Abstract

Mapping different structural forms of serotonin subtypes 5-HT1A–5-HT7 using a selective-specific ligand with good pharmacokinetics and brain permeability can open avenues for personalized medication in depressed population. Herein, the selective 5-HT1A/7 antagonist, modified for enhanced brain permeation, is developed as a homobivalent ligand, (6-AcBTZ)2DTPA. After in-depth computational studies to probe the binding mechanism, two-step synthesis lead to (6-AcBTZ)2DTPA. Biocompatibility studies indicated cytocompatibility with 3.6–1.64% cell death (0.1 mM–1 pM) and hemocompatibility with 2.33% hemolysis of human erythrocytes. When 99mTc-radiolabeled in a quantitative yield (98%), a stable preparation was obtained with 7.4 and 3.5% dissociation upon incubation with human serum and excess cysteine. The single-photon-emission computed tomography (SPECT) tracer 99mTc-(6-AcBTZ)2DTPA showed biphasic clearance (t1/2, distribution = 0.5 min and t1/2, elimination = 482 min) and maximum brain uptake of 0.42 ± 0.02% ID/g with the regional localization (hippocampus: 11.38% ID/g; cortex: 26.42% ID/g; cerebellum: 25.23% ID/g). Thus, the 99mTc-metal-based SPECT neurotracer holds potential for neuroreceptor mapping.
Introduction
There is a growing concern related to the increasing share of neurological disorders leading to the disability in population and associated emotional-financial burden on the society.1 Depression and anxiety are among the most common psychological consequences of neurological disorders, and when unmanaged, these bear a detrimental effect on the treatment management of a neurological disorder.2 Biochemically, depression and anxiety involve a complex interplay of neurotransmitters and neuroreceptors. The most popular theory of depression, “the monoamine hypothesis”, postulates that the reduction of serotonin transmission forms the underlying basis for depression. Thus, the theory brings a serotonergic pathway as the key target in the management of depression. The reduction of serotonergic signal transmission can be attributed to depletion of serotonin neurotransmitters or other mechanisms involving serotonin receptors such as alterations in receptor distribution and impaired receptor function.3,4 The role of serotonin receptors, particularly the subtype 5-HT1A, is well established as a modulator of various physiological functions and highly implicated in depression and anxiety.3−5 A report summarizing postmortem analysis of depressed models indicates (1) decreased density and affinity of 5-HT1A receptors in the hippocampus, amygdala, and cortex of depressed/stressed suicide victims and (2) reduced gene expression of 5-HT1A receptors in the receptor-rich regions in patients with no or on different antidepressant treatment regimens.4 Not surprisingly, 5-HT1A has been the key target for designing antidepressants and anxiolytics.4−7 Another subtype of 5-HT receptors discovered recently and speculated to have central role in depression is 5-HT7.6,7 The antidepressant-like behavior induced in animal models after blockading the 5-HT7 receptor and the high affinity of antidepressants, for example, the tricyclics and selective serotonin reuptake inhibitor (SSRI)-based ligands, toward 5-HT7 has lead researchers to consider the 5-HT7 receptor as a therapeutic target for depression.6−8 Emerging reports also point to the interplay and cross-talk of 5-HT1A with 5-HT7 in the prognosis of the disorders, although the exact mechanisms of action are yet to be established.7,9,10 These are highly coexpressed as homodimers (5-HT1A–5-HT1A and 5-HT7–5-HT7) and heterodimers (5-HT1A–5-HT7) both in vitro and in vivo, as seen in Förster Resonance Energy Transfer and coimmunoprecipitation studies.9,10 In depression and anxiety, modulation of receptor signaling by heterodimerization plays an important role. It is hypothesized that, under normal physiology, the density of postsynaptic dimeric 5-HT1A receptors is dominant.9 However, in depression or anxiety, the increased presynaptic 5-HT1A–5-HT7 heterodimers over the postsynaptic receptor density may alter the 5-HT1A expression, thereby affecting the feedback mechanism and, ultimately, the serotonin signaling.9,10 Overall, the heterodimers play a crucial role in maintaining 5-HT1A-mediated signaling and internalization under pathological conditions. The new treatment strategy for depression using designed multiple ligands proposes the antagonism of 5-HT1A/7 receptors.6−8
Considering the influence of dimerization on receptor-mediated signaling, quantification of dimeric receptors has become the most studied pharmacological mark for the diagnosis or treatment of depression/anxiety. Receptor mapping using molecular imaging, namely, positron emission tomography (PET) and single-photon-emission computed tomography (SPECT) modalities, can provide information related to functional occupancy, structural forms, and distribution of neuroreceptors.8,11−14 The success relies on the selectivity of the receptor-targeted imaging probes (radioligands). Targeting dimeric receptors requires ligands with two units of pharmacophore separated by an optimum linker length.15,16 Such ligands conform to the bivalent ligand approach coined by Portoghese et al.17,18 This approach has been extensively validated for designing selective and specific ligands for δ-κ (opioid), δ-μ (opioid), δ-CB1 (opioid-cannabinoid1), CXCR4 (chemokine), and 5-HT1A and 5-HT4 (serotonin) receptors.19−24 Similar endeavors have been efficiently employed by our own group for dimeric 5-HT1A receptors, wherein the ligand binds to the dimeric receptor through bivalent interactions and thus enhances selective drug actions.22,23 However, reports for bivalent ligand for dimeric receptors of 5-HT are a handful, with major emphasis on selectivity only for 5-HT1A receptors. As per our knowledge, there has been no report on the development of molecular probes for 5-HT7 dimers, although molecular probes for monomeric receptors exist. Prominent examples for molecular imaging of monomeric 5-HT1A and 5-HT7 are [11C]WAY100635, [18F]MPPF, [18F]FCWAY, [11C]trans-MeFWAY, [18F]BMPPSiF, and [68Ga]DO3A-butyl-MPP and [18F]2FP3, [11C]DR4446, [11C]CIMBI 717, and biaryl derivatives, respectively.14
A majority of the probes are PET-based probes radiolabeled using 11C or 18F. The on-site production, short half-life (11C, 18F), and the complex chemistry required to develop a PET radiopharmaceutical limit its adoption despite providing a higher resolution for receptor imaging. On the other hand, metal-based radiotracers offer functional and economic versatility. Pre-eminent metal-based radionuclides (99mTc (SPECT) and 68Ga (PET)) are generator-based, which allows elution of radionuclides on an as-needed basis. Second, since the principles of targeting and metal loading remain the same, the same ligand can be used for SPECT, PET, and even MRI or optical imaging by merely changing the metal ion that is compatible with the chelator. Among the polyamino-polycarboxylic acyclic chelators, diethylenetriaminepentaacetic acid (DTPA) has been the choice due to fast and high yielding radiolabeling. Despite the advantages, only a few metal-based radioprobes, especially for neuroreceptors, have been successful, with 99mTc-TRODAT for dopamine transporters being the prominent example.12 Following a similar pursuit, the presented work was focused on the design and synthesis of a metal-labeled radioligand for targeting a dimeric system of 5-HT1A–5-HT7 receptors using the bivalent ligand approach and validation through SPECT imaging. The ligand has been investigated for its receptor binding, biocompatibility, stability, and neuroimaging applications using computational and biological evaluations. Receptor distribution and affinity of the synthesized radioligand were established via regional localization and radioligand binding studies.
Results and Discussion
Ligand Design and Development
Choice of the Pharmacophore
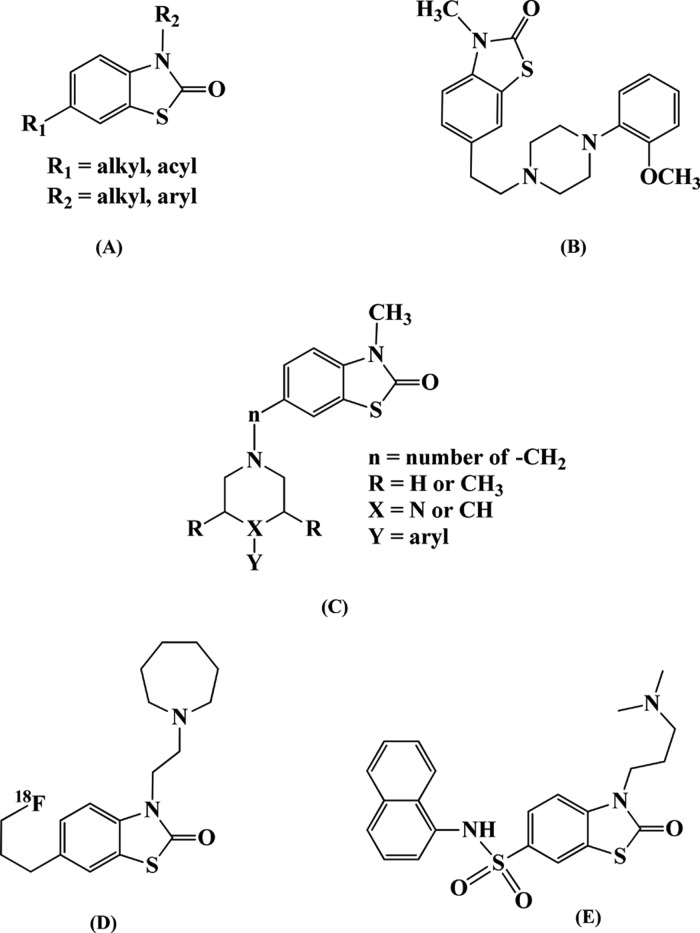
The structural similarity between 5-HT1A and 5-HT7 receptors has showed cross-reactivity among the ligands synthesized for these receptors.25 Among the various heterocyclic pharmacophores reported for 5-HT1A and 5-HT7 receptors, benzothiazolone (BTZ) derivatives gave the highest selectivity and affinity, owing to their ability to form H-bonding and side-chain hydrophobic interactions with these receptors through carbonyl oxygen and the BTZ ring.23,26−28 Recently, our research group has developed a BTZ-based homobivalent ligand, 99mTc-(BTZ)2DTPA, with a binding affinity of nanomolar concentration for 5-HT1A and 5-HT7 receptors.23 Following the lead, in this work, we chose a substituted BTZ pharmacophore to design a high-affinity ligand. We selected a 3,6-disubstituted BTZ scaffold originally reported by Taverne et al. and Mouithys-Mickalad et al. for 5-HT1A receptors.29,30 So far, prominent disubstituted modifications of 6-acyl/sulfonyl/alkyl-3-aryl/alkyl-2(3H)-BTZ have been carried out with end applications for selective 5-HT1A, 5-HT6, D2, and σ1 receptor-targeting ligands, yet no ligand has been synthesized for 5-HT7 receptors (Figure 1).29−34 Promising results in terms of better receptor binding, radioligand localization in specific brain regions, and antianxiety, antipsychotic, and anticonvulsant properties were quoted for 6-acyl-3-alkyl-2(3H)-BTZ derivatives. Considering the metabolic stability of acetyl functionality in human serum, thereby increasing the circulation time and high receptor-binding affinities of 3-alkylamine-BTZ derivatives, we modified the 6- and 3-positions of 6-acyl-3-alkyl-2(3H)-BTZ scaffold with 6-acetyl and 3-propylamine functionalities, where the terminal 3-alkylamine group would further conjugate with the DTPA chelator to facilitate bivalent ligand binding. As the ligands synthesized for 5-HT1A–5-HT7 receptors show cross-reactivity, we expect the designed bivalent ligand to exhibit selectivity and high affinity for the targeted serotonin receptor subtypes. DTPA-conjugated 6-acetyl-3-propyl-2(3H)-BTZ ((6-AcBTZ)2DTPA) is the first bivalent ligand that has a different scaffold from the other reported 5-HT1A–5-HT7 receptor radioligands, so we believe that the evaluations performed with this ligand may set up beneficial information about 5-HT1A–5-HT7 receptor binding.
Figure 1.
Structure of 3,6-disubstituted benzothiazolone derivatives reported for (B, C) 5-HT1A, (E) 5-HT6, (B) D2, and (A, C, D) σ1 receptors.
Pharmacophore Arrangement
Since our aim was to develop a bivalent ligand using a modified pharmacophore, we had to optimize two parameters: (i) the end-to-end ligand distances of the two units of the pharmacophores in the bivalent ligand and (ii) the position of the acetyl group in the modified pharmacophore, which can influence the receptor binding affinity of a ligand by modifying binding interactions. Based on the reports of bivalent ligands for receptor targeting,19−24 we exploited DTPA as both the chelator and linker. To ascertain the position and relative affinities of acetyl functionalization in BTZ, an in silico library of four compounds was generated and evaluated. The calculated end-to-end distance of designed ligands was found in the range of 21–26 Å, indicating good dimeric receptor-bridging ability (Figure 2). The relation between the position of acetyl substitution and binding affinity of individual ligands was analyzed by performing docking studies onto the monomeric and homo- and heterodimeric receptor models, and the relative affinities were determined on the basis of G-Score values (Table 1). Postdocking analysis of receptor–ligand complexes provided receptor–ligand interactions and the relative orientations of the ligands in the binding grooves of monomeric and dimeric receptors (see the Supporting Information).
Figure 2.

End-to-end distances of four derivatives of the (AcBTZ)2DTPA molecule.
Table 1. G-Score Values (kcal/mol) of Molecules Obtained with Dimeric and Monomeric Receptor Systems.
| 5-HT1A–5-HT7 heterodimer |
||||||
|---|---|---|---|---|---|---|
| molecule | 5-HT1A-5–HT1A homodimer | 5-HT7–5-HT7 homodimer | 5-HT1A pocket | 5-HT7 pocket | 5-HT1A monomer | 5-HT7 monomer |
| (7-AcBTZ)2DTPA | –1.7 | –2.4 | –2.1 | –4.1 | –2.1 | –2.8 |
| (6-AcBTZ)2DTPA | –4.0 | –5.6 | –2.8 | –5.0 | –5.1 | –6.3 |
| (5-AcBTZ)2DTPA | –4.8 | –3.0 | 0.6 | –2.5 | –3.0 | –1.8 |
| (4-AcBTZ)2DTPA | –6.2 | –3.4 | –1.1 | –0.8 | –3.8 | –0.5 |
| SB-269970 | –5.6 | –6.8 | ||||
| WAY-100635 | –4.8 | –5.0 | ||||
Docking with the Heterodimeric 5-HT1A–5-HT7 Receptor Model
Among the four designed ligands, molecule 2 showed the highest binding in the 5-HT1A (G-Score = −2.8) and 5-HT7 (G-Score = −5.0) pockets. Molecule 1 interacted with the amino acid residues of ECL2 (extra cellular loop 2) via H-bond and salt bridges in the 5-HT1A binding pocket. Albeit acquiring a U-shape orientation, molecule 1 could not bind properly as the two acetyl groups at the 7-position prohibited it to enter into the binding pocket. Likewise, it could also not bind to the 5-HT7 binding pocket. The N-terminal residues along with the three ECLs (ECL1–ECL3) of the 5-HT7 receptor unit gripped molecule 1 in the ECL region, which forbid it to reach the binding groove, and thus indicated low binding. Unlike molecule 1, the presence of acetyl groups at the 6-position in molecule 2 facilitated its binding to both binding pockets. Residues Lys191 and Leu380 interacted with the proton of the −NH2 group and oxygen of the −COOH group of DTPA in the 5-HT1A binding pocket, whereas residues Arg350, Gln235, Asn226, and Phe343 interacted through H-bonding with carbonyl oxygen of the BTZ ring, the −COOH group of DTPA, acetyl group, the proton of −CONH, and the π–π stacking with the two aromatic rings of BTZ moiety in the 5-HT7 binding pocket. These interactions suggested better binding of molecule 2. Molecule 3 showed an analogous trend to that of molecule 1. It was oriented in a distorted U-shape conformation with BTZ rings projected in the opposite direction. The H-bond (Asn226, Arg350, Gln235), salt bridge (Arg350), π–cation (Phe237), and π–π stacking (Phe343) interactions were observed with the O-atom of the acetyl group, BTZ, carboxylate oxygen, the protonated tertiary N-atom of DTPA, and the thiazolone ring of BTZ moiety. These interactions together pulled the bulky molecule deeper inside the binding groove, resulting into a strained conformation of the receptor–ligand complex. The small-size cavity further enhanced postcomplexation conformational strain, which leads to low binding of molecule 3. Molecule 4 gave resembling interactions as noted for molecule 3. The only H-bond interaction was with Thr288 via carbonyl oxygen of the BTZ ring, suggesting a low G-Score value for 5-HT1A binding. A further reduced G-Score value of −0.8 noted for the 5-HT7 pocket indicated a lack of interactions necessary for better binding. Overall, molecule 2 showed better binding.
Docking with Homodimeric 5-HT1A–5-HT7 Receptor Models
Molecules 1 and 2 were binding better at the two binding pockets as compared to molecules 3 and 4, which could only bind at one of the pockets in the homodimers despite obtaining higher G-Score values as compared to molecules 1 and 2. When docked with monomeric 5-HT1A and 5-HT7 receptor models, binding of molecule 2 was found best with G-Score values of −5.1 and −6.3, respectively. Additionally, binding of molecule 2 was congruent with reference ligands SB-269970 and WAY-100635 (data not shown), which further suggested the potent nature of molecule 2. The respective 2D and 3D ligand interaction diagrams (LIDs) are provided in the Supporting Information.
Docking with Technetium Complex of 2
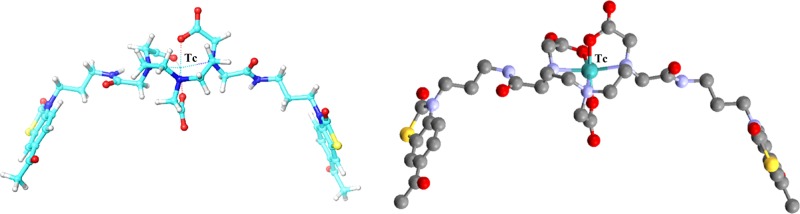
As molecule 2 showed better binding for targeted receptors and our ultimate aim was to load the ligand with metal radionuclide 99mtechnetium, it was further complexed with technetium to analyze any post-metal complexation variation. The DFT-optimized Tc-(6-AcBTZ)2DTPA complex (Figure 3) was docked analogously onto the dimeric and monomeric 5-HT1A–5-HT7 receptors. A majority of the interactions of (6-AcBTZ)2DTPA and Tc-(6-AcBTZ)2DTPA were found identical in all the receptor systems (Table 2). This suggested no considerable change in the binding upon metal complexation (see the Supporting Information). In addition, we observed increased G-Score values with homodimeric 5-HT1A–5-HT1A and 5-HT7–5-HT7 receptors, namely, from −4.0 to −4.3 and −5.6 to −7.2, respectively. Similarly, binding with heterodimeric 5-HT1A–5-HT7 receptors was also enhanced upon technetium complexation with the increased G-Score values from −2.8 to −3.6 and −5.0 to −5.1 in the binding pockets of 5-HT1A and 5-HT7 receptors, respectively (see the Supporting Information). These results encouraged molecule 2 as the best found ligand, and so, it was synthesized and radiolabeled for further evaluations.
Figure 3.
DFT-optimized structure of Tc-(6-AcBTZ)2DTPA using (left) Maestro and (right) Mercury tools, wherein Tc-metal occupies an octahedral geometry with DTPA.
Table 2. Interaction Analysis of Receptor–Ligand Interactions of (6-AcBTZ)2DTPA and Tc-(6-AcBTZ)2DTPA with 5-HT1A–5-HT7 Dimeric and Monomeric Receptorsa.
| receptor system | hydrophobic interactions | π–π stacking and π–cationinteractions | polar interaction | charged interactions | H-bond and salt bridge |
|---|---|---|---|---|---|
| 5-HT1A–5-HT1A homodimer | chain A: Trp102, Leu104, Pro184, Leu99, Val107, Leu95, Ala94, Pro91, Ile47, Leu43, Leu41 | chain A: Asn100, Thr103, Gln106, Thr108, Ser40 | chain A: Lys101, Asp185 | chain A: Lys101 | |
| chain B: Leu43, Ala94, Leu95, Val98, Leu99, Leu104, Pro184, Ile47 | chain B: Gln36, Ser40, Thr103 | chain B: Lys101, Asp185 | chain B: Gln36 | ||
| 5-HT7–5-HT7 homodimer | chain A: Tyr76, Ile74, Ile144, Trp148, Ile149 | chain B: Tyr249 | chain A: Asn75, Gln73, Thr141, His152 | chain A: Lys147, Arg78, Asp142 | chain A: Asn75, Lys147, Trp148, Ile149, His152, Gly14 |
| chain B: Phe237, Ile241, Tyr242, Ala245, Phe352, Ala349, Leu346, Leu345, Tyr249, Tyr249, Leu256 | chain B: Thr348, Ser253 | ||||
| 5-HT1A–5-HT7 heterodimer | |||||
| 5-HT1A pocket | Phe370, Cys371, Leu380, Leu381, Ile384, Ile385, Tyr96, Pro348, Ala383, Leu388, Val367, Ile189, Ala186 | Phe370, Hip193 | Thr196, Ser374, Ser190, Thr188, Asn100, Gln97, Thr379, Hie193, Ser190, Ser182 | Arg181, Asp180, Glu179, Hip193, Asp192, Lys191, Asp183 | Thr188, Leu380, Lys191 |
| 5-HT7 pocket | Val230, Leu232, Phe237, Pro351, Trp364, Leu363, Pro362, Ile361, Cys360, Val225, Cys354, Ile233, Tyr239, Val163, Phe344, Phe343, Ala247 | Phe343 | Asn226, Ser234, Gln235, Thr240, Thr244, Ser243, Ser347 | Asp227, Asp228, Lys229, Asp236, Arg350, Arg367, Glu365, | Leu363, Asn226, Gln235, Arg350 |
| 5-HT1A monomer | Phe370, Val367, Ile363, Trp387, Ile384, Ala383, Leu381, Leu380, Pro378, Ala186, Cys187, Ile189, Tyr96 | Thr188, Thr379, Asn100, Gln97, Ser182, Ser190, Ser374 | Asp185, Lys191, Lys101, Asp180, Glu179, Arg181, Asp192, Hip193 | Leu381, Tyr96, Hip193, Lys191, Ile189, Cys187, Asp180 | |
| 5-HT7 monomer | Val230, Leu232, Ile233, Phe237, Val163, Cys166, Phe343, Phe344, Pro351, Cys354, Cys360, Ile361, Leu363, Val225, Phe343, Ala247, Tyr239 | Phe343 | Asn226, Ser234, Gln235, Thr240, Ser243, Thr244, Ser347 | Glu366, Arg350, Asp236, Asp228, Asp227 | Arg350, Cys354, Gln235, Ser243 |
Italic text: interactions observed with Tc-(6-AcBTZ)2DTPA; bold normal text: common interactions between (6-AcBTZ)2DTPA and Tc-(6-AcBTZ)2DTPA ligands; normal text: interactions of (6-AcBTZ)2DTPA.
Chemistry
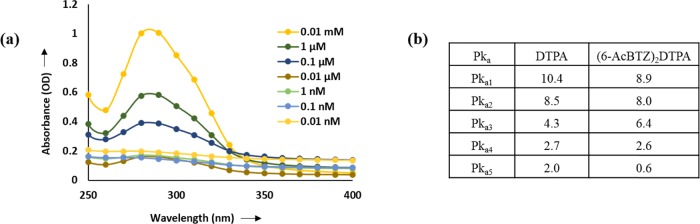
The ligand (6-AcBTZ)2DTPA was synthesized as shown in Scheme 1. Briefly, 6-acetylbenzothiazole was reacted with 3-bromopropylamine hydrochloride in an equimolar amount to yield the primary amine intermediate. The amine was precipitated as its hydrochloride salt and characterized as such using spectroscopic techniques. The appearance of NMR signals at δH 1.97–2.06, 3.24–3.29, 3.90–3.94 (−CH2), 4.03–4.07 (−NH2), 7.13–7.16, and 7.72–7.75 ppm (aromatic −CH) suggested product formation. The HPLC-purified amine and DTPA dianhydride were then reacted in 2:1 molar ratio in the presence of triethylamine (pH 8.0) in inert conditions to yield the desired bis-conjugated bivalent ligand (6-AcBTZ)2DTPA. It was then precipitated using chilled ether and further purified using HPLC to give the final product in 91–92% yield. The disappearance of amine protons (4.03–4.07 ppm) and the appearance of signals at 2.44–3.45 ppm for methylene protons of DTPA confirmed the desired product. UV–Vis data showed a broad peak in the wavelength range of 260–330 nm, indicating the successful conjugation of amine to DTPA. The broadened absorption was attributed to the overlapping of π–π* transition of amide (220–260 nm) and carbonyl groups of 6-acetylbenzothiazolone moiety (280–320 nm), giving rise to mixed energy levels upon excitation in the wavelength range of 250–400 nm (Figure 4a).35 Lower protonation constants were observed for the synthesized bivalent ligand as compared to DTPA,36 which suggested fast ionization and quick delivery of acidic protons in the aqueous solution and thus promoted an adequate metal complexation of the ligand (Figure 4b).
Scheme 1. Synthesis of (6-AcBTZ)2DTPA Using the Bivalent Ligand Approach.
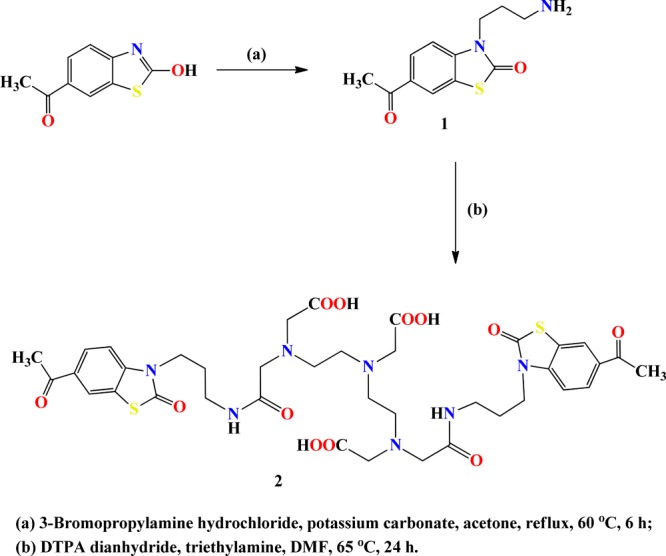
Figure 4.
Physicochemical characterizations: (a) UV–Vis and (b) potentiometric titration of the (6-AcBTZ)2DTPA ligand.
Biocompatibility
MTT Assay
No cellular cytotoxicity was noted in the concentration range of 0.1 mM–1 pM on the normal HEK-293 cell line. The maximum cell death at this concentration range was only 3.6 to 1.64% with reference to control (24 h). The extended follow-up (48 and 72 h) in the similar concentration range resulted in cell death in the range of 6.52 to 3.69% and 6.64 to 17.98%, respectively (Figure 5a). We observed optimum percent cell viability even upon extended drug treatment, suggesting a substantial biocompatible window of this ligand for its useful application in medical imaging.
Figure 5.
Biocompatibility, stability, and pharmacokinetics studies: (a) MTT assay and (b) hemolysis study, (c) radiolabeling efficiency in saline, (d) cysteine challenge test, (e) human serum stability test, and (f) blood kinetics evaluation studies.
Hemocompatibility
The blood compatibility of the synthesized ligand is essential for diagnostic purposes as a majority of the imaging agents are administered intravenously, and so, the hemocompatibility was analyzed by performing hemolysis study on fresh human blood samples. A transparent and achromatic supernatant indicated no hemolytic effects or erythrocyte destruction upon incubation with the ligand (6-AcBTZ)2DTPA. Hemoglobin measurements at 540 nm for the developed ligand showed only 2.33% erythrocyte destruction until 4 h, suggesting no appreciable hemolytic effects, which, in turn, indicate no influence on the structure of biomembranes, thereby ensuring a discernible compatibility with blood cells (Figure 5b).37
Radiolabeling and Optimization of Stability Parameters
The synthesized ligand was radiolabeled with Na[99mTcO4] (pertechnetate) using the direct radiolabeling method, wherein pertechnetate was reduced with an acidic SnCl2 solution (pH 7.4) to obtain a maximum radiolabeling efficiency. Radiolabeling parameters, namely, incubation time, SnCl2 concentration, and pH of labeling, were optimized to ensure maximum radioconjugation of the (6-AcBTZ)2DTPA ligand into the 99mTc-(6-AcBTZ)2DTPA radioligand (see the Supporting Information). Optimum radiolabeling was achieved using 100 μg/mL SnCl2 (pH = 6.5 ± 0.3) at 10 min of incubation. The biological stability of 99mTc-(6-AcBTZ)2DTPA was estimated under physiological conditions. When incubated with saline, 99mTc-(6-AcBTZ)2DTPA was found stable for up to 24 h, with a maximum radiochemical purity of 98–99% respectively (Figure 5c). The radioligand was further incubated with a freshly prepared human serum sample at physiological conditions to determine its in vitro stability, where we found it stable for up to 24 h with only 7.4% dissociation of the radiolabeled compound (Figure 5e). The trans-complexation stability was also evaluated by challenging 99mTc-(6-AcBTZ)2DTPA with cysteine (25–100 mM). The radioligand exhibited only 0.72, 2.35, and 3.53% trans-complexation of 99mTc-radioisotope upon challenging it with 25, 50, and 100 mM concentrations of cysteine, respectively. Approximately 96% radioactivity was found intact to the radioconjugate even after the 2 h challenge test with 100 mM cysteine at 37 °C (Figure 5d).
Pharmacokinetics
Longer blood residence time due to nonspecific interactions of the radioligand with blood components has been an interfering parameter in the development of an effective radiopharmaceutical for medical imaging purposes. Thus, to understand the blood clearance pattern of 99mTc-(6-AcBTZ)2DTPA, blood kinetics experiment was conducted. Quick washout of the radioligand was noted as 87.84 ± 0.42% of the administered radioactivity washed out from circulation within 30 min post injection (p.i.). Only 4.87 ± 0.22 and 2.05 ± 0.06% radioactivity values were retained in the circulation after 3 and 24 h respectively, reflecting biphasic clearance with the biological half-lives of t1/2, fast = 0.5 min and t1/2, slow = 8 h and 2 min (or 482 min, Figure 5f).
BBB Permeability
Targeting a neuroreceptor in the central nervous system requires permeation through the blood–brain barrier (BBB). Brain permeability of a molecule is governed by various parameters, with lipophilicity of the molecule being one of the major determinants. The lipophilicity of a molecule can be determined with the help of octanol–water partition coefficient (log Po/w). Ideally, molecules with log P values of 1.0–3.5 may be better able to show BBB permeability and high brain uptake, although exceptions exist.38 The theoretical log P values calculated from MolInspiration and Schrödinger tools were −1.93 and −2.10, respectively, whereas experimental log P was found to be −0.36 at pH 7.4. The negative log P is attributed to the highly hydrophilic nature of the DTPA linker due to the presence of carboxylic groups. An additional increment in the value of log P was observed upon radiolabeling with 99mTc at physiological pH, which was found to be 0.58 (average of three experiments) somewhat lower than the ideal range. Log P is one of the predictive criteria for efficient BBB permeation; therefore, it may not always be predictive of positive in vivo brain uptake.39 The increase in log P is credited to the formation of a new chemical entity with distinct pharmacological profile after radiolabeling, with reference to the original molecule.22 However, the log P of acetylated derivative was less than the non-acetylated 99mTc-(BTZ)2DTPA analog (log P = 1.19),23 as earlier reported by our group for 5-HT1A–5-HT7 receptors. This attributed from the hydrophilic contribution of the acetyl group present in 99mTc-(6-AcBTZ)2DTPA as compared to the non-acetylated 99mTc-(BTZ)2DTPA radiopharmaceutical.
Preclinical Evaluations
In Vivo Dynamic SPECT Imaging
The brain penetration, distribution, and clearance through various organs of the developed radiotracer were analyzed by dynamic SPECT scans of normal New Zealand rabbits. Images analyzed after 30 min p.i. (on-bed injection) showed brain uptake as early as 60 s p.i. The maximum brain uptake was obtained within 2 min p.i., which retained until 4 min p.i. following a slow washout, suggesting no non-specific binding or a noticeable retention in the normal brain (Figure 6).
Figure 6.
Dynamic SPECT scan and time–activity curve of 99mTc-(6-AcBTZ)2DTPA.
Biodistribution Studies
The SPECT scan observations were then corroborated through the biodistribution studies, where radioactivity accumulation was expressed as the percentage of injected radioactivity dose/gram of the tissues. The highest brain accumulation of 0.42 ± 0.02% ID/g was noted at 15 min p.i., which was found comparable to the well-known metal-based radiotracer 99mTc-TRODAT-1 (dopamine transporters; brain uptake: 0.40% ID/g).12 Other promising 99mTc-labeled radiopharmaceuticals have also shown brain uptake in the 0.2–1.4% ID/g range.13 The uptake of 1.10 ± 0.04% ID/g at 30 min p.i. relative to 3.19 ± 0.14% ID/g at 2 min p.i. in blood indicated a high blood pool activity along with a quick washout analogous to the observations of the pharmacokinetics experiment. The heart uptake of 2.54 ± 0.11% ID/g at 2 min p.i. is attributed to distribution and regulation of 5-HT1A–5-HT7 receptors in circadian rhythm; thus, subsequent binding of the compound was observed in the heart.40−45 The activity accumulation in the lungs, intestine, stomach, and spleen is credited to the peripheral expression of these serotonin receptor subtypes in the non-neuronal tissues of these organs.40−45 Major activity uptake of 20.14 ± 0.91, 18.89 ± 0.77, 15.96 ± 0.53, 15.31 ± 0.62, and 14.20 ± 0.58% ID/g and 8.32 ± 0.34, 6.33 ± 0.27, 6.20 ± 0.27, 6.04 ± 0.24, and 5.15 ± 0.21% ID/g were observed in the liver and kidney, respectively, at 2, 5, 10, 15, and 30 min p.i. The highest radioactivity accumulation observed in the liver followed by kidney indicated the lipophilic nature of the synthesized compound. It also suggested the combined hepatobiliary-renal excretion mode for the radiotracer partly due to the coexisting lipophilic–hydrophilic nature of the acetyl-substituted acid-conjugated biomolecule (Figure 7).
Figure 7.

Biodistribution studies of 99mTc-(6-AcBTZ)2DTPA.
Regional Uptake Studies
The specific localization of the 99mTc-(6-AcBTZ)2DTPA radioligand was evaluated using regional brain uptake studies in the post-mortem brain of female Balb/c mice. The regional uptake of the intravenous (i.v.) administered radiotracer was expressed as % ID/g of brain regions in comparison to the total brain uptake. Appreciated regional activity accumulation in the hippocampus (11.38%, 30 min p.i.), cortex (26.42%, 15 min p.i.), and cerebellum (25.23%, 15 min p.i.) indicated specific localization of the radioligand in 5-HT1A–5-HT7 receptor-rich regions of the brain (Figure 8a).40−47 Persistence in hippocampal, cortical, and cerebral regions was observed for up to 30 min p.i., which indicated high affinity for the 5-HT1A and 5-HT7 receptors. The Kd values were 0.75 and 6.59 nM, respectively, for the 5-HT1A and 5-HT7 receptors. Binding with the striatum and the rest of brain regions was accredited to nonspecific binding and tissue heterogeneity, which was substantiated by a decline of activity accumulation in these regions. The selective binding toward targeted receptors was further justified by achieving 4.25-, 8.07-, and 17.10-fold maximum radiotracer accumulation in the hippocampus, cortex, and cerebellum at 30, 10, and 2 min p.i., respectively, with respect to the striatum (Figure 8b). Significant brain/blood ratio of 0.33 (30 min p.i.) indicated brain penetration, as has been supported by SPECT images and the biodistribution observations, which exhibited a noticeable permeation through BBB and brain uptake of 99mTc-(6-AcBTZ)2DTPA.
Figure 8.
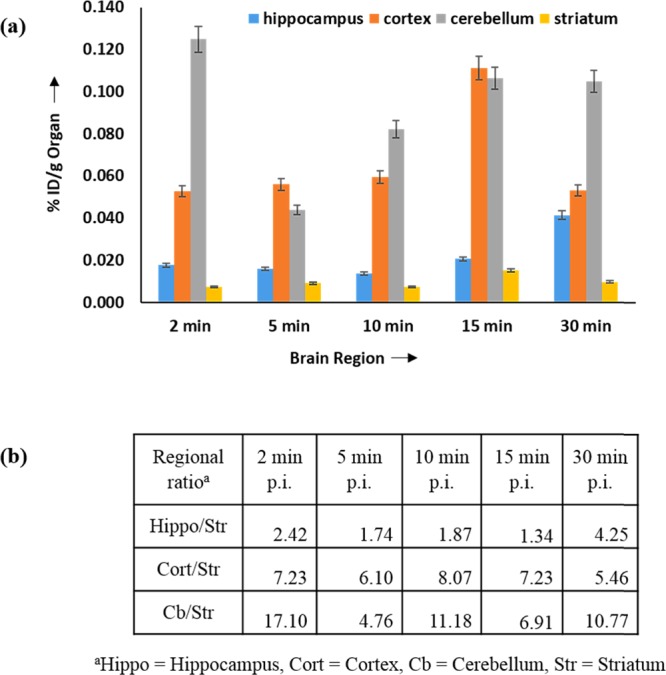
(a) Regional localization and (b) selectivity studies of 99mTc-(6-AcBTZ)2DTPA.
Conclusions
A homobivalent radioligand was developed using structure-based drug design methodology. The influence of substitution position of the acetyl group on the BTZ pharmacophore was analyzed via receptor–ligand interactions with dimeric and monomeric 5-HT1A–5-HT7 receptor forms. Biocompatibility and stability of ligand in the unlabeled and 99mTc-labeled forms were analyzed thoroughly. BBB permeation (log P = 0.58) of 99mTc-(6-AcBTZ)2DTPA was noted in SPECT scans. The synthesized radiotracer showed comparable maximum brain uptake to that of well-reported and clinically used metallo-radiopharmaceutical 99mTc-TRODAT-1, suggesting promising candidature for nuclear medicine imaging applications. Optimum radioactivity accumulation observed in receptor-rich regions showed significant brain uptake and specific localization of the homobivalent radiotracer for the targeted receptors. We introduced acetylation to have metabolic stability in human serum, increased circulation time, and high receptor-binding affinities. However, we observed reduced total brain accumulation (0.42 ± 0.02% ID/g) for the acetylated 99mTc-(6-AcBTZ)2DTPA radiotracer as compared to the non-acetylated 99mTc-(BTZ)2DTPA (2.08 ± 0.08% ID/g) radiopharmaceutical, which occurred due to the decreased lipophilicity, as indicated in log P. We also observed a difference in the regional uptake for the acetylated radiotracer (hippocampus: 11.38% ID/g; cortex: 26.42% ID/g) as compared to the non-acetylated radiotracer23 (hippocampus: 41.83% ID/g; cortex: 23.56% ID/g). Nevertheless, lower BBB permeation and decreased brain uptake emphasize on further structural modifications for this radiotracer.
Experimental Section
In Silico Studies
The molecular docking studies were carried using Schrödinger software suite.48 The studies were mainly centered on identifying the structure-based receptor–ligand interactions and corresponding binding affinities in the active sites of the 5-HT1A and 5-HT7 receptors. The homology models of 5-HT1A–5-HT7 receptors were generated using the well-reported protocols (see the Supporting Information).49,50 Preparation of ligands and the structure optimization parameters of the best found metal-based ligand are reported elsewhere.23
Chemicals
The required chemicals were procured from Sigma-Aldrich/Merck/Fisher Scientific and used directly without any purification. Reactions needing anhydrous conditions were carried under continuous supply of nitrogen gas and using oven-dried glasswares. Reaction progress was monitored using silica gel thin-layer chromatography (SG-TLC; F254, Merck-Germany) and visualized in either iodine-impregnated silica or a UV–Vis chamber. Instant thin-layer chromatography (ITLC; Paul German, USA) was used for determining the extent of radioconjugation and percent radiochemical purity of the 99mTc-labeled radiocomplex. 99mTc was purchased from Regional Centre for Radiopharmaceuticals (northern region), BRIT, DAE, India.
Animal Models
The required animal experiments were duly approved and conducted in accordance with the guidelines of the Institutional Animal Ethics Committee (IAEC) of INMAS (registration no. 8/GO/a/99/CPCSEA). New Zealand rabbits weighing 2.5–3.0 kg were used for blood kinetics and SPECT scans. The biodistribution and regional uptake studies were performed on the normal female BALB/c mice weighing 25–30 g. The animals were received from a stock colony, which was maintained at the INMAS animal facility, which were fed ad libitum with continuous water supply. The habitat temperatures were maintained at 22 ± 2 °C with the natural daytime light followed by no light post 19 h until dawn.
Chemical Synthesis
6-Acetyl-3-(3-aminopropyl)benzo[d]thiazol-2(3H)-one (1)
The equimolar amounts of 6-acetyl-2-hydroxybenzothiazole (or 6-acetyl-benzo[d]thiazol-2-ol, 500 mg, 2.59 mmol) and K2CO3 (357.65 mg, 2.59 mmol) were mixed well in CH3COCH3 (30 mL), and the reaction mixture was heated to attain a temperature of 60 °C. 3-Bromopropylamine hydrochloride solution (566.53 mg, 2.59 mmol) prepared in CH3COCH3 was then added dropwise to the above solution. The reaction mixture was refluxed and stirred for the next 6 h. Reaction progress was accessed by disappearance of the reactant in TLC, where chloroform/methanol (9:1) solution was used as the solvent system. After reaction completion, unreacted salts were filtered, and the filtrate was evaporated to dryness. The resulting oily residue was mixed with diethyl ether (30 mL), and the amine product was precipitated as its hydrochloride salt using dropwise addition of hydrochloric acid (concentrated, 1 N). Diethyl ether was decanted off, and the precipitated light yellow solid was dried under reduced pressure to get the final product (631.5 mg, 97.6%). δH (300 MHz; D2O): 1.97–2.06 (2H, m, CH2), 3.01 (3H, s, CH3), 3.28 (2H, t, J = 4.5 Hz, CH2), 3.92 (2H, t, J = 5.1 Hz, CH2), 4.06 (2H, t, J = 2.1 Hz, NH2), 7.13–7.16 (1H, dd, J = 6.6 Hz, CH), 7.72–7.75 (2H, d, J = 4.2 Hz, 2CH). δC (75 MHz; D2O): 26.0 (CH3), 36.5 (CH2), 39.9 (CH2), 51.6 (CH2), 111.4 (CH), 122.3 (CH), 123.3 (CH), 127.9 (quart C), 131.7 (quart C), 140.2 (quart C), 172.9 (CO), 200.9 (CO). ESI-MS(+): m/z [M + H]+ calcd (found), 250.0776 (250.9). HR-MS: m/z [M + H]+ calcd (found), 250.0064 (250.0072). rt = 4.105 min.
15-(6-Acetyl-2-oxobenzo[d]thiazol-3(2H)-yl)-3-(2-((3-(6-acetyl-2-oxobenzo[d]thiazol-3(2H)-yl)propyl)amino)-2-oxoethyl)-6,9-bis(carboxymethyl)-11-oxo-3,6,9,12-tetraazapentadecan-1-oic Acid (2)
The solution of compound 1 (139.97 mg, 0.58 mmol) dissolved in dry DMF (5 mL) was added dropwise into the stirring solution of DTPA dianhydride (100 mg, 0.28 mmol) also dissolved in anhydrous DMF (10 mL). The reaction mixture was then heated up to 60 °C, refluxed, and stirred for 24 h in an inert atmosphere, wherein reaction pH was maintained at 8.0 with the help of triethylamine (TEA). Reaction progress was monitored through TLC by using a solution of water–0.01% TFA/MeOH (7:3). After reaction completion, the solvent was evaporated to dryness, the obtained residue was dissolved in the minimum volume of CH3OH, and ice-chilled ether (30 mL) was added to precipitate. The expected compound was obtained as a yellow solid. The product was purified using HPLC (219.8 mg, 91.6%). δH (300 MHz; D2O): 1.82–1.99 (4H, m, 2 × CH2), 2.44 (2H, s, CH2), 2.66 (2H, s, CH2), 2.68–3.02 (4H, m, 2 × CH2), 3.13–3.18 (4H, m, 2 × CH2), 3.29 (6H, s, 3 × CH2), 3.41–3.50 (6H, m, 3 × CH2), 3.83 (8H, s, 4 × CH2), 6.99–7.18 (1H, m, CH), 7.51–7.55 (1H, m, CH), 7.74–8.03 (4H, m, 4 × CH). δC (75 MHz; D2O): 26.0 (CH2, CH3), 40.6 (CH2), 42.3 (CH2), 46.7 (CH2), 49.4 (CH2), 52.6 (CH2), 54.7 (CH2), 57.3 (CH2), 58.6 (CH2), 111.0 (CH), 122.3 (CH), 123.2 (CH), 127.8 (CH), 131.6 (quart C), 140.5 (quart C), 164.5 (CO), 167.9 (CO), 170.4 (CONH), 171.9 (CONH), 172.3 (COOH), 172.7 (COOH), 174.2 (COOH). HR-MS: m/z [M – H]+ calcd (found), 856.2646 (856.2790). HR-MS: m/z [M + H + Na]+ calcd (found), 881.2700 (881.8383). rt = 9.264 min.
UV–Vis and Potentiometric Studies
Product formation was assured by the appearance of amide and carbonyl excitation wavelengths of the ligand upon bifunctional conjugation with DTPA. The λmax of the ligand was determined on the Biotek Synergy H4 hybrid multiplate reader instrument, and potentiometric titrations were performed as reported previously.23
Hemolysis and MTT Assays
These assays were performed as reported previously,23 and the average values of three experiments were reported as final values.
Radiolabeling of (6-AcBTZ)2DTPA
The radioisotope 99mTc was eluted as reported previously.23 Briefly, freshly prepared solution of 99mTcO4– (74–110 MBq, 2–3 mCi) was added into the mixture of lyophilized (6-AcBTZ)2DTPA ligand (1 mg) and stannous chloride (100 μg, prepared in N2-purged 0.1 N HCl solution) in deionized water (0.5 mL). The reaction pH was maintained in the range of 6.5–7.0. The final radiocomplex was purified using the C-18 Sep-Pak cartridge. Percent labeling efficiency of 99mTc-(6-AcBTZ)2DTPA was analyzed using SG-ITLC (Paul Gelman, USA) in (i) 100% CH3COCH3 and (ii) C5H5N/CH3COOH/H2O (3:5:1.5, v/v/v) as per the standard reported radiolabeling and purification procedures.22,23
Partition Coefficient (Log Po/w)
Partition coefficient of 99mTc-(6-AcBTZ)2DTPA was determined using the protocol reported previously.23 Briefly, 100 μL of the radiocomplex (100 μM) was added to 100 μL of octanol in a centrifuge, vortexed at room temperature, and centrifuged at 3000 rpm for 10 min. Radioactivity counts of 50 μL of individual layers were measured, and the log Po/w of the 99mTc-labeled radioligand was calculated by dividing radioactivity counts of octanol to the aqueous fraction. The partition coefficient of the unlabeled compound was determined using UV–Vis spectrophotometry. Similar protocol was followed, except the radioactivity part. Optical density (OD) values of equal volumes (50 μL) of organic and inorganic phases were measured on a Biotek Synergy H4 hybrid multiplate reader. Values of log Po/w were determined by taking the ratio of OD values obtained for octanol and water fractions. The average of the three experiments was reported.
Stability in Human Serum
Similar procedure was followed as reported previously.23 Briefly, 99mTc-(6-AcBTZ)2DTPA (100 μL, 3.35 MBq) was incubated with serum (900 μL) under similar environmental conditions for 24 h. Precipitation of serum proteins was achieved by collecting aliquots (100 μL) of the mixture at the incubation intervals of 0.5, 1, 2, 3, 4, and 24 h, respectively, followed by washing with ethanol (200 μL) and centrifugation at 4 °C and 300g for 20 min. The radioactivity values of respective supernatant solutions were recorded on a well-type γ-counter. Washed precipitates (ethanol, 2 × 1 mL) were measured for counts compared with those of supernatants, and the percent association with serum proteins was determined. Intact radioconjugation was determined by counting the radioactivity of supernatant solutions using ITLC-SG strips (100% CH3COCH3) at the incubation intervals of 0.5–24 h. Serum stability was reported (average of three experiments) as the plot of intact percent radiolabeling efficiency versus incubation time with human serum.
Cysteine Challenge Study
The radioligand 99mTc-(6-AcBTZ)2DTPA (100 μL, 3.25 MBq) was incubated with cysteine (25, 50, and 100 mM) at 37 °C for up to 2 h. Fifty microliters of aliquots from individual radiomixture was pipetted out and spotted on ITLC-SG strips (0.1 M PBS solution, pH 7.4). The dried ITLC-SG strips were cut and measured for radioactivity counts in the corresponding portions to calculate the fraction of radioligand chelated to the three concentrations of cysteine.
Pharmacokinetics
The blood kinetics of the radioligand was evaluated in normal New Zealand rabbits weighing approximately 2.2–2.6 kg as reported previously.23 Briefly, blood samples (200 μL) of the i.v. administered radioligand (200 μL, 37 MBq) were withdrawn beginning from 5 min–24 h, and the radioactivity counts of the aliquots were counted on the well-type γ-counter. The decay-corrected dose at different time intervals were then evaluated by assuming the whole-body blood volume as 7% of the total body weight of rabbits. The final data was reported as the percentage radioactivity remaining in blood at different time intervals.
SPECT Scans
The SPECT images were acquired on Siemens γ-camera (Symbia True Point Dual Head). A healthy rabbit of 2.5 kg was anesthetized using diazepam, followed by i.v. administration of 99mTc-(6-AcBTZ)2DTPA (100 μL, 3.7 MBq) through the ear vein. The whole-body dynamic scans were acquired for 30 min after the on-bed administration of 99mTc-(6-AcBTZ)2DTPA. Circulation of the radiotracer was observed by analyzing the scintigraphy after 30 min p.i., and the time points for tissue distribution studies were estimated.
Biodistribution Studies
Tissue distribution of the radiotracer was evaluated on normal female BALB/c mice (20–25 g, n = 15, 3). One hundred microliters of radiotracer (3.68 MBq) was i.v.-administered through tail, and mice were sacrificed at 2, 5, 10, 15, and 30 min p.i. The organs were harvested, washed, and bottled to expel the excess liquid. The radioactivity in individual organ was counted on the well-type γ-counter. Radioactivity uptake in the individual organ was calculated and reported as the % ID/g of the organ. Experiments were performed in triplicates.
Regional Uptake Evaluation
The analogous protocol was performed as described above. Briefly, 5.2 MBq of radiotracer was i.v.-injected via tail vein into the BALB/c mice (female, n = 15, group = 3, 20–25 g). The mice were euthanized using overdose of isoflurane inhalation and sacrificed at the time intervals of 2, 5, 10, 15, and 30 min p.i. The brains were then taken out, kept in ice, washed with chilled saline, and weighed on the analytical balance. The radioactivity uptake was then counted on the γ-ray counter. Brain regions (hippocampus, striatum, cortex, cerebellum, and the rest of the brain) were dissected, weighed, and measured for activity, and the regional activity accumulation of 99mTc-(6-AcBTZ)2DTPA was then determined by taking the decay correction factor into account.
Radioligand Binding Assay
The receptor binding of 99mTc-(6-AcBTZ)2DTPA was determined using the radioligand binding assays, as reported previously.23
5-HT1A Receptor
Briefly, the binding assay was performed in the final volumes of 1000 μL, which contained Tris buffer, hippocampus-membrane homogenate (61.48 μg/mL protein), and varying radioligand concentrations. The nonspecific binding was evaluated in the presence of 10 mM serotonin. Specific binding was determined as the difference between the nonspecific binding and total radioligand binding. The experiment was performed in triplicates (20 °C, 120 min), and incubation was halted by immediate filtration through GF/B filters. These filters were quickly washed with ice-cold buffer (2 mL), and the activity counts were measured on a well-type γ-counter.
5-HT7 Receptor
Triplicate experiments were performed in the final volumes of 1.0 mL, which contained the Tris buffer, cortex-membrane homogenate (2.63 mg/mL protein), and varying radioligand concentrations. These samples were then vortexed and incubated (20 °C, 60 min). The nonspecific binding was determined by an excess amount of serotonin (100-fold). Filtration and radioactivity measurements of individual samples were performed similarly, as described above.
Acknowledgments
The work was supported by INMAS-DRDO, Ministry of Defence, India (project INM-311) and INMAS-SAMEER-MEITY joint project. The authors acknowledge the Director of INMAS for his encouragements. We greatly endorse the Department of Chemistry and SCFBio of IIT Delhi for providing the necessary instrumentation facilities and the supercomputing support for the DFT calculations.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00633.
Calculated distances of designed ligands; 2D and 3D LIDs; 1H/13C-NMR and LR-MS/HR-MS data; HPLC profiles; optimized radiolabeling parameters; EZ-TLC of 99mTc-(6-AcBTZ)2DTPA; whole-body SPECT images; activity uptake in the rest of brain regions; Scatchard plots; and brief details of computational methodology, instrumentation, cell culture, hemolysis assay formula, radiochemical purity, and optimization of radiolabeling parameters (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Geneva: World Health Organization . Depression and Other Common Mental Disorders: Global Health Estimates; (2017). Licence: CC BY-NC-SA 3.0 IGO.
- Kanner A. M. Is major depression a neurologic disorder with psychiatric symptoms?. Epilepsy Behav. 2004, 5, 636–44. 10.1016/j.yebeh.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Kerr C. W. The Serotonin Theory of Depression. Jefferson J. Psychiatry 1994, 12, 1–14. 10.29046/JJP.012.1.001. [DOI] [Google Scholar]
- Savitz J.; Lucki I.; Drevets W. C. 5-HT1A Receptor Function in Major Depressive Disorder. Prog. Neurobiol. 2009, 88, 17–31. 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia A. L.; Newman-Tancredi A.; Leonardo E. D. 5-HT1A receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology 2014, 231, 623–636. 10.1007/s00213-013-3389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund P. B. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology 2009, 206, 345–354. 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O.; Pappa E.; Konradsson-Geuken Å.; Ögren S. O. The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front. Pharmacol. 2015, 6, 1–17. 10.3389/fphar.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A. Targeting the Serotonin 5-HT7 Receptor in the Search for Treatments for CNS Disorders: Rationale and Progress to Date. CNS Drugs 2015, 29, 265–275. 10.1007/s40263-015-0236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumenko V. S.; Popova N. K.; Lacivita E.; Leopoldo M.; Ponimaskin E. G. Interplay between Serotonin 5-HT1A and 5-HT7 Receptors in Depressive Disorders. CNS Neurosci. Ther. 2014, 20, 582–590. 10.1111/cns.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner U.; Zeug A.; Woehler A.; Niebert M.; Dityatev A.; Dityateva G.; Gorinski N.; Guseva D.; Abdel-Galil D.; Fröhlich M.; Döring F.; Wischmeyer E.; Richter D. W.; Neher E.; Ponimaskin E. G. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. 10.1242/jcs.101337. [DOI] [PubMed] [Google Scholar]
- Hirvonen J.; Karlsson H.; Kajander J.; Lepola A.; Markkula J.; Rasi-Hakala H.; Någren K.; Salminen J. K.; Hietala J. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int. J. Neuropsychopharmacol. 2008, 11, 465–476. 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- Meegalla S. K.; Plossl K.; Kung M.-P.; Chumpradit S.; Stevenson D. A.; Kushner S. A.; McElgin W. T.; Mozley P. D.; Kung H. F. Synthesis and characterization of technetium-99m-labeled tropanes as dopamine transporter-imaging agents. J. Med. Chem. 1997, 40, 9–17. 10.1021/jm960532j. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S.; Kaul A.; Hazari P. P.; Mishra A. K. Mapping neuroreceptors with metal-labeled radiopharmaceuticals. Med. Chem. Commun. 2017, 8, 855–870. 10.1039/C6MD00610H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari P. P.; Pandey A.; Chaturvedi S.; Mishra A. K. New trends and current status of positron-emission tomography and single-photon-emission computerized tomography radioligands for neuronal serotonin receptors and serotonin transporter. Bioconjugate Chem. 2017, 28, 2647–2672. 10.1021/acs.bioconjchem.7b00243. [DOI] [PubMed] [Google Scholar]
- Kane R. S. Thermodynamics of Multivalent Interactions: Influence of the Linker. Langmuir 2010, 26, 8636–8640. 10.1021/la9047193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata J.; Juneja A.; Diestler D. J.; Knapp E. W. Influence of spacer–receptor interactions on the stability of bivalent ligand-receptor complexes. J. Phys. Chem. B 2012, 116, 2595–2604. 10.1021/jp211383s. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S.; Larson D. L.; Yim C. B.; Sayre L. M.; Ronsisvalle G.; Lipkowski A. W.; Takemori A. E.; Rice K. C.; Tam S. W. Stereostructure-activity relationship of opioid agonist and antagonist bivalent ligands. Evidence for bridging between vicinal opioid receptors. J. Med. Chem. 1985, 28, 1140–1141. 10.1021/jm00147a002. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S.; Larson D. L.; Sayre L. M.; Yim C. B.; Ronsisvalle G.; Tam S. W.; Takemori A. E. Opioid agonist and antagonist bivalent ligands. The relationship between spacer length and selectivity at multiple opioid receptors. J. Med. Chem. 1986, 29, 1855–1861. 10.1021/jm00160a010. [DOI] [PubMed] [Google Scholar]
- Xie Z.; Bhushan R. G.; Daniels D. J.; Portoghese P. S. Interaction of Bivalent Ligand KDN21 with Heterodimeric δ-κ Opioid Receptors in Human Embryonic Kidney 293 Cells. Mol. Pharmacol. 2005, 68, 1079–1086. 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- Le Naour M.; Akgün E.; Yekkirala A.; Lunzer M. M.; Powers M. D.; Kalyuzhny A. E.; Portoghese P. S. Bivalent Ligands That Target μ Opioid (MOP) and Cannabinoid1 (CB1) Receptors Are Potent Analgesics Devoid of Tolerance. J. Med. Chem. 2013, 56, 5505–5513. 10.1021/jm4005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T.; Nomura W.; Narumi T.; Masuda A.; Tamamura H. Bivalent Ligands of CXCR4 with Rigid Linkers for Elucidation of the Dimerization State in Cells. J. Am. Chem. Soc. 2010, 132, 15899–15901. 10.1021/ja107447w. [DOI] [PubMed] [Google Scholar]
- Singh N.; Hazari P. P.; Prakash S.; Chuttani K.; Khurana H.; Chandra H.; Mishra A. K. A homodimeric bivalent radioligand derived from 1-(2-methoxyphenyl)piperazine with high affinity for in vivo 5-HT1A receptor imaging. Med. Chem. Commun. 2012, 3, 814–823. 10.1039/c2md20062g. [DOI] [Google Scholar]
- Jha P.; Chaturvedi S.; Kaul A.; Pant P.; Anju; Pal S.; Jain N.; Mishra A. K. Design, physico-chemical and pre-clinical evaluation of a homo-bivalent 99mTc-(BTZ)2DTPA radioligand for targeting dimeric 5-HT1A/5-HT7 receptors. New J. Chem. 2018, 42, 15032–15043. 10.1039/C8NJ00089A. [DOI] [Google Scholar]
- Soulier J.-L.; Russo O.; Giner M.; Rivail L.; Berthouze M.; Ongeri S.; Maigret B.; Fischmeister R.; Lezoualc’h F.; Sicsic S.; Berque-Bestel I. Design and Synthesis of Specific Probes for Human 5-HT4Receptor Dimerization Studies. J. Med. Chem. 2005, 48, 6220–6228. 10.1021/jm050234z. [DOI] [PubMed] [Google Scholar]
- Leopoldo M.; Lacivita E.; Berardi F.; Perrone R.; Hedlund P. B. Serotonin 5-HT7 receptor agents: structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol. Ther. 2011, 129, 120–148. 10.1016/j.pharmthera.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa M. A.; Salerno L.; Modica M. N.; Pittalà V.; Romeo G.; Amato M. E.; Nowak M.; Bojarski A. J.; Mereghetti I.; Cagnotto A.; Mennini T. Synthesis of new arylpiperazinylalkylthiobenzimidazole, benzothiazole, or benzoxazole derivatives as potent and selective 5-HT1A serotonin receptor ligands. J. Med. Chem. 2008, 51, 4529–4538. 10.1021/jm800176x. [DOI] [PubMed] [Google Scholar]
- Salerno L.; Pittalà V.; Modica M. N.; Siracusa M. A.; Intagliata S.; Cagnotto A.; Salmona M.; Kurczab R.; Bojarski A. J.; Romeo G. Structure-activity relationships and molecular modeling studies of novel arylpiperazinylalkyl 2-benzoxazolones and 2-benzothiazolones as 5-HT(7) and 5-HT(1A) receptor ligands. Eur. J. Med. Chem. 2014, 85, 716–726. 10.1016/j.ejmech.2014.08.023. [DOI] [PubMed] [Google Scholar]
- Medina R. A.; Sallander J.; Benhamú B.; Porras E.; Campillo M.; Pardo L.; López-Rodríguez M. L. Synthesis of new serotonin 5-HT7 receptor ligands. Determinants of 5-HT7/5-HT1A receptor selectivity. J. Med. Chem. 2009, 52, 2384–2392. 10.1021/jm8014553. [DOI] [PubMed] [Google Scholar]
- Taverne T.; Diouf O.; Depreux P.; Poupaert J. H.; Lesieur D.; Guardiola-Lemaître B.; Renard P.; Rettori M.-C.; Caignard D.-H.; Pfeiffer B. Novel Benzothiazolin-2-one and Benzoxazin-3-one Arylpiperazine Derivatives with Mixed 5HT1A/D2 Affinity as Potential Atypical Antipsychotics. J. Med. Chem. 1998, 41, 2010–2018. 10.1021/jm970298c. [DOI] [PubMed] [Google Scholar]
- Mouithys-Mickalad A.; Poupaert J. H.; Spampinato S.; Lesieur D. Synthesis and Pharmacological Evaluation of 6-Piperidino- and 6-Piperazinoalkyl-2(3H)-benzothiazolones as Mixed σ/5-HT1A Ligands. Bioorg. Med. Chem. Lett. 2002, 12, 1149–1152. 10.1016/S0960-894X(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Larchanche P.-E.; Ultré V.; le Broc D.; Ballandone C.; Furman C.; Dallemagne P.; Melnyk P.; Carato P. 6-Sulfonylbenzothiazolones as potential scaffolds for the design of 5-HT6 ligands. Eur. J. Med. Chem. 2015, 92, 807–817. 10.1016/j.ejmech.2015.01.052. [DOI] [PubMed] [Google Scholar]
- James M. L.; Shen B.; Zavaleta C. L.; Nielsen C. H.; Mesangeau C.; Vuppala P. K.; Chan C.; Avery B. A.; Fishback J. A.; Matsumoto R. R.; Gambhir S. S.; McCurdy C. R.; Chin F. T. New Positron Emission Tomography (PET) Radioligand for Imaging σ-1 Receptors in Living Subjects. J. Med. Chem. 2012, 55, 8272–8282. 10.1021/jm300371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucar H.; Cacciaguerra S.; Spampinato S.; van derpoorten K.; Isa M.; Kanyonyo M.; Poupaert J. H. 2(3H)-benzoxazolone and 2(3H)-benzothiazolone derivatives: Novel, potent and selective σ1 receptor ligands. Eur. J. Pharmacol. 1997, 335, 267–273. 10.1016/S0014-2999(97)01248-X. [DOI] [PubMed] [Google Scholar]
- Ucar H.; van derpoorten K.; Cacciaguerra S.; Spampinato S.; Stables J. P.; Depovere P.; Isa M.; Masereel B.; Delarge J.; Poupaert J. H. Synthesis and Anticonvulsant Activity of 2(3H)-Benzoxazolone and 2(3H)-Benzothiazolone Derivatives. J. Med. Chem. 1998, 41, 1138–1145. 10.1021/jm970682+. [DOI] [PubMed] [Google Scholar]
- Tian F.; Jiang X.; Dou X.; Wu Q.; Wang J.; Song Y. Design and synthesis of novel adenine fluorescence probe based on Eu(III) complexes with dtpa-bis(guanine) ligand. Spectrochim. Acta, Part A 2017, 179, 194–200. 10.1016/j.saa.2017.02.048. [DOI] [PubMed] [Google Scholar]
- Chauhan K.; Datta A.; Adhikari A.; Chuttani K.; Singh A. K.; Mishra A. K. 68Ga based probe for Alzheimer’s disease: synthesis and preclinical evaluation of homodimeric chalcone in β-amyloid imaging. Org. Biomol. Chem. 2014, 12, 7328–7337. 10.1039/C4OB00941J. [DOI] [PubMed] [Google Scholar]
- American Society for Testing and Materials F756–00 . Standard Practice for Assessment of Hemolytic Properties of Materials ;Annual Book of ASTM Standards, 2000, 1–5. [Google Scholar]
- Levin V. A. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 1980, 23, 682–684. 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- Laruelle M.; Slifstein M.; Huang Y. Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol. Imaging Biol. 2003, 5, 363–375. 10.1016/j.mibio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Plenevaux A.; Weissmann D.; Aerts J.; Lemaire C.; Brihaye C.; Degueldre C.; Le Bars D.; Comar D.; Pujol J.-F.; Luxen A. Tissue distribution, autoradiography, and metabolism of 4-(2′-Methoxyphenyl)-1-[2′ -[N-2″-Pyridinyl-p-[18F]Fluorobenzamido]ethyl]piperazine (p-[18F]MPPF), a new serotonin 5-HT1A antagonist for positron emission tomography. J. Neurochem. 2000, 75, 803–811. 10.1046/j.1471-4159.2000.0750803.x. [DOI] [PubMed] [Google Scholar]
- Heimbold I.; Drews A.; Syhre R.; Kretzschmar M.; Pietzsch H. J.; Johannsen B. A novel technetium-99m radioligand for the 5-HT1A receptor derived from desmethyl-WAY-100635 (DWAY). Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 82–87. 10.1007/s00259-001-0660-x. [DOI] [PubMed] [Google Scholar]
- Defraiteur C.; Lemaire C.; Luxen A.; Plenevaux A. Radiochemical synthesis and tissue distribution of p-[18F]DMPPF, a new 5-HT1A ligand for PET, in rats. Nucl. Med. Biol. 2006, 33, 667–675. 10.1016/j.nucmedbio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hauser S. R.; Hedlund P. B.; Roberts A. J.; Sari Y.; Bell R. L.; Engleman E. A. The 5-HT7 receptor as a potential target for treating drug and alcohol abuse. Front. Neurosci. 2015, 8, 448. 10.3389/fnins.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson E. L.; Durkin M. M.; Bard J. A.; Zgombick J.; Branchek T. A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-HT7 receptor in rat brain. Br. J. Pharmacol. 1996, 117, 657–666. 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Cora F. J.; Pazos A. Autoradiographic distribution of 5-HT7 receptors in the human brain using [3H]mesulergine: comparison to other mammalian species. Br. J. Pharmacol. 2004, 141, 92–104. 10.1038/sj.bjp.0705576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osredkar D.; Krzan M. Expression of serotonin receptor subtypes in rat brain and astrocyte cell cultures: an age and tissue-dependent process. Period. Biol. 2009, 111, 129–135. [Google Scholar]
- Geurts F. J.; De Schutter E.; Timmermans J. P. Localization of 5-HT2A, 5-HT3, 5-HT5A and 5-HT7 receptor-like immunoreactivity in the rat cerebellum. J. Chem. Neuroanat. 2002, 24, 65–74. 10.1016/S0891-0618(02)00020-0. [DOI] [PubMed] [Google Scholar]
- Prime version 2.2, Lig prep version 2.7, Glide version 6.0; L. L. C. Schrödinger: NY, New York, 2014.
- Paila Y. D.; Tiwari S.; Sengupta D.; Chattopadhyay A. Molecular modeling of the human serotonin 1A receptor: role of membrane cholesterol in ligand binding of the receptor. Mol. BioSyst. 2011, 7, 224–234. 10.1039/C0MB00148A. [DOI] [PubMed] [Google Scholar]
- Jha P.; Chaturvedi S.; Swastika; Pal S.; Jain N.; Mishra A. K. Improvising 5-HT7R homology model for design of high affinity ligands: model validation with docking, embrace minimization, MM-GBSA, and molecular dynamic simulations. J. Biomol. Struct. Dyn. 2017, 36, 2475–2494. 10.1080/07391102.2017.1359907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.