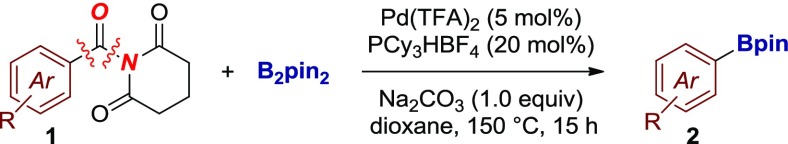Abstract
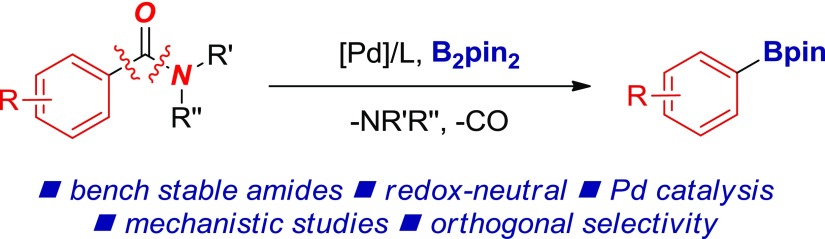
The development of transition-metal-catalyzed borylation reactions is of significant importance for the fields of organic synthesis and medicinal chemistry because of the versatility of organoboron functional groups. Herein, we report the direct decarbonylative borylation of amides by highly selective carbon–nitrogen bond cleavage by palladium catalysis. The approach capitalizes on the ground-state destabilization of the amide bond in N-acyl glutarimides to achieve Pd-catalyzed insertion into the amide N–C bond and decarbonylation (deamidation). Mechanistic studies and the utility of this methodology in orthogonal sequential cross-couplings of robust, bench-stable amides are reported.
1. Introduction
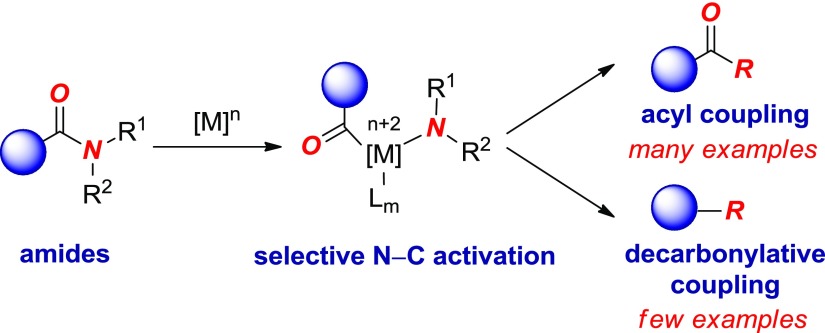
Transition-metal-catalyzed cross-coupling of amides has recently emerged as a powerful platform for the functionalization of the traditionally inert amide bond (nN → πC=O* donation, barrier to rotation in planar amides of 15–20 kcal/mol).1−3 The capacity of the amide bond to support selective insertion of a transition metal into the N–C bond by ground-state destabilization4 enables an array of new approaches for the synthesis of important motifs in organic synthesis.5 In this context, there are two general pathways for metal-catalyzed cross-coupling of amides (Figure 1): (i) direct acyl coupling and (ii) decarbonylative cross-coupling, involving formation of the acyl metal complex and CO extrusion to afford aryl-metal intermediates6 fulfilling the criteria of classical metal-catalyzed cross-coupling reactions.3
Figure 1.
Acyl and decarbonylative cross-coupling of amides.
Unfortunately, although great progress has been made in acyl cross-couplings of the amide bond,7 the corresponding decarbonylative manifold remains much less developed.8−15 In particular, palladium-catalyzed decarbonylative cross-couplings have remained a challenging goal, with very few examples of such reactions (cf. Ni) reported to date.14 The versatility of Pd catalysis,16 including the broad industrial use of Pd-catalyzed processes,17 makes this approach attractive in decarbonylative amide bond cross-coupling.
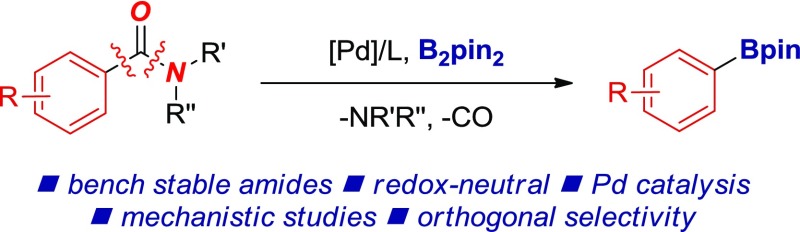
Herein, we report the direct decarbonylative borylation of amides by highly selective carbon–nitrogen bond cleavage by palladium catalysis (Figure 2).18 The method represents a rare example of using less nucleophilic Pd (cf. Ni) to promote efficient metal insertion into the amide N–C bond and decarbonylation.6 The method capitalizes on the ground-state destabilization of the amide bond in N-acyl glutarimides4 to achieve high selectivity of the decarbonylation. Mechanistic studies and the utility of this methodology in orthogonal sequential cross-couplings of robust, bench-stable amides are reported. This user-friendly methodology can be used for the construction of the organoboron functional group from bench-stable amides using commercially available and air-stable reagents.19
Figure 2.
Palladium-catalyzed redox-neutral decarbonylative borylation of amides (this study).
The reaction is fundamentally different from decarbonylative borylation of carboxylic acids reported by our group:18f (1) the present study addresses activation of a N–C versus O–C bond; (2) the underlying activation principle hinges upon amide bond twist and geometric distortion of the amide bond2b,4 (cf. steric-control of regioselectivity and reversible insertion); (3) the catalyst system radically differs in the use of a mono- versus bidentate phosphine ligand; (4) amides can be prepared from fundamentally different precursors than carboxylic acids, including primary amides. Principally, the method constitutes the first example of Pd-catalyzed synthesis of aryl boronates from amides, and as such represents a potentially significant advance in the construction of C–B bonds by N–C decarbonylative coupling akin to the classic Miyaura borylation of aryl halides and pseudohalides.20 Given the prevalence of amides in organic synthesis and medicinal chemistry, potential future applications range from Pd-catalyzed functionalization of biomolecules to the synthesis of specialty and bulk chemicals.
2. Results and Discussion
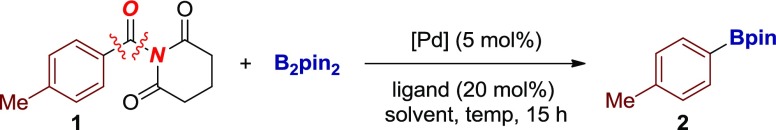
Drawing from our studies in the decarbonylative cross-coupling of amides,6a,9a,14b,14c we envisioned that Pd-catalyzed decarbonylative borylation of rotationally inverted N-acyl glutarimides could be achieved by applying a catalyst system that would favor decarbonylation. We commenced our studies by investigating the cross-coupling of electronically unbiased 1-(4-toluoyl)-N-glutarimide with bis(pinacolato)diboron as the boron source (Table 1).20
Table 1. Optimization of Pd-Catalyzed Decarbonylative Borylation of Amidesa.
| entry | catalyst | ligand | base | yieldb (%) |
|---|---|---|---|---|
| 1 | Pd(OAc)2 | PPh3 | Na2CO3 | 15 |
| 2 | Pd(OAc)2 | P(4-MeO-C6H4)3 | Na2CO3 | 27 |
| 3 | Pd(OAc)2 | P(4-CF3-C6H4)3 | Na2CO3 | 12 |
| 4 | Pd(OAc)2 | PCyPh2 | Na2CO3 | 24 |
| 5 | Pd(OAc)2 | PCy2Ph | Na2CO3 | 28 |
| 6 | Pd(OAc)2 | PCy3HBF4 | Na2CO3 | 49 |
| 7 | Pd(OAc)2 | Dppb | Na2CO3 | 5 |
| 8 | Pd(OAc)2 | Dppp | Na2CO3 | 25 |
| 9 | Pd(OAc)2 | Pn-Bu3HBF4 | Na2CO3 | <5 |
| 10 | Pd(OAc)2 | Xphos | Na2CO3 | <5 |
| 11 | Pd(OAc)2 | SPhos | Na2CO3 | <5 |
| 12 | Pd(OAc)2 | PCy3HBF4 | NaOAc | 44 |
| 13 | Pd(OAc)2 | PCy3HBF4 | KOAc | 15 |
| 14 | Pd(OAc)2 | PCy3HBF4 | K2CO3 | 36 |
| 15 | Pd(OAc)2 | PCy3HBF4 | K3PO4 | 34 |
| 16 | Pd(OAc)2 | PCy3HBF4 | Li2CO3 | 20 |
| 17 | Pd(OAc)2 | PCy3HBF4 | NaOt-Bu | 16 |
| 18 | Pd(CH3CN)2Cl2 | PCy3HBF4 | Na2CO3 | 55 |
| 19 | Pd(PPh3)2Cl2 | PCy3HBF4 | Na2CO3 | 52 |
| 20 | Pd(acac)2 | PCy3HBF4 | Na2CO3 | 47 |
| 21 | Pd(TFA)2 | PCy3HBF4 | Na2CO3 | 64 |
| 22 | Pd(dba)2 | PCy3HBF4 | Na2CO3 | 58 |
| 23 | Pd2(dba)3 | PCy3HBF4 | Na2CO3 | 56 |
| 24 | PEPPSI-IPr | PCy3HBF4 | Na2CO3 | <5 |
| 25c | Pd(TFA)2 | PCy3HBF4 | Na2CO3 | 58 |
| 26d | Pd(TFA)2 | PCy3HBF4 | Na2CO3 | 69 |
| 27e | Pd(TFA)2 | PCy3HBF4 | Na2CO3 | 60 |
| 28f | Pd(TFA)2 | PCy3HBF4 | Na2CO3 | 77 |
Conditions: amide (1.0 equiv), B2pin2 (2.0 equiv), [Pd] (5 mol %), ligand (20 mol %),base (2.0 equiv), dioxane(0.25 M), 150 °C, and 15 h.
GC/1H NMR yields.
B2pin2 (1.2 equiv).
Na2CO3 (1.0 equiv).
Na2CO3 (0.5 equiv).
B2pin2 (1.2 equiv), Na2CO3 (1.0 equiv). See the Supporting Information for details.
Although initial attempts were unsuccessful, after very extensive survey of the reaction conditions, we identified a catalyst system that provided a significant improvement in the cross-coupling, furnishing the desired decarbonylative borylation product in 77% yield (Table 1, entries 1–28). Several optimization results are worth noting. The choice of the phosphane ligand had a significant effect on the cross-coupling (entries 1–11). We identified PCy3 as the preferred ligand for this transformation, presumably because of facile activation of the N–C bond to form the acyl-metal intermediate. In the evaluation of different bases, Na2CO3 proved optimal (entries 12–17). Various Pd catalysts were tested, and Pd(TFA)2 showed the best activity (entries 18–24). Further improvement of the reaction efficiency was achieved by careful adjustment of the reagent stoichiometry (entries 25–28), presumably to match decarbonylation with the transmetalation step. Importantly, the reaction proceeds with a complete selectivity for N–C(O) acyl bond decarbonylation, with products corresponding to the acyl coupling and unselective cleavage of the endocyclic C–O bonds not observed. Furthermore, as an important synthetic advantage, the method utilizes commercially available, bench-stable reagents and does not require preparation of the activated boron source or co-catalytic metal additives.9b,9f Furthermore, we note that the cross-coupling of N-benzoylsuccinimide proceeds in an unoptimized 45% yield.8c,8d At this stage of reaction development, other amides have not been tested.
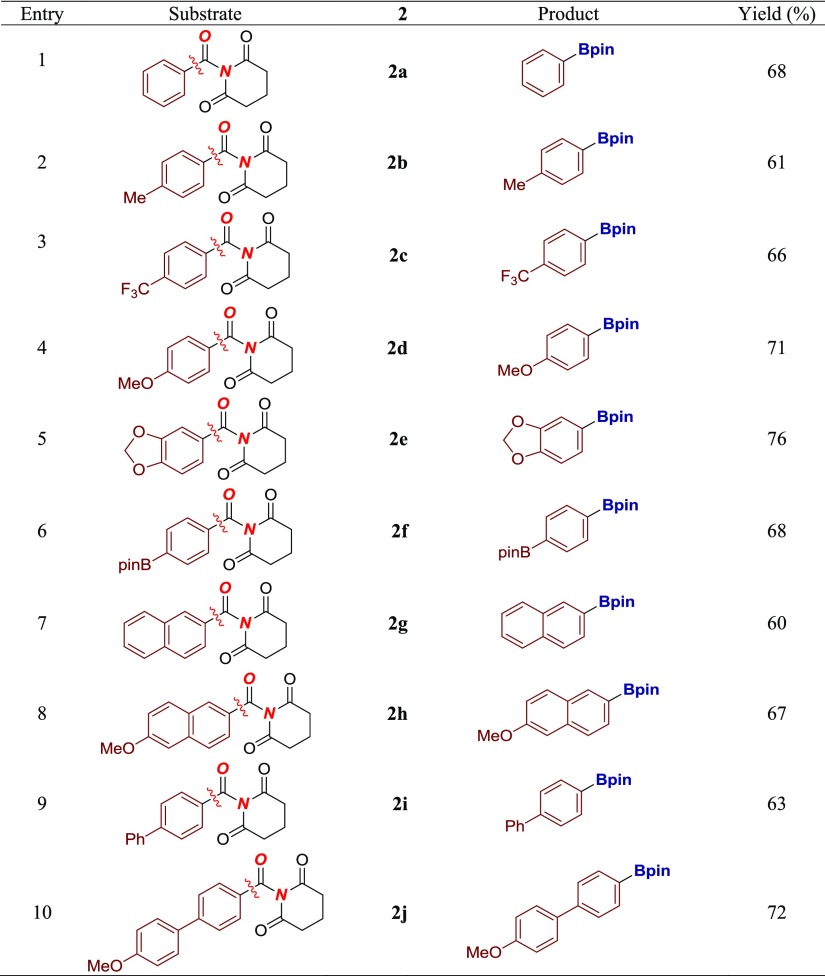
With the optimal conditions for cross-coupling, the scope of the reaction was briefly investigated (Table 2). We were pleased to find that the reaction readily tolerates unbiased neutral (entries 1–2), electron-withdrawing (entry 3), and electron-donating (entry 4) arenes, affording the desired organoboranes in 61–71% yields. In contrast, Ni-catalyzed cross-couplings are often limited to the use of conjugated π-systems to favor insertion/decarbonylation.6b,9c Electron-donating oxygen heterocycles that are important in medicinal chemistry applications such as dioxolane (entry 5) are well tolerated. Furthermore, amides containing Bpin bonds are also competent substrates (entry 6), resulting in formal double borylation by exploiting orthogonal electrophilicity of aryl halide and amide functional groups. Notably, conjugated arenes that are typically prone to reduction under decarbonylative conditions also delivered the corresponding borylated products in good yields (entries 7–8). Finally, the method could be applied to biaryl amides to generate the desired conjugated adducts, containing synthetically useful C–B handle for further cross-coupling functionalization (entries 9–10, vide infra).
Table 2. Pd-Catalyzed Decarbonylative Borylation of Amidesa.
Conditions: amide (1.0 equiv), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), Na2CO3 (1.0 equiv), dioxane (0.25 M), 150 °C, and 15 h. Isolated yields. See the Supporting Information for full details.
While the method establishes an important precedent in the Pd-catalyzed borylation by selective N–C bond cleavage of amides, several limitations should be noted. (1) At the present stage, the method is not compatible with heteroaromatic amides (<20% yield) and ortho-substituted amides (<5% yield). (2) Aliphatic amides undergo decarbonylation/β-hydride elimination to give olefins. This is in agreement with a recent report by Shi and co-workers on Ni-catalyzed retro-hydroamidocarbonylation of aliphatic amides.9g,9h (3) In general, halogens are not compatible with the decarbonylative cross-coupling manifold of amides. We further note that primary and secondary amides are unreactive under the present conditions, while N-methoxy amides undergo decomposition by the N–O cleavage pathway. Meta-substituted amides have not been tested because they are not at the conjugating position. Studies to address these limitations and the design of more selective catalyst systems are currently underway.
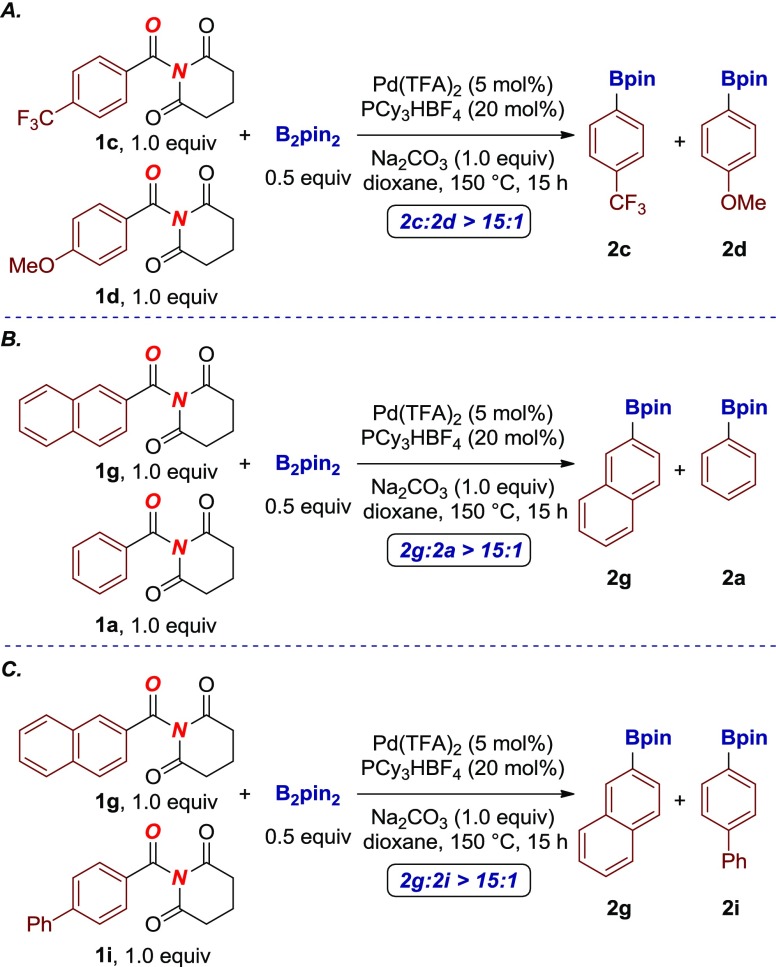
To gain insight into the reaction mechanism, intermolecular competition experiments were conducted (Scheme 1). To this end, intermolecular competition experiments with 4-substituted amides revealed that electron-deficient amides react preferentially, consistent with facility of metal insertion (Scheme 1A).4 Further competition experiments established that conjugated π-systems such as naphthalene couple preferentially (Scheme 1B). Furthermore, competition experiments established that π-conjugated arenes react preferentially over biaryls (Scheme 1C).6b
Scheme 1. Intermolecular Competition Experiments in Decarbonylative Borylation of Amides.
Overall, the mechanistic studies highlight that (i) electrophilicity of the amide bond and (ii) capacity of the acyl-metal intermediate to influence the relative reactivity of amides in this decarbonylative (deamidative) cross-coupling.
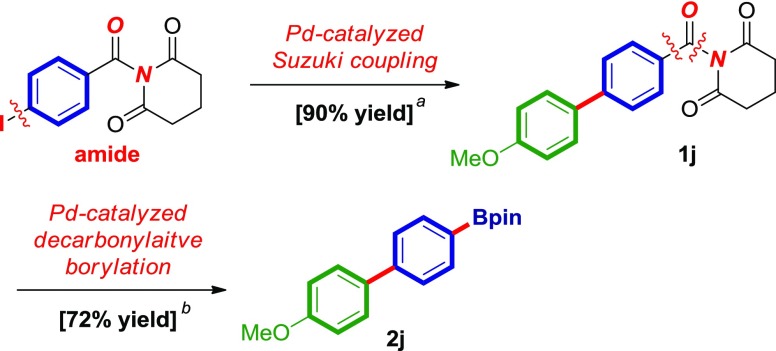
In recent years, orthogonal cross-couplings have been an area of significant interest due to permitting versatile disconnections in organic synthesis.21 An attractive feature of the present methodology is the enabling nature for orthogonal cross-couplings (Scheme 2). As shown in Scheme 2, the amide bond in N-acyl glutarimides displays orthogonal electrophilic reactivity to the traditional Pd-catalyzed cross-couplings.3,19,20 The amide group is not reactive toward Pd(0)-orthogonal Suzuki–Miyaura cross-coupling reactions, highlighting a powerful opportunity for sequential Pd-catalyzed C(sp2)–C(sp2) cross-coupling/Pd-catalyzed decarbonylative cross-coupling in organic synthesis.
Scheme 2. Orthogonal Cross-Coupling/Decarbonylative Borylation of Amides by Pd-Catalysis.
Conditions: (a) Pd(OAc)2, 4-MeO-C6H4-B(OH)2, Na2CO3, EtOH:H2O, 23 °C. (b) B2pin2, Pd(TFA)2, PCy3HBF4, Na2CO3, dioxane, and 150 °C.
3. Conclusions
In summary, we have documented the first palladium-catalyzed decarbonylative borylation of amides by selective carbon–nitrogen cleavage for the synthesis of versatile organoboranes. The method exploits the ground-state destabilization of the amide bond in N-acyl glutarimides to achieve Pd-catalyzed insertion into the amide N–C bond and decarbonylation. Although the yields are good to modest, the reaction is synthetically useful because it employs commercially available and air-stable reagents. Furthermore, the method sets an important precedent for decarbonylative borylation of amides using versatile Pd-catalysis. Moreover, the potential of the amide N–C bond disconnection has been demonstrated in orthogonal cross-couplings using palladium. Additional studies directed at decarbonylative cross-coupling of amide electrophiles, including extension to other nucleophiles, as well as structural mechanistic investigations and studies on the development of improved reaction conditions and catalyst systems are in progress in our laboratory and will be reported in due course.
4. Experimental Section
4.1. General Methods
All compounds reported in the manuscript are commercially available or have been previously described in literature unless indicated otherwise. All experiments involving palladium were performed using standard Schlenk techniques under an argon or a nitrogen atmosphere unless stated otherwise. All amides were prepared by standard methods.14c1H NMR and 13C NMR data are given for all compounds in the Experimental Section for characterization purposes. 1H NMR, 13C NMR and HRMS data are reported for all new compounds. All products have been previously reported, unless stated otherwise. Spectroscopic data matched literature values. General methods have been published.14c
4.2. Synthesis of Starting Materials
Amides 1a–1j have been reported.14c The synthesis of amide 1j by Suzuki–Miyaura cross-coupling of 1-(4-iodobenzoyl)piperidine-2,6-dione has been published.14c
4.3. General Procedure for Decarbonylative Borylation of Amides
An oven-dried vial equipped with a stir bar was charged with an amide substrate (neat, 1.0 equiv), B2pin2 (1.2 equiv), Na2CO3 (1.0 equiv), Pd(TFA)2 (5 mol %), and PCy3HBF4 (20 mol %), placed under a positive pressure of argon, and subjected to three evacuation/backfilling cycles under high vacuum. Dioxane (0.25 M) was added with vigorous stirring at room temperature, the reaction mixture was placed in a preheated oil bath at 150 °C, and stirred for the indicated time at 150 °C. After the indicated time, the reaction mixture was cooled down to room temperature, diluted with CH2Cl2 (10 mL), filtered, and concentrated. A sample was analyzed by 1H NMR (CDCl3, 500 MHz) and gas chromatography–mass spectrometry (GC–MS) to obtain conversion, yield, and selectivity using internal standard and comparison with authentic samples. Purification by chromatography on silica gel (hexanes/ethyl acetate) afforded the title products. Caution: Reactions involving high pressure must be carried out in a well-ventilated hood with appropriate pressure vessels, pressure relief equipment, and/or blast shields.
4.4. Representative Procedure for Decarbonylative Borylation of Amides
4.4.1. 1.0 mmol Scale
An oven-dried vial equipped with a stir bar was charged with 1-(4-methylbenzoyl)piperidine-2,6-dione (neat, 1.0 mmol, 231.3 mg, 1.0 equiv), bis(pinacolato)diboron (1.2 mmol, 304.8 mg, 1.2 equiv), sodium carbonate (1.0 mmol, 106.0 mg, 1.0 equiv), Pd(TFA)2 (5 mol %, 16.6 mg), and PCy3HBF4 (20 mol %, 73.6 mg), placed under a positive pressure of argon, and subjected to three evacuation/backfilling cycles under high vacuum. Dioxane (4.0 mL) was added with vigorous stirring at room temperature, the reaction mixture was placed in a preheated oil bath at 150 °C, and stirred for 15 h at 150 °C. After the indicated time, the reaction mixture was cooled down to room temperature, diluted with CH2Cl2 (10 mL), filtered, and concentrated. A sample was analyzed by 1H NMR (CDCl3, 500 MHz) and GC–MS to obtain conversion, yield, and selectivity using internal standard and comparison with authentic samples. Purification by chromatography on the silica gel (hexanes/ethyl acetate) afforded the title product in yield 61% (133.5 mg) as a white solid. Characterization data are included in the section below.
4.5. General Procedure for Selectivity Studies
An oven-dried vial equipped with a stir bar was charged with two amide substrates (1.0 equiv each), B2pin2 (0.5 equiv), Na2CO3 (1.0 equiv), Pd(TFA)2 (5 mol %), and PCy3HBF4 (20 mol %), placed under a positive pressure of argon, and subjected to three evacuation/backfilling cycles under high vacuum. Dioxane (0.25 M) was added with vigorous stirring at room temperature, the reaction mixture was placed in a preheated oil bath at 150 °C, and stirred for the indicated time at 150 °C. After the indicated time, the reaction mixture was cooled down to room temperature, diluted with CH2Cl2 (10 mL), filtered, and concentrated. The sample was analyzed by 1H NMR (CDCl3, 500 MHz) and GC–MS to obtain conversion and yield using internal standard and compared with authentic samples.
4.6. Characterization Data of Cross-Coupling Products
4.6.1. 4,4,5,5-Tetramethyl-2-phenyl-1,3,2-dioxaborolane(Table 2, 2a)18e
According to the general procedure, the reaction of amide 1a (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), and Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 68% yield (27.7 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 7.82 (d, J = 7.5 Hz, 2H), 7.46 (t, J = 7.0 Hz, 1H), 7.37 (t, J = 7.5 Hz, 2H), 1.35 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 134.74, 131.26, 127.71, 83.78, 24.89.
4.6.2. 4,4,5,5-Tetramethyl-2-(p-tolyl)-1,3,2-dioxaborolane (Table 2, 2b)18e
According to the general procedure, the reaction of amide 1b (1.0 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), and Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 61% yield (133.5 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 7.70 (d, J = 7.0 Hz, 2H), 7.19 (d, J = 7.0 Hz, 2H), 2.37 (s, 3H), 1.34 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 141.54, 134.94, 128.66, 83.76, 25.00, 21.87.
4.6.3. 4,4,5,5-Tetramethyl-2-(4-(trifluoromethyl)phenyl)-1,3,2-dioxaborolane (Table 2, 2c)18e
According to the general procedure, the reaction of amide 1c (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 66% yield (35.9 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 7.91 (d, J = 7.5 Hz, 2H), 7.61 (d, J = 7.5 Hz, 2H), 1.36 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 135.14, 132.96 (q, JF = 32.5 Hz), 124.46 (q, JF = 3.8 Hz), 124.28 (q, JF = 271.2 Hz), 84.42, 25.00. 19F NMR (471 MHz, CDCl3): δ −63.04.
4.6.4. 2-(4-Methoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Table 2, 2d)18e
According to the general procedure, the reaction of amide 1c (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), and Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 71% yield (33.2 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 7.76 (d, J = 8.5 Hz, 2H), 6.90 (d, J = 8.5 Hz, 2H), 3.83 (s, 3H), 1.34 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 162.28, 136.64, 113.44, 83.68, 55.23, 25.00.
4.6.5. 2-(Benzo[d][1,3]dioxol-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Table 2, 2e)9f
According to the general procedure, the reaction of amide 1e (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), and Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 76% yield (37.7 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 7.36 (d, J = 7.7 Hz, 1H), 7.24 (s, 1H), 6.83 (d, J = 7.6 Hz, 1H), 1.33 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 150.29, 147.32, 129.85, 114.07, 108.42, 100.86, 83.84, 24.97.
4.6.6. 1,4-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene (Table 2, 2f)18c
According to the general procedure, the reaction of amide 1f (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), and Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 68% yield (44.9 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 7.80 (s, 4H), 1.35 (s, 24H). 13C NMR (125 MHz, CDCl3): δ 134.02, 83.99, 25.02.
4.6.7. 4,4,5,5-Tetramethyl-2-(naphthalen-2-yl)-1,3,2-dioxaborolane (Table 2, 2g)18c
According to the general procedure, the reaction of amide 1g (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 60% yield (30.5 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 8.37 (s, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.82–7.79 (m, 3H), 7.52–7.46 (m, 2H), 1.39 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 136.37, 135.14, 132.93, 130.52, 128.75, 127.81, 127.09, 127.08, 125.90, 84.02, 25.03.
4.6.8. 2-(6-Methoxynaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Table 2, 2h)18a
According to the general procedure, the reaction of amide 1h (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), and Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 67% yield (38.0 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 8.29 (s, 1H), 7.80 (d, J = 8.5 Hz, 1H), 7.78 (d, J = 8.5 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.13 (d, J = 9.0 Hz, 2H), 3.93 (s, 3H), 1.39 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 158.66, 136.56, 136.12, 131.25, 130.38, 128.51, 126.04, 118.81, 105.75, 83.93, 55.43, 25.07, 25.01.
4.6.9. 2-([1,1′-Biphenyl]-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Table 2, 2i)18a
According to the general procedure, the reaction of amide 1i (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), and Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 63% yield (35.3 mg) as a white solid. 1H NMR (500 MHz, CDCl3) δ: 7.89 (d, J = 8.0 Hz, 2H), 7.65–7.59 (m, 4H), 7.44 (t, J = 7.5 Hz, 2H), 7.36 (t, J = 7.5 Hz, 1H), 1.37 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 144.03, 141.16, 135.39, 128.90, 127.69, 127.37, 126.60, 83.97, 67.24, 25.03.
4.6.10. 2-(4′-Methoxy-[1,1′-biphenyl]-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Table 2, 2j)18c
According to the general procedure, the reaction of amide 1j (0.20 mmol), B2pin2 (1.2 equiv), Pd(TFA)2 (5 mol %), PCy3HBF4 (20 mol %), and Na2CO3 (1.0 equiv) in dioxane (0.25 M) for 15 h at 150 °C afforded after work-up and chromatography the title product in 72% yield (44.6 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 7.86 (d, J = 8.0 Hz, 2H), 7.57 (d, J = 3.0 Hz, 2H), 7.56 (d, J = 4.0 Hz, 2H), 6.98 (d, J = 8.5 Hz, 2H), 3.85 (s, 3H), 1.36 (s, 12H). 13C NMR (125 MHz, CDCl3): δ 159.54, 143.61, 135.39, 133.65, 128.40, 126.13, 114.36, 83.92, 55.51, 25.03.
Acknowledgments
Rutgers University and the NSF (CAREER CHE-1650766) are gratefully acknowledged for support. The 500 MHz spectrometer used in this study was supported by the NSF-MRI grant (CHE-1229030).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00081.
1H and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Greenberg A.; Breneman C. M.; Liebman J. F.. The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Materials Science, 1st ed.; Wiley-VCH: New York, 2003. [Google Scholar]; b Pattabiraman V. R.; Bode J. W. Rethinking Amide Bond Synthesis. Nature 2011, 480, 471–479. 10.1038/nature10702. [DOI] [PubMed] [Google Scholar]; c Ruider S. A.; Maulide N. Strong Bonds Made Weak: Towards the General Utility of Amides as Synthetic Modules. Angew. Chem., Int. Ed. 2015, 54, 13856–13858. 10.1002/anie.201508536. [DOI] [PubMed] [Google Scholar]
- For reviews on N–C functionalization, see:; a Meng G.; Shi S.; Szostak M. Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. 10.1055/s-0036-1588080. [DOI] [Google Scholar]; b Liu C.; Szostak M. Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N–C Amide Bond Activation. Chem.—Eur. J. 2017, 23, 7157–7173. 10.1002/chem.201605012. [DOI] [PubMed] [Google Scholar]; c Takise R.; Muto K.; Yamaguchi J. Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev. 2017, 46, 5864–5888. 10.1039/c7cs00182g. [DOI] [PubMed] [Google Scholar]; d Dander J. E.; Garg N. K. Breaking Amides using Nickel Catalysis. ACS Catal. 2017, 7, 1413–1423. 10.1021/acscatal.6b03277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Science of Synthesis: Cross-Coupling and Heck-Type Reactions, 1st ed.; Molander G. A., Wolfe J. P., Larhed M., Eds.; Thieme: Stuttgart, 2013. [Google Scholar]; b Metal-Catalyzed Cross-Coupling Reactions and More, 1st ed.; de Meijere A., Bräse S., Oestreich M., Eds.; Wiley: New York, 2014. [Google Scholar]; c New Trends in Cross-Coupling, 1st ed.; Colacot T. J., Ed.; The Royal Society of Chemistry: Cambridge, 2015. [Google Scholar]
- Pace V.; Holzer W.; Meng G.; Shi S.; Lalancette R.; Szostak R.; Szostak M. Structures of Highly Twisted Amides Relevant to Amide N–C Cross-Coupling: Evidence for Ground-State Amide Destabilization. Chem.—Eur. J. 2016, 22, 14494–14498. 10.1002/chem.201603543. [DOI] [PubMed] [Google Scholar]; and references cited therein
- a Roughley S. D.; Jordan A. M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]; b Kaspar A. A.; Reichert J. M. Future directions for peptide therapeutics development. Drug Discovery Today 2013, 18, 807–817. 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]; c Marchildon K. Polyamides - Still Strong After Seventy Years. Macromol. React. Eng. 2011, 5, 22–54. 10.1002/mren.201000017. [DOI] [Google Scholar]; d Chen Y.; Turlik A.; Newhouse T. R. Amide α,β-Dehydrogenation Using Allyl-Palladium Catalysis and a Hindered Monodentate Anilide. J. Am. Chem. Soc. 2016, 138, 1166–1169. 10.1021/jacs.5b12924. [DOI] [PubMed] [Google Scholar]
- a Liu C.; Szostak M. Decarbonylative Cross-Coupling of Amides. Org. Biomol. Chem. 2018, 16, 7998–8010. 10.1039/c8ob01832d. [DOI] [PubMed] [Google Scholar]; b Guo L.; Rueping M. Cross-Couplings: Decarbonylative Cross-Couplings: Nickel Catalyzed Functional Group Interconversion Strategies for the Construction of Complex Organic Molecules. Acc. Chem. Res. 2018, 51, 1185–1195. 10.1021/acs.accounts.8b00023. [DOI] [PubMed] [Google Scholar]
- Shi S.; Nolan S. P.; Szostak M. Well-Defined Palladium(II)-NHC Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective N-C/O-C Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. 10.1021/acs.accounts.. [DOI] [PubMed] [Google Scholar]
- For representative acyl coupling, see:; a Hie L.; Fine Nathel N. F.; Shah T. K.; Baker E. L.; Hong X.; Yang Y.-F.; Liu P.; Houk K. N.; Garg N. K. Conversion of amides to esters by the nickel-catalysed activation of amide C-N bonds. Nature 2015, 524, 79–83. 10.1038/nature14615. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lei P.; Meng G.; Shi S.; Ling Y.; An J.; Szostak R.; Szostak M. Suzuki-Miyaura cross-coupling of amides and esters at room temperature: correlation with barriers to rotation around C-N and C-O bonds. Chem. Sci. 2017, 8, 6525–6530. 10.1039/c7sc02692g. [DOI] [PMC free article] [PubMed] [Google Scholar]; and references cited therein. For cross-coupling of N-acyl-succinimides, see:; c Osumi Y.; Liu C.; Szostak M.; N-Acylsuccinimides N-Acylsuccinimides: twist-controlled, acyl-transfer reagents in Suzuki-Miyaura cross-coupling by N-C amide bond activation. Org. Biomol. Chem. 2017, 15, 8867–8871. 10.1039/c7ob02269g. [DOI] [PubMed] [Google Scholar]; For a recent pertinent review on acyl-couplings, see:; d Buchspies J.; Szostak M. Recent Advances in Acyl Suzuki Cross-Coupling. Catalysts 2019, 9, 53. 10.3390/catal9010053. [DOI] [Google Scholar]; and references cited therein
- For representative decarbonylative coupling, see:; a Shi S.; Meng G.; Szostak M. Synthesis of Biaryls through Nickel-Catalyzed Suzuki-Miyaura Coupling of Amides by Carbon-Nitrogen Bond Cleavage. Angew. Chem., Int. Ed. 2016, 55, 6959–6963. 10.1002/anie.201601914. [DOI] [PubMed] [Google Scholar]; b Hu J.; Zhao Y.; Liu J.; Zhang Y.; Shi Z. Nickel-Catalyzed Decarbonylative Borylation of Amides: Evidence for Acyl C–N Bond Activation. Angew. Chem., Int. Ed. 2016, 55, 8718–8722. 10.1002/anie.201603068. [DOI] [PubMed] [Google Scholar]; c Dey A.; Sasmal S.; Seth K.; Lahiri G. K.; Maiti D. Nickel-Catalyzed Deamidative Step-Down Reduction of Amides to Aromatic Hydrocarbons. ACS Catal. 2017, 7, 433–437. 10.1021/acscatal.6b03040. [DOI] [Google Scholar]; d Yue H.; Guo L.; Liao H.-H.; Cai Y.; Zhu C.; Rueping M. Catalytic Ester and Amide to Amine Interconversion: Nickel-Catalyzed Decarbonylative Amination of Esters and Amides by C–O and C–C Bond Activation. Angew. Chem., Int. Ed. 2017, 56, 4282–4285. 10.1002/anie.201611819. [DOI] [PubMed] [Google Scholar]; e Yue H.; Guo L.; Lee S.-C.; Liu X.; Rueping M. Selective Reductive Removal of Ester and Amide Groups from Arenes and Heteroarenes through Nickel-Catalyzed C–O and C–N Bond Activation. Angew. Chem., Int. Ed. 2017, 56, 3972–3976. 10.1002/anie.201612624. [DOI] [PubMed] [Google Scholar]; f Rueping M.; Lee S. C.; Guo L.; Yue H.; Liao H. H. Nickel-Catalyzed Decarbonylative Silylation, Borylation, and Amination of Arylamides via a Deamidative Reaction Pathway. Synlett 2017, 28, 2594–2598. 10.1055/s-0036-1591495. [DOI] [Google Scholar]; g Hu J.; Wang M.; Pu X.; Shi Z. Nickel-catalysed retro-hydroamidocarbonylation of aliphatic amides to olefins. Nat. Commun. 2017, 8, 14993. 10.1038/ncomms14993. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a pertinent review on decarbonylation of aliphatic carboxylic acids, see:; h Zhang X.; Jordan F.; Szostak M. Transition-metal-catalyzed decarbonylation of carboxylic acids to olefins: exploiting acyl C-O activation for the production of high value products. Org. Chem. Front. 2018, 5, 2515–2521. 10.1039/c8qo00585k. [DOI] [Google Scholar]
- For representative tandem coupling, see:Walker J. A.; Vickerman K. L.; Humke J. N.; Stanley L. M. Ni-Catalyzed Alkene Carboacylation via Amide C-N Bond Activation. J. Am. Chem. Soc. 2017, 139, 10228–10231. 10.1021/jacs.7b06191. [DOI] [PubMed] [Google Scholar]; and references cited therein
- For a biomimetic esterification by N–C activation, see:Wybon C. C. D.; Mensch C.; Hollanders K.; Gadais C.; Herrebout W. A.; Ballet S.; Maes B. U. W. Zn-Catalyzed tert-Butyl Nicotinate-Directed Amide Cleavage as a Biomimic of Metallo-Exopeptidase Activity. ACS Catal. 2018, 8, 203–218. 10.1021/acscatal.7b02599. [DOI] [Google Scholar]
- For a chromium-catalyzed N–C activation, see:Chen C.; Liu P.; Luo M.; Zeng X. Kumada Arylation of Secondary Amides Enabled by Chromium Catalysis for Unsymmetric Ketone Synthesis under Mild Conditions. ACS Catal. 2018, 8, 5864–5868. 10.1021/acscatal.8b01380. [DOI] [Google Scholar]
- For a cobalt-catalyzed esterification by N–C activation, see:Bourne-Branchu Y.; Gosmini C.; Danoun G. Cobalt-Catalyzed Esterification of Amides. Chem.—Eur. J. 2017, 23, 10043–10047. 10.1002/chem.201702608. [DOI] [PubMed] [Google Scholar]
- For leading examples of Pd-catalyzed decarbonylative coupling, see:; a Liu L.; Zhou D.; Liu M.; Zhou Y.; Chen T. Palladium-Catalyzed Decarbonylative Alkynylation of Amides. Org. Lett. 2018, 20, 2741–2744. 10.1021/acs.orglett.8b00949. [DOI] [PubMed] [Google Scholar]; b Liu C.; Szostak M. Decarbonylative Phosphorylation of Amides by Palladium and Nickel Catalysis: The Hirao Cross-Coupling of Amide Derivatives. Angew. Chem., Int. Ed. 2017, 56, 12718–12722. 10.1002/anie.201707102. [DOI] [PubMed] [Google Scholar]; c Shi S.; Szostak M. Decarbonylative Cyanation of Amides by Palladium Catalysis. Org. Lett. 2017, 19, 3095–3098. 10.1021/acs.orglett.7b01199. [DOI] [PubMed] [Google Scholar]
- For select examples of metal-free acyl N–C bond activation, see:; a Verho O.; Pourghasemi Lati M.; Oschmann M. A Two-Step Procedure for the Overall Transamidation of 8-Aminoquinoline Amides Proceeding via the Intermediate N-Acyl-Boc-Carbamates. J. Org. Chem. 2018, 83, 4464–4476. 10.1021/acs.joc.8b00174. [DOI] [PubMed] [Google Scholar]; b Wu H.; Guo W.; Daniel S.; Li Y.; Liu C.; Zeng Z. Fluoride-Catalyzed Esterification of Amides. Chem.—Eur. J. 2018, 24, 3444–3447. 10.1002/chem.201800336. [DOI] [PubMed] [Google Scholar]; c Li G.; Szostak M. Highly Selective Transition-Metal-Free Transamidation of Amides and Amidation of Esters at Room Temperature. Nat. Chem. 2018, 9, 4165. 10.1038/s41467-018-06623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; and references cited therein
- Johansson-Seechurn C. C. C.; Kitching M. O.; Colacot T. J.; Snieckus V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed. 2012, 51, 5062–5085. 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- a Torborg C.; Beller M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. 10.1002/adsc.200900587. [DOI] [Google Scholar]; b Magano J.; Dunetz J. R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- For selected examples of decarbonylative borylation, see:; a Guo L.; Rueping M. Functional Group Interconversion: Decarbonylative Borylation of Esters for the Synthesis of Organoboronates. Chem.—Eur. J. 2016, 22, 16787–16790. 10.1002/chem.201604504. [DOI] [PubMed] [Google Scholar]; b Pu X.; Hu J.; Zhao Y.; Shi Z. Nickel-Catalyzed Decarbonylative Borylation and Silylation of Esters. ACS Catal. 2016, 6, 6692–6698. 10.1021/acscatal.6b01956. [DOI] [Google Scholar]; c Ochiai H.; Uetake Y.; Niwa T.; Hosoya T. Rhodium-Catalyzed Decarbonylative Borylation of Aromatic Thioesters for Facile Diversification of Aromatic Carboxylic Acids. Angew. Chem., Int. Ed. 2017, 56, 2482–2486. 10.1002/anie.201611974. [DOI] [PMC free article] [PubMed] [Google Scholar]; For selected examples of decarboxylative borylation, see:; d Li C.; Wang J.; Barton L. M.; Yu S.; Tian M.; Peters D. S.; Kumar M.; Yu A. W.; Johnson K. A.; Chatterjee A. K.; Yan M.; Baran P. S. Decarboxylative borylation. Science 2017, 356, eaam7355 10.1126/science.aam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Candish L.; Teders M.; Glorius F. Transition-Metal-Free, Visible-Light-Enabled Decarboxylative Borylation of Aryl N-Hydroxyphthalimide Esters. J. Am. Chem. Soc. 2017, 139, 7440–7443. 10.1021/jacs.7b03127. [DOI] [PubMed] [Google Scholar]; For a recent study on decarbonylative borylation of carboxylic acids, see:; f Liu C.; Ji C.-L.; Hong X.; Szostak M. Palladium-Catalyzed Decarbonylative Borylation of Carboxylic Acids: Tuning Reaction Selectivity by Computation. Angew. Chem., Int. Ed. 2018, 57, 16721–16726. 10.1002/anie.201810145. [DOI] [PubMed] [Google Scholar]
- Lennox A. J. J.; Lloyd-Jones G. C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. 10.1039/c3cs60197h. [DOI] [PubMed] [Google Scholar]
- Ishiyama T.; Murata M.; Miyaura N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. 10.1021/jo00128a024. [DOI] [Google Scholar]
- Afagh N. A.; Yudin A. K. Chemoselectivity and the Curious Reactivity Preferences of Functional Groups. Angew. Chem., Int. Ed. 2010, 49, 262–310. 10.1002/anie.200901317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.