Abstract
Background
Increased risk of acute exacerbation of chronic obstructive pulmonary disease (COPD) has been reported in patients who are overweight and obese. However, the effects of body fat in patients with normal or low body mass index (BMI) and COPD remain unknown. This study aimed to examine the association between acute exacerbations of COPD and the lean-to-fat (LTF) ratio in patients with a normal or low BMI.
Material/Methods
Patients with COPD (n=68) underwent assessment of body composition, in whom 43 cases had a normal BMI (18.5 to 25 kg/m2) and 14 cases were underweight (<18.5 kg/m2). Patients with COPD were treated according to current clinical guidelines and underwent regular follow-up for one year. Acute exacerbations of COPD were recorded.
Results
BMI, the fat-free mass index (FFMI), skeletal muscle mass index (SMMI), and LTF ratio had no significant effect of the risk of acute exacerbations of COPD in the whole study cohort, but a low LTF ratio was significantly associated with reduced risk of acute exacerbations of COPD in the subgroup with a BMI<25 kg/m2 (OR=4.528; P<0.05). The Fat Mass Index (FMI) had a protective effect in the whole cohort (OR=0.292; P=0.024) and in the subgroup with BMI <25 kg/m2 (OR=0.253, P=0.049). The cumulative incidence of acute exacerbations of COPD was significantly increased in the patients with a high LTF ratio in the whole cohort (P=0.047) and in the subgroup with BMI <25 kg/m2 (P=0.014).
Conclusions
In patients with BMI <25 kg/m2, the LTF ratio was positively correlated with the risk of occurrence of acute exacerbations of COPD.
MeSH Keywords: Body Composition; Disease Progression; Pulmonary Disease, Chronic Obstructive
Background
Worldwide, chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality that results in increasing economic and social burden [1,2]. An acute exacerbation of COPD is defined as an acute worsening of respiratory symptoms that results in the requirement for additional therapy [3]. Recently, with the aim of achieving more effective clinical management, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) proposed a new grading system for patient groups (ABCD), based on patient symptoms and their history of acute exacerbations of COPD in the previous year [1].
Current clinical guidelines for the management of COPD include monitoring nutritional status as an important part of the routine evaluation of patients with COPD. Surrogate measures, such as the use of body mass index (BMI), gives no indication of body composition, muscle mass, the proportion of fat to muscle, or the patient nutritional status. Recently, several studies have shown that parameters of body composition, including the use of the fat-free mass index (FFMI), have been shown to be associated with exercise capacity, disease severity, mortality, and quality of life in patients with COPD [4–9].
Weight loss and depletion of muscle mass are common systemic manifestations in COPD and have been reported to be associated with disease prognosis [10]. It has been shown that both overweight and obesity, as determined using the BMI, increase the severity of symptoms in patients with COPD [11]. However, during the past decade, and contrary to previous findings, overweight and obese patients with COPD were shown to have lower mortality [12]. Previous studies on the impact of body composition on acute exacerbation of COPD are limited and the findings are contradictory [13,14]. Also, existing studies have been undertaken mainly in Europea or the US, where overweight and obesity are prevalent, and there have been few studies that have investigated Asian patients who generally have a lower BMI.
Therefore, this study aimed to examine the association between acute exacerbations of COPD and the lean-to-fat (LTF) ratio in patients with a normal or low BMI in a Chinese patient population.
Material and Methods
Study design
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2017-017), and all the patients provided written informed consent before recruitment to the study.
Patients with chronic obstructive pulmonary disease (COPD) in the retrospective cohort were consecutively enrolled from the outpatient department of the First Affiliated Hospital of Soochow University, between 1 August 2016 to 30 July 2017. The inclusion criteria were: (1) diagnosis of COPD with a ratio of forced expiratory volume in one second by forced vital capacity (FEV1/FVC) less than 70% after administration of a bronchodilator, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [1], and stable COPD. The exclusion criteria included pulmonary infection (<4 weeks), bronchial reversibility (>12% after administration of β-agonists), other conditions known to affect muscle or cause dyspnea, including acute or chronic heart failure, renal failure, hepatic failure, cancer, diabetes, and severe arthritis, edema, dehydration, and drugs known to affect fluid balance.
Patients who had been enrolled into the retrospective study were invited to participate in a prospective study, and received treatment according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [1], and underwent regular follow-up for one year. Treatment included inhaled salmeterol/fluticasone or budesonide/formoterol (ICS+LABA), tiotropium bromide powder (LAMA), salmeterol/fluticasone or budesonide/formoterol and tiotropium bromide powder (ICS+LABA+LAMA) [1]. If an acute exacerbation of COPD occurred, additional treatment included systemic corticosteroids, and if an infection was present, antimicrobial therapy [1].
The results of body composition measurements, pulmonary function tests, the modified Medical Research Council (mMRC) grading system, the 6-minute walking distance (6MWD), and any history of acute exacerbations of COPD in the previous year before inclusion were recorded for all patients of the retrospective cohort at the time of inclusion. Patients underwent regular follow-up and monitoring of drug treatment at two-monthly follow-up in the outpatient clinic, or during hospital or physician visits when an acute exacerbation of COPD occurred in the following one year after being enrolled in the study.
Assessment of body composition
Weight and height were assessed with the patient being barefoot and wearing light clothes. The body mass index (BMI) was calculated as weight/height2 (kg/m2). According to the criteria of the World Health Organization (WHO) [15], patients were divided into four categories, obese (BMI >30 kg/m2), overweight (BMI, 25–30 kg/m2), normal weight (BMI, 18.5–25 kg/m2), and underweight (BMI <18.5 kg/m2). Body composition was assessed using a BCA-2A bioelectrical impedance body composition analyzer (Tsinghua Tongfang Co., Ltd., Beijing, China). Body weight, fat-free mass (FFM), fat mass (FM), skeletal muscle mass (SMM) and other body composite variables were measured.
The fat-free mass index (FFMI) was calculated as the FFM/height2. The skeletal muscle mass index (SMMI) was calculated as the SMM/height2. The lean-to-fat (LTF) ratio was calculated as the FFM/FM [16]. Because overweight and obese people in China account for a minority of the population, a subgroup of underweight and normal patients was identified with a BMI <25 kg/m2, to determine the role of body composition that was more representative of the Chinese population.
Pulmonary function tests
All the enrolled patients underwent spirometry using a Master screen-PFT system (Vyaire, Mettawa, Ill, USA), and the forced expiratory volume during one second (FEV1) and forced vital capacity (FVC) were measured, according to the American Thoracic Society (ATS) consensus guidelines [17,18]. The FEV1, FEV1/FVC, and the ratio of FEV1 to predicted FEV1 (FEV1%) after inhaling bronchodilators were recorded. According to the category of airflow limitation in COPD (based on FEV1 after the administration of a bronchodilator) in patients with FEV1/FVC <0.70, stable COPD was divided into four subgroups: mild (GOLD 1), ≥80% of predicted FEV1; moderate (GOLD 2), 50–80% of predicted FEV1; severe (GOLD 3), 30–50% of predicted FEV1; very severe (GOLD 4), <30% of predicted FEV1.
The 6-minute walking distance (6MWD)
All enrolled patients performed a 6-minute walking distance (6MWD) test, according to American Thoracic Society (ATS) criteria [17,18]. The 6MWD was used to evaluate exercise capacity and the distance in meters was recorded for each patient.
Dyspnea during activities of daily living (ADL)
As recommended by GOLD [1], the modified Medical Research Council (mMRC) questionnaire for the assessment of symptoms of COPD was used [19]. In this study, the eight-item unidimensional COPD Assessment Test (CAT) measure of health status impairment in COPD was not used because of its complexity and subjective nature [20].
Assessment of acute exacerbation risk for patients with stable COPD
Based on the mMRC score and history of moderate to severe exacerbations and/or episodes of hospitalization in the previous year, the patients in the retrospective cohort were classified into GOLD A, B, C, and D subgroups [1]. Patients in group C and D were considered to be at high risk for an acute exacerbation of COPD, and group A and B were considered as at low risk. When follow-up was completed, each subject in the prospective cohort was also evaluated by ABCD assessment based on the data in the second year. An updated ABCD assessment for stable COPD was used, as follows: A. mMRC 0–1, 0–1 exacerbation that did not lead to hospital admission in the previous year; B. mMRC ≥2, 1–2 exacerbations not leading to hospital admission in the previous year; C. mMRC 0–1, ≥2 exacerbation or ≥1 leading to hospital admission in the previous year; D: mMRC ≥2, ≥2 exacerbation or ≥1 leading to hospital admission in the previous year.
Statistical analysis
Numerical variables were shown as the mean ± standard deviation (SD), or the median and the interquartile range (IQR), and compared using the Kruskal-Wallis test. Categorical variables were expressed as the frequency and compared using the chi-squared (χ2) test. The occurrence of acute exacerbation of COPD was calculated by the Kaplan-Meier method and compared by the log-rank test. The impact of covariates was analyzed either by adjusted logistic regression for the risk of acute exacerbation of COPD, or by Cox proportional hazard model for the cumulative occurrence of acute exacerbation of COPD. All tests were performed with a two-tailed P-value (α=0.05). Statistical analysis was conducted using SPSS version 19 (SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered to be statistically significant.
Results
There were 68 patients with chronic obstructive pulmonary disease (COPD) who were enrolled in the retrospective study. The majority of patients were men (n=66), and there were two women. Using the body mass index (BMI), most patients in the study were underweight (BMI <18.5 kg/m2) (n=14), and of normal weight (BMI, 18.5–25 kg/m2) (n=43), and only two patients were obese (BMI >30 kg/m2), and nine patients were overweight (BMI, 25–30 kg/m2). Of the 68 cases in the retrospective study, 50 cases participated in the prospective or one-year follow-up study. The baseline characteristics of both the retrospective cohort and prospective cohort are shown in Table 1.
Table 1.
Patient characteristics in the retrospective and prospective cohorts.
| Retrospective cohort (n=68) | Prospective cohort (n=50) | P- value | ||
|---|---|---|---|---|
| Age (years) | 68 (54–85) | 68 (54–85) | 0.505 | |
| Gender | 1.000 | |||
| Male | 66 | 48 | ||
| Female | 2 | 2 | ||
| Smoking history | ||||
| Former smokers | 19 | 16 | ||
| Current smokers | 22 | 12 | ||
| Never smokers | 27 | 22 | ||
| FEV1% | 49.71±19.36 | 48.35±16.76 | 0.614 | |
| GOLD 1 | 3 | 1 | 0.780 | |
| GOLD 2 | 29 | 20 | ||
| GOLD 3 | 27 | 24 | ||
| GOLD 4 | 9 | 5 | ||
| 6-min walking distance (m) | 417±93 | 428±8 | 0.544 | |
| mMRC | ||||
| 0–1 | 33 | 24 | 1.000 | |
| ≥2 | 35 | 26 | ||
| Exacerbations in the last year (times) | 1 (0–4) | 1 (1–3) | 1.000 | |
| ≥2 or ≥1 leading to hospital admission | 23 | 37 | ||
| 0 or 1 (not leading to hospital admission) | 45 | 13 | ||
| Exacerbations in the next year (times) | 0 (0–5) | |||
| ≥2 or ≥1 leading to hospital admission | 17 | |||
| 0 or 1 (not leading to hospital admission) | 33 | |||
| ABCD assessment | 0.846 | |||
| A | 26 | 18 | ||
| B | 19 | 15 | ||
| C | 7 | 6 | ||
| D | 16 | 11 | ||
| High-risk (grade C and D) | 23 | 17 | ||
| Low-risk (grade A and B) | 45 | 33 | ||
| BMI (kg/m2) | (mean ±SD) | 21.9±3.8 | 21.8±4.0 | 0.824 |
| (median, IQR) | 21.9 (19.5–24.0) | 21.9 (18.1–24.0) | ||
| FFMI (kg/m2) | (mean ±SD) | 17.2±2.1 | 17.2±2.2 | 0.846 |
| (median, IQR) | 17.2 (16.0–18.4) | 17.3 (15.5–18.5) | ||
| SMMI (kg/m2) | (mean ±SD) | 11.6±1.4 | 11.6±1.5 | 0.934 |
| (median, IQR) | 9.5 (10.8–12.3) | 11.5 (10.6–12.4) | ||
| FMI (kg/m2) | (mean ±SD) | 4.5±2.4 | 4.4±2.4 | 0.933 |
| (median, IQR) | 4.4 (2.7–5.9) | 4.4 (2.4–6.0) | ||
| LTF | (median, IQR) | 3.9 (2.9–6.2) | 3.8 (2.9–6.9) | 0.673 |
COPD – chronic obstructive pulmonary disease; mMRC – modified Medical Research Council; BMI – body mass index; SMMI – skeletal muscle mass index; FFMI – fat-free mass index; LTF – lean-to-fat; SD – standard deviation; IQR – interquartile range; GOLD – Global Initiative for Chronic Obstructive Lung Disease.
The impact of body composition on the risk of acute exacerbation of COPD for the retrospective cohort was analyzed by adjusting the parameters of age, gender, smoking history, the percentage of forced expiratory volume during one second (FEV1%), the modified Medical Research Council (mMRC) grade, and the six-minute walking distance (6MWD) (Table 2). The BMI, the fat-free mass index (FFMI), the skeletal muscle mass index (SMMI), and the lean-to-fat (LTF) ratio showed no significant correlation with acute exacerbation of COPD. However, and increased LTF ratio showed a minimally significant association with an increased risk of an acute exacerbation of COPD (P=0.072). In the subgroup with a BMI <25 kg/m2, patients with a low LTF ratio showed a significantly reduced risk of acute exacerbation of COPD (OR=4.528; P<0.05). The FMI showed a significant protective effect and reduced risk of acute exacerbation of COPD in the whole cohort (OR=0.292; P=0.024) and in the lower BMI subgroup (OR=0.253; P=0.049).
Table 2.
Risk factor analysis in the retrospective cohort.
| Cohort (n=68) | Underweight cohort BMI <25 kg/m2 (n=57) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| BMI | 0.290 | 0.064–1.316 | 0.109 | 0.206 | 0.040–1.074 | 0.061 |
| FFMI | 0.589 | 0.129–2.679 | 0.493 | 0.457 | 0.089–2.336 | 0.457 |
| SMMI | 0.545 | 0.124–2.388 | 0.545 | 0.433 | 0.088–2.129 | 0.303 |
| FMI | 0.292 | 0.100–0.850 | 0.024* | 0.253 | 0.664–0.996 | 0.049* |
| LTF | 3.362 | 0.899–12.569 | 0.072 | 4.528 | 1.066–19.232 | 0.041* |
P<0.05.
BMI – body mass index; SMMI – skeletal muscle mass index; FFMI – fat-free mass index; LTF – lean-to-fat; OR – odds ratio; CI – confidence interval.
In the prospective cohort, during the one-year follow-up, there was a similar effect of the LTF ratio on the risk of an acute exacerbation of COPD in the lower BMI subgroup (OR=8.971; P=0.034) rather than in the whole cohort (Table 3). Also, the LTF ratio showed a significant impact on the occurrence of an acute exacerbation of COPD (OR=3.686; P=0.023), particularly in the lower BMI subgroup (OR=19.874; P=0.004) (Table 4). However, the FMI had no impact on the risk of an acute exacerbation of COPD or its occurrence in the prospective cohort, possibly due to the limited patient sample size used in this study.
Table 3.
Risk factor analysis for predicting the risk of an acute exacerbation (AE) of chronic obstructive pulmonary disease (COPD) in the prospective cohort.
| Cohort (n=50) | Underweight cohort BMI <25 kg/m2 (n=41) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| BMI | 0.651 | 0.084–5.015 | 0.680 | 0.822 | 0.089–7.594 | 0.863 |
| FFMI | 1.081 | 0.136–8.618 | 0.941 | 1.341 | 0.149–12.108 | 0.794 |
| SMMI | 1.047 | 0.133–8.228 | 0.966 | 1.281 | 0.146–11.239 | 0.823 |
| FMI | 0.879 | 0.624–1.229 | 0.461 | 1.490 | 0.189–11.770 | 0.705 |
| LTF | 9.307 | 0.905–95.737 | 0.061 | 8.971 | 1.179–68.271 | 0.034* |
P<0.05.
BMI – body mass index; SMMI – skeletal muscle mass index; FFMI – fat-free mass index; LTF – lean-to-fat; OR – odds ratio; CI – confidence interval.
Table 4.
Analysis of risk factors for the prediction of the occurrence of acute exacerbation (AE) of chronic obstructive pulmonary disease (COPD) in the prospective cohort.
| Cohort (n=50) | Underweight cohort BMI <25 kg/m2 (n=41) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| BMI | 1.866 | 0.577–6.170 | 0.294 | 1.622 | 0.368–7.150 | 0.523 |
| FFMI | 1.768 | 0.584–5.349 | 0.313 | 1.418 | 0.318–6.314 | 0.647 |
| SMMI | 1.718 | 0.566–5.215 | 0.339 | 1.376 | 0.301–6.302 | 0.681 |
| FMI | 0.440 | 0.135–1.431 | 0.173 | 0.181 | 0.032–1.032 | 0.054 |
| LTF | 3.686 | 1.196–11.353 | 0.023* | 19.874 | 2.613–151.170 | 0.004** |
P<0.05;
P<0.01.
BMI – body mass index; SMMI – skeletal muscle mass index; FFMI – fat-free mass index; LTF – lean-to-fat; OR – odds ratio; CI – confidence interval.
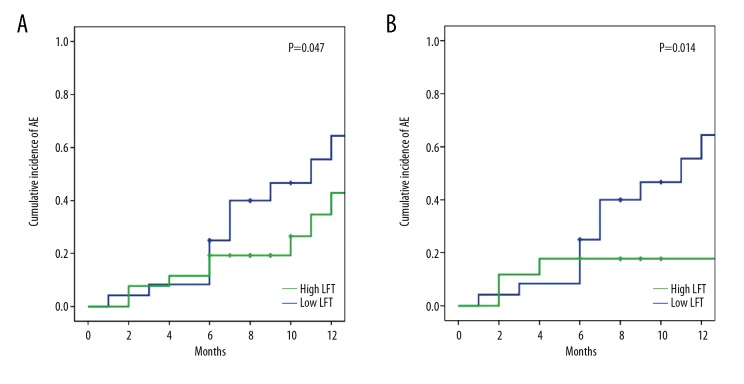
Based on these results above, in the prospective cohort of patients with COPD who were followed-up for one year, the cumulative incidence of acute exacerbation events differed in terms of the LTF ratio. In the whole cohort, the cumulative incidence of acute exacerbation events was 64.4±12.7% for patients with a high LTF ratio (>the median of 3.84), which was significantly increased when compared with the patients with a low LTF ratio (42.9±12.8%; P=0.047) (Figure 1A), and the difference was more significant in the lower BMI subgroup (64.4±12.7% vs. 45.1±17.0%; P=0.014) (Figure 1B).
Figure 1.
Cumulative incidence of acute exacerbations (AEs) of chronic obstructive pulmonary disease (COPD) in the high and low lean-to-fat (LTF) ratio subgroups. (A) Cumulative incidence of acute exacerbations (AEs) of chronic obstructive pulmonary disease (COPD) of the prospective cohort in the high lean-to-fat (LTF) ratio subgroup and the low lean-to-fat (LTF) ratio subgroup. (B) Cumulative incidence of acute exacerbations (AEs) of chronic obstructive pulmonary disease (COPD) of the subjects with a body mass index (BMI) <25 kg/m2 in the high lean-to-fat (LTF) ratio subgroup and the low lean-to-fat (LTF) ratio subgroup.
Discussion
Nutritional status is an important determinant of the extrapulmonary effects in patients with chronic obstructive pulmonary disease (COPD) [1]. Body mass index (BMI) is a well-established determinant of prognosis in patients with COPD, and increased weight and obesity have been reported to be associated with increased all-cause mortality and COPD-related mortality, independent of the severity of COPD [5]. However, BMI does not provide complete information about body composition and changes in body fat in patients with COPD, and BMI mainly indicates nutritional status.
Bioelectrical impedance analysis is a simple, inexpensive, quick and non-invasive technique for assessing body composition, and can be used in patients with COPD to evaluate the systemic effects of the disease [21]. Recent studies have shown that the fat-free mass index (FFMI) is an even more important determinant of prognosis in moderate to severe COPD than BMI [6], and is a significant indicator of exercise capacity, disease severity, quality of life, and prognosis in patients with COPD [22–24]. As a relative measure of body composition, the lean-to-fat (LTF) ratio is calculated by dividing lean body mass by fat mass. The use of the LTF ratio has substantive analytic advantages because it is independent of body size and is not colinear with height. Previous studies have shown that LTF is more closely related to functional limitation than lean body mass alone, and is more closely associated with walking speed and self-reported functional limitation in elderly adults than lean mass or fat mass [25,26]. Studies on the relationship between FMI and COPD was limited, but obesity has been considered to be an independent risk factor for reduced survival [27], as well as for co-morbidity in patients with COPD [28].
However, the distribution of body composition varies greatly in the populations of different countries. The prevalence of obesity in native Asian populations is much lower than Europeans and North Americans, where obesity is fairly common, and where 54% of patients with COPD have been reported to have a BMI >30 kg/m2 [16,17]. Existing epidemiologic data and studies on the prognostic value of body composition in patients with COPD have been mainly evaluated in populations living in Europe and the United States. Data on the relationship between body composition and COPD in countries with low BMI of the population is very limited. Also, most previously published studies have been aimed at investigating the correlation between body compositions and exercise capacity, pulmonary function tests, patient mortality, and quality of life, while the impact of body composition on the risk of acute exacerbation in COPD has been limited, especially in non-overweight and non-obese patients with stable COPD.
In the present study, most of the patients had a low BMI. The study findings showed that the FFMI, skeletal muscle mass index (SMMI), and the LTF ratio were not significantly associated with past or future risk of acute exacerbation of COPD, but that the LTF ratio showed a predictive trend. On further analysis, the LTF ratio represented an independent predictor for the risk of acute exacerbation of COPD in the subgroups of low BMI <25 kg/m2 in both the retrospective cohort and the prospective cohorts at one-year follow-up. A low value of the LTF ratio may predict a reduced risk of an acute exacerbation of COPD, which is a finding that appears contradictory with previous studies [22–24]. However, the LTF ratio may be an indicator that fat mass is a protective factor for acute exacerbation of COPD, especially for non-overweight and non-obese patients. This finding was confirmed in the retrospective cohort. The finding of a significant correlation between acute exacerbation of COPD in the previous and following year in this study was supported by the findings from a previously published study [29], However, the FMI was not an independent predictor for the future development of an acute exacerbation of COPD.
The potential protective role of fat mass on clinical outcome for patients with COPD is in contrast with the known association between obesity and reduced life expectancy [30]. The phenomenon of an ‘obesity paradox’ in human disease [12] has also been identified in several other chronic diseases [31], but the pathophysiological mechanisms underlying this phenomenon remain to be identified. Several recent studies have also reported a significant protective effect of overweight and obesity on all-cause mortality, indicating that also in COPD, an obesity paradox may be present [32–34]. However, the findings from these previous studies were based on obese subjects. It may be speculated that in non-obese individuals, a low LTF ratio and increased fat mass may prevent patients with COPD from acute exacerbations of COPD, while repeated exacerbations are also accompanied by an impaired energy balance that has a negative effect on body composition [14]. However, the correlation between body composition and acute exacerbation events in COPD remains inconclusion and further long-term prospective studies are needed with larger patient numbers. The findings and conclusion from the present study were limited by the relatively small study size, the varied clinical course of the patients who were included, the variable severity of COPD and the varied patient treatment regimes. Future well-designed, large-scale, multicenter clinical studies are required to further investigate the role of body composition in the clinical progression of COPD.
Conclusions
The findings of this study showed that although there was no association of body mass index (BMI), fat-free mass index (FFMI), and skeletal muscle mass index (SMMI), the lean-to-fat (LTF) ratio was significantly correlated with the risk of acute exacerbations of chronic obstructive pulmonary disease (COPD) in patients with a BMI <25 kg/m2. Therefore, in this group of patients, body fat might have a protective role. These findings require investigation with further clinical studies.
Footnotes
Source of support: This study was supported by the Peoples’ Livelihood Science and Technology of Suzhou Science and Technology Demonstration Engineering (No. SS201813)
Conflict of interest
None.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–82. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurst JR, Wedzicha JA. What is (and what is not) a COPD exacerbation: Thoughts from the new GOLD guidelines. Thorax. 2007;62:198–99. doi: 10.1136/thx.2007.077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mostert R, Goris A, Weling-Scheepers C, et al. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 94:859–67. doi: 10.1053/rmed.2000.0829. 200. [DOI] [PubMed] [Google Scholar]
- 5.Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–61. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 6.Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: Findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 7.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Luo Y, Zhou L, Li Y, et al. Fat-free mass index for evaluating the nutritional status and disease severity in COPD. Respir Care. 2016;61:680–88. doi: 10.4187/respcare.04358. [DOI] [PubMed] [Google Scholar]
- 9.Ingadottir AR, Beck AM, Baldwin C, et al. Two components of the new ESPEN diagnostic criteria for malnutrition are independent predictors of lung function in hospitalized patients with chronic obstructive pulmonary disease (COPD) Clin Nutr. 2018;37(4):1323–31. doi: 10.1016/j.clnu.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Choudhury G, Rabinovich R, MacNee W. Comorbidities and systemic effects of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:101–30. doi: 10.1016/j.ccm.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Zammit C, Liddicoat H, Moonsie I, Makkeer H. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335–43. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spelta F, Fratta Pssini AM, Cazzoletti L, Ferrari M. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord. 2018;23:15–22. doi: 10.1007/s40519-017-0456-z. [DOI] [PubMed] [Google Scholar]
- 13.Horadagoda C, Dinihan T, Roberts M, Kairaitis K. Body compositon and micronutrient deficiencies in patients with an acute exacerbation of chronic obstructive pulmonary disease. Intern Med J. 2017;49:1057–63. doi: 10.1111/imj.13453. [DOI] [PubMed] [Google Scholar]
- 14.Rubinsztajn R, Przybyłowski T, Maskey-Warzęchowska M, et al. Effect of exacerbation frequency on body composition and serum ghrelin and adiponectin concentrations in patients with chronic obstructive pulmonary disease. Pol Arch Med Wewn. 2014;124(7–8):403–9. [PubMed] [Google Scholar]
- 15.Akram D-S, Astrup AV, Atinmo T, et al. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 16.Eisner MD, Blanc PD, Sidney S, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8:7. doi: 10.1186/1465-9921-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. Standardization of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society Statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–17. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher CM. Standardized questionnaire on respiratory symptoms: A statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score) BMJ. 1960;2:1662. [Google Scholar]
- 20.Karloh M, Fleig Mayer A, et al. The COPD assessment test: What do we know so far?: A systematic review and meta-analysis about clinical outcomes prediction and classification of patients into GOLD stages. Chest. 2016;149:413–25. doi: 10.1378/chest.15-1752. [DOI] [PubMed] [Google Scholar]
- 21.Celli B, Cote C, Marin J. The body mass index, airflow obstruction, dyspnea and exercise capacity index in COPD. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 22.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 23.Ischaki E, Papatheodorou G, Gaki E, et al. Body mass and fat free mass indices in COPD: Relation with variables expressing disease severity. Chest. 2007;132:164–69. doi: 10.1378/chest.06-2789. [DOI] [PubMed] [Google Scholar]
- 24.Slinde F, Gronberg A, Engstrom CP, et al. Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir Med. 2005;99:1004–9. doi: 10.1016/j.rmed.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–21. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 26.Tager IB, Haight T, Sternfeld B, et al. Effects of physical activity and body composition on functional limitation in the elderly: Application of the marginal structural model. Epidemiology. 2004;15:479–93. doi: 10.1097/01.ede.0000128401.55545.c6. [DOI] [PubMed] [Google Scholar]
- 27.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 28.Franssen FM, O’Donnell DE, Goossens GH, et al. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63:1110–17. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 29.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 30.Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–42. doi: 10.1097/MCO.0b013e3281a30594. [DOI] [PubMed] [Google Scholar]
- 32.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–87. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 33.Divo MJ, Cabrera C, Casanova C, et al. Comorbidity distribution, clinical expression and survival in COPD patients with different body mass index. Chronic Obstr Pulm Dis. 2014;1:229–38. doi: 10.15326/jcopdf.1.2.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi Y, Hasegawa W, Yasunaga H, et al. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int J Chron Obstruct Pulmon Dis. 2014;9:1337–46. doi: 10.2147/COPD.S75175. [DOI] [PMC free article] [PubMed] [Google Scholar]