicts K69, K285, K286, K515, K518 to be the potential SUMOylation sites. However, it should be noted that SUMOylation can also occur at lysine residues outside conventional motifs and the presence of conventional motifs do not guarantee the SUMOylation. The mapping of SUMO-conjugation sites on hOAT3 is currently underway in our laboratory.
PKA, like other protein kinases, regulates the target protein substrates by either directly phosphorylating the target protein or phosphorylating a protein that is associated with the target protein. Whether PKA directly phosphorylates OATs or phosphorylates an OAT-interacting protein would be an interesting topic for future investigation. In addition, protein phosphorylation has been revealed to play a role in regulating SUMOylation in some proteins, where it could stimulate or inhibit substrate SUMOylation depending on what the substrate is (15, 33–35). Therefore, if hOAT3 is a substrate of PKA phosphorylation, a co-regulation between phosphorylation and SUMOylation may also exist.
IGF-1 plays significantly roles in growth, development, and metabolism (36, 37). The abnormalities in the IGF-1 have been reported to be related to the development of several diseases, such as Laron syndrome and Acromegaly (38, 39). And a synthetic analog of IGF-1, Mecasermin, has been approved by the both U S Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of growth failure (40). In our current work, IGF-1 significantly enhanced OAT3 transport activity through PKA pathway (Fig. 9a) as well as OAT3 SUMOylation (Fig. 9b). Renal cortical slices from normal rats and diabetic rats have been utilized as tools for the studies involving the roles of PKA signaling pathway induced by hormones in OAT3. (23, 32). However, none of these studies illustrated the relationship between PKA stimulated SUMOlyation and OAT3 function in vivo. Therefore the in vivo investigations about SUMOlyation and OAT3 activity will be established in the future. As mentioned above, the abnormalities in the IGF-1 have been linked to the development of Laron syndrome. Further studies investigating the activity, expression and SUMOylatin of OAT3 in kidney from Laron mouse model would be particularly interesting.
In addition, our findings about the IGF-1 regulation on OAT3 through PKA signaling are consistent with a remote sensing and signaling model for transporters (41). Based on such a model, transporters in networks are regulated by hormones and growth factors, and effectively communicate among one another. In doing so, these transporters coordinately maintain the solute balance between multiple organs and therefore system homeostasis. Hormones/growth factors, released from one organ under the influence of the stimuli/environmental changes, enter the blood stream, and then reach to the target organs and exert their regulatory functions on transporters through cell signaling. Consistent with this general model, our data support that IGF-1, which is produced primarily by the liver under the stimuli, arrives at the kidney through blood stream, and then binds to its receptors and up-regulates OAT3 through PKA signaling.
Our study clearly illustrates PKA signaling on OAT3 regulation. By comparing our in vitro results with the metabolomics analyses of the Oat3 Knockout mice (42, 43), we observe an interesting connection here. In OAT3 knockout mice, many metabolites accumulated, including gentisate and bile acids which are capable of activating G-protein-coupled receptors (GPCRs). Following the activation of GPCRs, the secondary messenger cAMP level maybe elevated leading to the activation of PKA signaling pathway. Based on our data, PKA activation increased OAT3 surface expression and transporter activity. Therefore, the interesting connection is that metabolite variations contributed by the OAT3 reduction form a negative feedback loop to up-regulate OAT3 expression and function through PKA signaling.
Conclusion:
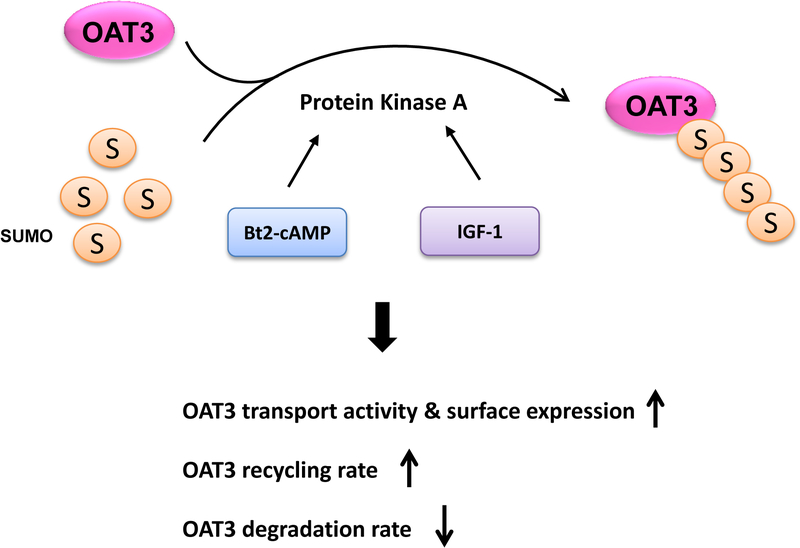
We provided the first demonstration that PKA stimulated hOAT3 expression and transport activity by altering the trafficking kinetics of hOAT3 possibly through the crosstalk between SUMOylation and ubiquitination (Fig. 10).
Fig. 10.
The role of PKA in OAT3 transport activity, trafficking and SUMOylation. S: SUMO, IGF-1: insulin-like growth factor 1
Acknowledgement
This work was supported by grants (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000).
We would like to thank Dr. Jorge A Iñiguez-Lluhí for his generous gifts of HA-tagged SUMO1, SUMO2, SUMO3, and Ubc9 plasmids.
Footnotes
Conflict of Interest
The authors have declared that there is no conflict of interest
References
- 1.Structure You G., function, and regulation of renal organic anion transporters. Med Res Rev. 2002;22(6):602–16. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 2.Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38(7–8):889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618(2):185–93. [DOI] [PubMed] [Google Scholar]
- 4.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31(1):1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 5.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76(3):481–90. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terada T, Inui K. Gene expression and regulation of drug transporters in the intestine and kidney. Biochem Pharmacol. 2007;73(3):440–9. doi: 10.1016/j.bcp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem. 2008;283(47):32570–9. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Pan Z, You G. Regulation of human organic anion transporter 4 by protein kinase C and NHERF-1: altering the endocytosis of the transporter. Pharm Res. 2010;27(4):589–96. doi: 10.1007/s11095-009-9983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Suh W, Pan Z, You G. Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3. Int J Biochem Mol Biol. 2012;3(2):242–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Xu D, Zhang J, Zhang Q, Fan Y, Liu C, You G. PKC/Nedd4–2 Signaling Pathway Regulates the Cell Surface Expression of Drug Transporter hOAT1. Drug Metab Dispos. 2017;45(8):887–95. doi: 10.1124/dmd.117.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol. 2010;11(12):861–71. doi: 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulrich HD. The fast-growing business of SUMO chains. Mol Cell. 2008;32(3):301–5. doi: 10.1016/j.molcel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Plant LD, Dementieva IS, Kollewe A, Olikara S, Marks JD, Goldstein SA. One SUMO is sufficient to silence the dimeric potassium channel K2P1. Proc Natl Acad Sci U S A. 2010;107(23):10743–8. doi: 10.1073/pnas.1004712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 2005;121(1):37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Rajan S, Dickson LM, Mathew E, Orr CM, Ellenbroek JH, Philipson LH, et al. Chronic hyperglycemia downregulates GLP-1 receptor signaling in pancreatic beta-cells via protein kinase A. Mol Metab. 2015;4(4):265–76. doi: 10.1016/j.molmet.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JS, Saunier EF, Akhurst RJ, Derynck R. The type I TGF-beta receptor is covalently modified and regulated by sumoylation. Nat Cell Biol. 2008;10(6):654–64. doi: 10.1038/ncb1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Z, El Far O, Betz H, Scheschonka A. Pias1 interaction and sumoylation of metabotropic glutamate receptor 8. J Biol Chem. 2005;280(46):38153–9. doi: 10.1074/jbc.M508168200. [DOI] [PubMed] [Google Scholar]
- 18.Minami S, Ito K, Honma M, Ikebuchi Y, Anzai N, Kanai Y, et al. Posttranslational regulation of Abcc2 expression by SUMOylation system. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G406–13. doi: 10.1152/ajpgi.90309.2008. [DOI] [PubMed] [Google Scholar]
- 19.Giorgino F, de Robertis O, Laviola L, Montrone C, Perrini S, McCowen KC, et al. The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells. Proc Natl Acad Sci U S A. 2000;97(3):1125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulrich HD. Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest. Trends Cell Biol. 2005;15(10):525–32. doi: 10.1016/j.tcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2(2):233–9. [DOI] [PubMed] [Google Scholar]
- 22.Duan P, Li S, You G. Regulation of human organic anion transporter 4 by parathyroid hormone-related protein and protein kinase A. Int J Biochem Mol Biol. 2012;3(3):322–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Barros SA, Srimaroeng C, Perry JL, Walden R, Dembla-Rajpal N, Sweet DH, et al. Activation of protein kinase Czeta increases OAT1 (SLC22A6)- and OAT3 (SLC22A8)-mediated transport. J Biol Chem. 2009;284(5):2672–9. doi: 10.1074/jbc.M808078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soodvilai S, Chatsudthipong V, Evans KK, Wright SH, Dantzler WH. Acute regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol. 2004;287(5):F1021–9. doi: 10.1152/ajprenal.00080.2004. [DOI] [PubMed] [Google Scholar]
- 25.Subramaniam S, Shahani N, Strelau J, Laliberte C, Brandt R, Kaplan D, et al. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. J Neurosci. 2005;25(11):2838–52. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, You G. SGK1/Nedd4–2 signaling pathway regulates the activity of human organic anion transporters 3. Biopharm Drug Dispos. 2017;38(8):449–57. doi: 10.1002/bdd.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Xu D, Toh MF, Pao AC, You G. Serum- and glucocorticoid-inducible kinase SGK2 regulates human organic anion transporters 4 via ubiquitin ligase Nedd4–2. Biochem Pharmacol. 2016;102:120–9. doi: 10.1016/j.bcp.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Li S, Patterson C, You G. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Mol Pharmacol. 2013;83(1):217–24. doi: 10.1124/mol.112.082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan P, Li S, You G. Angiotensin II inhibits activity of human organic anion transporter 3 through activation of protein kinase Calpha: accelerating endocytosis of the transporter. Eur J Pharmacol. 2010;627(1–3):49–55. doi: 10.1016/j.ejphar.2009.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagos Y, Hundertmark P, Shnitsar V, Marada VV, Wulf G, Burckhardt G. Renal human organic anion transporter 3 increases the susceptibility of lymphoma cells to bendamustine uptake. Am J Physiol Renal Physiol. 2015;308(4):F330–8. doi: 10.1152/ajprenal.00467.2014. [DOI] [PubMed] [Google Scholar]
- 31.Chioukh R, Noel-Hudson MS, Ribes S, Fournier N, Becquemont L, Verstuyft C. Proton pump inhibitors inhibit methotrexate transport by renal basolateral organic anion transporter hOAT3. Drug Metab Dispos. 2014;42(12):2041–8. doi: 10.1124/dmd.114.058529. [DOI] [PubMed] [Google Scholar]
- 32.Phatchawan A, Chutima S, Varanuj C, Anusorn L. Decreased renal organic anion transporter 3 expression in type 1 diabetic rats. Am J Med Sci. 2014;347(3):221–7. doi: 10.1097/MAJ.0b013e3182831740. [DOI] [PubMed] [Google Scholar]
- 33.Muller S, Matunis MJ, Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 1998;17(1):61–70. doi: 10.1093/emboj/17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SH, Jaffray E, Senthinathan B, Hay RT, Sharrocks AD. SUMO and transcriptional repression: dynamic interactions between the MAP kinase and SUMO pathways. Cell Cycle. 2003;2(6):528–30. doi: 10.4161/cc.2.6.597. [DOI] [PubMed] [Google Scholar]
- 35.Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, et al. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol. 2003;23(8):2953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bach LA, Hale LJ. Insulin-like growth factors and kidney disease. Am J Kidney Dis. 2015;65(2):327–36. doi: 10.1053/j.ajkd.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Yakar S, Adamo ML. Insulin-like growth factor 1 physiology: lessons from mouse models. Endocrinol Metab Clin North Am. 2012;41(2):231–47, v. doi: 10.1016/j.ecl.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, et al. Expert consensus document: A consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10(4):243–8. doi: 10.1038/nrendo.2014.21. [DOI] [PubMed] [Google Scholar]
- 39.Janecka A, Kolodziej-Rzepa M, Biesaga B. Clinical and Molecular Features of Laron Syndrome, A Genetic Disorder Protecting from Cancer. In Vivo. 2016;30(4):375–81. [PubMed] [Google Scholar]
- 40.Bang P, Polak M, Woelfle J, Houchard A, Group EIRS. Effectiveness and Safety of rhIGF-1 Therapy in Children: The European Increlex(R) Growth Forum Database Experience. Horm Res Paediatr. 2015;83(5):345–57. doi: 10.1159/000371798. [DOI] [PubMed] [Google Scholar]
- 41.Wu W, Dnyanmote AV, Nigam SK. Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol Pharmacol. 2011;79(5):795–805. doi: 10.1124/mol.110.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bush KT, Wu W, Lun C, Nigam SK. The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J Biol Chem. 2017;292(38):15789–803. doi: 10.1074/jbc.M117.796516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu W, Jamshidi N, Eraly SA, Liu HC, Bush KT, Palsson BO, et al. Multispecific drug transporter Slc22a8 (Oat3) regulates multiple metabolic and signaling pathways. Drug Metab Dispos. 2013;41(10):1825–34. doi: 10.1124/dmd.113.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]