Abstract
Background:
Prevalence of migraine, as a chronic neurovascular disorder, was approximately 10.3 and 23.1% among men and women, respectively, mostly in people younger than 40 years old. Migraine is prevalent in different geographic areas worldwide. The present study was designed to compare the impact of intranasal lidocaine 4% and peppermint essential oil drop 1.5% on migraine attacks.
Methods:
In this double-blind, parallel, randomized controlled trial, 120 adult patients with a diagnosis of migraine based on the International Headache Society criteria were treated with intranasal lidocaine drop 4% or peppermint essential oil drop 1.5% or placebo. Patients expressed their symptoms 5 and 15 min after dripping, and if they still had a headache after 15 min, they were given the second dose. Patients with a second dose of medication, 15 min later recorded their headache rate. All patients recorded their symptoms after 30 min. Symptoms of the patients were followed by a researcher through the phone and in-person after 2 months. Then, the questionnaires were filled.
Results:
In the present study, there was a significant difference among groups in headache intensity after treatment (P < 0.001). In 40% of the patients in the peppermint oil and lidocaine groups, the intensity of headache decreased. In the placebo group, fewer patients responded highly to the treatment, whereas 41.5% of patients in the lidocaine group and 42.1% of patients in the peppermint oil group responded to the treatment considerably.
Conclusions:
Concerning the findings of the present study, nasal application of peppermint oil caused considerable reduction in the intensity and frequency of headache and relieved majority of patients' pain similar to lidocaine. On the basis of findings of this study, it can be concluded that nasal menthol, such as lidocaine, can be used to relieve migraine headaches.
Keywords: Headache, lidocaine, migraine attacks, peppermint oil
Introduction
Chronic diseases can affect the quality of life.[1] Severe headache is indeed one of the most common complaints in medical science.[2] More than 46% of humans experience at least one headache attack per year, and 64% experience at least one headache during their lifetime.[3] Prevalence of migraine as a chronic neurovascular disorder has been observed among men with an incidence of 10.3% and among women with an incidence of 23.1%, and mostly occurs among people <40 years old. Migraine is prevalent in different geographic areas across the world.[4] The clinical approach in migraine possibly includes prophylaxis and palliative treatment of attacks.[5] Formerly, to treat severe attacks of migraine, simple analgesics such as dopamine antagonists and serotonin agonists were applied; however, recently intravenous sodium valproate drug has also been considered in this field.[6] However, this drug has several side effects.[7] Other alternative sedatives include ergots, butalbital (including analgesic and other analgesic combinations), isometheptene, and nonsteroidal antiinflammatory drugs (NSAIDs).[8] Nowadays, using nasal medicines is considered to treat headache because it may be easier and more effective than other known treatment methods.[9] Nasal cycle circulates the delay in the treatment of the severe phase of migraine attack, which may occur through digestive system absorption. Therefore, patients who have nausea may prefer nonoral formulations due to reduction in the risk of vomiting and its efficacious nature.[10] Various studies have demonstrated that lidocaine 4% as a nasal medicine could reduce pain quickly. Other reports have also shown the pain relief with lidocaine drug.[11] Lidocaine can indeed reduce symptoms of cluster and migraine headaches quickly (between 15 s and 2 min).[12] Furthermore, some studies showed the ability of peppermint oil as an another effective factor in treating migraine headaches by relaxing muscles and reliving pain.[13] Results of these studies revealed that rubbing mint oil on forehead can be influential with the same effect as taking two acetaminophen pills.[14] Considering the presented models in migraine pathogenesis depending on the increased sensitivity of pre-cranial muscle, it was expected that peppermint oil could also be used to reduce the severity of migraine headache.[15,16] Various conditions such as anemia, uncontrolled diabetes, uncontrolled hypertension, vitamin D deficiency,[17,18,19,20] some organic diseases, and diet[21,22] may begin or aggravate the episodes of migraine. However, considering the side effects of drugs and the lack of proper treatment regimens encouraged to conduct this study for evaluating and comparing the efficacy of intranasal lidocaine drop 4% and peppermint essential oil drop 1.5% on migraine attacks.
Methods
Patients
A total of 120 adult patients with a migraine diagnosis based on the International Headache Society (HIS) criteria were enrolled into a randomized, double-blind, parallel, and controlled crossover study at the Shahrekord University of Medical Sciences, Shahrekord, Iran.
The study protocol was reviewed, approved, and monitored by the Local Ethics Committee of Shahrekord University of Medical Sciences (Registration number: IR.SKUMS.REC.1395.78). In addition, the consent forms were provided based on the standards of university and were filled by all participants of the study. The necessary information about the study was explained to all participants and all subjects filled the consent forms. All information was confidential and patients were allowed to exit from the study any time they like.
Inclusion criteria for the selection of patients with migraine diagnosis were approved by a neurologist. Participants were 20–40 years old, whom were referred to treat their acute migraine attacks. They should not have other diseases such as depression, anxiety, bipolar disorder and should not be using simulating drugs.[23] Moreover, they should not have respiratory disease such as asthma; however, it was possible to use NSAIDs prescribed by their doctors. Exclusion criteria were lack of patient tendency and failure to comply with doctor orders, application of simulating drugs more than 15 days per month, pregnancy or being pregnant during trial and incidence of drug side effect during intervention (itching, flushing, hives, shortness of breath caused by allergy to peppermint essence, or possible seizure caused by the use of lidocaine). Patients were selected after diagnosis and confirmation of their disease by neurologist using clinical symptoms.
Convenience sampling was used in this study after filling consent forms and questionnaires by participation. A total of 120 patients were included in the study (40 patients in each group). To collect data, first, 120 cards were provided by simple sampling method in which 40 numbers of A, 40 numbers of B, and 40 numbers of C were included. Then, participants were randomly divided into three groups of 40 each.
In this study, lidocaine 4% and peppermint oil 1.5% drops were used. Lidocaine 4% drop was made based on pharmacopoeia formulation of United States Pharmacopoeia (USP).[24] Peppermint essential oil drop was provided by Poursina Company, Iran, in pharmaceutical formulation laboratory of medical plants research center. Formulation of pharmaceutical combination was derived from Yurkasntai Company in France.[25] Patients in each three groups were not aware of obtained medicine. Moreover, characteristics of participants in all of the groups, including age, gender, headache type, severity, and prescribed medicine (NSAIDs), were collected by the physician. The patients were asked to use their usual medication (prescribed by their own physician) on time and if they have migraine severe attack, they will use the intervention treatment drug [Figure 1].
Figure 1.
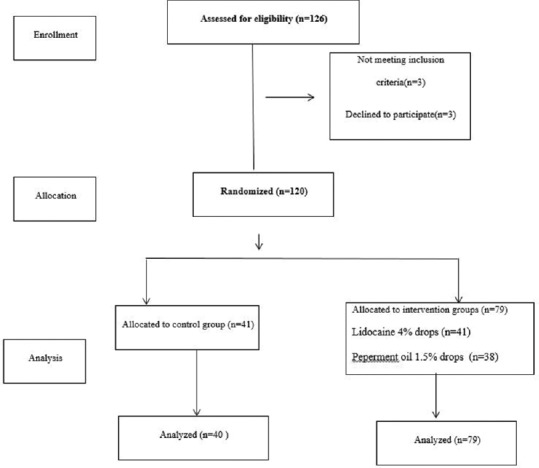
Flowchart of study design
Study protocol
The first group was given lidocaine drop 4%, second group was treated with peppermint essential oil drop of 1.5%, and for the control group, placebo treatment was supplied through intranasal method.[25] The study provided the following suggestions for the patients: As soon as they realize that headache is starting, use the given drop, lie down on their back, and while their shoulder is placed on the table edge, hang their head downward, turn their head 30° to the site of pain, and pour two drops of drug into their nose slowly. After dropping, lie on bed at least for 30 s.
Patients were asked to complete relative questionnaire regarding their headache severity in four scales before medicine consumption (None = 0; mild = 1; moderate = 2; and severe = 3). Patients were asked to convey their symptoms 5–15 min after drop pouring and if their headaches persisted after 15 min, they could apply a second dose of the medicine. The patients who received second dose of the medicine will note their degree of headache severity after 15 min too. In general, all patients recorded their symptoms after 30 min at home and their symptoms were followed by their physician after 2 months and questionnaires were filled.
Statistical analysis
Data were collected, and coding were analyzed by Statistical Package for the Social Sciences (SPSS) software version 18.0 (IBM, Chicago, IL, USA). Data analysis was carried out using descriptive statistics (frequency, percentage, mean, and standard deviation) and analytical statistics (Chi-square, one-way analysis of variance, and paired t-test).
Results
Participants of the study included 87 women and 33 men. Participants were assessed concerning distribution of age, gender, and type of consumed medicine before the study. Starting age of the headaches was ranged mostly from 11 to 30 years old. All patients were suffered at least from a migraine attack [Table 1]. Starting headache in the studied population in 53 patients (43.2%) was abrupt and in 66 patients (55%) was variant (day or night). In addition, duration of headache in 39 patients (32.5%) was a few minutes but a few hours in 33 patients (27.5%). Moreover, symptoms of the staring headache or symptoms at the time of headache were reported more than one case in 70 patients (58.3%) and included nausea, vomiting, and fear from the light.
Table 1.
Characteristics of the groups
| Variable | Placebo (n=41) frequency(%) |
Lidocaine (n=41) frequency(%) |
Peppermint oil(n=38) frequency(%) |
P |
|---|---|---|---|---|
| Gender | 0.713 | |||
| Female | 28(68.3) | 30(76.3) | 29(76.3) | |
| Male | 13(31.7) | 11(26.8) | 9(23.7) | |
| Starting age of headache | 0.419 | |||
| 11-20 | 11(26.8) | 19(46.3) | 15(39.5) | |
| 21-30 | 21(51.2) | 14(34.1) | 18(47.4) | |
| 31-40 | 9(22) | 7(17.1) | 4(10.5) | |
| More than 40 | 0(0) | 1(2.4) | 1(2.6) | |
| Age (year) (mean±SD) | 31.1±5.8 | 30.6±6.3 | 30.42±7.2 | 0.903 |
The descriptions of the headaches showed that headache was seen as dizziness and burning sensation in 18 patients (15.1%), as crusher in 59 patients (49.6%), and in 42 patients (53.3%) was sharp and in the form of spitting pain. The unilateral and bilateral headache in subjects indicated that the headache in 49 patients (40.8%) and 45 patients (37.5%) were bilateral and variant (sometimes unilateral and sometimes bilateral), respectively. Headache of 20 (25.3%), 13 (16.4%), 23 (29.1%), and 23 (29.1%) patients were in forehead, temple, behind the head, and unspecified, respectively. Results of family background in the studied population indicated that 95 (79.2%) patients had positive migraine family background.
Majority of patients [111 (92.5%) patients] did not state any special disease such as blood pressure, pulmonary disease, and other problems. Considering aggravating factors of headache, 74 patients (61.7%) stated more than one factor as a result of their headache aggravation. These factors may include stress, insomnia, and noise. Headache of 53 patients (44.2%) was relieved by resting and spontaneous treatment or using ordinary medicine; however, the headache of 47 patients (39.2%) was relieved through receiving special medicine for migraine relief.
There was a significant difference in impact of medicine on headache intensity among groups. In the lidocaine (41.5%) and peppermint essential oil (42.1%) groups, the majority of patients expressed a high level of headache reduction compared to the placebo group, with only 4.9% have a feeling of reduction in the severity of their headache (P < 0.0001) [Table 2].
Table 2.
Association between impact of medicine on pain intensity in groups
| Groups | Placebo (n=41) frequency (%) |
Lidocaine (n=41) frequency (%) |
Peppermint oil (n=38) frequency (%) |
P |
|---|---|---|---|---|
| Medicine impact | ||||
| Ineffective | 9 (22) | 9 (22) | 10 (26.3) | P<0.0001 |
| Inconsiderable | 14 (34.1) | 5 (12.2) | 0 (0) | |
| Average | 16 (39) | 10 (24.4) | 12 (31.6) | |
| High | 2 (4.9) | 17 (41.5) | 16 (42.1) |
There was also a significant difference in duration of medicine impact on headache among groups. In lidocaine (31.7%) and peppermint essential oil (44.7%) groups, the headache was relieved in majority of the patients, 5 min after receiving the drug in comparison with the placebo group of 7.3% relief (P < 0.01) [Table 3].
Table 3.
Association between impact of medicine on headache duration in groups
| Groups | Placebo (n=41) frequency (%) |
Lidocaine (n=41) frequency (%) |
Peppermint oil (n=38) frequency (%) |
P |
|---|---|---|---|---|
| Medicine impact | ||||
| Ineffective | 12 (29.3) | 11 (26.8) | 8 (21.1) | P<0.01 |
| 30 min later | 14 (34.1) | 6 (14.61) | 5 (13.2) | |
| 15 min later | 12 (29.3) | 11 (26.8) | 8 (21.1) | |
| 5 min later | 3 (7.3) | 13 (31.7) | 17 (44.7) |
Results of clinical outcome in studied subjects demonstrated that there was no significant difference between headache intensity and its impact on daily activities before intervention (P < 0.05). However, there were significant differences between headache intensity and their impacts on daily activities among three groups after intervention. In lidocaine (65.8%) and peppermint essential oil (57.9%) groups, majority of patients reported the impact of headache intensity on daily activities at zero and one levels but two and three levels in the placebo group (48.7%) (P < 0.01) [Table 4].
Table 4.
Association between impact of headache intensity on daily activities among groups after intervention
| Groups | Placebo (n=41) frequency (%) |
Lidocaine (n=41) frequency (%) |
Peppermint oil (n=38) frequency (%) |
P |
|---|---|---|---|---|
| Medicine impact | ||||
| Zero | 6 (4.9) | 13 (31.7) | 13 (34.2) | P<0.01 |
| One | 19 (46.3) | 14 (34.1) | 9 (23.7) | |
| Two | 14 (34.1) | 10 (24.4) | 16 (24.1) | |
| Three | 6 (14.6) | 4 (9.8) | 0 (0) |
Zero=No pain, One=There is pain but do not interact with daily activities, Two=Interacting with daily activities but not preventing activities, Three=Preventing daily activities
Findings of this study revealed that among the patients at third scale before the treatment, headache intensity did not prevent their daily activities after the treatment by peppermint oil drop (P < 0.01) [Table 5]. Comparison of the frequency of headaches showed that there was a significant difference in the average frequency of headache in the study groups, before and after intervention (P < 0.0001). However, there was no significant difference in the frequency of headaches between the groups before and after the intervention (P > 0.05) [Table 6].
Table 5.
Association between impact of headache intensity on daily activities before and after treatment in the peppermint oil group
| Before treatment After treatment |
Zero frequency (%) | One frequency (%) | Two frequency (%) | Three frequency(%) | P |
|---|---|---|---|---|---|
| Zero | 12 (15.4) | 2 (14.3) | 9 (81.8) | 0 (0) | P<0.01 |
| One | 3 (23.1) | 4 (28.6) | 2 (18.2) | 0 (0) | |
| Two | 8 (61.5) | 8 (57.1) | 0 (0) | 0 (0) | |
| Three | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total | 13 (100) | 14 (100) | 11 (100) | 0 (0) |
Zero=No pain, One=There is pain but do not interact with daily activities, Two=Interacting with daily activities but not preventing activities, Three=Preventing daily activities
Table 6.
Differences between before and after treatment regarding headache frequency in each group and comparison of all groups
| Menthol | Lidocaine | Placebo | P | |
|---|---|---|---|---|
| Before | 4.84±1.64 | 5.46±2.05 | 4.88±1.4 | 0.170 |
| After | 2.58±1.78 | 2.71±2.40 | 2.13±1.67 | 0.731 |
| P-value | P < 0.0001 | P < 0.0001 | P < 0.0001 | |
| Difference (before−after) | 2.26±2.19 | 2.76±2.60 | 1.95±1.67 | 0.248 |
Discussion
This study showed that there were significant differences among groups in terms of headache intensity after treatment (P < 0.001). In the peppermint oil and lidocaine groups, intensity of headache was reduced considerably in about 40% of patients. In addition, there were significant differences in terms of headache intensity among groups after the treatment. In the placebo group, fewer patients responded highly to the treatment, whereas 41.5% of the patients in the lidocaine group and 42.1% of the patients in the peppermint oil group responded to treatment considerably. In the study conducted by Robins, 27% of patients reported relative relief, 27% reported a slight relief, and 46% did not report any relief. The differences in this Robin's study can be related to varieties in headache type (cluster headache), distinctions in medicine type (spray), as well as subject of the study (men).[26] The study by Miles et al. reported that using lidocaine 4% at the time of aura could disappear headache in all patients, so it was assigned to zero or one intensity in visual analog scale.[23]
One of the limitations of this study was that the experiment did not include the placebo group. In this paper, researchers have mentioned that probable mechanism for impact of intranasal lidocaine might be reaching to internal spaces of nose through sphenopalatine ganglion and facial nerve branches.[23] In addition, the peppermint oil treatment resulted in control of headache intensity and regular activities of daily life without any limitation, while 25% of patients in the lidocaine group whose headaches prohibited their daily activities had limitations after treatment, and in the placebo group, 35.75% of patients had limitation in terms of daily activities due to their headache intensity before and after the treatment.
There were significant differences among groups in terms of the impact of medicine on headache duration. Only 7% of patients responded to medicine 5 min later in the placebo group, while the duration of medicine impact on headache was 31.7% in the lidocaine group and 44.7% in the peppermint oil group. In another study, Blanda et al. demonstrated that headache intensity was reduced 5 min later among 7% of patients who received lidocaine medicine and 14% of patients who obtained placebo.[27]
According to the previous studies, it was expected that intervention groups respond much better to the treatment, which might be due to the simultaneous consumption of prochlor perazine that could modify the impact of lidocaine. In fact, the obtained results of combination of lidocaine and intravenous prochlor perazine did not have any advantage compared to only prochlor perazine.[27] Furthermore, the differences between the results of this study and our results could be due to variety in scale of intensity measurement in both studies.
In another study, the results indicated that the headache was reduced considerably after the treatment (P < 0.0005) and migraine attacks were completely relieved in 12 patients with 8 patients who recovered 5 min later.[11] Of course, there was no control group in this study. In fact, as in cluster headache, conduction-blocking action on internal carotid, pterygo palatine, as well as cavernous sinus ganglia may result in rapid relief of the headache, which is an indicator of the point that involving parasympathetic activity as well as calling into question the primacy of the trigemino vascular system to that of vaso-parasympathetic afferents are the same in pain-evoking mechanisms in migraine and cluster headache. Moreover, in the study by Borhani et al. on the impact of skin contact of peppermint oil solution 10% on temple in severe attacks of migraine without aura, the results revealed that intensity of headache was decreased by 50% after 2 h and 25 min after peppermint oil contact and relieved completely after 7 h and 15 min.[28] The differences in this study referred to the type of skin in contact with peppermint oil and the type of nasal drop in our study as well as the subjects in our study. However, no study has been conducted in terms of frequency of migraine headache before and after treatment, concerning differences in terms of headache frequency before and after treatment. Our paper demonstrated that headache frequencies were decreased significantly among three groups through three types of treatments and there were no significant differences among the three groups concerning frequency reduction. This result could be contributed to the consumption of prescribed daily medicines.
Conclusions
It can be concluded from the results of the present study that peppermint oil and lidocaine caused considerable reduction in intensity and frequency of headache and relieved the pain of most patients after 5 min following the drug consumption.
Financial support and sponsorship
This work was supported by grant No. 2713 from Shahrekord University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank the Research Council of Shahrekord University of Medical Sciences.
References
- 1.Shahbazi K, Solati K, Hasanpour-Dehkordi A. Comparison of hypnotherapy and standard medical treatment alone on quality of life in patients with irritable bowel syndrome: A randomized controlled trial. J Clin Diagn Res. 2016;10:OC01–4. doi: 10.7860/JCDR/2016/17631.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: A meta-analysis. JAMA. 2012;307:1736–45. doi: 10.1001/jama.2012.505. [DOI] [PubMed] [Google Scholar]
- 3.Manzoni GC, Stovner LJ. Epidemiology of headache. Handb Clin Neurol. 2010;97:3–22. doi: 10.1016/S0072-9752(10)97001-2. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: A systematic review of population-based studies. Dev Med Child Neurol. 2010;52:1088–97. doi: 10.1111/j.1469-8749.2010.03793.x. [DOI] [PubMed] [Google Scholar]
- 5.Woldeamanuel Y, Rapoport A, Cowan R. The place of corticosteroids in migraine attack management: A 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia. 2015;35:996–1024. doi: 10.1177/0333102414566200. [DOI] [PubMed] [Google Scholar]
- 6.Edwards KR, Norton J, Behnke M. Comparison of intravenous valproate versus intramuscular dihydroergotamine and metoclopramide for acute treatment of migraine headache. Headache. 2001;41:976–80. doi: 10.1046/j.1526-4610.2001.01191.x. [DOI] [PubMed] [Google Scholar]
- 7.Coppola G, Schoenen J. Management of acute and chronic migraine. Curr Opin Support Palliat Care. 2012;6:177–82. doi: 10.1097/SPC.0b013e3283521dc3. [DOI] [PubMed] [Google Scholar]
- 8.Mueller LL. Diagnosing and managing migraine headache. J Am Osteopath Assoc. 2007;107(10 Suppl 6):ES10–6. [PubMed] [Google Scholar]
- 9.Djupesland PG, Messina JC, Mahmoud RA. Breath powered nasal delivery: A new route to rapid headache relief. Headache. 2013;53(Suppl 2):72–84. doi: 10.1111/head.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapoport AM, Bigal ME, Tepper SJ, Sheftell FD. Intranasal medications for the treatment of migraine and cluster headache. CNS Drugs. 2004;18:671–85. doi: 10.2165/00023210-200418100-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kudrow L, Kudrow DB, Sandweiss JH. Rapid and sustained relief of migraine attacks with intranasal lidocaine: Preliminary findings. Headache. 1995;35:79–82. doi: 10.1111/j.1526-4610.1995.hed3502079.x. [DOI] [PubMed] [Google Scholar]
- 12.Kligler B, Chaudhary S. Peppermint oil. Am Fam Physician. 2007;75:1027–30. [PubMed] [Google Scholar]
- 13.Kittrelle JP, Grouse DS, Seybold ME. Cluster headache: Local anesthetic abortive agents. Arch Neurol. 1985;42:496–8. doi: 10.1001/archneur.1985.04060050098017. [DOI] [PubMed] [Google Scholar]
- 14.Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F. Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci. 2014;5:1085–96. doi: 10.1021/cn500083e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zargaran A, Borhani-Haghighi A, Faridi P, Daneshamouz S, Kordafshari G, Mohagheghzadeh A. Potential effect and mechanism of action of topical chamomile (Matricaria chammomila L.) oil on migraine headache: A medical hypothesis. Med Hypotheses. 2014;83:566–9. doi: 10.1016/j.mehy.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Mirabi P, Alamolhoda SH, Esmaeilzadeh S, Mojab F. Effect of medicinal herbs on primary dysmenorrhoea-a systematic review. Iran J Pharm Res. 2014;13:757–67. [PMC free article] [PubMed] [Google Scholar]
- 17.Nasri H. The adverse effects of vitamin D deficiency on health. J Renal Endocrinol. 2017;3:e03. [Google Scholar]
- 18.Asgari A. Pathophysiology of epilepsy. Acta Persica Pathophysiol. 2016;1:e07. [Google Scholar]
- 19.Nasri H. On the occasion of world hypertension day; high blood pressure in geriatric individuals. J Ischemia Tissue Repair. 2017;1:e01. [Google Scholar]
- 20.Baradaran A. The role of biomarkers to detect progression of diseases. J Negat Results Clin Exp Stud. 2018;1:e05. [Google Scholar]
- 21.Seylanian-Toosi F, Boloursaz S, Abbasi B, Hekmat R, Mortazavi-Ardestani R, Mohajerzadeh MN. Joubert syndrome; misleading presentation of two cases as pseudo-tumor cerebri and literature review. J Renal Inj Prev. 2017;6:76–9. doi: 10.15171/jrip.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kafeshani M. Diet and immune system. Immunopathol Persa. 2015;1:e04. [Google Scholar]
- 23.Maizels M, Geiger AM. Intranasal lidocaine for migraine: A randomized trial and open-label follow-up. Headache. 1999;39:543–51. doi: 10.1046/j.1526-4610.1999.3908543.x. [DOI] [PubMed] [Google Scholar]
- 24.Spalding L. The Pharmacopoeia of the United States of America. Charles Ewer. 2005 [Google Scholar]
- 25.Keifer D, Ulbricht C, Abrams TR, Basch E, Giese N, Giles M, et al. Peppermint (Mentha Xpiperita) An evidence-based systematic review by the natural standard research collaboration. J Herb Pharmacother. 2008;7:91–143. doi: 10.1300/j157v07n02_07. [DOI] [PubMed] [Google Scholar]
- 26.Robbins L. Intranasal lidocaine for cluster headache. Headache. 1995;35:83–4. doi: 10.1111/j.1526-4610.1995.hed3502083.x. [DOI] [PubMed] [Google Scholar]
- 27.Blanda M, Rench T, Gerson LW, Weigand JV. Intranasal lidocaine for the treatment of migraine headache: A randomized, controlled trial. Acad Emerg Med. 2001;8:337–42. doi: 10.1111/j.1553-2712.2001.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 28.Haghighi A, Moatazediyan S, Pourmokhtary S, Khodaiy SH, Mohammady F, Rezaii R, et al. The effectof 10% solution of menthol in the temporal effect of skin application in acute migraine attacks without aura. Hormozgan Med J. 2008;12:83–8. [Google Scholar]


