Abstract
Communication with plants to understand their growth mechanisms and interaction with the surrounding environment may improve production yield in agriculture and facilitate prevention of plant diseases and negative influence of environmental stress. Typical sensing technologies in plant biology and precision agriculture largely rely on techniques with low spatial and temporal resolutions, and fail to continuously and precisely determine localized variation in leaf physiology and microenvironments. Here, techniques to develop a multifunctional stretchable leaf-mounted sensor have been developed to offer optimized adaptability to plant growth and monitor leaf physiological and environmental conditions in continuous and highly sensitive manners. The multifunctional leaf sensor contains multiple heterogeneous sensing elements made of metal, carbon nanotube matrix, and silicon, leading to temperature, hydration, light illuminance, and strain sensing capabilities on a leaf. Evaluation under a controlled environment indicates excellent precision and accuracy of the sensor compared to conventional devices. Furthermore, indoor and outdoor experiments have demonstrated the multifunctional monitoring ability of the sensor in real situations. The multifunctional stretchable sensor holds the promise to advance monitoring techniques in plant biology and precision agriculture, resulting in improved capability to record slow and subtle physiological changes in plants and plant/environment interaction.
Introduction
Plants contain more than 82% of mass of all life forms on earth and include 320 thousand species. They are not only the major determinants to earth atmosphere and climates but also primary resources for food, medicine, and energy. Better understating of growth mechanisms of plants and their interaction with surrounding environments is constantly haunting the botanists and biologists. Unlike the animal studies in which external or internal stimulants can be rapidly reflected by physiological signal changes and animal behaviors, the expression of plants is silent, involving slow and subtle changes that demand long-term and continuous observation. Many issues such as long-term plant response under multiple stress conditions,1,2 relationship between stress response and plant growth,3,4 and early detection of plant diseases5,6 remain unsolved, requiring quantitative monitoring of both plant physiology and environment conditions.
The state-of-the-art technologies in precision agriculture use spectroscopy,7−9 machine vision,10−12 and airborne/satellite surveys.13−15 However, these techniques are low in spatial or temporal resolutions or cannot provide timely response to events that influence plant physiology. Emerging technologies use leaf sensors that are either fixed on leaves with fixtures or placed in close proximity to closely monitor leaf physiology and environmental parameters such as water content,16,17 leaf elongation rates,18,19 chlorophyll,20,21 stomatal conductance,22,23 and temperature.24,25 However, the rigid configurations of these leaf sensors are mechanically incompatible to the soft and fragile natures of leaves. Thus, these sensors are only for discrete measurements. Recent developments of flexible electronics lead to the development of several flexible devices26−29 that can be attached on leaves to measure microclimate changes. The sensor substrates, which are typically made of polydimethylsiloxane (PDMS) and polyimide (PI), have much higher modulus than leaves, whereas the sensor structures lack of stretchable configurations, causing physical constraint to the mechanics of leaves and incompetence to perform long-term monitoring. Despite comprehensive parameters such as leaf elongation rate, water content, stomatal conductance, temperature, light, and humidity are crucial for botanic studies. A multifunctional sensor that can adapt to leaf growth and simultaneously monitor leaf physiology and microclimate has not yet been achieved.
Here, we develop an ultrathin and lightweight stretchable sensor with multiple heterogeneous sensing elements that conduct strain, impedance, temperature, and light intensity monitoring. The sensor, which possesses skin-like mechanical properties, only applied <170 μN contact pressure to the leaves and deforms more than 120% to adapt to the leaf growth. The sensor has been demonstrated to continuously and simultaneously measure plant physiology and environmental conditions for 2 days with little influence to the hosting plant after 45 days integration. A wireless sensing platform can transmit the sensing results to a distance larger than 100 m, demonstrating the capability to form sensor networks to facilitate large-scale plant research and precision agriculture. This paper offers innovative methods to quantify subtle plant physiological and environmental changes, offering crucial techniques that may eventually lead to better understanding about the growth mechanisms of plants and their interaction with the surrounding environment.
Results and Discussion
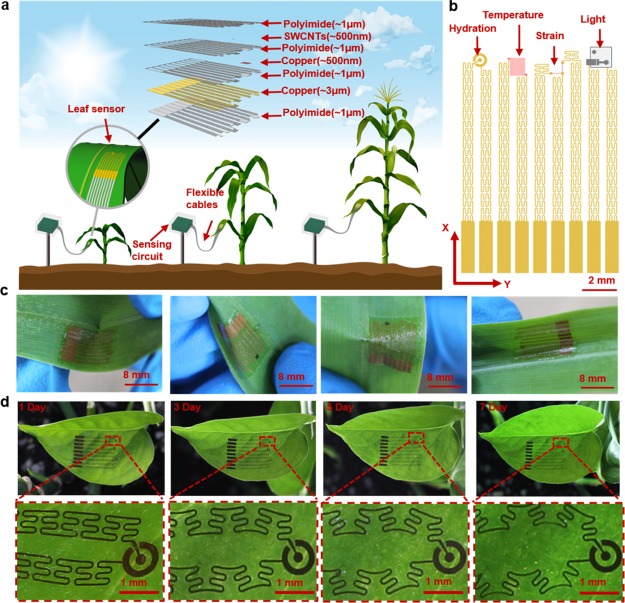
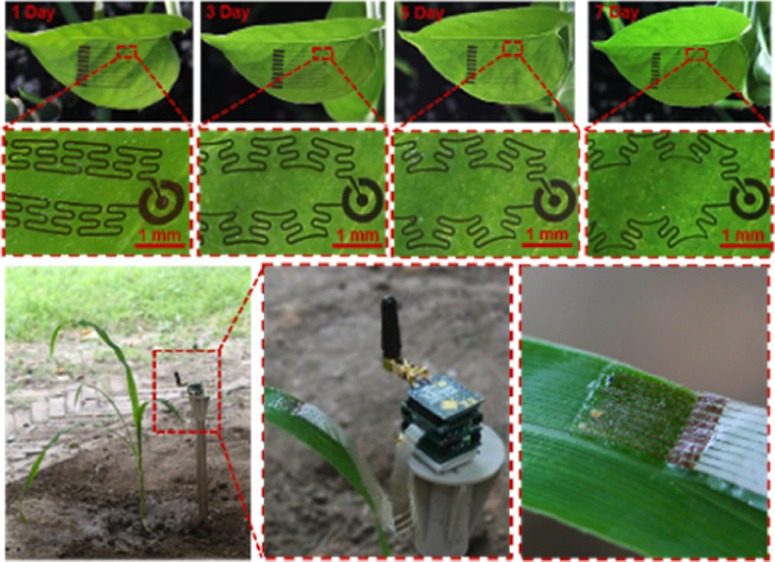
A typical sensor (Figure 1a) with a dimension of 13 000 × 8800 × 30 μm3 and a mass of 17 mg contains multiple heterogeneous sensing elements made of metal, carbon nanotube (CNT), and silicon. The sensor is fabricated by combining CMOS, printable electronics, and transfer printing techniques, leading to hydration, temperature, strain, and light illuminance sensing capabilities on leaves (Figure 1b). These elements are connected with meshed structures, forming an island-bridge configuration that can be engineered to optimize strain distribution within the ultrathin composition materials when being stretched. In addition, the self-similar serpentine interconnects that contain secondary hierarchy structures offer stretchability that allows the entire device to synchronize its growth with the hosting leaf. A perforated silicone membrane with an ultralow modulus (∼3.0 kPa) supports and protects the sensor while allowing light, gas, and water vapor to penetrate through.30 This membrane employs a healthcare-grade material with biocompatibility to enable excellent adhesion between the leaf and the sensor. The adhesion of the sensor may be subjected to the influence from rain and snow, causing the variation of adhesion forces. To quantify the influence, the adhesion forces between the sensors and leaves were characterized using a tensile tester by 180° peel tests. Sensors supported by a commercial fabric (10 × 70 mm2) were attached on leaves and were peeled off mechanically from the leaves at a speed of 50 mm·min–1. Peel forces in situations without and with immersing the sensors on leaves into water were obtained. As shown in Figure S1, the average peel forces are 0.32, 0.29, 0.28, and 0.27 N, respectively, corresponding to immersing times of 0, 10, 20, and 30 min. As the immersing time increased to 30 min, the average peel force only decreased by 0.05 N, indicating that the sensor still maintains good adhesion to the leaf under wet environment. The sensor measures comprehensive information for better understanding the effect of photosynthesis, plant nutrition, environmental stress, and transpiration on plant growth and crop yield.31 The entire sensor can withstand different deformations (Figure 1c) and grow together with the host leaf in a measurement period of 7 days (Figure 1d). In addition, the sensor only applies minimized contact pressure (<170 μN) to leaves with almost no physical constraint to leaf growth. Table 1 compares performances and functions of this sensor with other reported flexible leaf sensors. The leaf sensor in this work offers larger deformation and multifunctions that allow comprehensive assessment of relation between environmental conditions and plant growth, while minimizing the interference to the hosting plants.
Figure 1.
Schematics and demonstration of a multifunctional stretchable sensor on a leaf. (a) Schematic diagram and exploded view of a leaf sensor. (b) Top view of the leaf sensor. (c) A sensor can adapt to the morphology of a corn leaf and deform together with the leaf. (d) A sensor attached on a leaf grows together with the leaf throughout 7 days.
Table 1. Comparison of Flexible Leaf Sensors in Previous Studies.
| substrate | flexible/stretchable | functions | size (mm2) | thickness (μm) | refs |
|---|---|---|---|---|---|
| photo paper | flexible | temperature | >5 | >100 | (32) |
| resin | flexible | bioelectric potentials | >2 | >100 | (27) |
| PI/PET | flexible | drought stress | >1.1 | ∼100 | (33) |
| PI | flexible | relative humidity | ∼10 | >100 | (34) |
| PDMS | flexible/∼22% | temperature and humidity | ∼3 | >50 | (29) |
| Silbione RT Gel 4717 | flexible/up to 120% | temperature, light intensity, hydration, and leaf growth | ∼1.1 | ∼30 | this work |
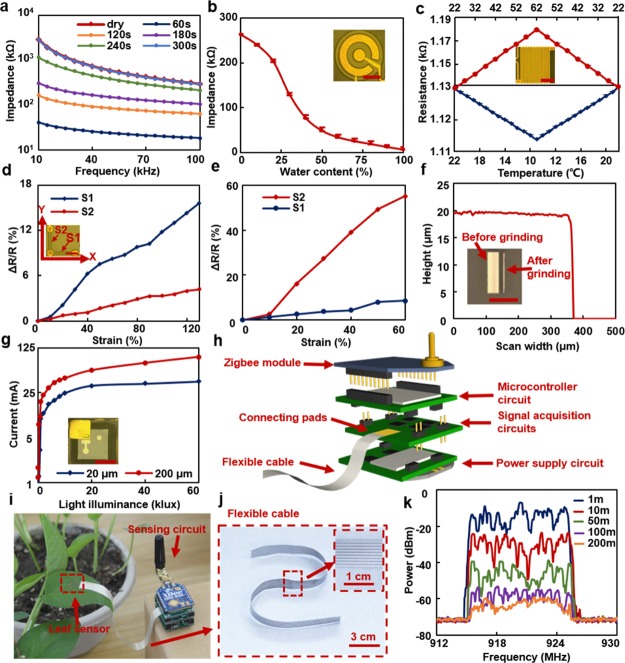
The multifunctional sensor has been evaluated under controlled indoor environments. All sensing elements were individually characterized and calibrated with known parameters. The characterization of the hydration sensing element was conducted by attaching a piece of filter paper with known water contents onto the sensor. The filter paper was first soaked with a saturated amount of water, followed by water evaporation over time, resulting in changes in hydration levels in the paper. Figure 2a shows varied impedance with changing hydration levels at different frequencies. Changes of impedance are 2.85 MΩ at 10 kHz and 0.29 MΩ at 100 kHz, indicating that the impedance measured at lower frequencies may offer better sensitivity and resolution. As subsequent experiments were conducted using a miniaturized measurement circuit (Figures 2h and S2), which has a fixed impedance measurement range from 200 to 600 kΩ. Therefore, to tolerate potential hydration variation and fabrication variation of different batches of devices, impedance was measured at a fixed frequency of 50 kHz, which offered both a large hydration measurement range and a relatively high measurement resolution. As shown in Figure 2b, more than 95% reduction in the magnitude of the impedance has been observed when the hydration levels change from 0 to 100% at a frequency of 50 kHz.
Figure 2.
Characterization of individual components and an experimental circuit. (a) Impedance measured by the hydration sensing element between 10 and 100 kHz with varied water contents. (b) Impedance values at 50 kHz with varied water contents. (c) Responses of the temperature sensing element to changing temperature from 9 to 62 °C. A strain sensing component was evaluated by stretching both sensors uniaxially along (d) x- and (e) y-directions. (f) Height and surface profile of the phototransistor measured by a surface profilometer after grinding. (g) Collector-emitter current of the phototransistor with changing light intensity from 0 to 100 klux (scale bars in figures b, c, d, f, and g represent 500 μm). (h) Exploded view of the wireless sensing circuit that consists of stacked printed circuit board (PCB) layers. (i) A sensing circuit is connected to a leaf sensor with (j) screen-printed flexible cable. (k) Signal power transmitted by a Zigbee module received in different distances.
A temperature sensing element that contains a meander structure has been calibrated using a Peltier heater that offers temperature ranging from 9 to 62 °C. As shown in Figure 2c, resistance increases by 4.3% as the temperature rises from 22 to 62 °C and decreases by 1.5% as the temperature drops from 22 to 9 °C. The result indicates that resistance of the temperature sensing element changes linearly with the temperature. The temperature coefficient of resistance α is determined to be ∼1100 ppm/°C, according to the linear relationship between resistance and temperature R = R0[1 + α(T – T0)], where R0 is the resistance at reference temperature of T0 and R is the resistance at temperature of T. The strain sensing element, which contains two perpendicular CNTs strain gauges (denoted as S1 and S2 in insets of Figure 2d,e), has been evaluated by stretching the sensing element uniaxially along x- and y-direction. The results of uniaxial strain sensing element exhibit strong orientation-dependent. As shown in Figure 2d, S1 exhibits 15.6% variation in resistance under 130% stretching along x-direction, whereas S2 varies only 4.2%. By contrast, a strain of 60% along y-direction leads to an increase in resistance of S2 by 55% and only 8.6% variation in S1 (Figure 2e). The varied gauge factors along different directions are caused by different clamping positions in x- and y-direction during the experiments, resulting in different sensor responses to the same levels of overall strain.
A silicon-based phototransistor has been evaluated in response to various environmental light intensity. The phototransistor was mechanically polished from a thickness of 200 μm to only 20 μm, resulting in a thin-film flexible phototransistor (Figure 2f). Sensor’s response to varied light intensity has been conducted before and after the thinning process, as indicated in Figure 2g. The collector-emitter current appears a logarithmic increase with increased light intensity from 0 to 60 klux when the collector voltage is fixed at 3.3 V. Comparing with the phototransistor before polishing, the thinning process leads to at least 41.0% reduction in the photoelectric current because of reduced numbers of charge carriers when the same amount of photonic energy is received. However, as long as the signal–noise ratio is allowed, the reduced photocurrent can be compensated by increasing the gain in the external sensing circuit. The measurement of illuminance is susceptible to various influencing factors such as wind, shadowing, and bending. Therefore, the time resolution of the sensor should be optimized to precisely record the influence from the above-mentioned factors, allowing better identification between the measurement abnormity and actual environmental light conditions. In addition, the sensor may need to be attached onto a proper leaf of a plant to reflect the general light exposure of the entire plant. To demonstrate applications of the multifunctional device for real-time monitoring, a miniaturized multifunctional sensing circuit equipped with wireless transfer capability was developed (Figure 2h). The circuit, which is 3 × 3 × 2.5 cm3 in its dimension, consists of four stacked PCBs and uses the Zigbee protocol to enable long-range data transmission. The wireless sensing circuit connected with a leaf sensor through a flexible cable (Figure 2i), which contains nine screen-printed silver conductors on a polyethylene terephthalate (PET) substrate (Figure 2j). Signal power received in varied distances to the sensing circuit were measured. The result demonstrates that the received power changes from ∼−15 to −65 dBm (Figure 2k) when the distance changes from 1 to 200 m, indicating that the system can work in a network with a node-to-node distance at least at 200 m, which are ideal in an open-field environment in precision agriculture applications. The communication distance can be further enhanced by adding radio-frequency power amplifiers on either the transmitting or receiving side.
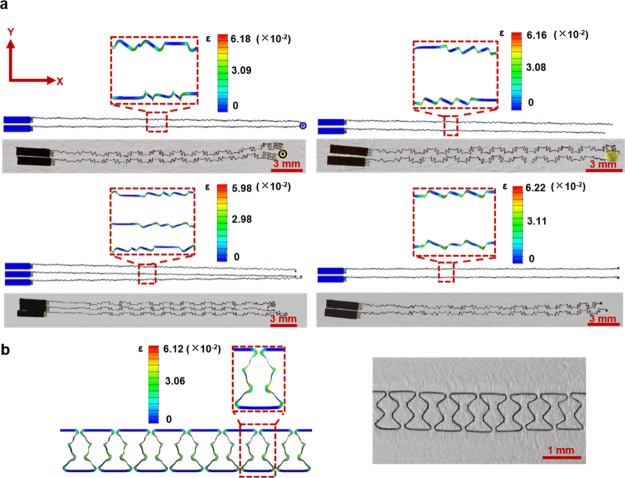
Mechanical deformation of the stretchable sensor under stretching was investigated by the FEA. Simulations of four sensing elements were conducted separately to reduce model sizes. As copper possesses lower fracture strain (∼6%)36 comparing to PI,37 the stretchability of the sensor is mainly determined by strain distributions in the copper layer. As shown in Figure 3a, the stretching causes both out-of-plane and in-plane bending of serpentine interconnects to release the strain. The maximum strain of the sensor (∼6.2%) is mainly located on arcs of the serpentine interconnects with the largest radius of curvature under 150% stretching in x-direction; the results are consistent with previous studies38 as well as device images obtained by applying strains through a mechanical stretcher. A maximum strain of ∼6.1% in response to 60% stretching in y-direction can also be observed on serpentine structures with the largest radius of curvature (Figure 3b). The maximum stretchability along the y-direction is, thus, determined to be 60%, which can be improved by introducing higher orders of self-similar serpentine interconnects.
Figure 3.
Mechanical study of the multifunctional stretchable sensor. Finite element analysis (FEA) and experimental images of serpentine interconnections under mechanical stretching (a) along y-direction with a strain of 150% and (b) along x-direction with a strain of 60%.
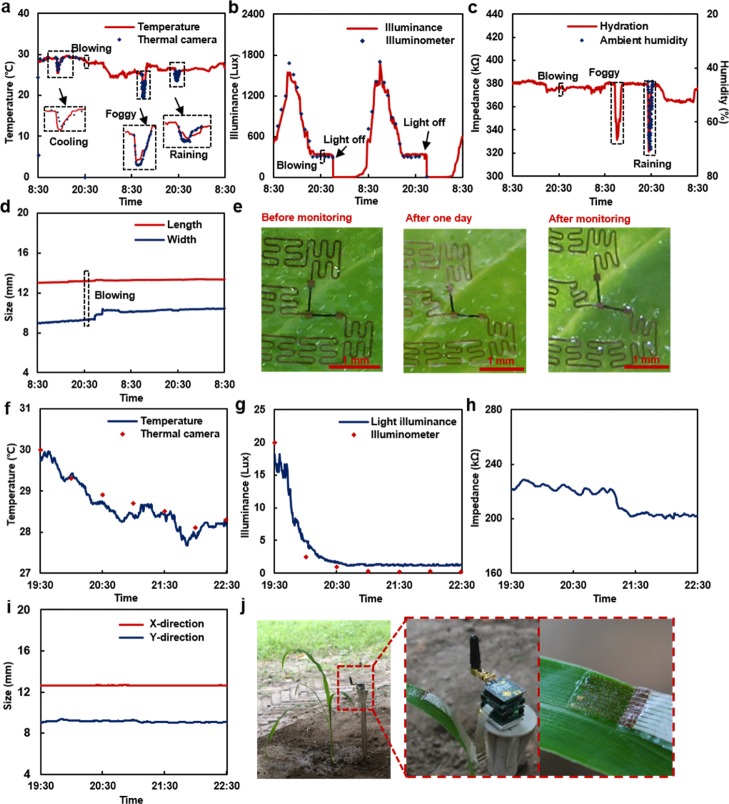
To demonstrate multifunctional monitoring ability of the stretchable sensor, experiments that measured leaf physiology continuously were first conducted in an indoor environment on a Scindapsus aureus (S. aureus) leaf in 2 days (Figure 4a–d). During the experiment, external conditions involving cooling, wind blowing, fog generation, water spraying, and light switching were deliberately introduced to evaluate sensor response. As shown in the temperature measurement result (Figure 4a), the sensor can timely respond to four different external conditions that induce rapid temperature changes as large as 4.9 °C in the local environment. In addition, the sensor is also able to monitor temperature changes that caused by natural conditions, resulting in a slow change in room temperature of 5.4 °C in a day. By considering the signal-to-noise ratio and resolution of the sensing circuit, the temperature sensing element can offer a temperature sensing resolution of 0.2 °C. During the environmental light illuminance measurement, the environmental lighting was controlled by in-door lighting and external sunlight. It can be observed that the sensor has very sensitive responses to both light sources (Figure 4b). Its response closely follow the variation of sunlight during the day and instantly adapt to the change in in-door lighting after switching off the light. External factors such as wind blowing can only cause less than 3.0% signal variation, which is negligible when considering the overall signal variation (Δ = 1678 lux) determined by environmental lighting. The hydration sensing element has also demonstrated stable response to slow hydration changes on the leaf as well as rapid environmental humidity changes caused by fog generation and water spraying (Figure 4c). In comparison, the sensor exhibits a negligible signal variation of less than 0.6% in response to the wind blowing event, which is considered barely alternate both environmental humidity and leaf hydration. A reference measurement that eliminated environmental influence using a leaf cutoff from the plant indicates that the impedance maintains increasing over a period of 3 days. The increase in impedance was mainly caused by the dehydration process of the leaf (Figure S3). The result of strain sensing indicates that the leaf grew steadily during the test (Figure 4d). It is noticeable that the growth of the leaf is mainly in the width (y-direction), causing large deformation (15.5%) of the sensor in the y-direction (Figure 4e) as compared with 2.4% strain in the x-direction. Again, the strain sensing element exhibits excellent robustness to withstand external wind blowing, as the resistance variation due to wind blowing is only 0.9%. It has been observed that the resistance of the sensor along the y-direction underwent a rapid increase on the second day between 23:00 PM and 05:00 AM, indicating a potential rapid leaf growth event during the night. The strain sensing results, as well as other experiments, conducted in-door demonstrate the multiparameter sensing capability of the sensor in response to various events. These parameters can be combined to determine the growth conditions of leaves and reveal factors that can influence leaf growth.
Figure 4.
Characterization of multifunctional leaf sensors indoor and outdoor. Results of (a) ambient temperature, (b) light intensity, (c) hydration, and (d) strains measured by a leaf sensor on a Scindapsus aureus leaf obtained in a period of 2 days. (e) Images of the strain sensor before and after the measurement are also shown. Synchronized measured results of (f) ambient temperature, (g) light intensity, (h) hydration, and (i) strain measured by a leaf sensor on a corn leaf. (j) Images of a multifunctional leaf sensor attached on a corn leaf and the measurement circuit during the outdoor measurement.
A similar leaf sensor was then attached on a leaf of a corn for preliminary outdoor experiments (Figure 4f–j). All data are synchronized to reveal potential influencing factors to the growth of the corn. The temperature variation measured by the sensor can closely follow the temperature measured by a thermal camera (Figure 4f). The decreased environmental temperature of 2.3 °C during the measurement period has been recorded. Correspondingly, the environmental light illuminance also follows the patterns similar to the temperature measurement. The complete sunset appears at around 8 PM indicated by changes in the environmental light illuminance changes from 18.2 to 1.1 lux (Figure 4g), which is also confirmed by referring to the local meteorological data. Because of the reduced sun exposure and temperature decrease, the transpiration effect within the leaf is believed to be reduced. As a result, the hydration within the leaf is increased as the impedance reduces from 228.3 to 200.2 kΩ (Figure 4h). A time delay can be observed between light and temperature decrease and hydration increase. This may be due to the biological response of corn and may be interesting to be further examined in future research. Despite changes in temperature, light illuminance, and hydration, the signals from the strain sensing element are very steady with noise less than 3% (Figure 4i). Because of the relative short measurement period, no significant changes in strains along both the x- and y-direction have been observed. The results from the outdoor experiments indicate that the leaf sensor can work in outdoor environments and multichannel sensing results can serve as important parameters for analyzing environmental influence and leaf biology. Outdoor experiments over an extended measurement period will be conducted in future research to reveal environmental and intrinsic factors that will influence the plant growth.
The biological effect of leaf sensors to leaves has been studied using the changes in the morphology of the leaf stomas, which are vital organs to regulate transpiration and gas exchange between leaves and atmosphere.39−41 Important indicators of leaf status such as stoma sizes and densities42−44 have been monitored using leaves of S. aureus with leaf sensors attached for time periods from 15 to 45 days. Leaf regions underneath and surrounding the sensors have been categorized as the experimental group and the control group, respectively. Figure 5a shows representative images of the experimental group (left) and the control group (right) of a leaf after 15-day sensor attachment. The growth of leaves leads to increased stoma sizes and decreased stoma densities both in the experimental group and the control group, as shown in Figures 5b,c as well as in Table S1. This phenomenon accords well with the relationship between stoma sizes and densities reported in previous paper.45 It is noticeable that both the length and the width have steadily increased with negligible difference between the experimental groups and the control groups (Figure 5b). However, the experimental group exhibits a larger reduction in the stomata density from 59.6 to 48.3 per mm2 as compared with only a change of 2.8 per mm2 in the control group (Figure 5c). The preliminary biological study has demonstrated that the fundamental functions of leaves maintain unchanged during the extended measurement period over 45 days. The existence of the leaf sensors may have an only minimum influence to the leaf growth as observed by the reduced stoma density. Further investigation will be conducted to supply an in-depth understanding of the mutual influence between leaves and leaf sensors.
Figure 5.

Study of the biological effect of the leaf sensor to hosting leaves. (a) Images of stomas of the experimental group (left) and the control group (right). Statistic results of changes in (b) stoma sizes and (c) density of experimental and control groups in 45 days.
Conclusions
Techniques for developing multifunctional stretchable sensors for continuous monitoring of long-term leaf physiology and microclimate have been presented. A representative sensing system has been constructed using ultrasoft and thin materials as well as stretchable structures, allowing maximum adaptability to surface morphology of the leaves. By introducing the island-bridge design with self-similar second-order serpentine structures, the stretchability of the sensor can be enhanced to match the growth of leaves, enabling unique capability for long-term monitoring that is compared differently than other flexible leaf sensors. In addition, multifunctional sensing elements offer the capability to tailor functions of the sensors according to the demands of agriculture production. The leaf sensor reshapes the appearance of agriculture sensing by allowing people to synchronize plant growth with both intrinsic and extrinsic conditions and advances both methodology and paradigm of flexible electronics and agriculture sensors. Similar stretchable sensors may be used to improve crop yield and quality through planning and managing agriculture resources effectively, leading to higher profits and sustainable agriculture. It is also feasible in the future investigation to integrate other sensing elements to monitor developments of plant diseases and insect pests, increasing the capability to prevent and minimize the negative effect on agriculture production. Last, these sensors may help to gain fundamental understandings of plant growth in different environments and knowledge to mitigate adverse environmental conditions for agriculture and forestry productions. For practical use, the density of sensors depends on specific applications. For small-scale botanical and biology studies in which precise physiological and environmental conditions of each plant are need, the sensors may be applied to each tested plant. In large-scale agricultural and industrial production, each sensor may cover a relatively large area of crops that are subjected to the same growth conditions. Several such sensors may be used to conduct mapping of the growth conditions of crops over large areas to obtain overall situation of the entire farmland. To optimize the leaf sensor, higher-order serpentine structures could be designed to improve the stretchability, and ultrathin elastomer materials could be utilized in the flexible cable to release the constraint on the sensor.
Experimental Section
Design of the Multifunctional Leaf Sensor
The hydration sensing element consists of an inner disk (120 μm in radius) surrounded by an open-ended annulus (250 μm in inner radius and 400 μm in outer radius), forming a planar capacitor whose dielectric permittivity is determined by leaf hydration. The temperature sensing element is made of an ultrathin copper film (thickness: 50 nm) patterned into a meander shape (width: 20μm) to achieve large resistances (1.0 kΩ) compared to serpentine interconnections (∼10 Ω). This sensing element is located in a neutral mechanical plane between top and bottom PI membranes (length: 1100 μm, wide: 900 μm) to minimize strains caused by leaf growth. Strain sensing elements contain two perpendicular strips (500 μm in length and 30 μm in width) made of single-walled CNTs (SWCNTs) and respond uniaxial strain induced by mechanical deformation because of leaf growth. The ambient light illuminance is measured by a SU-8 encapsulated phototransistor (1100 × 1100 × 30 μm3). The SU-8 encapsulation facilities assembly and interconnection of the phototransistor with the pre-defined copper electrodes on the leaf sensor and protects the thinned phototransistor from cracking. All elements are connected with screen-printed flexible Ag cables with a line width of 750 μm.
Thinning and Encapsulation of the Phototransistor
A bare die phototransistor (ST-0128, Opto Tech Corp.) was mechanically thinned using a grinding machine (UNIPOL-802, MTI Corp.) for 6 h, resulting in a reduction of thickness from 200 μm to approximately 20 μm to enable intimate contact with the leaf. As shown in Figure S4, the thin-film phototransistor was then transfer printed onto an uncured PI film on a sacrificial polymethyl methacrylate (PMMA, 950 PMMA A4, MicroChem Corp.) layer on a glass substrate. The PI film was then cured in a vacuum oven at 250 °C for 2 h. A layer of SU-8 (SU-8 2015, MicroChem Corp.) with a thickness of 25 μm was spin-coated and patterned to expose the emitter of the phototransistor and form a square encapsulation layer (1100 × 1100 μm2) that encloses the phototransistor. Magnetron sputtering and patterning of stacked layers of Ti/Cu/Ti/Au (5/500/5/50 nm) created interconnection between the emitter and the pre-defined electrode on the leaf sensor. Plasma etching of the PI film exposed the sacrificial PMMA, allowing releasing of packaged phototransistor through dissolving the PMMA in acetone. Finally, the PI film on the backside of the phototransistor was completely removed by plasma etching to complete the encapsulation process.
Fabrication of the Multifunctional Sensor
The fabrication process of a multifunctional leaf sensor started with spin-coating a layer of PI (1 μm in thickness) onto a copper foil (3 μm in thickness) (Figure S5). The PI-coated Cu foil was laminated onto a glass slide coating with PDMS (Sylgard 184, Dow Corning Corp.). The copper film was patterned into the hydration sensing element as well as serpentine interconnects, followed by coating an additional PI film to form an insulating layer. Plasma etching opened vias for electrical contact with the subsequent metallization layer on the PI film, followed by coating and patterning of Ti/Cu layers (2 nm/50 nm) to form the temperature sensing element. A third PI insulating layer was coated and etched to form vias, which allowed connection between drop-casted SWCNTs (1 μL aqueous dispersion, TNSR, Chengdu Organic Chemicals Co., Ltd.) with the serpentine interconnects. A final PI layer was then applied and patterned by plasma to complete the fabrication process. A water-soluble cellulose tape (ASWT-2, Aquasol Corp.) allowed picking up the resulting sensor from the PDMS substrate and transfer-printing the sensor onto a porous silicone elastomer membrane (Silbione RT GEL 4717 A&B, China National Bluestar (Group) Co., Ltd). Finally, a packaged phototransistor was transferred and electrically connected with the leaf sensor with silver epoxy (8000H, Shenzhen Sunflower Electronic Materials Co., Ltd.).
Multifunctional Wireless Sensing Circuit
The multifunctional wireless sensing circuit contains stacked layers of circuit boards (Figure S2). The impedance of the hydration sensing element was measured by an impedance converter (AD5933, Analog Devices Inc.), which supplied an input voltage at frequencies from 20 to 100 kHz and measured the magnitude and the phase of the return voltage. A bridge circuit connected with the temperature sensing element and converted resistance changes to voltage changes (Figure S6a). The strain sensing element was connected into a bridge circuit, which effectively eliminated influence from the temperature to the resistance (Figure S6b). The sensing circuit for the phototransistor supplied a voltage of 3.3 V to the collector of the phototransistor. The light-dependent collector-emitter current was converted into voltage values through a load resistance. A microcontroller (MCU, LPC1114, NXP Semiconductors Corp.) communicated with AD5933 via the I2C serial interface protocol and acquired signals of temperature, ambient light, and strain through three internal 10-bits analogue-to-digital converters. The digitalized data were packed into data packages and sent to a remote receiver connected with a computer through Zigbee (XBee-PRO S3B 900HP, Digi International Inc.). The bottom PCB managed power supplied by either a rechargeable battery or external power supply. The rechargeable battery had a capacity of 370 mAh, allowing continuous operation for 7 h under 165 mW/h power consumption. The middle PCB contained signal acquisition circuits as well as flexible cable connecting pads to allow connection with flexible cables. The top PCB mainly contained the MCU and the Zigbee module, which used a frequency band from 902 to 928 MHz to communicate with a remoted Zigbee module that was connected to a computer for data storage and display.
Finite Element Simulation
A FEA software ABAQUS was used to investigate the mechanical deformation of the sensor under stretching. Young’s moduli of Silbione, PI, Cu, Au, and CNT were set as 0.01, 2500, 119 000, 79 000, and 395 000 MPa, respectively. The Poisson’s ratio is 0.49 for Silbione and PI, 0.34 for Cu, 0.44 for Au, and 0.285 for CNT. Considering the small thickness of sensing elements and connections, they were modeled as skin layers on the surface of substrates to reduce computational load. Various composite material properties were assigned to the skin layers, depending on the location. For example, the central region of serpentine interconnects is a multilayer structure of PI/Cu/PI. Meanwhile, it is reasonable to assume that connections between the four sensing elements are very weak because of the softness of substrates. Displacement boundary conditions were imposed to stretch the sensor by 150% along the y-direction and 60% along the x-direction.
Study of Mutual Influence between the Sensor and the Leaf
Leaves of the experimental group and the control group were cut into 1.5 × 1.5 cm2 samples for the stomatal morphology analysis. The samples were immersed in sodium hypochlorite 20% at 30 °C for 1 h, followed by water rinsing. Epidermis and mesophylls of the samples were then segregated under an optical microscope (ST60, Sunny Optical Technology Co., Ltd.). The epidermis was stained in safranin for 5 min and dehydrated in ethanol. All images were captured through a microscope (VHX-1000, Keyence Corp.) at ×500 magnification (0.3185 mm–2 fields of view). The experimental group and the control group were taken at different times each contains 10 images. Stoma sizes and density were counted for all images with ImageJ.
Acknowledgments
This work is supported by the National Natural Science Foundation of China under grant no. 61604108, the Natural Science Foundation of Tianjin under grant no. 16JCYBJC40600, and the Independent Innovation Fund in Tianjin University. We would like to acknowledge Ok-Byol Ri from Institute of Plant Tissue Culture from the Academy of Biotechnology for guidance to conduct leaf biology study.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01035.
Biological effect study of the leaf sensor, impedance changes of a leaf cutoff from the S. aureus plant, encapsulation of phototransistor, fabrication processes for the multifunctional sensor, block diagram of the wireless sensing circuit, and schematic circuit diagrams (PDF)
Author Contributions
X.H. conceived, designed, and directed the project. Y.Z., S.G., and J.L. performed the experiments. J.Z. and H.C. supported and performed the mechanical simulation. Y.Z. performed the biological experiments. Y.Z. and X.H. wrote the paper. All authors analyzed the data, discussed the results, and commented on the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Holopainen J. K.; Gershenzon J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. 10.1016/j.tplants.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Bahuguna R. N.; Jagadish K. S.V. Temperature regulation of plant phenological development. Environ. Experim. Botany 2015, 111, 83–90. 10.1016/j.envexpbot.2014.10.007. [DOI] [Google Scholar]
- Hirayama T.; Shinozaki K. Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 2010, 61, 1041–1052. 10.1111/j.1365-313x.2010.04124.x. [DOI] [PubMed] [Google Scholar]
- Arnao M. B.; Hernández-Ruiz J. Functions of melatonin in plants: a review. J. Pineal Res. 2015, 59, 133–150. 10.1111/jpi.12253. [DOI] [PubMed] [Google Scholar]
- Martinelli F.; Scalenghe R.; Davino S.; Panno S.; Scuderi G.; Ruisi P.; Villa P.; Stroppiana D.; Boschetti M.; Goulart L. R.; Davis C. E.; Dandekar A. M. Advanced methods of plant disease detection. A review. Agron. Sustain. Develop. 2015, 35, 1–25. 10.1007/s13593-014-0246-1. [DOI] [Google Scholar]
- Mahlein A.-K. Plant Disease Detection by Imaging Sensors – Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Disease 2016, 100, 241–251. 10.1094/pdis-03-15-0340-fe. [DOI] [PubMed] [Google Scholar]
- Mulla D. J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. 10.1016/j.biosystemseng.2012.08.009. [DOI] [Google Scholar]
- He Y.; Huang M.; García A.; Hernández A.; Song H. Prediction of soil macronutrients content using near-infrared spectroscopy. Comput. Electron. Agric. 2007, 58, 144–153. 10.1016/j.compag.2007.03.011. [DOI] [Google Scholar]
- Zhang Q.; Li Q.; Zhang G. Rapid Determination of Leaf Water Content Using VIS/NIR Spectroscopy Analysis with Wavelength Selection. Spectrosc. Int. J. 2012, 27, 93. 10.1155/2012/276795. [DOI] [Google Scholar]
- Chen Y.-R.; Chao K.; Kim M. S. Machine vision technology for agricultural applications. Comput. Electron. Agric. 2002, 36, 173–191. 10.1016/s0168-1699(02)00100-x. [DOI] [Google Scholar]
- Mccarthy C. L.; Hancock N. H.; Raine S. R. Applied machine vision of plants: a review with implications for field deployment in automated farming operations. Int. Serv. Robot. 2010, 3, 209–217. 10.1007/s11370-010-0075-2. [DOI] [Google Scholar]
- Heldman D. R.; Moraru C. I.. Encyclopedia of Agricultural, Food, and Biological Engineering; Heldman D. R., Moraru C. I.; Taylor & Francis, 2007; Vol. 18 (8), 34. [Google Scholar]
- Yang C.; Everitt J. H.; Du Q.; Luo B.; Chanussot J. Using High-Resolution Airborne and Satellite Imagery to Assess Crop Growth and Yield Variability for Precision Agriculture. Proc. IEEE 2013, 101, 582–592. 10.1109/jproc.2012.2196249. [DOI] [Google Scholar]
- Primicerio J.; Di Gennaro S. F.; Fiorillo E.; Genesio L.; Lugato E.; Matese A.; Vaccari F. P. A flexible unmanned aerial vehicle for precision agriculture. Precision Agriculture 2012, 13, 517–523. 10.1007/s11119-012-9257-6. [DOI] [Google Scholar]
- Metternicht G. Vegetation indices derived from high-resolution airborne videography for precision crop management. Int. J. Remote Sens. 2003, 24, 2855–2877. 10.1080/01431160210163074. [DOI] [Google Scholar]
- Atherton J. J.; Rosamond M. C.; Zeze D. A. A leaf-mounted thermal sensor for the measurement of water content. Sens. Actuators A 2012, 187, 67–72. 10.1016/j.sna.2012.06.021. [DOI] [Google Scholar]
- Afzal A.; Mousavi S. F.; Khadem M. Estimation of Leaf Moisture Content by Measuring the Capacitance. J. Agric. Sci. Technol. 2010, 12, 339–346. [Google Scholar]
- Hsiao T. C.; Acevedo E.; Henderson D. W. Maize Leaf Elongation: Continuous Measurements and Close Dependence on Plant Water Status. Science 1970, 168, 590–591. 10.1126/science.168.3931.590. [DOI] [PubMed] [Google Scholar]
- Nagelmüller S.; Kirchgessner N.; Yates S.; Hiltpold M.; Walter A. Leaf Length Tracker: a novel approach to analyse leaf elongation close to the thermal limit of growth in the field. J. Exp. Bot. 2016, 67, 1897–1906. 10.1093/jxb/erw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demotes-Mainard S.; Boumaza R.; Meyer S.; Cerovic Z. G. Indicators of nitrogen status for ornamental woody plants based on optical measurements of leaf epidermal polyphenol and chlorophyll contents. Sci. Hortic. 2008, 115, 377–385. 10.1016/j.scienta.2007.10.006. [DOI] [Google Scholar]
- Miao Y.; Mulla D. J.; Randall G. W.; Vetsch J. A.; Vintila R. Combining chlorophyll meter readings and high spatial resolution remote sensing images for in-season site-specific nitrogen management of corn. Prec. Agric. 2009, 10, 45–62. 10.1007/s11119-008-9091-z. [DOI] [Google Scholar]
- Verhoef A. The effect of temperature differences between porometer head and leaf surface on stomatal conductance measurements. Plant, Cell Environ. 1997, 20, 641–646. 10.1111/j.1365-3040.1997.00098.x. [DOI] [Google Scholar]
- Monteith J. L.; Campbell G. S.; Potter E. A. Theory and performance of a dynamic diffusion porometer. Agric. Forest Meteor. 1988, 44, 27–38. 10.1016/0168-1923(88)90031-7. [DOI] [Google Scholar]
- Palazzari V.; Mezzanotte P.; Alimenti F.; Fratini F.; Orecchini G.; Virili M.; Mariotti C.; Roselli L.. Leaf compatible “eco-friendly” temperature sensor clip for high density monitoring wireless networks. Microwave Symposium, 2016; pp 1–4.
- Sahatiya P.; Puttapati S. K.; Srikanth V. V. S. S.; Badhulika S. Graphene-based wearable temperature sensor and infrared photodetector on a flexible polyimide substrate. Flex. Print. Electron. 2016, 1, 025006. 10.1088/2058-8585/1/2/025006. [DOI] [Google Scholar]
- Daskalakis S. N.; Goussetis G.; Assimonis S. D.; Tentzeris M. M.; Georgiadis A. A uW Backscatter-Morse-Leaf Sensor for Low-Power Agricultural Wireless Sensor Networks. IEEE Sensors J. 2018, 18, 7889. 10.1109/jsen.2018.2861431. [DOI] [Google Scholar]
- Tago S.; Ochiai T.; Suzuki S.; Hayashi M.; Kondo T.; Fujishima A. Flexible Boron-Doped Diamond (BDD) Electrodes for Plant Monitoring. Sensors 2017, 17, 1638. 10.3390/s17071638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahatiya P.; Puttapati S. K.; Srikanth V. V. S. S.; Badhulika S. Graphene-based wearable temperature sensor and infrared photodetector on a flexible polyimide substrate. Flexible Printed Electron. 2016, 1, 025006. 10.1088/2058-8585/1/2/025006. [DOI] [Google Scholar]
- Nassar J. M.; Khan S. M.; Villalva D. R.; Nour M. M.; Almuslem A. S.; Hussain M. M. Compliant plant wearables for localized microclimate and plant growth monitoring. npj Flex. Electron. 2018, 2, 24. 10.1038/s41528-018-0039-8. [DOI] [Google Scholar]
- Jang K. I.; Sang Y. H.; Sheng X. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 2014, 5, 4779. 10.1038/ncomms5779. [DOI] [PubMed] [Google Scholar]
- Legris M.; Klose C.; Burgie E. S.; Rojas C. C. R.; Neme M.; Hiltbrunner A.; Wigge P. A.; Schäfer E.; Vierstra R. D.; Casal J. J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. 10.1126/science.aaf5656. [DOI] [PubMed] [Google Scholar]
- Daskalakis S. N.; Collado A.; Georgiadis A.; Tentzeris M. M. In Backscatter morse leaf sensor for agricultural wireless sensor networks, 2017 IEEE SENSORS, Oct 29–Nov 1, 2017; pp 1–3.29780437
- Im H. L. S.; Lee S.; Naqi M.; Lee C.; Kim S. Flexible PI-Based Plant Drought Stress Sensor for Real-Time Monitoring System in Smart Farm. Electronics 2018, 7, 114. 10.3390/electronics7070114. [DOI] [Google Scholar]
- Oren S.; Wang Z.; Wang X.; Tabassum S.; Jiao Y.; Montgomery B. J.; Neihart N.; Mcninch C. M.; Schnable P. S.; Liang D. In Tracking of water movement dynamics inside plants using leaf surface humidity sensors. IEEE International Conference on Nano/micro Engineered & Molecular Systems, 2017.
- Davis J. R.Copper and Copper Alloys; ASM International, 2001. [Google Scholar]
- Zhang Y.; Wang S.; Li X.; Fan J. A.; Xu S.; Song Y. M.; Choi K.-J.; Yeo W.-H.; Lee W.; Nazaar S. N.; Lu B.; Yin L.; Hwang K.-C.; Rogers J. A.; Huang Y. Experimental and theoretical studies of serpentine microstructures bonded to prestrained elastomers for stretchable electronics. Adv. Funct. Mater. 2014, 24, 2028–2037. 10.1002/adfm.201302957. [DOI] [Google Scholar]
- Fan Z.; Zhang Y.; Ma Q.; Zhang F.; Fu H.; Hwang K.-C.; Huang Y. A finite deformation model of planar serpentine interconnects for stretchable electronics. Int. J. Solids Struct. 2016, 91, 46–54. 10.1016/j.ijsolstr.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar G. D.; Sharkey T. D. Stomatal conductance and photosynthesis. Ann. Rev. Plant Physiol. 1982, 33, 317–345. 10.1146/annurev.pp.33.060182.001533. [DOI] [Google Scholar]
- Hetherington A. M.; Woodward F. I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901. 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Franks P. J.; Farquhar G. D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2006, 143, 78–87. 10.1104/pp.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M.; Underwood W.; Koczan J.; Nomura K.; He S. Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Franks P. J.; Beerling D. J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. 2009, 106, 10343–10347. 10.1073/pnas.0904209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allègre M.; Daire X.; Héloir M.-C.; Trouvelot S.; Mercier L.; Adrian M.; Pugin A. Stomatal deregulation inPlasmopara viticola-infected grapevine leaves. New Phytol. 2007, 173, 832–840. 10.1111/j.1469-8137.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- de Boer H. J.; Price C. A.; Wagner-Cremer F.; Dekker S. C.; Franks P. J.; Veneklaas E. J. Optimal allocation of leaf epidermal area for gas exchange. New Phytol. 2016, 210, 1219–1228. 10.1111/nph.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.