Abstract
Background and Objective:
The Asian continent appears to be a growing destination for conducting cost-effective clinical trials, utilizing the available pool of treatment naïve subjects. This study aims to determine the growth rate of clinical trials in Asia.
Methods:
A database review was conducted. Registered clinical trials conducted in selected countries in Asia, Europe, Australia, and North America between 2008 and 2017 were searched from the International Clinical Trial Registry Platform. Compound Annual Growth Rate was determined for registered clinical trials.
Results:
During the 10-year period, a total of 125,918 registered clinical trials were conducted in Asia. There was a 7-fold increase in the number of registered clinical trials in Asia. More trials were registered in Japan than any other Asian country (30.8%). The average annual increase in the number of registered trials was generally higher in Asia than the United States of America, Canada, EU countries, or Australia. Iran recorded the highest average annual increase in the number of all clinical trials in Asia (41.9%). The number of pediatric clinical trials in Iran also increased annually by an average of 30.4%, more than any other country included in this study.
Conclusions:
Clinical trials recruitment in Asia is increasing faster than Europe, North America, and Australia. Lower trial cost and large patient pool may be contributory to this increase.
Keywords: Asia, clinical trials, database, pediatric
INTRODUCTION
Clinical trials are integral to the drug development process, however not all clinical trials are aimed to evaluate the safety or efficacy of new drugs or provide evidence for obtaining market authorization. Clinical trials, however, provide the highest level of evidence for the efficacy and safety of drugs if properly implemented.[1] The extent to which clinical trials are conducted is a reflection of the level of advancement that exists within a health-care system.[1] More clinical trials are conducted in emerging markets in Asia than the United States of America (USA) or Europe.[2] This shift could be attributed to the potential for large patient recruitment, reduction in operational costs, proliferation of contract research organizations, improved regulatory environment and improved clinical trials capacity in these countries.[3] In the USA and Europe, approximately 35% of trial delays occur as a result of patient recruitment, and one-fifth of the trials in these countries do not enroll a sufficient number of subjects.[4] Consequently, more USA and European registered clinical trials are inclusive of the developing countries to expedite the recruitment process and avoid drug approval delays.[5]
Since 2005, the International Committee of Medical Journal Editors mandated the registration of clinical trials in public registries as a condition for consideration for publication[2] and in 2006, the World Health Organization (WHO) established the International Clinical Trial Registry Platform (ICTRP).[6] In this study, we described the growth rate of clinical trials in selected Asian countries and compared them with selected countries in Europe, Australia, and North America.
METHODS
A search of the WHO's ICTRP was carried out to identify all clinical trials carried out in the selected countries between January 1, 2008, and December 31, 2017. The database aggregates all clinical trials data from around the world and is inclusive of 16 national and regional databases.[7] All Asian countries with national databases represented on the ICTRP were included in the study and compared with eight selected countries in Europe, North America, and Australia. All clinical trials involving both adults and children were extracted; a further extraction of only pediatric clinical trials in the respective countries was carried out.
The annual increase of trials in each country was estimated using the Compound Annual Growth Rate (CAGR) formula:
CAGR = (Pend/Pstart)(1/N)−1 × 100
Where Pstart is the number of registered trials for the 1st year reported and Pend is the number of registered trials for the last year reported; N is the number of years between the 1st and last year of reporting. The regional distribution of trials was expressed as a proportion.
RESULTS
During the period under review, a total of 125,918 clinical trials were conducted in Asia. These constituted about 28.5% of all registered clinical trials. The USA accounted for 24% of all registered clinical trials. In Asia, 30.8% of the trials were conducted in Japan, 21.1% in China and 14.3% in India. Over the 10-year period between 2008 and 2017, there was a 7-fold increase in the number of registered clinical trials that recruited in Asia. Likewise, there was a 6-fold increase in pediatric clinical trials over the same period. The majority of the selected Asian countries recorded higher annual increases in the number of clinical trials than Australia, Europe, and North America.
Between 2008 and 2017, the average annual increase in the number of all clinical trials conducted in Iran was 41.9%, 27.1% in Sri Lanka, 23.3% in China, 21.3% in India, 18.4% in Japan, 14.7% in Thailand, 12.9% in Korea, and 8.4% in Malaysia [Figure 1]; compared to 7.3% in Australia, 2.8% in Canada, and 1.4% in the USA [Figure 2]. The average yearly change in clinical trials conducted in Europe varied between a decrease of 0.8% in Italy and an increase of 8.8% in the Netherlands. Similarly, the number of pediatric clinical trials increased annually by an average of 30.4% in Iran, 24.2% in China, 21.1% in India, 17.5% in Malaysia, 15.2% in Japan and 10.1% in Korea [Figure 3]; while pediatric trials conducted in Europe increased marginally between 1.4% in Italy and 5.1% in the UK. Similarly, only marginal annual increases in the USA (1.6%) and Canada (2.6%) were observed [Figure 4].
Figure 1.
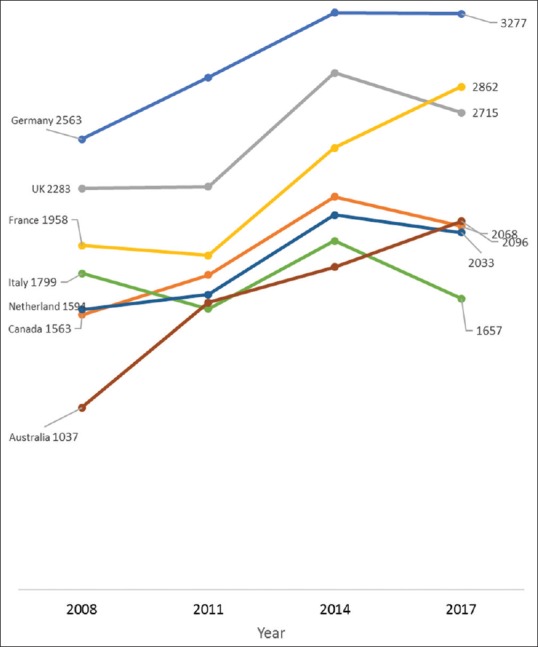
Annual changes in the number of all trials recruiting in Europe, Australia, and Canada
Figure 2.
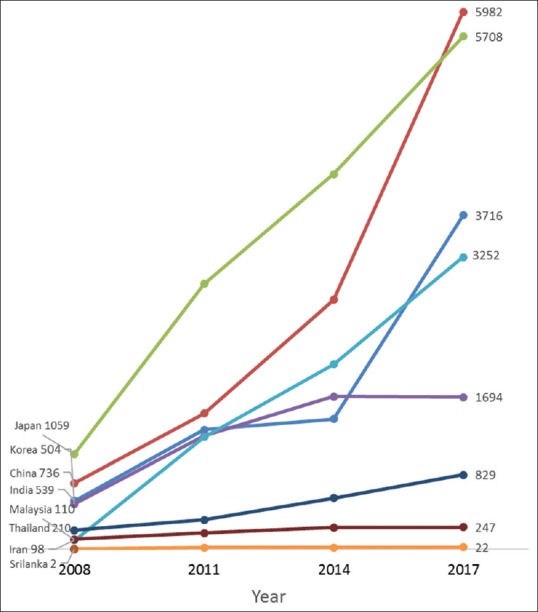
Annual changes in the number of all trials recruiting in Asia
Figure 3.
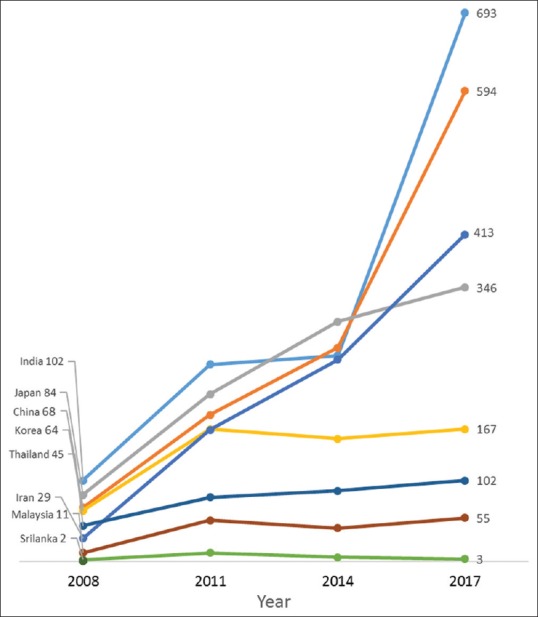
Annual changes in the number of pediatric trials recruiting in Asia
Figure 4.
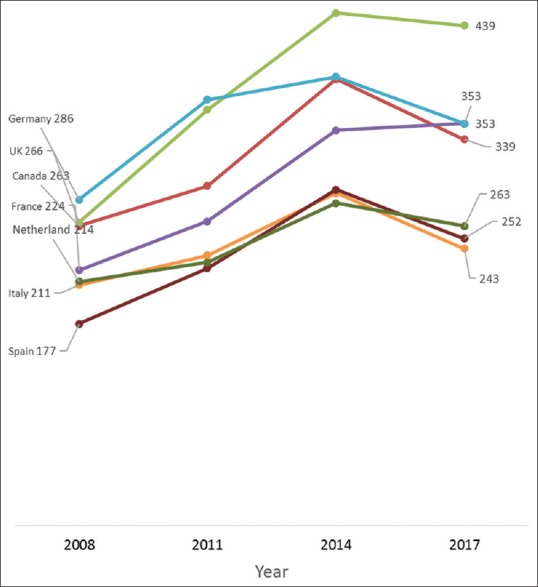
Annual changes in the number of pediatric trials recruiting in Europe, Australia, and Canada
DISCUSSION
This study shows that clinical trials recruitment has increased in Asia over the last decade, compared to several countries in Europe, Australia, and North America. The largest annual increases were seen in Iran, China, and India. Increases were also observed for trials involving children. Although the increase in the number of clinical trials involving children mirrored the trend for all (adults and pediatrics) trials, the proportion of pediatric trials for each year did not change extensively in the respective countries. Despite regulatory incentives,[8,9] several factors including, ethical considerations, parents’ reluctance to enroll their children and restrictive regulatory oversight have impeded the growth of pediatric clinical trials.[10]
The rise in the number of clinical trials in Asia may be attributed to several factors including, the presence of heterogeneous population groups and the availability of a large number of treatment naïve patients.[11,12] Furthermore, the high incidence of some diseases in some Asian countries makes them the preferred recruitment site for treatment trials for such diseases. For example, pharmaceutical companies would be more likely to carry out drug trials for the treatment of liver cancer or gastroesophageal cancer in Korea or China, respectively, because these countries provide a large pool of patients for conducting clinical trials for these diseases.[2,11] Asia is also the most populated continent in the world, with more than half of the world's population.[13,14,15] In addition, the clinical trial cost in Asia is about 30%–40% lower than the USA and the EU,[2] with the combined cost for each patient per visit in China, India, and Thailand nearly equivalent to per patient per visit cost in the USA alone.[2]
Individual countries in Asia have also devised strategies to encourage clinical trials. According to a report by the Iranian Clinical Trial Committee, the increase in the number of clinical trials in the country is attributable to the formation of several knowledge-based companies, established with the help of the Iranian government.[16] Thus, the number of such companies increased from 135 in 2014–1610 by the end of 2015.[17] Such companies were given tax and custom exemptions to encourage productivity and profitability.[17] About 4000 research projects are reviewed annually by the Iranian National and Regional Ethical Bodies; 30%–50% of which were clinical trials.[18] Similarly, the China Food and Drug Administration (CFDA) has also devised ways of expediting clinical trial registration and approval. One of which is the four-color light strategy, which color codes clinical trials based on their importance.[19] Thus, trials that focus on the treatment of severe or life-threatening conditions such as AIDS and cancer are granted a fast track designation for early approvals. Furthermore, the CFDA clinical trials record was migrated onto an electronic platform and sponsors were banned for up to 3 years for falsifying clinical trials data.[19] In the last decade, South Korea has also strengthened clinical trials by establishing the Korea National Enterprise for Clinical Trials.[20]
Although clinical trials in Asia is on the rise, several challenges such as inadequate workforce,[19] uncertain regulatory environment, infrastructural challenges,[21] and ethical challenges[22] still persists. For example, the upsurge in the number of clinical trial applications in China after 2008 resulted in delays in the approval process due to inadequate resources and staffing.[19] The WHO has also highlighted the ethical challenge of the establishment of hospitals by pharmaceutical companies in India, which may significantly influence trial results.[22]
The WHO ICTRP registry is inclusive of trials from several countries; it is, however, likely to be biased toward countries and regions that regularly supply trial data. In order to minimize this bias, we have only included countries that provided regular data to the registry. To the best of our knowledge, this is the first study to compare the growth rate of clinical trials in Asia with other continents.
CONCLUSIONS
We conclude that clinical trials conducted in Asia are increasing faster than Europe, North America, and Australia. Lower trial cost, large patient pool, and each countries incentives for clinical trials may be contributory to this increase.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ali S, Alghamdi MA, Alzhrani JA, De Vol EB. Magnitude and characteristics of clinical trials in the kingdom of Saudi Arabia: A cross-sectional analysis. Contemp Clin Trials Commun. 2017;7:126–9. doi: 10.1016/j.conctc.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan F. Asia: Preferred Destination for Clinical Trials-A Frost and Sullivan White Paper. [Last accessed on 2018 Jun 24]. Available from: https://www.novotechcro.com/sites/default/files/170217_FrostSullivan_Asia%20white%20paper_full.pdf .
- 3.Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Dis. 2008;7:13–4. [Google Scholar]
- 4.Rajadhyaksha V. Conducting feasibilities in clinical trials: An investment to ensure a good study. Perspect Clin Res. 2010;1:106–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmer LO, Nolen TL, Pramanpol S, Wallace D, Walker ME, Pappas P, et al. International collaboration between US and Thailand on a clinical trial of treatment for HIV-associated cryptococcal meningitis. Contemp Clin Trials. 2010;31:34–43. doi: 10.1016/j.cct.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viergever RF, Li K. Trends in global clinical trial registration: An analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open. 2015;5:e008932. doi: 10.1136/bmjopen-2015-008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ICTRP Search Portal. [Last accessed on 2018 May 25]. Available from: http://www.apps.who.int/trialsearch/AdvSearch.aspx .
- 8.European Public Health Alliance. The EU Adopts a Regulation on Medicines for Children. [Last accessed on 2018 Jun 25]. Available from: http://www.epha.org/a/1451 .
- 9.National Institutes of Health. NIH Policy and Guidelines on the Inclusion of Children as Participants in Research Involving Human Subjects. [Last accessed on 2018 Jun 25]. Available from: http://www.grants.nih.gov/grants/guide/noticefiles/not98-024.html .
- 10.Bavdekar SB. Pediatric clinical trials. Perspect Clin Res. 2013;4:89–99. doi: 10.4103/2229-3485.106403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yathindranath S, Kureishi A, Singh S, Yeow S, Geng G, Wai K, et al. Evolution of the clinical trial landscape in Asia Pacific. Open Access J Clin Trials. 2014;4:75–84. [Google Scholar]
- 12.Karlberg JP. The establishment of emerging trial regions. Clin Trial Mag. 2011;4:7–23. [Google Scholar]
- 13.World Bank. Population, Total. 2017. [Last accessed on 2018 Jun 25]. Available from: https://www.data.worldbank.org/indicator/SP.POP.TOTL .
- 14.Naions. Countries by Continents (Countries of Asia) 2017. [Last accessed on 2018 Jun 29]. Available from: http://www.nationsonline.org/oneworld/asia.htm .
- 15.Rahman S, Majunder MA. Managing clinical trials in Asia: Issues, threats, opportunities and approaches. S E Asia J Public Health. 2012;2:80–4. [Google Scholar]
- 16.Hosseini SA, Darbooy S, Salimi A. Regulatory aspects of clinical trials in Iran: Third year report of clinical trial committee in food and drug organization. Iran J Public Health. 2013;42:102–6. [PMC free article] [PubMed] [Google Scholar]
- 17. [Last accessed on 2018 Jun 30];Techrasa. Growth of Knowledge-Based Companies in Iran. http://www.techrasa.com/2016/11/01/growth-knowledge-based-companies-iran . [Google Scholar]
- 18.Hosseini SA, Juibary AG. Clinical trials in Iran; biannual report of clinical trial committee in food and drug organization, ministry of health and medical education of Iran. Arch Iran Med. 2012;15:52–4. [PubMed] [Google Scholar]
- 19.Zhou Q, Chen XY, Yang ZM, Wu YL. The changing landscape of clinical trial and approval processes in china. Nat Rev Clin Oncol. 2017;14:577–83. doi: 10.1038/nrclinonc.2017.10. [DOI] [PubMed] [Google Scholar]
- 20.KoNECT. What We Do. 2017. [Last accessed on 2018 Jun 30]. Available from: http://www.en.konect.or.kr/KoNECT/What.htm .
- 21.Pacific Bridge Medical 2016 Update on Clinical Trial Procedures in Asia. 2016. [Last accessed on 2018 Jun 30]. Available from: http://www.pacificbridgemedical.com/publication/2016-update-clinical-trial-procedures-asia .
- 22.Clinical Trials in India: Ethical concerns. Bull World Health Organ. 2008;86:581–2. doi: 10.2471/BLT.08.010808. [DOI] [PMC free article] [PubMed] [Google Scholar]


