Abstract
Composite films of proteins and polysaccharides have a broad range of biomedical and food packaging applications, in which they are frequently exposed to fluid environments with varying ionic strengths. In the present work, we report the behavior of biopolymer films derived from chitosan (Ch), gelatin (GEL), and Ch/GEL mixture in salt solutions with varying concentrations and ion charges. The swelling and dissolution of the Ch films reduced with increasing salt concentration due to the polyelectrolyte behavior of this biopolymer, while the GEL films displayed a polyampholyte behavior, in which film swelling and dissolution were enhanced in salt solutions. Composite Ch/GEL films followed the behavior of GEL. The release of small ionic and zwitter-ionic molecules from the films was enhanced in ionic solutions due to the screened attraction between these molecules and the polymer matrix. These results provide insight into the behavior of protein/polysaccharide films in varying ionic environments, thus enabling enhanced design of biomaterials for a broad range of applications.
Introduction
Composite polysaccharide–protein films are used for food packaging, cell culture, tissue engineering, and drug delivery. In particular, chitosan/gelatin (Ch/GEL) films are utilized as edible and biodegradable coatings,1,2 wound dressings,3 skin tissue engineering scaffolds,4 and transdermal drug delivery patches.5 Both biopolymers are cost-efficient, biocompatible, and biodegradable. For packaging and coatings applications, both polymers exhibit good film-forming properties, while, in addition, Ch provides antimicrobial and antioxidant activities, as well as a decrease in oxygen permeability.6 In tissue regeneration applications, GEL promotes cell adhesion, proliferation, and migration, thus enhancing wound recovery and tissue growth,4 while Ch offers antimicrobial properties7 and promotes wound recovery.8 Composite Ch/GEL films can also be used as “active” materials for the delivery or absorption of small molecules in wound dressings,2 active food packaging, and edible films.9
Composite biopolymer films are frequently exposed to fluid environments with varying ionic strengths.10,11 Under these conditions, film performance is closely related to its swelling and dissolution behavior. In particular, depending on the pH, GEL containing amino acids with positive (amine) and negative (carboxyl) ionizable groups behaves as a polyampholyte,12 while Ch is a copolymer of glucosamine (containing ionizable amine groups) and n-acetyl glucosamine; thus, the latter behaves as a polyelectrolyte.13
Due to the polyelectrolyte and polyampholyte nature of Ch and GEL, respectively, they exhibit distinct properties in ionic solutions. A decrease in swelling with an increasing ionic strength of the solution is expected for Ch films, since the repulsion between charged amine groups of the polymer is screened in ionic solutions.14−16 In contrast, swelling of GEL close to its isoelectric point is enhanced due to the weaker ionic cross-linking between the charged amine and carboxyl groups.17 Far away from the isoelectric point, screening of the repulsion between likely charged carboxyl or amino groups results in a GEL polyelectrolyte behavior, in which the degree of swelling decreases with an increasing salt concentration.
In the composite Ch/GEL films, ionic interactions between the positively charged amine groups of Ch and the negatively charged carboxyl groups of GEL are complemented by the formation of ion couples between the amine and carboxylic groups of GEL and hydrogen bonds between the hydroxyl, amine, and carboxyl groups of the respective polymers.18,19 In general, a polyampholyte behavior is expected; however, when positively charged amine groups of Ch are present in excess, divalent anions may cross-link them and cause reduced swelling of the films.20 The complex behavior of the Ch/GEL films in ionic solutions is further complicated when they are used for the delivery of small ionic molecules.
In the present work, we examined the dissolution, swelling, and change in the structure of Ch, GEL, and Ch/GEL composite films in ionic solutions with varying salt concentrations and ion charges. In addition, we explored the effect of varying ionic strength of the solution on the release of small charged molecules such as rhodamine B (RhB) and eosin Y (EosY) from the Ch, GEL, and Ch/GEK films. This work provides insight into the nature of interactions in composite protein–polysaccharide films in ionic media and thus broadens their applications.
Experimental Section
Materials
Chitosan was supplied by Kimica Marine Biopolymers (LLWP). Gelatin type B, glacial acetic acid, NaCl, Na2SO4, CaCl2, o-phthalaldehyde, N-acetyl-l-cysteine, ethanol, sodium tetraborate decahydrate, Folin’s phenol reagent, CuSO4, Na·K tartrate, rhodamine B (RhB), and eosin Y (EosY) were purchased from Sigma-Aldrich Canada.
Film Preparation
All films were prepared by solution-casting on a glass slide or into a poly(tetrafluoroethylene) (PTFE) mold. Solutions of Ch, GEL, and Ch/GEL mixtures were prepared with a solid content of 2.5% (w/v), with Ch/GEL weight ratios of 1:0, 1:12, and 0:1, respectively. The final Ch solution contained 60% (w/w Ch) acetic acid and the final Ch/GEL solution contained 20% (w/w Ch) acetic acid.
Film Dissolution and Swelling
The pH of salt solutions used in the present work were in the range of 5.5–6.5 (measured using a pH meter (EcoMet, P25)). Since film swelling and dissolution in ionic solutions are close-to-concurrent processes, we determined each of these characteristics separately. To characterize the swelling and dissolution of the films after their 1 h incubation in the solutions, 650 μL of Ch, Ch/GEL, or GEL solution was cast onto 18 mm diameter glass coverslips and dried at room temperature for 24 h. A dry film was weighed and submerged into 50 mL of salt solution (NaCl, Na2SO4, or CaCl2) with concentrations varying from 0 to 0.8 M. After 1 h, the film was removed from the solution, excess water was carefully removed using a filter paper, and the film was immediately weighed. Subsequently, it was dried for 24 h in a vacuum oven at 70 °C and reweighed. These procedures were repeated at least three times. The fraction of the film dissolved, MD, was determined as
| 1 |
where Wd is the weight of the film after 1 h incubation in the solution and Wi is the initial weight of the film (both weights were determined after drying the film in a vacuum oven at 70 °C for 24 h).
The swelling ratio, Q, was determined as
| 2 |
where Ws is the weight of the film swollen for 1 h.
Film Dissolution under Agitation
To characterize polymer dissolution in water or NaCl solutions under agitation, the films were prepared by casting 12 mL of Ch, Ch/GEL, or GEL solution into a PTFE mold (2 mm × 6 cm × 6 cm) and drying at room temperature for 24 h. The dry films were cut into 1 cm × 1 cm squares (14 mg), placed in 20 mL of 0–0.8 M NaCl solution, and stirred at 100 rpm. At different time intervals, a sample of solution was removed with a syringe and filtered through a filter with a pore size of 0.45 μm. The amount of dissolved GEL and/or Ch was determined using the colorimetric Lowry21 and o-phthalaldehyde assay.22
Lowry assay was used to determine the GEL dissolution from GEL and Ch/GEL films. A supernatant solution (300 μL) was mixed with 1.5 mL of Lowry reagent solution, incubated for 10 min at 25 °C, mixed with 150 μL of diluted Folin’s phenol reagent, and incubated for 30 min at 25 °C. Absorbance of the solution was measured at 750 nm using a Varian Cary 50 UV–visible spectrometer. Lowry reagent solution and diluted Folin’s phenol reagent were prepared immediately prior to their use by mixing 50 mL of 2% Na2CO3 in 0.1 M NaOH with 1 mL of 0.5% CuSO4·5H2O in 1% Na·K tartrate, and 1 mL of Folin’s phenol reagent with 1 mL of deionized (DI). An intensity–concentration calibration curve was generated using aqueous solutions with five GEL concentrations (Figure S1).
o-Phthalaldehyde assay was used to determine the dissolution of Ch and Ch/GEL films. A supernatant solution (1 mL) was mixed with 1 mL of the reagent solution of o-phthalaldehyde and incubated for 1 h at 25 °C. Absorbance of this solution was measured at 340 nm. o-Phthalaldehyde reagent solution was prepared immediately prior to its use by adding 200 μL of 0.11 M o-phthalaldehyde and 0.071 M N-acetyl-l-cysteine solutions in ethanol to 5 mL of 0.2 M borate buffer at pH 8.9. Two separate absorbance intensity–concentration calibration curves were generated using 0–0.8 M NaCl solutions with varying GEL and Ch concentrations in Figure S2. To determine the concentration of Ch and GEL following the dissolution of Ch/GEL films, the concentration of GEL was measured using Lowry assay and converted to the corresponding absorbance using the o-phthalaldehyde assay intensity–concentration calibration curve for GEL. This absorbance was subtracted from the absorbance obtained using the o-phthalaldehyde assay and the remaining absorbance value was used to calculate the Ch concentration.
Scanning Electron Microscopy (SEM)
After removal from the solution, the films were frozen in liquid propane and lyophilized for 2 days. The films were fractured and gold-coated using a SC7640 high-resolution sputter coater (QuorumTechnologies) for 30 s at 2.0 kV, and their cross sections were imaged using an FEI Quanta FEG 250 SEM.
Release of Ionic Molecules from Films
Rhodamine B (RhB) or eosin Y (EosY) were mixed with solutions of Ch, GEL, or Ch/GEL at 1 mM concentration. Films were prepared by casting 650 μL of Ch, Ch/GEL, or GEL solution onto 18 mm diameter glass coverslips and dried at room temperature for 24 h. The dry films were submerged into 20 mL of 0–0.8 M NaCl solution. At different time intervals, 500 μL of the supernatant was removed, filtered (0.45 μm pore size), and mixed with 500 μL of PBS buffer (pH 7.4). The mixture was analyzed using a Varian Cary 5000 UV–visible spectrometer at 554 and 512 nm for RhB and EosY, respectively.
Results
Chitosan Films
Figure 1 shows the variation in the fraction of the Ch film dissolved, MD, and the degree of film swelling upon its incubation in NaCl, CaCl2, and Na2SO4 solutions. In Figure 1a, the values of MD for the Ch films are plotted vs the concentrations of NaCl, Na2SO4, and CaCl2 solutions. Comparison of film dissolution in the solutions of salts of monovalent (Na+, Cl–) and divalent ions (SO42–, Ca2+) ions revealed a consistent trend: the extent of Ch film dissolution decreased with increasing salt concentration. The value of MD reduced in the sequence NaCl > CaCl2 > Na2SO4 (clearly observed for 0.2 and 0.4 M salt solutions). For 0.8 M salt solutions, the fraction of the film dissolved reduced to ∼1%.
Figure 1.
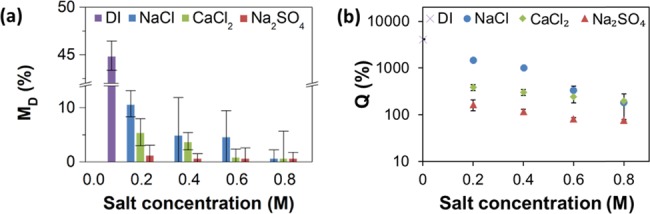
Stability of Ch films in salt solutions. (a) Variation in the fraction of the film dissolved (MD) and (b) the degree of swelling (Q) after 1 h film incubation in water (DI) and NaCl, Na2SO4, and CaCl2 solutions, plotted as a function of salt concentration. The error bars show the standard deviation obtained in three independent experiments.
Figure 1b shows the variation in the swelling ratio, Q, after Ch film incubation for 1 h in NaCl, CaCl2, and Na2SO4 solutions with the concentration in the range of 0–0.8 M. The trend in the variation in Q correlated with the change in film dissolution (shown in Figure 1a): in all salt solutions, the swelling of the Ch films decreased with increasing salt concentration. In particular, in NaCl solutions, film swelling decreased exponentially with concentration, that is, from 4120 in water to 181% in 0.8 M NaCl solution, respectively. For NaCl and CaCl2 solutions with the same salt concentration, higher values of Q were measured for NaCl solutions. In the Na2SO4 solution, the values of Q were consistently lower than those in the solutions containing Cl– anions. While the effect of the variation in the ionic strength of salt solutions is shown in Figure S3, Supporting Information, we note that for the three salt solutions at the ionic strength of 0.6 M (for 0.6 M NaCl, 0.2 M Na2SO4, and 0.2 M CaCl2 solutions), the variation of Q showed a trend that was similar to film dissolution (Figure 1a): film exposure to the solutions containing Cl– anions resulted in similar Q values (332 vs 386% for NaCl and CaCl2 solutions, respectively), while exposure to Na2SO4 solution showed a significantly lower Q value of 164%.
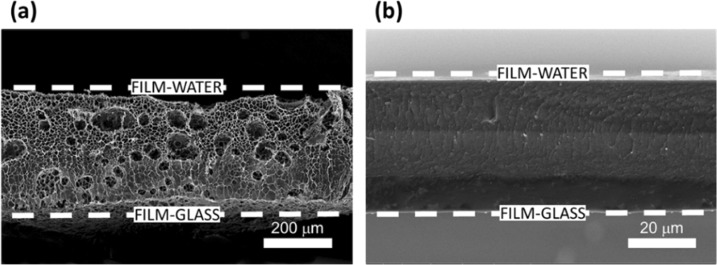
Figure 2 shows the morphology of the cross section of the Ch films swollen in water and in a 0.8 M NaCl solution. The swelling time was limited to 30 s to minimize the effect of film dissolution. The water-swollen film (Figure 2a) exhibited large pores and thickness of ∼300 μm, which was significantly larger than 29 μm of the film prior to swelling experiments (Figure S3). Furthermore, Figure 2a shows a gradient in the Ch film structure: the size of the pores decreased from the film–water interface (bottom) toward the film–glass interface. In contrast, the Ch film exposed to 0.8 M NaCl solution showed an insignificant change in structure or thickness, in comparison with an original film (Figure 2b). In contrast to the Ch film incubated in water, swelling in 0.8 M NaCl solution resulted in a minor change in film thickness from 29 to 39 μm, and the structure of the film did not appear to be porous under the magnification used.
Figure 2.
SEM images of the Ch films after 30 s incubation in (a) water and (b) 0.8 M NaCl solution.
Gelatin Films
Figure 3 shows the variation in the fraction of the GEL film dissolved and the degree of film swelling upon incubation in NaCl, CaCl2, and Na2SO4 solutions. In Figure 3a, the values of MD for the GEL films are plotted vs the concentrations of NaCl, Na2SO4, and CaCl2 solutions. For the films incubated in NaCl solutions, the changes in film dissolution, in comparison with films incubated in water, were not statistically significant (p > 0.05). The dissolution of the GEL films in the solutions containing anions of SO42– reduced with increasing salt concentration. Film dissolution in solutions containing Ca2+ cations drastically increased with increasing salt concentration (and in 0.8 M CaCl2 showed MD value of 79%).
Figure 3.
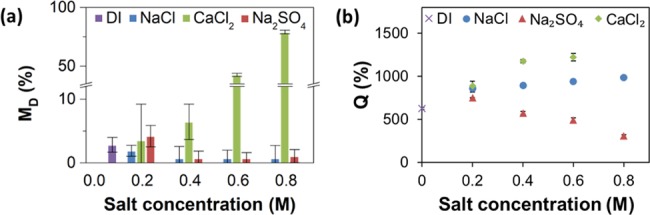
Stability of GEL films in ionic solutions. (a) Variation in the fraction of the film dissolved (MD) and (b) the degree of swelling (Q) after 1 h film incubation in water (DI) and in NaCl, Na2SO4, and CaCl2 solutions, plotted as a function of salt concentration. Due to the complete dissolution of the GEL films in 0.8 M CaCl2 solution, no swelling data are shown for CaCl2 at that concentration. The error bars show the standard deviation for three independent experiments.
Figure 3b shows the variation in the swelling ratio, Q, after the GEL film incubation for 1 h in NaCl, CaCl2, and Na2SO4 solutions. The variation in Q showed a strong dependence on the type of solution it was exposed to. The value of Q increased from 630% in water to 985% in the 0.8 M NaCl solution. In solutions containing Ca2+ cations, the degree of film swelling increased from 630% (water) to 1220% in 0.6 M CaCl2 solution, that is, it was significantly higher than that in NaCl solutions. When comparing film swelling in these solutions at the ionic strength of 0.6 M, the Q values for the GEL films exposed to NaCl and CaCl2 solutions were close, that is, 940 and 890%, respectively, while in solutions containing SO42– anions, the film swelling first increased from 630% (water) to 750% in 0.2 M CaSO4 solution and then decreased to 306% in 0.8 M solution. The effect of the variation in the ionic strength of salt solutions is shown in Figure S4, Supporting Information.
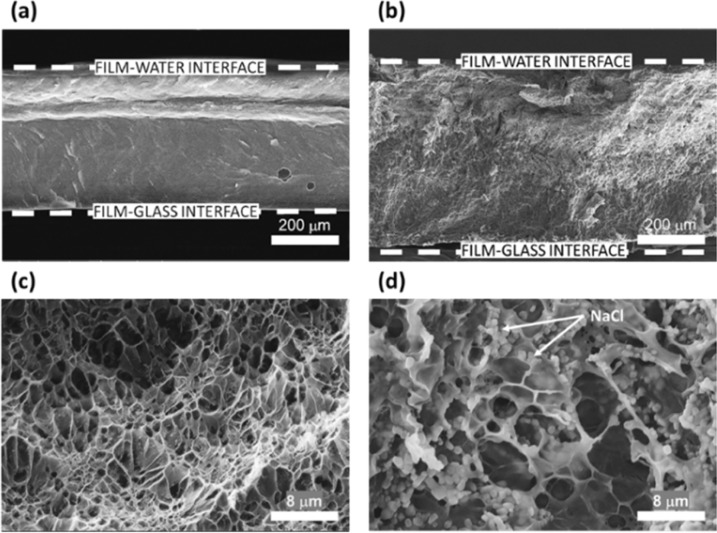
Figure 4 shows the morphology of the cross section of the GEL films swollen in water and 0.8 M NaCl solution. The thickness of the GEL film increased 10-fold after its incubation in water (Figures 4a and S4). Small pores were observed in this film under high magnification (Figure 4c). The film exposed to a 0.8 M NaCl solution (Figure 4b) was ∼14-fold thicker than the original GEL film. Notably, larger pores were observed in this film, in comparison with the film swollen in water (Figure 4d). In addition, small NaCl particles were embedded in the film, indicating deep solution penetration in the film and a greater degree of swelling.
Figure 4.
SEM images of GEL films swollen for 1 h in (a, c) water and (b, d) 0.8 M NaCl solution.
Composite Ch/Gel Films
Figure 5 illustrates the swelling and dissolution behavior of the composite films prepared at the Ch/GEL mass ratio of 1:12 and exposed to NaCl, CaCl2, and Na2SO4 solutions. The variation in the fraction of the film dissolved was similar to that of the GEL films due to the large fraction of this polymer in the film. For the films incubated in NaCl solutions, the change in MD was not statistically significant (p > 0.05) in comparison with MD in water. The dissolution of the films in solutions containing Ca2+ cations increased with increasing salt concentration, e.g., from 6.5 to 25.6% for the films incubated in water and 0.8 M CaCl2 solution, respectively. For the films incubated in solutions containing SO42– anions, the dissolution of the films noticeably decreased with increasing salt concentration. The effect of the variation in the ionic strength of the salt solutions is shown in Figure S5, Supporting Information.
Figure 5.
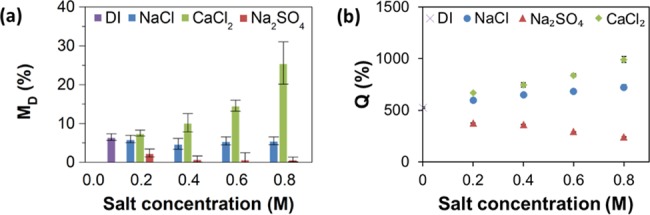
Stability of Ch/GEL films in ionic solutions. (a) Variation in the fraction of the Ch/GEL film dissolved (MD) and (b) the degree of swelling (Q) after 1 h film incubation in water (DI) and in NaCl, Na2SO4, and CaCl2 solutions, plotted as a function of salt concentration. The error bars show the standard deviation obtained in three independent experiments.
Figure 5b shows the variation in the degree of swelling, Q, for the Ch/GEL films incubated in NaCl, Na2SO4, and CaCl2 solutions. In solutions containing the monovalent anion Cl–, the swelling of the Ch/GEL films increased with increasing salt concentration and was stronger in CaCl2 than in NaCl solution. At an ionic strength of 0.6, the Q values for the Ch/GEL films exposed to NaCl and CaCl2 solutions were similar, i.e., 682 and 668%, respectively, and in solutions containing SO42– anions, the swelling of the Ch/GEL films decreased with increasing salt concentration.
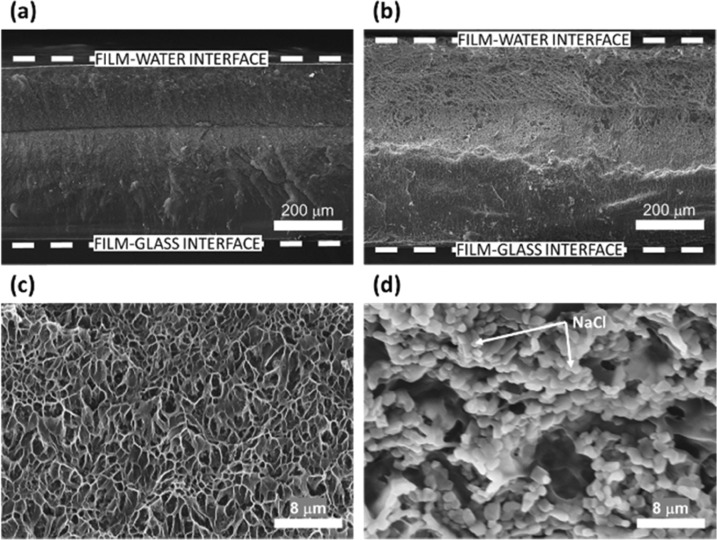
Figure 6 shows the morphology of the cross section of the composite Ch/GEL films swollen in water and 0.8 M NaCl solution (Figure 6a,c,b,d respectively). After incubation in water, the film had the thickness of 505 μm, that is, 8-fold larger than that of the Ch/GEL film prior to its exposure to water (Figure S5). Similar to that of the GEL film, the Ch/GEL film swollen in water had small pores (Figure 6c). After incubation in a 0.8 M NaCl solution (Figure 6b), the film exhibited stronger swelling than that in water to reach the thickness of 620 μm (Figure 6a). Interestingly, in both cases, a horizontal boundary was observed in the film. Above the boundary (closer to the film/solution interface), the film was more porous than at the bottom film region, which was caused by the front of water diffusing in the film. Overall, the Ch/GEL films showed the trend characteristic of the GEL films, that is, a stronger degree of swelling and a more porous structure after incubation in 0.8 M NaCl solution than in water.
Figure 6.
SEM images of the Ch/GEL films swollen for 1 h in (a, c) water and (b, d) 0.8 M NaCl solution.
Film Dissolution under Agitation
The behavior of biopolymer films in ionic solutions under the action of mechanical forces may be important for film function.23 For example, mastication and the peristaltic contraction of the esophagus and stomach expose the polymer coating on orally administered tablets to mechanical stress and affect the dissolution, swelling, and release of a drug.24 In our work, film stability under the action of mechanical forces was determined under the agitation of the solution at 100 rpm.
Figure 7a shows the variation in the fraction of the film dissolved for the Ch films incubated under agitation in 0–0.8 M NaCl solutions. Film dissolution was tested after 1 h (Figure S6); however, an invariant MD value was reached already after 10 min due to the enhanced transport properties. Figure 7a shows a trend observed under static conditions: with an increasing salt concentration, MD for the Ch films drastically reduced. In particular, after 2 min incubation, the value of MD was 71 and 0.35% for water and 0.8 M NaCl solution, respectively. At longer incubation times, the Ch films exposed to solutions with the concentration of up to 0.4 M NaCl exhibited close-to-complete (>90%) dissolution, while in 0.6 and 0.8 M NaCl solutions, the films preserved 13% (±2.8) and 0.85% (±0.11) of their weight, respectively.
Figure 7.
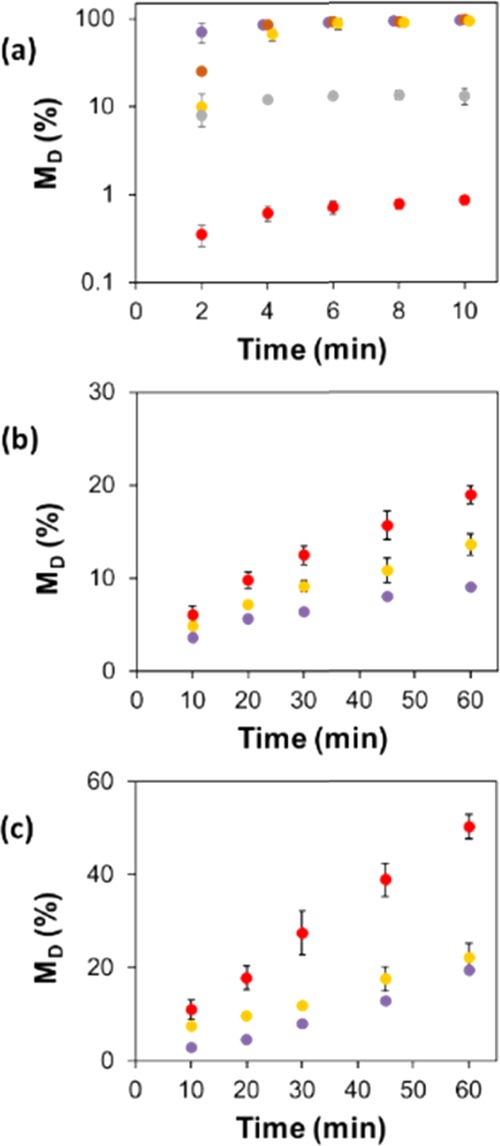
Temporal variation in the fraction of (a) Ch, (b) GEL, and (c) Ch/GEL dissolved under agitation at 100 rpm in solutions with varying NaCl concentration: (purple circle solid) 0 M, (brown circle solid) 0.2 M, (yellow circle solid) 0.4 M, (gray circle solid) 0.6 M, and (red circle solid) 0.8 M. The error bars show the standard deviation obtained in three independent experiments.
For the GEL films, the value of MD increased over the 1 h time interval in NaCl solutions and with increasing NaCl concentration (Figure 7b). Composite Ch/GEL films exhibited a trend characteristic for the GEL films: the value of MD increased over 1 h time interval in NaCl solutions and with increasing NaCl concentration (Figure 7c).
Release of Low-Molecular-Weight Ionic Molecules from the Films
Biopolymer films such as protein and polysaccharide coatings are extensively used for the encapsulation and delivery of functional ingredients. For example, Ch and GEL films are utilized in the treatment of wounds and infections for the delivery of zwitterionic drugs such as ciprofloxacin25 and ofloxacin26 and anionic drugs such as diclofenac sodium27 and ibuprofen.28 In addition, Ch films are used to encapsulate anionic antioxidants and antimicrobial components, e.g., malic and citric acids29 for food packaging applications. Due to the ionic nature of proteins and many polysaccharides, the release of the ionic functional ingredients depends on their interactions with the biopolymer matrices. Furthermore, the release may occur in ionic environments, e.g., in the salty solutions produced from high-salt content foods,11 drinking water, and gastric fluid. The ionic strength of these solutions may influence the release of small molecules incorporated in the films.30
We explored the release of the zwitterionic dye rhodamine B (RhBD) and the anionic dye eosin Y (EosYD) from the Ch, GEL, and Ch/GEL films into the solutions with varying NaCl concentration. The release of RhB and EosY from the Ch films increased with increasing salt concentration (Figure 8a,b, respectively). In particular, the cumulative release of the zwitterionic RhB after 1 h was 27 and 93% in water and 0.4 M NaCl solution, respectively. The release of the anionic EosY was significantly lower, that is, 0.4 and 9.3% in water and 0.8 M NaCl, respectively.
Figure 8.
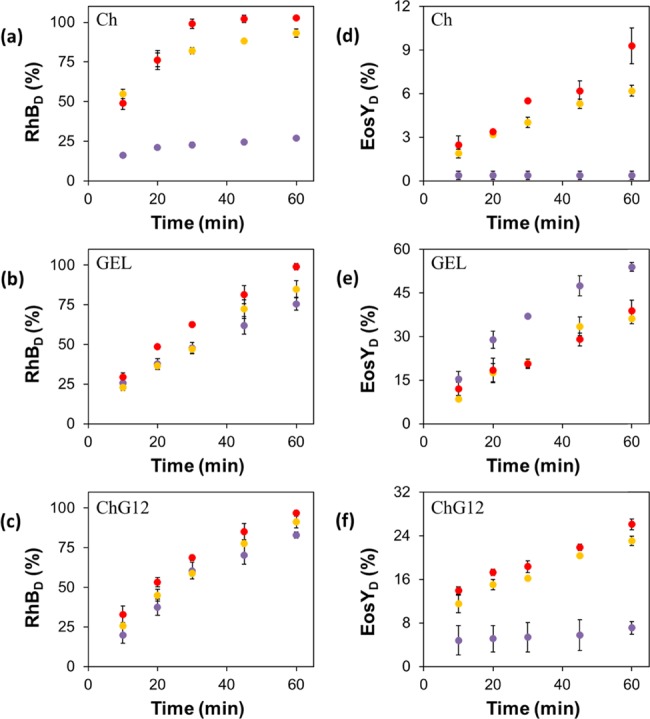
Temporal variation in the fraction of cationic dye RhB (RhBD) and anionic dye EosY (EosYD) released from the (a, d) Ch, (b, e) GEL, and (c, f) Ch/GEL films, respectively, in solutions with varying NaCl concentrations: (purple circle solid) 0 M, (yellow circle solid) 0.4 M, and (red circle solid) 0.8 M. The error bars show the standard deviation obtained in three independent experiments.
The release of RhB and EosY from the GEL films showed a different trend: the release of RhB increased and EosY decreased in NaCl solutions in comparison with water. For the release of RhB, this trend was less pronounced than that for the Ch films, with 75 and 99% release in water and 0.8 M NaCl solution, respectively. The release of EosY from the GEL films was greater than from the Ch films, with 54 and 38% release in water and 0.8 M NaCl solution, respectively.
Interestingly, the release of the dyes from the Ch/GEL films showed the trend that was similar to that of the Ch films: the release of RhB and EosY increased with increasing NaCl concentration. Notably, the release of EosY was intermediate between those of the GEL and Ch films, with a minimum release of 7% and a maximum release of 26% in water and 0.8 M NaCl solution, respectively.
Discussion
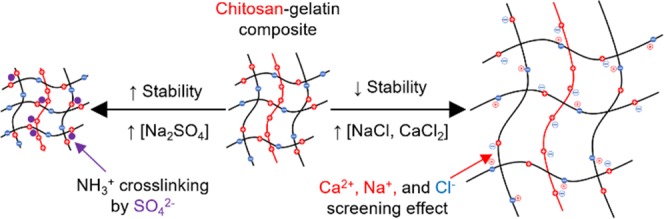
The swelling and dissolution properties of Ch, GEL, and Ch/GEL films originated from the balance between the osmotic pressure and the elasticity of the polymer network. The increase in salt concentration in the solution (or increase in its ionic strength) resulted in an increase in the osmotic pressure, thus favoring film swelling. The elasticity depended on nonionic and ionic interactions between the polymer charged groups as well as the interactions of these groups with ions in the solution. Chitosan is a weak polyelectrolyte (base) with pKa ∼6.3, while gelatin is a polyampholyte with a weak acid (pKa = 4.3) and weak base behavior (pKa ∼ 6.3). In the pH range of 5.5–6.5 of the salt solutions used in the present work, the carboxylic and amine groups of the biopolymers were ionized. Figure 9 illustrates the variation in the repulsive forces acting between the positively charged groups of polyelectrolyte and the attractive forces acting between the charged groups of GEL polyampholyte. The decrease in the swelling and dissolution of the Ch films with increasing ionic strength originated from its polyelectrolyte nature, that is, the screened electrostatic repulsion of the primary amine groups,14,15 and thus polymer densification.31,32 The penetration of water molecules in the film favored its swelling and dissolution in water at pH 7.0 and was suppressed in salt solutions. Notably, in addition to the screening of the repulsive interactions between the protonated amine groups, in Na2SO4 solutions, SO42– anions could act as cross-linkers of the protonated amine groups, thus further restricting the ability of the films to swell and solubilize. Thus, at the same ionic strength of NaCl, CaCl2, and Na2SO4 solutions, the dissolution and swelling of the Ch films were strongly suppressed.
Figure 9.
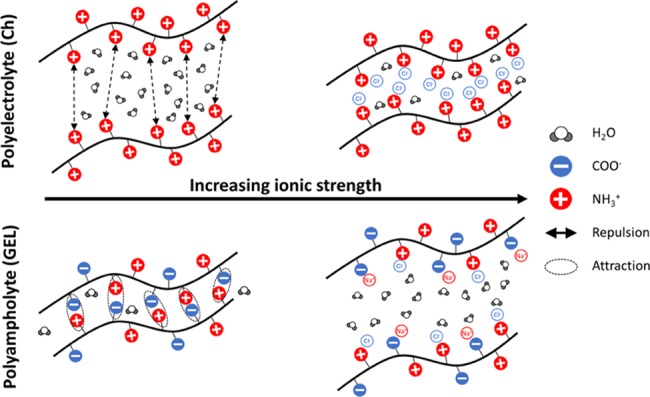
Illustration of the ionic interactions of Ch polyelectrolyte and GEL polyampholyte in water and ionic solutions. The ionic repulsion between NH3+ groups of Ch (polyelectrolyte) and attraction between COO– and NH3+ groups of GEL (polyampholyte) is screened with increasing ionic strength, thus resulting in contraction and expansion of the polymer network, respectively.
The enhanced swelling of the GEL films with increasing NaCl concentration was caused by interactions of the amine and carboxyl groups of this biopolymer (in approximately 36 mmol33 and 100–115 mmol per 100 g, respectively, density)34 and thus the polyampholyte nature of this biopolymer. As the isoelectric point of GEL of 4.7–5.3,34 both positive NH3+ and negative COO– groups coexisted on the GEL molecules (although in a different number), thus forming ion couples (Figure 9, bottom). In salt solutions, attractive interactions were screened by the Na+ and Cl– counterions, thus resulting in enhanced water penetration between polymer chains and enhanced swelling.35
The swelling and dissolution behavior of the GEL films in CaCl2 and Na2SO4 solutions depended on the interactions between Ca2+ and SO42– ions with water molecules and with GEL. The change in these properties with increasing CaCl2 and Na2SO4 concentrations resembled the trend of the Hoffmeister series.36 The introduction of Ca2+ ions resulted in the “salting in” effect, that is, an increase in protein solubility in water, while SO42– ions led to the “salting out” effect, that is, a decrease in protein solubility in the corresponding solutions.37 Thus, a comparison of the film behavior in solutions of the same ionic strength was not straightforward.
The similarity between the swelling and the dissolution properties of the Ch/GEL and GEL films was attributed to the dominating effect of the GEL component and the formation of the polyampholyte–polyelectrolyte complex between Ch and GEL due to interactions between the amino groups of Ch and the carboxyl groups of GEL.38 As a result, increased swelling was observed for the Ch/GEL films with increasing NaCl content. A lower degree of swelling than that for the GEL films in water was attributed to the formation of ionic cross-links between the amine groups of Ch and the excess deprotonated carboxyl groups of GEL.
Agitation resulted in an increase in the rate of dissolution of all films, as it reduced film integrity and provided convective current of the dissolution medium, thus enhancing transport properties.24,38,39 This effect was observed for all of the films and all of the solutions studied in the present work.
Interestingly, the trends observed for the release of RhB and EosY were the result of the ionic interactions between the ionic groups of the polymer and the dyes,40 rather than interpolymer interactions: for Ch films, the enhanced dye release with increasing NaCl concentration was not expected from the trends observed for the swelling and dissolution of these films. We ascribe this difference to the attraction of the amine groups of Ch and the negatively charged carboxyl groups of RhB41 or carboxyl and phenol groups of EosY.20 When this attraction was screened by Na+ and Cl– ions, the release of dye molecules was enhanced with increasing NaCl concentration. Notably, EosY is a divalent anion, which could cross-link the protonated amine groups of Ch, thus causing a significantly weaker release, in comparison with RhB.
In the GEL films containing RhB, in the pH range of salt solutions, attraction existed between amine and carboxyl groups of GEL and RhB.42 Screening of this attraction by Na+ and Cl– ions enhanced the release of RhB dye with increasing NaCl concentration. The opposite trend observed for the release of EosY originated from the dominant negative GEL charge,43 and therefore, an ionic repulsion between deprotonated carboxyl groups of GEL and EosY. This ionic repulsion was screened in NaCl solutions, and the release of EosY was suppressed at increased NaCl content.
Similar to GEL films, the attraction between RhB and charged amine and carboxyl groups of Ch/GEL films was screened in NaCl solutions, which resulted in greater RhB release with increasing NaCl concentration. Conversely, although Ch/GEL films were predominantly composed of GEL, the screening of attraction between the carboxylic and amine groups of Ch and GEL43 resulted in enhanced release of EosY from these films with increasing NaCl concentration.
Conclusions
We conducted a comprehensive study of the swelling and dissolution properties of the Ch and GEL films, as well as the release of small ionic molecules from these films in ionic solutions. The distinct swelling and dissolution behavior of the Ch and GEL films stemmed from their polyelectrolyte and polyampholyte behavior, respectively. For the Ch films, an increasing ionic strength of salt solutions resulted in the screening of the electrostatic repulsion between positively charged amine groups, thus resulting in decreased swelling and dissolution. For the GEL films, an increasing ionic strength of salt solutions resulted in screened attraction between ionized carboxyl and amine groups, thus resulting in enhanced swelling and dissolution (a polyampholyte behavior). The stability of the composite Ch/GEL films was dominated by the GEL behavior. The swelling and dissolution of these films in solutions containing Ca2+ and SO42– ions was related to the solubility of GEL in the corresponding solutions and resembled the trend of the Hoffmeister series. Agitation increased the rate of dissolution of all films. The release of RhB and EosY molecules from the film was governed by their interactions with the ionic groups of the biopolymers. It was enhanced with increasing NaCl concentration for all films, with the exception of EosY–GEL films. For this dye, screening of an ionic repulsion between GEL and EosY by Na+ and Cl– ions suppressed the dye release. These results provide enhanced understanding of the factors influencing the properties of a biopolymer film in ionic solutions, thereby enabling the development of biopolymer films with applications in ionic media.
Acknowledgments
The authors thank NSERC Canada, Ontario Center of Excellence and SunCayr, Ltd. for financial and technical support. E.K. thanks Canada Research Chair (Tier 1) program (NSERC Canada). M.A. thanks NSERC Vanier Canada Graduate Scholarship.
Glossary
Abbreviations
- Ch
chitosan
- GEL
gelatin
- EosY
eosin Y
- RhB
rhodamine B
- SEM
scanning electron microscopy
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00251.
Calibration graphs for Lowry and o-phthalaldehyde assays, SEM images, dissolution and swelling of biopolymer films, and dissolution of Ch films with agitation at 100 rpm (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by NSERC Canada Engage grant, Ontario Center of Excellence (OCE) and SunCayr, Ltd.
The authors declare no competing financial interest.
Supplementary Material
References
- Vartiainen J.; Vähä-Nissi M.; Harlin A. Biopolymer Films and Coatings in Packaging Applications—A Review of Recent Developments. Mater. Sci. Appl. 2014, 5, 708–718. 10.4236/msa.2014.510072. [DOI] [Google Scholar]
- Vieira M. G. A.; da Silva M. A.; dos Santos L. O.; Beppu M. M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. 10.1016/j.eurpolymj.2010.12.011. [DOI] [Google Scholar]
- Sezer A. D.; Cevher E. Biopolymers as Wound Healing Materials: Challenges and New Strategies. Biomater. Appl. Nanomed. 1992, 383–414. 10.5772/25177. [DOI] [Google Scholar]
- Thein-Han W. W.; Saikhun J.; Pholpramoo C.; Misra R. D. K.; Kitiyanant Y. Chitosan–gelatin Scaffolds for Tissue Engineering: Physico-Chemical Properties and Biological Response of Buffalo Embryonic Stem Cells and Transfectant of GFP–buffalo Embryonic Stem Cells. Acta Biomater. 2009, 5, 3453–3466. 10.1016/j.actbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Paul S.; Jayan A.; Sasikumar C. S. Physical, Chemical and Biological Studies of Gelatin/Chitosan Based Transdermal Films with Embedded Silver Nanoparticles. Asian Pac. J. Trop. Dis. 2015, 5, 975–986. 10.1016/S2222-1808(15)60968-9. [DOI] [Google Scholar]
- Khwaldia K.; Arab-Tehrany E.; Desobry S. Biopolymer Coatings on Paper Packaging Materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. 10.1111/j.1541-4337.2009.00095.x. [DOI] [PubMed] [Google Scholar]
- Kong M.; Chen X. G.; Xing K.; Park H. J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Dai T.; Tanaka M.; Huang Y. Y.; Hamblin M. R. Chitosan Preparations for Wounds and Burns: Antimicrobial and Wound-Healing Effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitoyannis I. S.; Nakayama A.; Aiba S.-i Chitosan and Gelatin Based Edible Films: State Diagrams, Mechanical and Permeation Properties. Carbohydr. Polym. 1998, 37, 371–382. 10.1016/S0144-8617(98)00083-6. [DOI] [Google Scholar]
- Patel S.; Srivastava S.; Singh M. R.; Singh D. Preparation and Optimization of Chitosan/gelatin Films for Sustained Delivery of Lupeol for Wound Healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. 10.1016/j.ijbiomac.2017.10.056. [DOI] [PubMed] [Google Scholar]
- Mehyar G. F.; Al Nabulsi A. A.; Saleh M.; Olaimat A. N.; Holley R. A. Effects of Chitosan Coating Containing Lysozyme or Natamycin on Shelf-Life, Microbial Quality, and Sensory Properties of Halloumi Cheese Brined in Normal and Reduced Salt Solutions. J. Food Process. Preserv. 2018, 42, e13324 10.1111/jfpp.13324. [DOI] [Google Scholar]
- Gómez-Guillén M. C.; Gimenez B.; Lopez-Caballero M. E.; Montero M. P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocolloids 2011, 25, 1813–1827. 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- Rinaudo M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- Bhuvaneshwari S.; Sruthi D.; Sivasubramanian V.; Kalyani N.; Sugunabai J. Development and Characterization of Chitosan Film. Int. J. Eng. Res. Appl. 2011, 1, 292–299. [Google Scholar]
- Kim K. M.; Son J. H.; Kim S.-K.; Weller C. L.; Hanna M. A. Properties of Chitosan Films as a Function of pH and Solvent Type. J. Food Sci. 2006, 71, E119–E124. 10.1111/j.1365-2621.2006.tb15624.x. [DOI] [Google Scholar]
- Llanos J. H. R.; Vercik L.; Vercik A. Physical Properties of Chitosan Films Obtained after Neutralization of Polycation by Slow Drip Method. J. Biomater. Nanobiotechnol. 2015, 6, 276–291. 10.4236/jbnb.2015.64026. [DOI] [Google Scholar]
- Qiao C.; Cao X. Swelling Behavior of Physically Cross-Linked Gelatin Gels in Varied Salt Solutions. J. Macromol. Sci., Part B: Phys. 2014, 53, 1609–1620. 10.1080/00222348.2013.837302. [DOI] [Google Scholar]
- Yin Y. J.; Li Z. Y.; Sun Y. B.; Yao K. D. A Preliminary Study on Chitosan/Gelatin Polyelectrolyte Complex Formation. J. Mater. Sci. 2005, 40, 4649–4652. 10.1007/s10853-005-3929-9. [DOI] [Google Scholar]
- Insua I.; Wilkinson A.; Fernandez-Trillo F. Polyion Complex (PIC) Particles: Preparation and Biomedical Applications. Eur. Polym. J. 2016, 81, 198–215. 10.1016/j.eurpolymj.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling X.; Zu-Yu Y.; Chao Y.; Hua-Yue Z.; Yu-Min D. Swelling Studies of Chitosan/gelatin Films Cross-Linked by Sulfate. Wuhan Univ. J. Nat. Sci. 2004, 9, 247–251. 10.1007/BF02830611. [DOI] [Google Scholar]
- Lowry O. H.; Rosenbrough N. J.; Farr A. L.; Randall R. J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [PubMed] [Google Scholar]
- Larionova N. I.; Zubaerova D. K.; Guranda D. T.; Pechyonkin M. A.; Balabushevich N. G. Colorimetric Assay of Chitosan in Presence of Proteins and Polyelectrolytes by Using O-Phthalaldehyde. Carbohydr. Polym. 2009, 75, 724–727. 10.1016/j.carbpol.2008.10.009. [DOI] [Google Scholar]
- Bala R.; Khanna S.; Pawar P.; Arora S. Orally Dissolving Strips: A New Approach to Oral Drug Delivery System. Int. J. Pharm. Invest. 2013, 3, 67. 10.4103/2230-973X.114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q.; Ye A.; Lad M.; Dalgleish D.; Singh H. Behaviour of Whey Protein Emulsion Gel during Oral and Gastric Digestion: Effect of Droplet Size. Soft Matter 2014, 10, 4173–4183. 10.1039/c4sm00598h. [DOI] [PubMed] [Google Scholar]
- Hima B. T.; Vidyavathi M.; Kavitha K.; Sastry T.; Suresh K. R. Preparation and Evaluation of Ciprofloxacin Loaded Chitosan-Gelatin Composite Films for Wound Healing Activity. Int. J. Drug Delivery 2010, 2, 173–182. 10.5138/ijdd.2010.0975.0215.02027. [DOI] [Google Scholar]
- de Oliveira Fulgêncio G.; Viana F. A. B.; Silva R. O. S.; Lobato F. C. F.; Ribeiro R. R.; Fanca J. R.; Byrro R. M. D.; Faraco A. A. G.; da Silva Cunha-Júnior A. Mucoadhesive Chitosan Films as a Potential Ocular Delivery System for Ofloxacin: Preliminary in Vitro Studies. Vet. Ophthalmol. 2014, 17, 150–155. 10.1111/vop.12140. [DOI] [PubMed] [Google Scholar]
- Ahmed M. G.; Harish N. M.; Charyulu R. N.; Prabhu P. Formulation of Chitosan-Based Ciprofloxacin and Diclofenac Film for Periodontitis Therapy. Trop. J. Pharm. Res. 2009, 8, 33–41. 10.4314/tjpr.v8i1.14710. [DOI] [Google Scholar]
- Li H.; Cheng F.; Gao S.; Wu Z.; Dong L.; Lin S.; Luo Z.; Li X. Preparation, Characterization, Antibacterial Properties, and Hemostatic Evaluation of Ibuprofen-Loaded Chitosan/Gelatin Composite Films. J. Appl. Polym. Sci. 2017, 134, 45441. 10.1002/app.45441. [DOI] [Google Scholar]
- Li M.; Wang W.; Fang W.; Li Y. Inhibitory Effects of Chitosan Coating Combined with Organic Acids on Listeria Monocytogenes in Refrigerated Ready-to-Eat Shrimps. J. Food Prot. 2013, 76, 1377–1383. 10.4315/0362-028X.JFP-12-516. [DOI] [PubMed] [Google Scholar]
- Dakhara S.; Anajwala C. Polyelectrolyte Complex: A Pharmaceutical Review. Syst. Rev. Pharm. 2010, 1, 121. 10.4103/0975-8453.75046. [DOI] [Google Scholar]
- Drozdov A. D.; declaville Christiansen J. Modeling the Effects of pH and Ionic Strength on Swelling of Polyelectrolyte Gels. J. Chem. Phys. 2015, 142, 114904 10.1063/1.4914924. [DOI] [PubMed] [Google Scholar]
- Al-Remawi M. Properties of Chitosan Nanoparticles Formed Using Sulfate Anions as Crosslinking Bridges. Am. J. Appl. Sci. 2012, 9, 1091–1100. 10.3844/ajassp.2012.1091.1100. [DOI] [Google Scholar]
- Kuijpers A. J.; Engbers G. H. M.; Krijgsveld J.; Zaat S. A. J.; Dankert J.; Feijen J. Cross-Linking and Characterisation of Gelatin Matrices for Biomedical Applications. J. Biomater. Sci., Polym. Ed. 2000, 11, 225–243. 10.1163/156856200743670. [DOI] [PubMed] [Google Scholar]
- Djagny K. B.; Wang Z.; Xu S. Gelatin: A Valuable Protein for Food and Pharmaceutical Industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. 10.1080/20014091091904. [DOI] [PubMed] [Google Scholar]
- Gao M.; Gawel K.; Stokke B. T. Polyelectrolyte and Antipolyelectrolyte Effects in Swelling of Polyampholyte and Polyzwitterionic Charge Balanced and Charge Offset Hydrogels. Eur. Polym. J. 2014, 53, 65–74. 10.1016/j.eurpolymj.2014.01.014. [DOI] [Google Scholar]
- Okur H. I.; Hladílková J.; Rembert K. B.; Cho Y.; Heyda J.; Dzubiella J.; Cremer P. S.; Jungwirth P. Beyond the Hofmeister Series: Ion-Specific Effects on Proteins and Their Biological Functions. J. Phys. Chem. B 2017, 121, 1997–2014. 10.1021/acs.jpcb.6b10797. [DOI] [PubMed] [Google Scholar]
- Salis A.; Ninham B. W. Models and Mechanisms of Hofmeister Effects in Electrolyte Solutions, and Colloid and Protein Systems Revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. 10.1039/C4CS00144C. [DOI] [PubMed] [Google Scholar]
- Qiao C.; Ma X.; Zhang J.; Yao J. Molecular Interactions in Gelatin/Chitosan Composite Films. Food Chem. 2017, 235, 45–50. 10.1016/j.foodchem.2017.05.045. [DOI] [PubMed] [Google Scholar]
- Sievens-Figueroa L.; Pandya N.; Bhakay A.; Keyvan G.; Michniak-Kohn B.; Bilgili E.; Davé R. N. Using USP I and USP IV for Discriminating Dissolution Rates of Nano- and Microparticle-Loaded Pharmaceutical Strip-Films. AAPS PharmSciTech 2012, 13, 1473–1482. 10.1208/s12249-012-9875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. F.; Chiu R. J. Investigation of Charge Effects on Drug Release Behavior for Ionic Thermosensitive Hydrogels. Mater. Sci. Eng., C 2002, 20, 161–166. 10.1016/S0928-4931(02)00027-9. [DOI] [Google Scholar]
- Wang Q.; Dong Z.; Du Y.; Kennedy J. F. Controlled Release of Ciprofloxacin Hydrochloride from Chitosan/Polyethylene Glycol Blend Films. Carbohydr. Polym. 2007, 69, 336–343. 10.1016/j.carbpol.2006.10.014. [DOI] [Google Scholar]
- Pittman J. L.; Gilman S. D.; Schrum K. F. On-Line Monitoring of Electroosmotic Flow for Capillary Electrophoretic Separations. Analyst 2001, 126, 1240–1247. 10.1039/b103316f. [DOI] [PubMed] [Google Scholar]
- Mao J.; Kondu S.; Ji H. F.; McShane M. J. Study of the Near-Neutral PH-Sensitivity of Chitosan/Gelatin Hydrogels by Turbidimetry and Microcantilever Deflection. Biotechnol. Bioeng. 2006, 95, 333–341. 10.1002/bit.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.