Abstract
Fused nitrogen heterocyclesnamely, pyrazolo[3,4-d]pyridazin-7(6H)-ones have been obtained by exploiting the 1,3-dipolar nature of N-arylsydnones, from hydrazones of 3-aryl-4-acetylsydnones via the Vilsmeier–Haack strategy. Facile intramolecular nucleophilic addition followed by CO2 elimination under reflux or upon microwave irradiation was presented. Plausible mechanisms for the formation of the title compounds are proffered. Structure confirmatory evidence came from single-crystal X-ray crystallography.
Introduction
Mesoionic compounds are the five-membered heterocycles that cannot be represented by either a polar or covalent structure. Sydnones are the class of compounds that are mesoionic comprising nitrogen and oxygen atoms in the ring.1−3 These are used as useful synthons in various reactions, particularly in 1,3-dipolar cycloaddition to yield a variety of nitrogen heterocycles. Huisgen and co-workers in 1962 reported a pioneering work of formation of pyrazoles by thermal 1,3-dipolar cycloaddition between sydnones as dipoles and alkynes as dipolarophiles. These may be considered as 1,3-dipoles that upon photolysis, yield nitrile imine intermediates or in the thermal reactions, act as cyclic azomethine imines. With acetylenic dipolarophiles, they undergo such cycloaddition reaction under thermal4,5 or photochemical conditions6−8 to yield pyrazole and/or pyrazolines. 3,4-Disubstituted sydnones upon photochemical reaction yield fused pyrazole derivatives.9 Sydnone can also undergo transformations and delivers pyrazole via a [4 + 2] cycloaddition–retrocycloaddition with the expulsion of carbon dioxide. Nonsymmetrical alkynes resulted in the formation of two pyrazole products, with less or negligible regioselectivity.10 A proper insight about the reactivity and recent advances of the popular mesoionic compound, namely, N-arylsydnone, has been extensively reviewed. Two recent reviews discuss the strategies involved in the sydnone–alkyne cycloaddition12 and also their synthetic applications.13
More than a century has passed since α-diazo derivatives of pyrazole have been discovered, but the five-membered heterocycle, pyrazole, has continued to be a star molecule because of its great interest in various fields.14 Pyrazole and their derivatives possess a wide range of bioactivities.15−21 Pyridazine and their analogues were thought worthwhile to study because they are available as a number of marketed drugs such as Hydralazine,22 Minaprine,23 and Cefozopran.24 It has also been reported that pyridazine derivatives display anticancer,25 anti-HIV,26 antihypertensive,27 antimicrobial, and antifungal activities.28−30
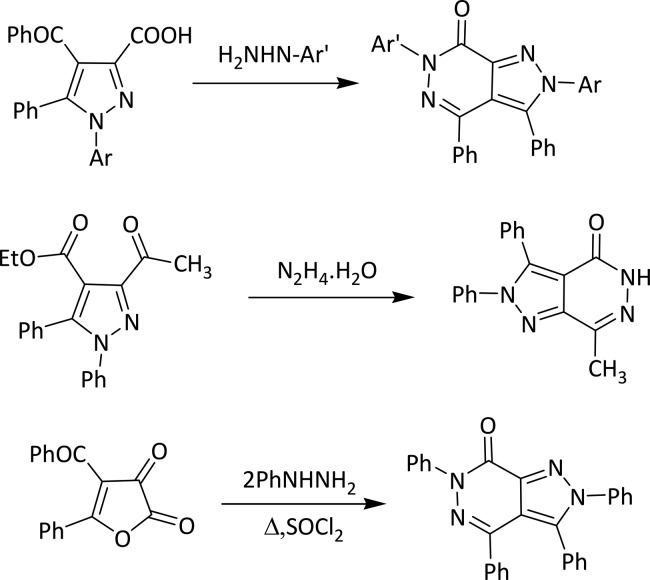
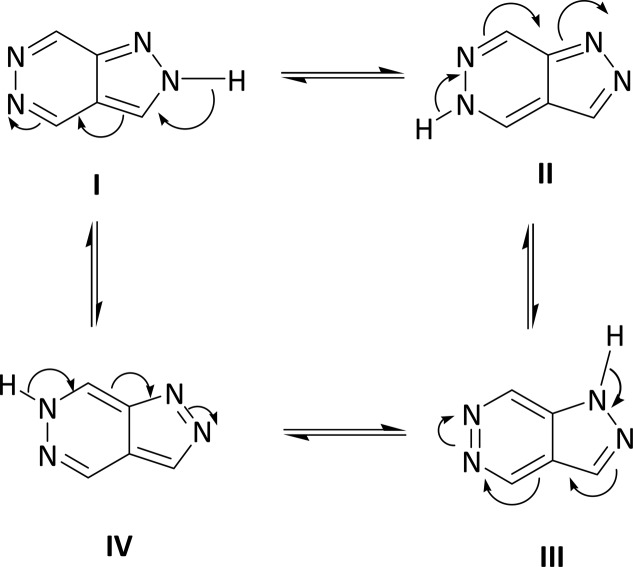
The combination of two pharmacophores into a single molecule is an established methodology for obtaining a potent molecule. Consequently, a design of efficient methods for the assembly of these fused nitrogen heterocycles is of considerable interest, and many such approaches have been discussed in the literature. A useful method for the preparation of pyrazole and pyrazolopyridazine derivatives is the nucleophilic addition of hydrazines to 4-acyl-furandiones in boiling benzene.31 Pyrazolopyridazine/ones have been obtained by various methods, that is, (i) pyrazole-3-carboxylic acid with 2,5-dichlorophenylhydrazine (4),32 (ii) 1-phenyl-4-(2-thienylcarbonyl)-pyrazole-3-carboxylate (5),33 and (iii) 4-benzoyl-5-phenyl-2,3-dihydro-2,3-furandione (6)34 (Scheme 2). Three position isomers have been reported by different methods of synthesis such as pyrazolo[1,2-a]pyridazine-5,8-diones, pyrazolo[3,4-d]pyridazinones, and pyrazolo[4,3-c]pyridazines.35 One of the isomers, namely, pyrazolo[3,4-d]pyridazines, exhibits four tautomeric structures as shown in Scheme 1.
Scheme 2. Previous Reported Synthesis of Pyrazolopyridazines/ones (Yield = <70%).
Scheme 1. Tautomeric Structures of Pyrazolo[3,4-d]pyridazine.
Several derivatives of pyrazolopyridazines exhibit potential activities such as antifungal,36 antibacterial,37 anti-inflammatory, analgesic, antihypoxic, antipyretic agents,38 and antimicrobial actions.39 Also, some pyrazolopyridazine derivatives are reported to be inhibitors of cyclin-dependent kinase 1 (CDK1),40 phosphodiesterase 4 (PDE4) inhibitors, which are widely used in therapeutic treatment of inflammatory and immune disorders,41 including glycogen synthase kinase 3 (GSK-3) inhibitors.42
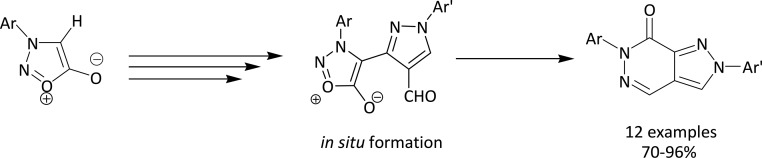
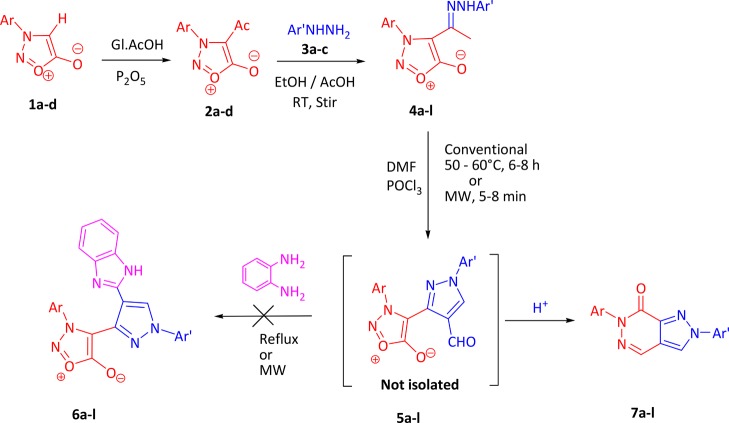
During the course of our studies directed toward the design and synthesis of heterocycles employing a ring transformation of sydnones, we have recently reported the newer heterocyclic scaffolds using 3-arylsydnones 1a–d as synthons.43−47 We, herein, report the serendipitous formation of pyrazolo[3,4-d]pyridazin-7(6H)-ones (7a–l) from hydrazone of 3-aryl-4-acetylsydnones 2a–d by intramolecular nucleophilic addition–elimination. The synthetic route for the synthesized compounds is outlined in Scheme 3.
Scheme 3. Synthetic Route for Title Compounds 7a–l.
7a: Ar = −C6H5, Ar′ = −C6H5; 7b: Ar = −C6H5, Ar′ = 4-Cl–C6H4; 7c: Ar = −C6H5, Ar′ = 4-Br–C6H4; 7d: Ar = 4-CH3O–C6H4, Ar′ = −C6H5; 7e: Ar = 4-CH3O–C6H4, Ar′ = 4-Cl–C6H4; 7f: Ar = 4-CH3O–C6H4, Ar′ = 4-Br–C6H4; 7g: Ar = 4-Cl–C6H4, Ar′ = −C6H5; 7h: Ar = 4-Cl-C6H4, Ar′ = 4-Cl–C6H4; 7i: Ar = 4-Cl–C6H4, Ar′ = 4-Br–C6H4; 7j: Ar = 4-H3C–C6H4, Ar′ = −C6H5; 7k: Ar = 4-H3C–C6H4, Ar′ = 4-Cl–C6H4; 7l: Ar = 4-H3C–C6H4, Ar′ = 4-Br–C6H4.
Results and Discussion
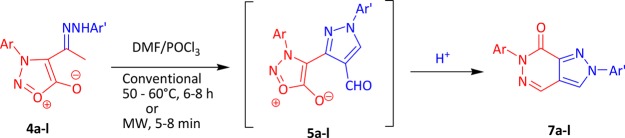
Initially, our aim was cyclization of hydrazones 4a–l by the Vilsmeier–Haack reaction to 3-(3-arylsydnon-4-yl)-1-aryl-1H-pyrazole-4-carbaldehydes 5a–l, which was further planned to convert into benzimidazole derivatives 6a–l by condensing with o-phenylenediamines. However, we did not get the expected aldehydes 5a–l but ended up with an unexpected product. For example, when a hydrazone 4a was reacted with DMF and POCl3 at 50–60 °C, the product upon mass spectral analysis exhibited a molecular ion peak at 288 (m/z) instead of 332 (m/z), which is expected for a benzimidazole derivative 6a. We analyzed the product for the possible structure by spectral analyses (IR, 1H and 13C NMR, GC–MS) as well as by single-crystal X-ray studies and were successful in establishing the structure of the compound as 7a. The unusual reaction of forming fused ring, namely, pyrazolo[3,4-d]pyridazin-7(6H)-ones 7a–l via the Vilsmeier–Haack reagent, and an optimized condition under both conventional and microwave irradiation were established. This occurred by elimination of CO2 and water via acid-catalyzed intramolecular nucleophilic addition–elimination in 3-aryl-4[(1-aryl-4-formyl-pyrazol-3-yl)]-sydnones 5a–l, which was not isolated.
The reaction mixture was irradiated by microwave irradiation for 150 W at 120 °C. Interestingly, we observed the completion of the reaction within 5–8 min with excellent yields (94–96%) for all the title compounds 7a–l (Table 1). Compared to conventional (6–7 h), microwave irradiation greatly reduced the reaction time from 6–7 h to 5–8 min. The yield of the product was also increased up to 94%. This report for pyrazolopyridazin-7-one analogues 7a–l is more advantageous than other reported methods32−34 as the synthetic method includes the shorter reaction time, easy workup, and excellent yields by using DMF/POCl3 as a solvent. The proposed mechanism of conversion of hydrazone of 4-acetyl-3-arylsydnones 4a–l to pyrazolo[3,4-d]pyridazin-7(6H)-ones 7a–l is presented in Scheme 4. It is also interesting to note that 3-aryl-4-substituted sydnones have been used for ring transformation into various heterocycles.11 This is the first protocol being reported for the ring transformation of hydrazone of 4-acetyl-3-arylsydnones 4a–l upon the Vilsmeier–Haack reaction to ring transform into pyrazolo[3,4-d]pyridazin-7(6H)-ones 7a–l directly in excellent yields.
Table 1. Synthesis of Fused Pyrazolopyridazin-7-ones (7a–l) from Hydrazones (3a–c) and DMF/POCl3 under Optimized Conditions.
| thermal |
microwave |
|||||
|---|---|---|---|---|---|---|
| product | Ar 3(a–d) | Ar′ 4(a–c) | time (h) | yielda (%) | time (min) | yielda (%) |
| 7a | –C6H5 | –C6H5 | 5 | 74 | 5 | 90 |
| 7b | –C6H5 | -4ClC6H4 | 5 | 76 | 5 | 94 |
| 7c | –C6H5 | -4BrC6H4 | 6 | 70 | 5 | 92 |
| 7d | 4-CH3OC6H4 | –C6H5 | 6 | 74 | 7 | 95 |
| 7e | 4-CH3OC6H4 | -4ClC6H4 | 5 | 72 | 7 | 95 |
| 7f | 4-CH3OC6H4 | -4BrC6H4 | 5 | 70 | 7 | 95 |
| 7g | -4ClC6H4 | –C6H5 | 7 | 73 | 6 | 96 |
| 7h | -4ClC6H4 | -4ClC6H4 | 7 | 75 | 6 | 92 |
| 7i | -4ClC6H4 | -4BrC6H4 | 8 | 71 | 6 | 95 |
| 7j | 4-CH3C6H4 | –C6H5 | 6 | 70 | 8 | 91 |
| 7k | 4-CH3C6H4 | -4ClC6H4 | 6 | 71 | 8 | 94 |
| 7l | 4-CH3C6H4 | -4BrC6H4 | 7 | 73 | 8 | 96 |
Isolated yield.
Scheme 4. Plausible Mechanism for Formation of Pyrazolo[3,4-d]pyridazin-7(6H)-ones 7a–l.
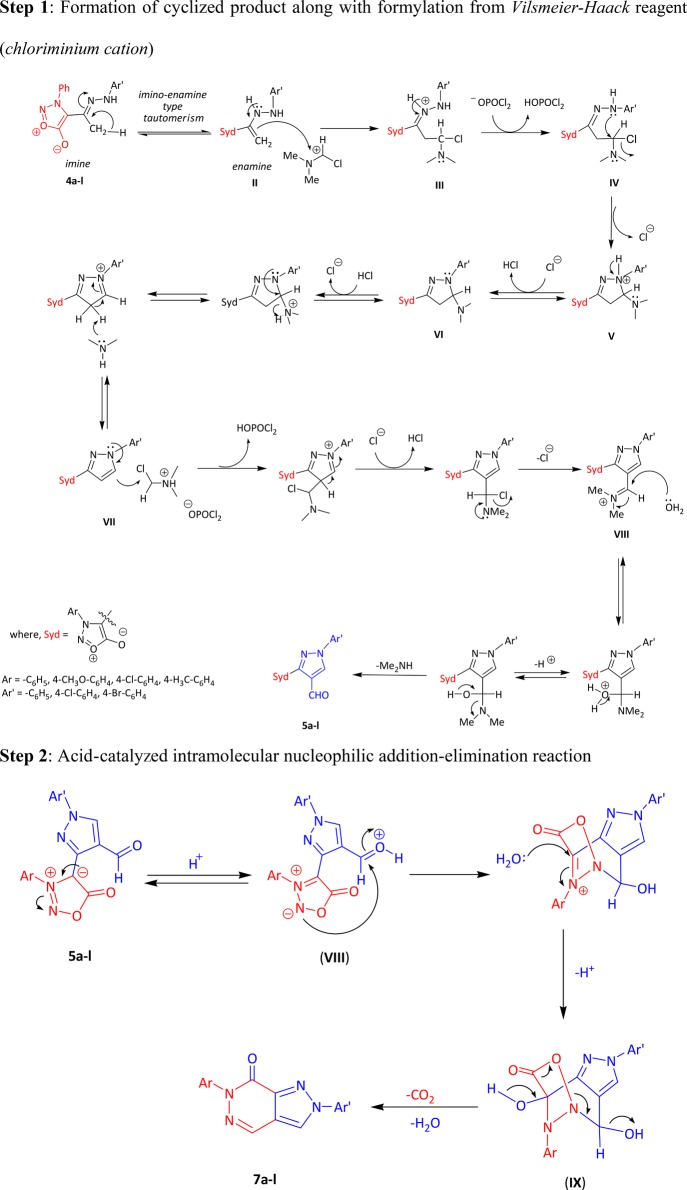
The reaction followed a simple concerted mechanism. The mechanism of transformation of hydrazone of 3-arylsydnones 1a–d to pyrazolo[3,4-d]pyridazin-7(6H)-ones (7a–l) occurred in three steps. In the first step, the Vilsmeier–Haack reagent I is formed in situ by the reaction of POCl3 and DMF. This attacks on CH2 of tautomer II to form intermediate III in step 2. Intermediate III undergoes elimination of HCl to form intermediate IV, which further undergoes removal of dimethyl amine followed by the attack of the Vilsmeier–Haack reagent I once again, which forms an aldehyde 5a–l that was not possible to isolate. The reaction continued further as in the third step of Scheme 3 as intramolecular acid-catalyzed nucleophilic addition–elimination, which occurs through a protonated carbonyl group of aldehyde on pyrazole VIII. This is followed by elimination of CO2 (cycloreversion) and water molecule from IX that gave the final compound 7a–l.
The structures of pyrazolo[3,4-d]pyridazin-7(6H)-ones 7a–l were confirmed by spectroscopic techniques, namely, IR, 1H and 13C NMR, GC–MS, single-crystal X-ray structure studies, and elemental analyses. The compounds have shown a strong adsorption band for carbonyl of pyridazinone and C=N of pyrazole ring at 1630–1679 and 1528–1609 cm–1, respectively. In the case of 1H NMR spectral analyses, the compounds exhibited a singlet in the range 9.94–10.06 ppm for pyridazin-7-one (C3–H) ring and pyrazole (C4–H) at 8.42–9.72 ppm. The aromatic protons of all phenyl rings appeared as multiplets in the range 7.36–7.70 ppm. In the case of 13C NMR spectral study, compounds have shown the number of signals, which are consistent with number of magnetically nonequivalent carbon atoms in the compound, and in mass spectra, the synthesized compounds have shown the molecular ion peaks at their respective m/z values.
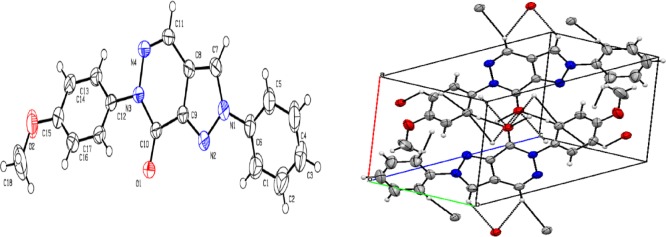
The crystalline nature of the compound is characterized by long range well-defined three-dimensional orders. An ORTEP view of the compound 7d is presented in Figure 1. The crystal data and refinements are presented in Table S1 in Supporting Information. The X-ray structure clearly suggested that compound 7d crystallized as a triclinic system.
Figure 1.
ORTEP structure of compound 7d (displacement ellipsoids are drawn at the 50% probability level) and packing structure of compound 7d.
Conclusions
In summary, we have successfully established a fused ring for the first time from the sydnone in which the acid catalyzed intramolecular nucleophilic addition–elimination reaction provides an efficient means for the preparation of novel fused 2,6-diaryl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-ones 7a–l under a microwave-assisted condition in good yields. Formation of cyclized products along with formylation and intramolecular nucleophilic addition followed by an elimination sequence of reactions was presented. The other features of this protocol include mild conditions, short reaction time, and easy workup.
Experimental Section
General Information
All the reagents and chemicals were purchased from Spectrochem and Sigma-Aldrich. Melting points were measured in open capillaries and are uncorrected. The IR spectra were recorded by a Nicolet Impact 410 FTIR spectrometer using KBr pellets (range 4000–500 cm–1). The 1H NMR spectra were recorded at 400 MHz on a Bruker Avance FT NMR spectrometer in DMSO-d6 solvent with tetramethylsilane (TMS) as internal standard. 13C NMR spectra were recorded at 100 MHz on a Bruker Avance FT NMR spectrometer in DMSO-d6 solvent with TMS as internal standard. The mass spectra were recorded on Shimadzu GC–MS operating at 70 eV. Thin-layer chromatography (TLC) was performed on 0.20 mm Aluchrosep silica gel 60 F254 plates (SD Fine, Mumbai). Synthesis by microwave irradiation was carried out using a CEM Discover microwave synthesizer equipped with an IR sensor to monitor reaction temperatures.48
Procedure for the Synthesis of 4-Acetyl-3-arylsydnones (2a–d)49
To a suspension of 5.20 g (0.0369 mol) of phosphorous pentoxide in dry xylene (20 mL) was added 2.00 g (0.0123 mol) of 3-arylsydnones 1a–d in a two-necked RB flask equipped with a reflux condenser with a calcium chloride drying tube. The stirred mixture was heated to reflux on a water bath. Glacial acetic acid of about 0.80 mL (0.0123 mol) was added dropwise, and the reaction mixture was refluxed for 6 h during which the resultant clear brown solution turned black. After completion of the reaction, the reaction mixture was cooled to room temperature. Xylene was then decanted, and the remaining black residue was extracted twice with xylene (2 × 10 mL). Combined washings and decanted xylene were evaporated to obtain a pale yellow solid and recrystallized using ethanol to obtain pure compounds (2a–d).
Procedure for the Synthesis of 1-(3-Arylsydnon-4-yl)ethylidene)-2-phenylhydrazines (4a–l)49
4-Acetyl-3-arylsydnones 2a–d (1.0 g, 0.005 mol) were dissolved in ethanol (10 mL), and about 3–4 drops of acetic acid were added. Aryl hydrazines 3a–c (0.50 mL, 0.0049 mol) were then added, and the reaction mixture was refluxed for 2 h. The progress of the reaction was monitored by TLC, and after completion of the reaction, the separated solid was filtered, dried, and recrystallized from ethanol to obtain the compounds 4a–l.
Procedure for the Synthesis of 2,6-Diaryl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-ones (7a–l)
DMF (1.16 mL, 0.015 mmol) in an RB flask was cooled to 0 °C, and POCl3 (3.92 mL, 0.042 mmol) was added dropwise with constant stirring. To this adduct, 1-(3-arylsydnon-4-yl)ethylidene)-2-phenylhydrazines 4a–l (2.0 g, 0.006 mmol) was added and again stirred for 10 min. The resulting solution was heated on a water bath for 10–12 h. After completion of the reaction, the reaction mixture was cooled to RT and poured into crushed ice. The pale yellow solid separated was filtered and washed with water to obtain the compounds 7a–l, which was recrystallized from ethanol.
Procedure for Microwave-Assisted Synthesis of 2,6-Diphenyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-ones (7a–l)
In a sealed glass tube vial (30.0 mL), DMF (0.19 mL, 0.0025m mol) was taken and cooled to 0 °C, and POCl3 (0.65 mL, 0.007 mol) was added dropwise with constant stirring. To this, 1-(3-arylsydnon-4-yl)ethylidene)-2-phenylhydrazines 4a–l (0.5 g, 0.0016 mol) was added and again stirred for 10 min. The sealed vessel with the reaction mixture was prestirred for 30 s at room temperature. The reaction mixture was then irradiated by 150 W at 120 °C for about 5–8 min at medium stirring. The completion of the reaction was monitored by TLC using hexane:ethylacetate (7:3 drops) as an eluent. The reaction mixture was then quenched into crushed ice, and the crude product obtained was filtered, washed, and dried. It was recrystallized from ethanol to obtain pure crystals of the compounds 7a–l.
4-Acetyl-3-arylsydnone (2a)
Pale yellow solid; mp 141–143 °C; IR (KBr; ν̅max, cm–1): 1750 cm–1 (C=O), 1H NMR (400 MHz, DMSO-d6, ppm): 7.57–7.69 (m, 5H, Ar-H), 2.32 (s, 3H, −CH3); 13C NMR (100 MHz, DMSO-d6, ppm): 183.9, 166.6, 135.5, 132.5, 129.7, 125.87, 107.5, 28.1. MS-EI (m/z): 204 [M]+; analysis calcd (%) for C10H8N2O3: C, 58.82; H, 3.95; N, 13.72. Found: C, 58.81; H, 3.97; N, 13.73.
1-(3-Arylsydnon-4-yl)ethylidene)-2-(4-bromopheny)-hydrazine (4c)
Yellow crystalline solid; mp 162–164 °C; IR (KBr; ν̅max, cm–1): 1660 cm–1 (C=N), 1745 cm–1 (C=O); 1H NMR (400 MHz, DMSO-d6, ppm): 2.92 (s, 3H, −CH3), 6.14–6.16 (d, 2H, Ar-H), 7.04–7.07 (d, 2H, Ar-H), 7.61–7.64 (m, 5H, Ar-H), 9.48 (s, 1H, N–H). 13C NMR (100 MHz, DMSO-d6, ppm): 166.8, 144.6, 136.9, 131.7, 131.5, 130.3, 130.0, 125.8, 115.0, 110.8, 108.3, 31.2. MS-EI (m/z): 372 [M]+, 374 [M]+2; analysis calcd (%) for C16H13N4BrO2: C, 51.49; H, 3.51, N, 15.01. Found: C, 51.50; H, 3.50; N, 15.02.
2,6-Diphenyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7a)
Light yellow solid; mp 136–138 °C; IR (KBr; ν̅max, cm–1): 1673 (pyridazin, C=O), 1525 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.89 (s, 1H, pyridazin C–H), 9.24 (s, 1H, pyrazolo C–H), 8.61 (d, 1H, J = 7.8 Hz, Ar-H), 8.04 (d, 1H, J = 8 Hz, Ar-H), 7.70–7.37 (m, 6H, Ar-H), 7.13 (d, 1H, J = 8.8 Hz, Ar-H), 7.03 (d, 1H, J = 8.8 Hz, Ar-H); 13C NMR (100 MHz, DMSO-d6, ppm): 185.4 (pyridazin C=O), 154.9, 139.1, 131.3, 131.0, 129.7, 129.4, 128.8, 128.0, 126.6, 122.5, 119.8, 118.7; MS-EI (m/z): 288 [M]+; analysis calcd (%) for C17H12N4O: C, 70.82; H, 4.20; N, 19.43. Found: C, 70.85; H, 4.24; N, 19.46.
2-(4-Chlorophenyl)-6-phenyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7b)
Yellow solid; mp 158–160 °C; IR (KBr; ν̅max cm–1): 1675 (pyridazin, C=O), 1531 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.89 (s, 1H, pyridazin C–H), 9.17 (s, 1H, pyrazolo C–H), 7.51–7.70 (m, 9H, Ar-H); 13C NMR (100 MHz, DMSO-d6, ppm): 185.6 (pyridazin, C=O), 166.8, 160.1, 137.5, 134.8, 132.9, 132.8, 130.1, 128.2, 126.1, 125.7, 124.1, 121.2; MS-EI (m/z): 324 [M]+2, 322 [M]+; analysis calcd (%) for C17H11N4ClO: C, 63.26; H, 3.44; N, 17.36. Found: C, 63.32; H, 3.47; N, 17.44.
2-(4-Bromophenyl)-6-phenyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7c)
Yellow solid; mp 166–168 °C; IR (KBr; ν̅max, cm–1): 1630 (pyridazin, C=O), 1554 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.71 (s, 1H, pyridazin C–H), 9.00 (s, 1H, pyrazolo C–H), 7.33–7.52 (m, 9H, Ar-H); 13C NMR (100 MHz, DMSO-d6, ppm): 183.2 (pyridazin C=O), 164.5, 157.9, 136.4, 132.4, 130.5, 130.4, 127.8, 123.7, 123.4, 121.8, 118.9, 99.7; MS-EI (m/z): 368 [M]+2, 366 [M]+; analysis calcd (%) for C17H11N4BrO: C, 55.61; H, 3.02; N, 15.26. Found: C, 55.64; H, 3.05; N, 15.29.
6-(4-Methoxyphenyl)-2-phenyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7d)
Chrome yellow solid; mp 168–170 °C; IR (KBr; ν̅max, cm–1): 1672 (pyridazin, C=O), 1525 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 10.04 (s, 1H, pyridazin C–H), 8.44 (s, 1H, pyrazolo C–H), 7.53–7.48 (m, 5H, Ar-H), 7.01 (d, 2H, J = 6.8 Hz, Ar-H), 6.98 (d, 2H, J = 7.2 Hz, Ar-H), 3.90 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6, ppm): 184.2 (pyridazin C=O), 162.2, 138.62, 130.5, 129.8, 129.7, 128.4, 127.2, 126.3, 124.3, 121.1, 119.5, 114.6, 55.8 (−OCH3); MS-EI (m/z): 318 [M]+; analysis calcd (%) for C18H14N4O2: C, 67.91; H, 4.43; N, 17.60. Found: C, 67.95; H, 4.45; N, 17.63.
2-(4-Chlorophenyl)-6-(4-methoxyphenyl)-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7e)
Off-white solid; mp 155–157 °C; IR (KBr; ν̅max, cm–1): 1666 (pyridazin, C=O), 1532 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.85 (s, 1H, pyridazin C–H), 9.20 (s, 1H, pyrazolo C–H), 7.40–7.71 (m, 6H, Ar-H), 7.01–7.07 (d, 2H, Ar-H), 3.77 (s, 3H, −OCH3); 13C NMR (100 MHz, DMSO-d6, ppm): 185.5 (pyridazin C=O), 162.2, 138.8, 137.5, 133.0, 132.9, 130.2, 128.0, 127.2, 124.2, 121.3, 115.1, 101.8, 56.3 (−OCH3); MS-EI (m/z): 354 [M]+2, 352 [M]+; analysis calcd (%) for C18H13N4ClO2: C, 61.28; H, 3.71; N, 15.88. Found: C, 61.32; H, 3.75; N, 15.94.
2-(4-Bromophenyl)-6-(4-methoxyphenyl)-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7f)
Yellow solid; mp 158–160 °C; IR (KBr; ν̅max, cm–1): 1668 (pyridazin, C=O), 1528 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.85 (s, 1H, pyridazin C–H), 9.20 (s, 1H, pyrazolo C–H), 7.53–7.71 (m, 6H, Ar-H), 7.07–7.10 (d, 2H, Ar-H), 3.75 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6, ppm): 186.8 (pyridazin C=O), 163.5, 140.1, 138.8, 134.2, 134.1, 131.4, 129.3, 128.4, 125.4, 122.6, 116.4, 103.0, 57.6 (−OCH3); MS-EI (m/z): 398 [M]+2, 396 [M]+; analysis calcd (%) for C18H13N4BrO2: C, 54.43; H, 3.30; N, 14.10. Found: C, 54.51; H, 3.36; N, 14.17.
6-(4-Chlorophenyl)-2-phenyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7g)
White solid; mp 152–154 °C; IR (KBr; ν̅max, cm–1): 1679 (pyridazin, C=O), 1589 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.94 (s, 1H, pyridazin C–H), 9.46 (s, 1H, pyrazolo C–H), 8.62 (d, 2H, J = 8.4 Hz, Ar-H), 8.32 (d, 2H, J = 8 Hz, Ar-H), 7.70–7.38 (m, 5H, Ar-H); 13C NMR (100 MHz, DMSO-d6, ppm): 183.1 (pyridazin C=O), 169.6, 163.2, 139.9, 132.7, 126.6, 126.5, 126.1, 124.7, 122.2, 119.4, 106.2, 95.8; MS-EI (m/z): 324 [M]+2, 322 [M]+; analysis calcd (%) for C17H11N4ClO: C, 63.26; H, 3.44; N, 17.36. Found: C, 63.32; H, 3.47; N, 17.41.
2,6-Bis(4-chlorophenyl)-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7h)
Yellow solid; mp 148–150 °C; IR (KBr; ν̅max, cm–1): 1669 (pyridazin, C=O), 1556 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.86 (s, 1H, pyridazin C–H), 9.18 (s, 1H, pyrazolo C–H), 7.53–7.67(m, 6H, Ar-H), 7.36–7.38 (d, 2H, Ar-H); 13C NMR (100 MHz, DMSO-d6, ppm): 185.2 (pyridazin C=O), 160.1, 143.0, 137.5, 133.0, 132.3, 130.5, 130.2, 125.4, 124.2, 121.3, 121.1, 106.1; MS-EI (m/z): 356 [M]+4, 354 [M]+2, 352 [M]+; analysis calcd (%) for C17H10N4Cl2O: C, 57.16; H, 2.82; N, 15.69. Found: C, 57.22; H, 2.91; N, 15.74.
2-(4-Bromophenyl)-6-(4-chlorophenyl)-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7i)
Chrome yellow solid; mp 154–156 °C; IR (KBr; ν̅max, cm–1): 1672 (pyridazin, C=O), 1567 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.89 (s, 1H, pyridazin C–H), 9.17 (s, 1H, pyrazolo C–H), 7.54–7.70 (m, 8H, Ar-H); 13C NMR (100 MHz, DMSO-d6, ppm): 185.6 (pyridazin C=O), 135.8, 133.0, 132.6, 130.1, 129.7, 126.8, 125.8, 124.1, 121.5, 121.2, 112.5, 105.3; MS-EI (m/z): 400 [M]+4, 398 [M]+2, 396 [M]+; analysis calcd (%) for C17H10N4Br2O: C, 50.84; H, 2.51; N, 13.95. Found: C, 50.90; H, 2.57; N, 13.98.
2-Phenyl-6-p-tolyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7j)
Off-white solid; mp 168–170 °C; IR (KBr; ν̅max, cm–1): 1664 (pyridazin, C=O), 1609 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 10.05 (s, 1H, pyridazin C–H), 8.44 (s, 1H, pyrazolo C-H), 7.48–7.34 (m, 5H, Ar-H), 7.32 (d, 2H, J = 7.4 Hz, Ar-H), 7.29 (d, 2H, J = 8 Hz, Ar-H), 2.40 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6, ppm): 184.3 (pyridazin C=O), 167.1, 142.8, 138.5, 130.4, 130.1, 129.8, 128.4, 124.7, 119.4, 119.1, 117.3, 101.2, 21.5 (CH3); MS-EI (m/z): 302 [M]+; analysis calcd (%) for C18H14N4O: C, 71.51; H, 4.67; N, 18.53. Found: C, 71.58; H, 4.75; N, 18.59.
2-(4-Chlorophenyl)-6-p-tolyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7k)
Off white solid; mp 147–149 °C; IR (KBr; ν̅max, cm–1): 1676 (pyridazin, C=O), 1558 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.85 (s, 1H, pyridazin C–H), 9.18 (s, 1H, pyrazolo C–H), 7.36–7.67 (m, 8H, Ar-H), 2.34 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6, ppm): 185.5 (pyridazin, C=O), 151.9, 143.0, 138.7, 137.5, 133.0, 132.3, 130.5, 130.2, 125.4, 124.2, 121.3, 102.4, 21.4 (CH3); MS-EI (m/z): 336 [M]+2, 334 [M]+; analysis calcd (%) for C18H13N4ClO: C, 64.19; H, 3.89; N, 16.64. Found: C, 64.23; H, 3.92; N, 16.66.
2-(4-Bromophenyl)-6-p-tolyl-2H-pyrazolo[3,4-d]pyridazin-7(6H)-one (7l)
Yellow solid; mp 186–188 °C; IR (KBr; ν̅max, cm–1): 1674 (pyridazin, C=O), 1531 (CN); 1H NMR (400 MHz, DMSO-d6, ppm): 9.86 (s, 1H, pyridazin C–H), 9.18 (s, 1H, pyrazolo C–H), 7.36–7.68(m, 8H, Ar-H), 2.34 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6, ppm): 185.5 (pyridazin C=O), 166.8, 143.0, 139.2, 137.9, 132.9, 132.3, 130.5, 125.4, 124.2, 121.6, 121.2, 101.8, 21.8 (−CH3); MS-EI (m/z): 380 [M]+2, 378 [M]+; analysis calcd (%) for C18H13N4BrO: C, 56.71; H, 3.44; N, 14.70. Found: C, 56.76; H, 3.49; N, 14.75.
Acknowledgments
The authors are thankful to the Indian Institute of Science, Bangalore (IISc) for carrying out NMR analyses and to UGC, New Delhi for the financial assistance under UPE program vide F. No. 14-2/2012 (NS/PE) dated: 22-01-2013. One of the authors (M.N.K.) acknowledges University Grants Commission (UGC), New Delhi for fellowship under UGC-UPE program. The authors are grateful to University Scientific Instrumentation Centre (USIC), Karnatak University, Dharwad for providing single-crystal X-ray, IR, and mass spectral data.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02013.
Scanned copies of 1H NMR and 13C NMR, mass spectra for newly synthesized compounds 7a–7l, ORTEP X-ray crystal structure, and CIF files of crystallographic data for 4a (PDF)
Author Contributions
M.N.K., S.K.J.S., P.K.B., A.A.K., and R.K.H. have equally contributed to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Stewart F. H. C. The Chemistry of Sydnones. Chem. Rev. 1964, 64, 129–147. 10.1021/cr60228a004. [DOI] [Google Scholar]
- Clapp L. B.1,2,3 and 1,2,4-Oxadiazoles. In Comprehensive Heterocyclic Chemistry; Katritzky A. R., Rees C. W., Eds.; Pergamon Press: Oxford, U.K., 1984; Vol. 6, pp 365–378. [Google Scholar]
- a Newton C. G.; Ramsden C. A. Meso-ionic heterocycles (1976-1980). Tetrahedron 1982, 38, 2965–3011. 10.1016/0040-4020(82)80186-5. [DOI] [Google Scholar]; b Browne D. L.; Taylor J. B.; Plant A.; Harrity J. P. A. Cross coupling of bromo sydnones: Development of a flexible route toward functionalized pyrazoles. J. Org. Chem. 2009, 74, 396–400. 10.1021/jo802240e. [DOI] [PubMed] [Google Scholar]; c Brown A. W.; Harrity J. P. A. Direct arylation of sydnones with aryl chlorides toward highly substituted pyrazoles. J. Org. Chem. 2015, 80, 2467–2472. 10.1021/acs.joc.5b00143. [DOI] [PubMed] [Google Scholar]; d Favre C.; de Cremoux L.; Badaut J.; Friscourt F. Sydnone reporters for highly fluorogenic copper-free click ligations. J. Org. Chem. 2018, 83, 2058–2066. 10.1021/acs.joc.7b03004. [DOI] [PubMed] [Google Scholar]; e Handa N. V.; Li S.; Gerbec J. A.; Sumitani N.; Hawker C. J.; Klinger D. Fully Aromatic High Performance Thermoset via Sydnone–Alkyne Ccloaddition. J. Am. Chem. Soc. 2016, 138, 6400–6403. 10.1021/jacs.6b03381. [DOI] [PubMed] [Google Scholar]
- Gribble G. W.Mesoionic Ring Systems. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, Padwa A., Pearson W. H., Eds.; Wiley & Sons: Hoboken, NJ, 2003; Vol. 59, pp 681–753. [Google Scholar]
- a Chang E.-M.; Chen T.-H.; Wong F. F.; Chang E.-C.; Yeh M.-Y. Convenient and efficient synthesis of pyrazole-based DHODase inhibitors from 3-Aryl-4-cyanosydnone. Synlett 2006, 6, 901–904. 10.1055/s-2006-939041. [DOI] [Google Scholar]; b Wu C.; Fang Y.; Larock R. C.; Shi F. Synthesis of 2H-Indazoles by the [3+2] cycloaddition of Arynes and Sydnones. Org. Lett. 2010, 12, 2234–2237. 10.1021/ol100586r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai G.; Puranik V. G.; Kalluraya B.; Hegde J. C. Exclusive formation of 1-aryl-3-(5-nitro-2-furyl)-4-aroylpyrazoles via regiospecific 1,3-dipolar cycloaddition of 3-arylsydnones with α,β-acetylenic ketones. Synth. Commun. 2007, 36, 1285–1290. 10.1080/00397910500518874. [DOI] [Google Scholar]
- Krauch C. H.; Kuhls J.; Piek H.-J. 4-Phenyl-Δ2-1.3.4-oxadiazolin-5-on aus N-phenylsydnon. Eine photochemische reaktion mit CO2. Tetrahedron Lett. 1966, 7, 4043–4048. 10.1016/S0040-4039(00)90284-3. [DOI] [Google Scholar]
- Akiko C.; Yoshiharu H.; Masaki O. The photochemical reactions of several mesoionic compounds. Bull. Chem. Soc. Jpn. 1970, 43, 2650. 10.1246/bcsj.43.2650. [DOI] [Google Scholar]
- a Meier H.; Heimgartner H.; Schmid H. Thermal and photochemically induced intramolecular 1,3-dipolar cycloaddition reactions of 4-phenyl-3-(2-allylphenyl)-sydnone. Helv. Chim. Acta 1977, 60, 1087–1090. 10.1002/hlca.19770600333. [DOI] [Google Scholar]; b Meier H.; Heimgartner H. Intramolecular 1,3-Dipolar Cycloaddition Reactions of Alkenyl-substituted 3,4-Diarylsydnones. Helv. Chim. Acta 1986, 69, 927–940. 10.1002/hlca.19860690421. [DOI] [Google Scholar]
- a Huisgen R.; Grashey R.; Gotthardt H.; Schmidt R. 1,3-Dipolar additions of sydnones to alkynes. A new route into the pyrazole series. Angew. Chem., Int. Ed. 1962, 1, 48–49. 10.1002/anie.196200482. [DOI] [Google Scholar]; b Huisgen R.; Gotthardt H. 1,3-Dipolar Cycloadditions, XXXIX. Kinetics and mechanism of cycloadditions of sydnones. Chem. Ber. 1968, 101, 1059–1071. 10.1002/cber.19681010343. [DOI] [Google Scholar]
- Browne D. L.; Harrity J. P. A. Recent developments in the chemistry of Sydnones. Tetrahedron 2010, 66, 553–568. 10.1016/j.tet.2009.10.085. [DOI] [Google Scholar]
- Decuypère E.; Plougastel L.; Audisio D.; Taran F. Sydnone-alkyne cycloaddition: Applications in synthesis and bioconjugation. Chem. Commun. 2017, 53, 11515–11527. 10.1039/C7CC06405E. [DOI] [PubMed] [Google Scholar]
- Hladíková V.; Váňa J.; Hanusek J. [3+2]-Cycloaddition reaction of sydnones with alkynes. Beilstein J. Org. Chem. 2018, 14, 1317–1348. 10.3762/bjoc.14.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fustero S.; Sánchez-Roselló M.; Barrio P.; Simón-Fuentes A. From 2000 to Mid 2010: A fruitful decade for the synthesis of pyrazoles. Chem. Rev. 2011, 111, 6984–7034. 10.1021/cr2000459. [DOI] [PubMed] [Google Scholar]
- Ouyang G.; Cai X.-J.; Chen Z.; Song B.-A.; Bhadury P. S.; Yang S.; Jin L.-H.; Xue W.; Hu D.-Y.; Zeng S. Synthesis and Antiviral Activities of Pyrazole Derivatives Containing an Oxime Moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. 10.1021/jf802489e. [DOI] [PubMed] [Google Scholar]
- Storer R.; Ashton C. J.; Baxter A. D.; Hann M. M.; Marr C. L. P.; Mason A. M.; Mo C.-L.; Myers P. L.; Noble S. A.; Penn C. R.; Weir N. G.; Woods J. M.; Coe P. L. The synthesis and antiviral activity of 4-fluoro-1-β-D-ribofuranosyl-1H-pyrazole-3-carboxamide. Nucleosides Nucleotides 1999, 18, 203–216. 10.1080/15257779908043068. [DOI] [PubMed] [Google Scholar]
- Gökhan-Kelekçi N.; Yabanoğlu S.; Kupeli E.; Salgin U.; Özgen O.; Uçar G.; Yeşilada E.; Kendi E.; Yeşilada A.; Bilgin A. A. A new therapeutic approach in Alzheimer disease: some novel pyrazole derivatives as dual MAO-B inhibitors and anti-inflammatory analgesics. Bioorg. Med. Chem. 2007, 15, 5775–5786. 10.1016/j.bmc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Kaushik D.; Khan S. A.; Chawla G.; Kumar S. N’-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]2/4-substituted hydrazides: synthesis and anticonvulsant activity. Eur. J. Med. Chem. 2010, 45, 3943–3949. 10.1016/j.ejmech.2010.05.049. [DOI] [PubMed] [Google Scholar]
- Balbi A.; Anzaldi M.; Macciò C.; Aiello C.; Mazzei M.; Gangemi R.; Castagnola P.; Miele M.; Rosano C.; Viale M. Synthesis and biological evaluation of novel pyrazole derivatives with anticancer activity. Eur. J. Med. Chem. 2011, 46, 5293–5309. 10.1016/j.ejmech.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Song H.; Liu Y.; Xiong L.; Li Y.; Yang N.; Wang Q. Design, Synthesis, and Insecticidal Activity of Novel Pyrazole Derivatives Containing α-Hydroxymethyl-N-benzyl Carboxamide, α-Chloromethyl-N-benzyl Carboxamide, and 4,5-Dihydrooxazole Moieties. J. Agric. Food Chem. 2012, 60, 1470–1479. 10.1021/jf204778v. [DOI] [PubMed] [Google Scholar]
- Chen H.; Li Z.; Han Y. Synthesis and Fungicidal Activity against Rhizoctonia solani of 2-Alkyl(alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (Thiadiazoles). J. Agric. Food Chem. 2000, 48, 5312–5315. 10.1021/jf991065s. [DOI] [PubMed] [Google Scholar]
- Taylor D. G. In Pharmacology of Antihypertensive Drugs; Scriabne A., Ed.; Raven Press: New York, 1980; p 407. [Google Scholar]
- Contreras J.-M.; Rival Y. M.; Chayer S.; Bourguignon J.-J.; Wermuth C. G. Aminopyridazines as Acetylcholinesterase Inhibitors. J. Med. Chem. 1999, 42, 730–741. 10.1021/jm981101z. [DOI] [PubMed] [Google Scholar]
- Kobayashi H.; Moritono S.; Hara K. Significant role of new Cephem antibiotics: focused on Cefozopran. Jpn. J. Antibiot. 1997, 50, 807–820. [PubMed] [Google Scholar]
- Rathish I. G.; Javed K.; Ahmad S.; Bano S.; Alam M. S.; Akhter M.; Pillai K. K.; Ovais S.; Samim M. Synthesis and evaluation of anticancer activity of some novel 6-aryl-2-(p-sulfamylphenyl)-pyridazin-3(2H)-ones. Eur. J. Med. Chem. 2012, 49, 304–309. 10.1016/j.ejmech.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Wang M.; Yao X.; Li Y.; Tan J.; Wang L.; Qiao W.; Geng Y.; Liu Y.; Wang Q. Design, synthesis and antiviral activity of novel pyridazines. Eur. J. Med. Chem. 2012, 54, 33–41. 10.1016/j.ejmech.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Mishra R.; Siddiqui A. A.; Husain A.; Rashid M.; Goda C. Design, synthesis and antihypertensive screening of novel pyridazine substituted s-triazin-2-imine/one/thione derivatives. J. Enzyme Inhib. Med. Chem. 2012, 28, 552–559. 10.3109/14756366.2012.656623. [DOI] [PubMed] [Google Scholar]
- Kandile N. G.; Mohamed M. I.; Zaky H.; Mohamed H. M. Novel pyridazine derivatives: Synthesis and antimicrobial activity evaluation. Eur. J. Med. Chem. 2009, 44, 1989–1996. 10.1016/j.ejmech.2008.09.047. [DOI] [PubMed] [Google Scholar]
- Althagafi I.; El-Sayed R. Synthesis, surface, and antimicrobial activity of hydrophobically thiadiazole, pyridazine, and pyrimidine systems based on surface active agents. J. Heterocycl. Chem. 2018, 55, 660–669. 10.1002/jhet.3084. [DOI] [Google Scholar]
- Sönmez M.; Berber İ.; Akbaş E. Synthesis, antibacterial and antifungal activity of some new pyridazinone metal complexes. Eur. J. Med. Chem. 2006, 41, 101–105. 10.1016/j.ejmech.2005.10.003. [DOI] [PubMed] [Google Scholar]
- a Akcamur Y.; Penn G.; Ziegler E.; Sterk H.; Kollenz G.; Peters K.; Peters E.-M.; von Schnering H. G. Reactions with cyclic oxalyl compounds, XXIV. For the reaction of 4-benzoyl-5-phenyl-furan-2,3-dione with phenylhydrazones and phenylhydrazine, respectively. Monatsh. Chem. 1986, 117, 231–245. 10.1007/BF00809443. [DOI] [Google Scholar]; b Akcamur Y.; Sener A.; Ipekoglu A. M.; Kollenz G. Functionalization and cyclization reactions of 4-benzoyl-1,5-diphenyl-1H-pyrazole-3-carboxylic Acid. J. Heterocycl. Chem. 1997, 34, 221–224. 10.1002/jhet.5570340133. [DOI] [Google Scholar]
- a Akbas E.; Aslanoǧlu F. Syntheses of some new 1H-pyrazole, pyridazin-3(2H)-one, and oxazin-4-one derivatives. Heteroat. Chem. 2006, 17, 8–12. 10.1002/hc.20170. [DOI] [Google Scholar]; b Abdelhamid A. O.; Al-Atoom A. A. Reactions With Hydrazonoyl Halides 45: Synthesis of Some New Triazolino[4,3-a]pyrimidines, Pyrazolo[3,4-d]pyridazines, Isoxazolo[3,4-d]pyridazines, and Thieno[2,3-b]pyridines. Synth. Commun. 2006, 36, 97–110. 10.1080/00397910500330593. [DOI] [Google Scholar]
- Shawali A. S.; Haboub A. J. M. Site-selectivity in hydrazinolysis of 3,4’-bis-(functionalized carbonyl)-4,3’-bis(pyrazolyl)ketones. A convenient synthesis of 4-(pyrazol-3-yl)-2H-pyrazolo[3,4-d]-pyridazines. ARKIVOC 2012, 2012, 301–311. 10.3998/ark.5550190.0013.525. [DOI] [Google Scholar]
- Akbas E.; Sener A. Microwave-assisted studies on the reactions of the 4-benzoyl-5-phenyl-2,3-dihydro-2,3-furandione and derivatives. Lett. Org. Chem. 2010, 7, 103–105. 10.2174/157017810790796381. [DOI] [Google Scholar]
- Li M.; Zhao B.-X. Progress of the synthesis of condensed pyrazole derivatives (from 2010 to mid-2013). Eur. J. Med. Chem. 2014, 85, 311–340. 10.1016/j.ejmech.2014.07.102. [DOI] [PubMed] [Google Scholar]
- Akbas E.; Berber I. Antibacterial and antifungal activities of new pyrazolo[3,4-d]pyridazin derivatives. Eur. J. Med. Chem. 2005, 40, 401–405. 10.1016/j.ejmech.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Bildirici I.; Şener A.; Tozlu İ. Further derivatives of 4-benzoyl-1,5-diphenyl-1H-pyrazole-3-carboxylic acid and their antibacterial activities. Med. Chem. Res. 2007, 16, 418–426. 10.1007/s00044-007-9082-z. [DOI] [Google Scholar]
- Al’-Assar F.; Zelenin K. N.; Lesiovskaya E. E.; Bezhan I. P.; Chakchir B. A. Synthesis and pharmacological activity of 1-hydroxy-, 1-amino-, and 1-hydrazino-substituted 2,3-dihydro-1H-pyrazolo[1,2-a]pyridazine-5,8-diones and 2,3-dihydro-1H-pyrazolo[1,2-b]phthalazine-5,10-diones. Pharm. Chem. J. 2002, 36, 598–603. 10.1023/A:1022665331722. [DOI] [Google Scholar]
- Mady M. F.; Saleh T. S.; El-Kateb A. A.; El-Rahman N. M. A.; El-Moez S. I. A. Microwave-assisted synthesis of novel pyrazole and pyrazolo[3,4-d]pyridazine derivatives incorporating diaryl sulfone moiety as potential antimicrobial agents. Res. Chem. Intermed. 2016, 42, 753–769. 10.1007/s11164-015-2054-x. [DOI] [Google Scholar]
- Braña M. F.; Cacho M.; García M. L.; Mayoral E. P.; López B.; de Pascual-Teresa B.; Ramos A.; Acero N.; Llinares F.; Muñoz-Mingarro D.; Lozach O.; Meijer L. Pyrazolo[3,4-c]pyridazines as Novel and Selective Inhibitors of Cyclin-Dependent Kinases. J. Med. Chem. 2005, 48, 6843–6854. 10.1021/jm058013g. [DOI] [PubMed] [Google Scholar]
- Biagini P.; Biancalani C.; Graziano A.; Cesari N.; Giovannoni M. P.; Cilibrizzi A.; Dal Piaz V.; Vergelli C.; Crocetti L.; Delcanale M.; Armani E.; Rizzi A.; Puccini P.; Gallo P. M.; Spinabelli D.; Caruso P. Functionalized pyrazoles and pyrazolo[3,4-d]pyridazinones: Synthesis and evaluation of their phosphodiesterase 4 inhibitory activity. Bioorg. Med. Chem. 2010, 18, 3506–3517. 10.1016/j.bmc.2010.03.066. [DOI] [PubMed] [Google Scholar]
- Witherington J.; Bordas V.; Haigh D.; Hickey D. M. B.; Ife R. J.; Rawlings A. D.; Slingsby B. P.; Smith D. G.; Ward R. W. 5-Aryl-pyrazolo[3,4-b]pyridazines: Potent Inhibitors of Glycogen Synthase Kinase-3 (GSK-3). Bioorg. Med. Chem. Lett. 2003, 13, 1581–1584. 10.1016/S0960-894X(03)00135-5. [DOI] [PubMed] [Google Scholar]
- Taj T.; Kamble R. R.; Kattimani P. P.; Badami B. V. Synthetic utility of sydnones: Synthesis of pyrazolines derivatized with 1,2,4-triazoles as antihyperglymic, antioxidant agents and their DNA cleavage study. Med. Chem. Res. 2012, 21, 3709–3719. 10.1007/s00044-011-9921-9. [DOI] [Google Scholar]
- Kattimani P. P.; Kamble R. R.; Kariduraganavar M. Y.; Dorababu A.; Hunnur R. K. Synthesis, characterization and in vitro anticancer evaluation of novel 1,2,4-triazolin-3-one derivatives. Eur. J. Med. Chem. 2013, 62, 232–240. 10.1016/j.ejmech.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Kattimani P. P.; Kamble R. R.; Dorababu A.; Hunnur R. K.; Kamble A. A.; Devarajegowda H. C. C5-Alkyl-1,3,4-Oxadiazol-2-ones undergo dealkylation upon nitrogen insertion to form 2H-1,2,4-triazol-3-ones: Synthesis of 1,2,4-triazol-3-one hybrids with triazolothiadiazoles and triazolothiadiazines. J. Heterocycl. Chem. 2017, 54, 2258–2265. 10.1002/jhet.2813. [DOI] [Google Scholar]
- Somagond S. M.; Kamble R. R.; Kattimani P. P.; Shaikh S. K. J.; Dixit S. R.; Joshi S. D.; Devarajegowda H. C. Design, docking, and synthesis of quinoline-2H-1,2,4-triazol-3(4H)-ones as potent anticancer and anti-tubercular agents. ChemistrySelect 2018, 3, 2004–2016. 10.1002/slct.201702279. [DOI] [Google Scholar]
- Shaikh S. K. J.; Sannaikar M. S.; Kumbar M. N.; Bayannavar P. K.; Kamble R. R.; Inamdar S. R.; Joshi S. D. Microwave-expedited green synthesis, photophysical, computational studies of Coumarin-3-yl-thiazol-3-yl-1,2,4-triazolin-3-ones and their anticancer activity. ChemistrySelect 2018, 3, 4448–4462. 10.1002/slct.201702596. [DOI] [Google Scholar]
- Kattimani P. P.; Kamble R. R.; Meti G. Y. Expedient synthesis of benzimidazoles using amides. RSC Adv. 2015, 5, 29447–29455. 10.1039/C5RA00021A. [DOI] [Google Scholar]
- Shinge P. S.; Mallur S. G.; Badami B. V. Termolecular One-Pot Synthesis of Symmetrical Azines of 4-Acetyl-3-arylsydnones. Hydrazone and Azine Derivatives of 4-Acetyl-3-arylsydnones, Their Spectral Characterization and Biological Properties. J. Indian Chem. Soc. 2005, 82, 659–664. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.