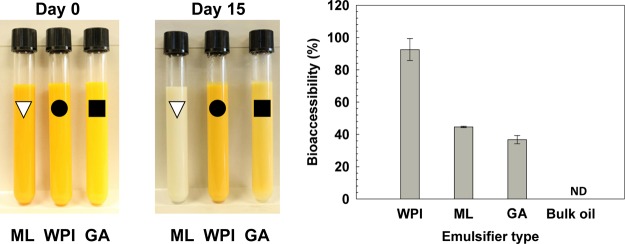
Abstract
The effect of natural emulsifiers (whey protein isolate, WPI; modified lecithin, ML; and gum arabic, GA) on the formulation, stability, and bioaccessibility of fucoxanthin-loaded oil-in-water (O/W) emulsions was determined in this study. The fine emulsions were prepared under high-pressure homogenization at 100 MPa for 4 passes, using 2 wt % WPI, ML, and GA, resulting in emulsions with the droplet sizes of 136, 140, and 897 nm, respectively. The chemical stability of fucoxanthin in the emulsions after long-term storage at ambient temperature decreased in the following order: WPI > GA > ML. The release of free fatty acids of fucoxanthin, studied by in vitro digestion, decreased in the following order: WPI > ML > GA > bulk oil. The bioaccessibility of fucoxanthin in emulsions stabilized by WPI, ML, and GA after in vitro digestion were 92.5 ± 6.8%, 44.6 ± 0.4, and 36.8 ± 2.5, respectively. These results indicate that natural emulsifier type and concentration used significantly affects the formulation, stability, lipid digestion, and fucoxanthin bioaccessibility, which may be ascribed to the different properties of each emulsifier. The bioaccessibility of fucoxanthin was improved by using emulsion-based delivery systems.
1. Introduction
With the constant pursuit of better life quality and healthier lifestyle, consumers are increasingly concerned about their health, and paying more attention to nutraceutical ingredients, such as vitamins and carotenoids. Fucoxanthin, a marine carotenoid found in brown seaweed, with a distinctive allenic bond in the 5,6-monoepoxide and hydroxyl groups, is an accessory pigment in the chloroplasts and is involved in photosynthesis.1 Fucoxanthin is known to possess many beneficial properties, including antioxidant,2,3 anticancer,4,5 anti-inflammatory,6 anti-obesity, and antidiabetic effects.7,8 A previous study investigated the effect of fucoxanthin supplementation, using ThinOgen, on body fat in overweight humans.9 The results revealed that subjects in the treatment group (fucoxanthin 2–4 mg/day intake) showed a significant reduction in weight, compared to that by subjects in the placebo group. Moreover, another research illustrated that consumption of fucoxanthin-fortified milk leads to enhanced bioaccessibility of fucoxanthin in in vitro and in vivo models.10 Therefore, fucoxanthin can be considered as a nutraceutical ingredient and can be utilized in the food industry and other fields to design new and improved nutraceuticals. However, as with other carotenoids, fucoxanthin is affected by light, oxygen, heat, and pH.11,12 Owing to its many limitations, such as its poor water-solubility (0.5 ppm), high melting point (166–168 °C), chemical variability, and low bioaccessibility,13,14 there are many challenges to its incorporation into nutraceutical products.
Food-grade emulsions are widely used in cosmetics, pharmaceuticals, foods, and beverages.15 Various studies have indicated that emulsions could improve the stability and the bioavailability of nutraceutical ingredients when incorporated into dispersed phase droplets. O/W emulsion-based delivery systems are quite suited to encapsulate lipophilic bioactive ingredients due to the dispersion of small lipid droplets in the continuous phase. These droplets can efficiently pass through the skin and enhance the penetration of the components.16,17
Previous studies have demonstrated successful encapsulation of highly purified fucoxanthin into nanoemulsions, nanoparticles, and other spray-dried powders.10,18−21 Most of them focused on the stability and bioaccessibility of fucoxanthin, which were affected by carrier oils (corn oil, medium chain triacylglycerol oil, orange flavor oil, and restructured lipids) and dispersions (whole milk and skimmed milk). Meanwhile, there is limited information about the comparison of various types of emulsifiers. For instance, fucoxanthin-loaded nanoemulsions were successfully prepared and characterized with Tween 80. Fucoxanthin-loaded nanoparticles of casein and chitosan showed improved bioavailability due to high absorption and entry into the blood.18 There are no reports about using crude fucoxanthin extract and different naturally occurring emulsifiers for the formulation of fucoxanthin-loaded emulsions.
This study aimed to utilize natural emulsifiers instead of synthetic surfactants to formulate emulsions, which are sustainable and label friendly. Natural emulsifiers are mainly classified into protein-based emulsifiers, phospholipid-based emulsifiers, and polysaccharide-based emulsifiers. It is an established fact that emulsifiers are crucial to the formulation and stability of emulsion-based systems and that they protect emulsions against destabilizing processes. In this study, we utilized three natural emulsifiers: whey protein isolate (WPI), modified lecithin (ML) and gum arabic (GA) to formulate emulsions. We also evaluated the effects of the emulsifier type and concentration on the physicochemical stability of emulsions encapsulating fucoxanthin. WPI (mainly consisting of β-lactoglobulin and α-lactalbumin) is widely used as a food-grade emulsifier in food and beverage products.16 Hydrophilically modified phospholipid, which is hydrolyzed by soy lecithin, was used in this study. ML has a low molecular weight (about 650 g/mol), and because of its zwitterionic nature, it can stabilize emulsions by electrostatic repulsions during processing and storage. In addition, GA is generally utilized as an amphiphilic polysaccharide in the food industry (in juices and candies). It is a soluble dietary fiber, with specific properties due to a complex mixture of polysaccharides and glycoproteins. In this study, we evaluated the effects of the emulsifier type and concentration, apart from homogenization parameters, on the formulation and stabilization of fucoxanthin-loaded O/W emulsions. Subsequently, the characteristics of the emulsions, including the volume mean diameter, droplet size distribution, physicochemical stability, and fucoxanthin retention, were investigated. Moreover, we also investigated the effects of different emulsifiers on the in vitro digestion behavior and bioaccessibility of fucoxanthin by evaluating the amount of free fatty acids (FFAs) released and fucoxanthin concentration in the micellar phase.
2. Results and Discussion
2.1. Influence of Emulsifier Type and Concentration on the Formulation of Fucoxanthin-Loaded O/W Emulsions
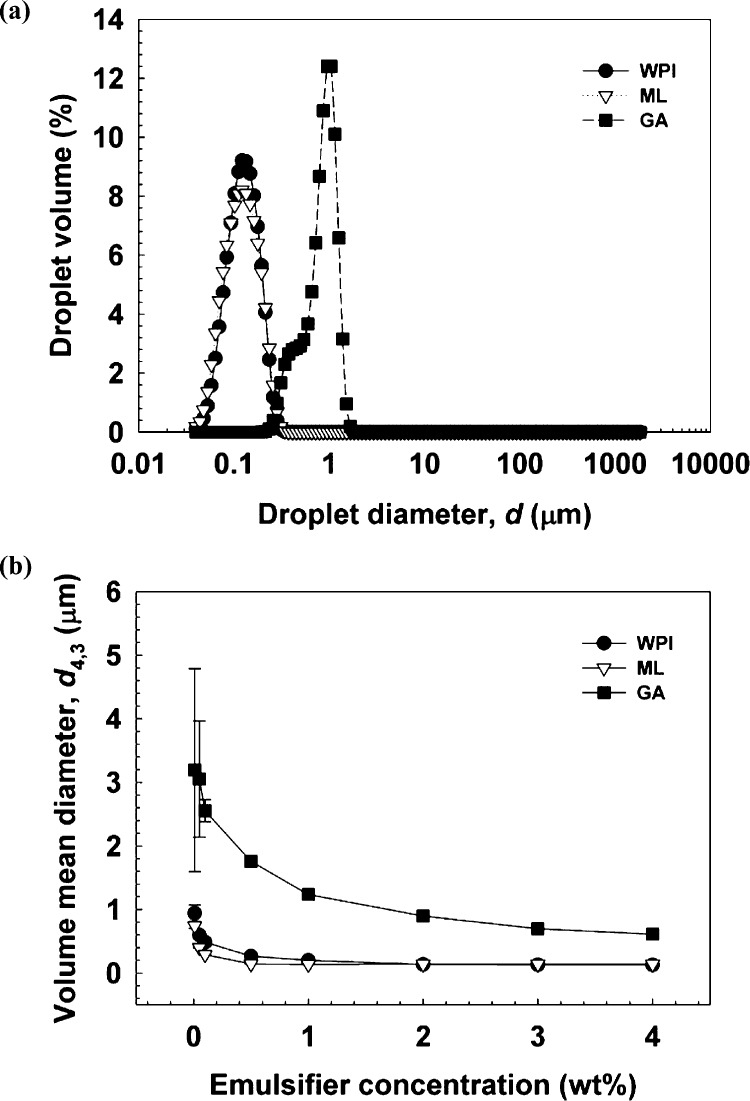
The influence of the type of emulsifier and their concentration on the formulation of fucoxanthin-loaded O/W emulsions, using standard homogenization conditions (100 MPa, 4 passes), was investigated. The droplet size and size distribution are presented in Figure 1a. The freshly prepared-emulsions stabilized by 2 wt % WPI, ML, and GA exhibited narrow droplet size distribution with small droplet sizes (d4,3) of = 136, 140, and 897 nm, respectively. The d4,3 of emulsions decreased with increasing concentration of the respective emulsifier. The decrease in d4,3 at low emulsifier concentrations (Figure 1b) can be attributed to the fact that there was not sufficient emulsifier available to adsorb at oil/water interface during homogenization.22 At high emulsifier concentrations, the d4,3 values remained constant because the disruptive energy of the homogenizer was not enough for further size reduction.23 Fine droplets with the minimum droplet diameters (d4,3) of ≈128 nm (WPI), ≈137 nm (ML), and ≈611 nm (GA), respectively, were obtained at high emulsifier concentrations (4 wt %). The difference in droplet size was likely due to the contribution of the different emulsifiers to the interfacial behavior. ML dissolved in Milli-Q water had smaller interfacial tension than Milli-Q water, which was favorable for formulating small droplets. In contrast, the higher interfacial tension for WPI and GA can be ascribed to their large-molecular structures, which prevent close packing of the points of contact at the interface.24 The coalescence of droplets occurred due to the splitting of droplets in a short period of time, in the homogenizer, which could not be fleetly stabilized by the large-molecule emulsifiers due to their low adsorption kinetics.25 For WPI and GA, a smaller droplet size of emulsions formulated was observed. This could be explained based on the structural change in WPI during homogenization. There might be more hydrophobic groups exposed by the protein chain due to unfolding during adsorption. GA has a 21–28 nm gyration radius of globular configuration, and it is not as good a protein in reducing interfacial tension.26 Therefore, under the same conditions, WPI may be faster and more efficient in covering the droplet surface than GA, thus resulting in the formulation of smaller droplets.
Figure 1.
Effect of different types of emulsifiers and their concentrations on the formulation of emulsions, encapsulating fucoxanthin, prepared by using high-pressure homogenization at 100 MPa for 4 passes. (a) Droplet size distribution of emulsions stabilized by ML (d4,3 = 140 nm), WPI (d4,3 = 136 nm), and GA (d4,3 = 897 nm). (b) d4,3 of emulsions stabilized by different concentrations of emulsifiers.
2.2. Influence of Homogenization Pressures and Number of Passes on the Formulation of Fucoxanthin-Loaded O/W Emulsions
Homogenization parameters, such as pressure and the number of passes, are very crucial for the size of droplets generated using high-pressure homogenization. In our study, the effect of different homogenization pressures (20–140 MPa, Figure 2a) and the number of passes (0–10 passes, Figure 2b) on the droplet size were evaluated by monitoring the d4,3 of the emulsions stabilized by 2 wt % of emulsifiers. Pass 0 refers to the use of only the rotor-stator homogenizer without high-pressure homogenizing. With increasing homogenization pressure and pass, the disruption energy increased together with a decrease in the d4,3.27 However, the d4,3 of GA-stabilized emulsions slightly increased from 760 to 904 nm. This phenomenon could be interpreted by the interdigitation of carbohydrates and protein denaturation in GA during the high-pressure process, which hindered the emulsifying capacity of GA.25 Meanwhile, after 80 MPa and 3 passes, there was no distinct change in d4,3. The d4,3 of WPI- or ML- stabilized emulsions decreased from 389 to 134 and 275 to 137 nm, respectively. Importantly, the chemical stability of fucoxanthin during the homogenization of emulsions, homogenized by different number of passes was also investigated. After the homogenization by using a rotor-stator homogenizer (pass 0, Figure 2b), the chemical stability of fucoxanthin during homogenization fell to nearly 65–80% which meant around 20–35% of fucoxanthin degraded in this process. Therefore, the first step in the homogenization process has a crucial effect on the degradation of fucoxanthin. On the other hand, there was a gradual decrease in the chemical stability of fucoxanthin during homogenization with increasing number of passes during the high-pressure homogenization process. Although the changes were minimal, the impact of this aspect should not be ignored. The chemical stability of fucoxanthin during homogenization in WPI-, ML-, or GA-stabilized fresh O/W emulsions were 72.7, 65.6, and 56.0%, respectively. This phenomenon can be explained by the following three factors: (i) during high-speed homogenization, the coarse emulsions were exposed to the atmosphere, and oxygen might be entrapped in the emulsions. (ii) After preparing the coarse emulsions, the dispersed phase was separated from the continuous phase rapidly. Even if high-speed homogenization can provide strong shear force, it is still a failure to make the emulsifiers to fully adsorb on the surface of droplets and formulate a better droplet form. (iii) During the high-pressure homogenization, because of the high energy input, free radicals may have been formed as fucoxanthin is sensitive to thermal energy.28
Figure 2.
Effect of homogenization pressure and number of passes on the formulation of emulsions. The d4,3 of emulsions formulated using different (a) pressure and (b) number of passes. The chemical stability during homogenization was also determined on emulsions homogenized by different number of passes. Pass 0 refers to the results of rotor-stator homogenization.
2.3. Storage Stability of Fucoxanthin in O/W Emulsions
The stability of the emulsion is a critical factor to determine the shelf-life of foods and beverages. Storage stability of fucoxanthin in emulsions stabilized by different types of emulsifiers (WPI, ML, or GA) was investigated during storage at 25 °C, up to 15 days. Because of the unsaturated structure, fucoxanthin is sensitive to heat, light, and oxidative degradation during processing and storage. Therefore, all samples were covered by aluminum foil and stored in the dark.
2.4. Physical Stability of Long-Term Storage
Figure 3 illustrates the results of d4,3 of fucoxanthin-loaded emulsions stabilized by different emulsifiers (WPI, ML, or GA) during storage up to 15 days, at 25 °C. During storage, emulsions showed excellent physical stability and there was no prominent broadening in d4,3, when stabilized by WPI or ML. WPI can form physically strong layers, which could avoid the coalescence of droplets by steric hindrance on the interface between oil and water.29 ML-stabilized emulsions contained tiny particles which could counter the gravitational force with Brownian motion. Furthermore, the droplets carry a negative charge, which might inhibit flocculation.30 Meanwhile, visible creaming occurred on top of the emulsion stabilized by GA. GA is an emulsifier with a large molecular structure and a high interfacial tension, which might result in relatively large droplets leading to a higher buoyancy force.
Figure 3.
Effect of different types of emulsifiers on the physical stability of fucoxanthin-loaded emulsions during 15 days of storage at 25 °C.
2.5. Chemical Stability of Fucoxanthin during Storage
The chemical stability of fucoxanthin during storage was measured by using eq 2. As shown in Figure 4, the chemical stability of fucoxanthin at day 0 was deemed as 100%, which decreased in all emulsions during storage. The chemical stability of fucoxanthin during storage of emulsions stabilized by ML or GA was lower than the detection limit at day 3 and day 10, respectively. After storage at 25 °C for 15 days, the chemical stability of fucoxanthin during storage decreased from 100 to 59.5% in WPI-stabilized emulsions and 55.5% in the case of bulk oil. The main factors that cause the degradation of fucoxanthin during storage are emulsifier type, surface area of the droplets, and the formation of free radicals during the high-pressure process.31 According to a previous study, the degradation of the carotenoid may be due to a chemical reaction on the surface, at the interface of oil and water, which may be slower in rate due to the smaller surface area.32 Although WPI- and ML-stabilized emulsions had larger surface area, the latter was easier to be oxidized and decomposed, owing to more contact between oil and water, resulting in fucoxanthin exposure to oxygen. ML is the small molecular emulsifier, whose molecular weight is around 650 g/mol. It can be closely contacted and adsorbed on the surface of oil drop with a thin layer. WPI contains cysteyl residues, thiol functional groups, and disulfide bonds, which might inhibit lipid oxidation by scavenging free radicals in emulsion systems.33 Additionally, the layer of adsorbed WPI might be considered as a thicker physical barrier than ML at the interface of oil and water and prevent fucoxanthin degradation.34 Therefore, WPI can alleviate the degradation of fucoxanthin because of its good antioxidant activity, as well as excellent emulsifying properties. Moreover, as a large molecular emulsifier, GA has higher interfacial tension. The droplet size was extremely larger in the GA-stabilized emulsion than in others. Although the surface area of droplets in GA-stabilized emulsion was smaller, it is not as good as WPI to prevent the degradation of fucoxanthin.
Figure 4.
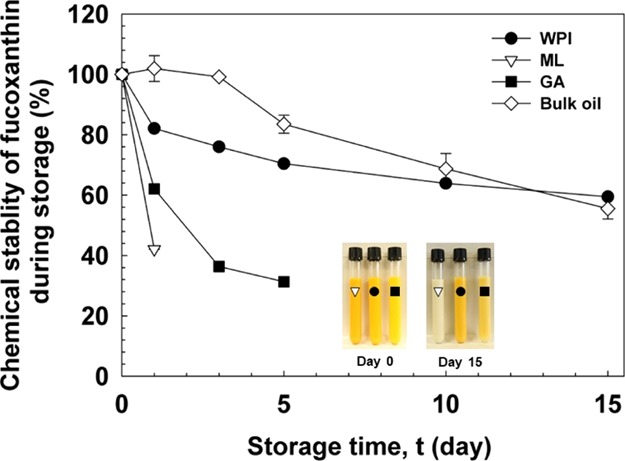
Effect of the type of emulsifiers on the chemical stability of fucoxanthin-loaded emulsions as compared to bulk oil, during 15 days of storage at 25 °C.
2.6. Lipid Digestion
The particle size drastically increased after the exposure of emulsions to the small intestinal phase, regardless of the emulsifier type. The initial d4,3 of emulsions stabilized by WPI, ML, or GA were around 136, 140, and 897 nm, which increased to 123, 108, and 121 μm, respectively, after digestion in the small intestinal phase (Figure 5). Other studies also suggest that the droplets in emulsions aggregate and are resistant to in vitro digestion.20,35,36 This phenomenon might be due to the lipid digestion products, such as colloidal particles, micelles, bilayers, liquid crystals, or vesicles, that are generated due to the hydrolysis of triacylglycerol molecules by lipases in the small intestinal phase.35,37 Notably, fatty acids, monoacylglycerols, and bile salts can produce mixed micelles in the small intestinal fluid.38,39 Moreover, the insoluble calcium-fatty acid soaps may be formulated during digestion.20 On the other hand, the interfacial and core characteristics may change and lead to droplet aggregation.
Figure 5.

The d4,3 of fucoxanthin-loaded emulsions formulated with different types of emulsifiers during in vitro digestion (initial and small intestine).
In the in vitro digestion study, the emulsifier type has an important influence on the lipid digestion rate and level.40 Lipid digestion was defined as FFA (%) values throughout digestion time (min) and monitored by a pH-stat method. Moreover, we compared the FFAs released in bulk oil and emulsions (Figure 6). For the bulk oil, we observed very little amount of initial FFAs being released (about 5.9%), which can be explained by the fact that most of the bulk oil cannot be digested. The release of FFAs in emulsions was quite faster and higher than that in the bulk oil because the surface area of emulsion droplets was larger than the one of bulk oil. The larger surface area can promote the interaction of oil droplets with the lipase to enhance rapid FFAs release.41 This also explained the reason why FFAs released in GA-stabilized emulsions were lower than those in emulsions formed by other emulsifiers. In previous studies, the FFAs in Tween 20- or Tween 80-stabilized emulsions were rapidly released. However, in our study, the FFAs release was slow during the digestion. The possible mechanism can be explained by the shielding effects of Ca2+, which were added in the digestion phase. Tween 20 and Tween 80 are nonionic emulsifiers, whereas WPI, ML, and GA are anionic emulsifiers. The anionic charged droplets can be aggregated or flocculated by the screening effect with the cationic Ca2+. Therefore, the rate of FFAs release observed in this study was much slower than previous studies.
Figure 6.
Effect of the types of emulsifiers on the FFAs released during in vitro small intestine digestion.
2.7. Chemical Stability and Bioaccessibility of Fucoxanthin during Digestion
In our study, the chemical stability of fucoxanthin during the digestion in WPI-stabilized emulsion was almost 100%, whereas the one in ML-stabilized emulsions was only 53.6% (Table 1). However, previous studies suggested no significant degradation of astaxanthin and β-carotene before and after digestion.40,42 This result has shown that the oxidation and degradation of the bioactive compound during digestion is highly dependent on the sample and emulsifier types. The chemical structure of fucoxanthin contains an allenic bond and conjugated carbonyl group, which provide the unique features of fucoxanthin. The chemical stability of astaxanthin and β-carotene was not as active as fucoxanthin. Moreover, WPI can act as an excellent emulsifier which alleviates the degradation of fucoxanthin. GA-stabilized emulsions had the large volume mean diameter and low FFAs released related to a large amount of oil floated on the digestive fluid, hence, it was difficult to detect the chemical stability of fucoxanthin after digestion. The same situation also occurred with the bulk oil.
Table 1. Effect of the Types of Emulsifiers on the Fucoxanthin Retention (Rf) in Every Step and the Bioaccessibility and Chemical Stability of Fucoxanthin-Loaded Emulsions during the Digestion Process as Compared with Bulk Oil.
| type | Rf in emulsion (%) | Rf in raw digesta (%) | Rf in micelles phase (%) | bioaccessibility (%) | chemical stability (%) |
|---|---|---|---|---|---|
| WPI | 72.7 ± 2.6a | 72.9 ± 0.1a | 68.4 ± 5.0a | 92.5 ± 6.8a | 100.3 ± 0.2a |
| ML | 65.6 ± 1.5b | 35.1 ± 5.7b | 29.3 ± 0.3b | 44.6 ± 0.4b | 53.6 ± 8.7b |
| GA | 56.0 ± 6.4c | 20.6 ± 1.4c | 36.8 ± 2.5c | ||
| bulk oil | 100 ± 1.3a | ND | ND |
All data are mean ± standard deviations.
a–e values with a different letter in the same row are significantly different (p < 0.05).
ND: not detected under this analysis condition.
The bioaccessibility of fucoxanthin was highly dependent on the type of emulsifier in the emulsions and was determined as the fraction of fucoxanthin merged with the mixed micelles in the micellar phase after digestion by using in vitro gastrointestinal digestion model. It is noticeable that the bioaccessibility of fucoxanthin was drastically improved by using emulsion-based delivery systems. The bioaccessibility of fucoxanthin in samples followed the order: WPI (92.5%) >ML (44.6%) > GA (36.8%) > bulk oil (ND), and correlated inversely with the order of the initial droplet size. Especially, the retention of fucoxanthin in the micelles of WPI-stabilized emulsions rose up to 68.4% which was drastically higher than those in the bulk oil. For emulsions, lipolysis was more rapid and sufficient with smaller initial droplets. Different studies also indicated that the initial droplet size of dispersion has a significant influence on the generation of micelles and the lower bioaccessibility of β-carotene with increasing initial droplet size.42,43
Although the high-pressure homogenization method can improve the bioaccessibility of fucoxanthin, its drawbacks should not be neglected. Table 1 shows that with further processing and digestion, the proportion of fucoxanthin retention decreased in every step. The reasons for the decrease of Rf, such as high energy input, exposure to oxygen, and some other reasons, have been discussed above. Therefore, further studies are needed to solve the issues of reducing the degradation of fucoxanthin during processing and digestion.
In the present study, fucoxanthin could be successfully incorporated into O/W emulsions which were stabilized by WPI, ML, or GA. WPI and ML were able to formulate emulsions with smaller droplets. In contrast, GA-stabilized emulsions resulted in larger droplets. The long-term storage experiments showed that all emulsions exhibited good physical stability during storage for 15 days at 25 °C. The Rf in emulsions was highly dependent on the natural emulsifier type. Emulsions stabilized by WPI had the highest retention of fucoxanthin, probably due to the antioxidant properties of WPI. The in vitro digestion experiments indicated that emulsion-based delivery systems could notably enhance the bioaccessibility of fucoxanthin compared to that by bulk oil. Moreover, the type of natural emulsifier has a major effect on the lipid digestion and bioaccessibility. The release of FFAs and bioaccessibility of fucoxanthin in WPI- or ML-stabilized emulsions were higher than in the case of GA-stabilized emulsions. This was attributed to the lipid digestion and highly relied on the initial droplet size along with the larger surface area which could increase the lipolysis rate and FFAs release. The findings reported herein can provide valuable information about the bioaccessibility of hydrophobic bioactive compounds, such as fucoxanthin, which can be improved by using emulsion-based delivery systems formulated through high-pressure homogenization method.
3. Materials and Methods
3.1. Materials
Fucoxanthin extract samples (ThinOgen fucoxanthin oil 5%, fucoxanthin purity 5% by HPLC) were kindly donated by BGG-Japan Co., Ltd. (Tokyo, Japan). The source of fucoxanthin extract was Laminaria saccharina (L.) Lamouroux (Alga Kombu)-Syn:Laminaria japonicaor Undaria pinnatifida, Harvey (Wakame Algae). Medium-chain triacylglycerol (MCT-7) oil was purchased from Taiyo Kagaku Co., Ltd. (Mie, Japan). The triacylglycerol in MCT was reported to contain around 25% capric acid and 75% caprylic acid and polyglyceryl-5-laurate. WPI was procured from Nichiga, Japan Garlic Co., Ltd. (Gunma, Japan). ML (SLP whitelyso) was purchased from Tsuji Oil Mills Co., Ltd. (Tokyo, Japan). ML is a mixture of different phospholipids and contains lysophosphatidylcholine (18–30%), phosphatidylinositol (10–20%), phosphatidylcholine (2–8%), phosphatidylethanolamine (1–7%), and phosphatidic acid (0–5%). GA was purchased from Fujifilm Wako Pure Chem., Co. (Osaka, Japan). All other chemicals used were of analytical grade. Double distilled water (Milli-Q water) was used for the preparation of all solutions and emulsions.
3.2. Formulation of Fucoxanthin-Loaded O/W Emulsions
The dispersed phase was prepared by dispersing 4 wt % fucoxanthin extract in MCT oil and stirred at ambient temperature, overnight, to ensure that fucoxanthin completely dissolved. The samples were refined by passing through a poly(tetrafluoroethylene) (PTFE) syringe filter (0.45 μm) to eliminate undissolved particles. The continuous phases were prepared by dissolving 0.01–4 wt % emulsifiers (WPI, ML, or GA) in Milli-Q water and 0.02 wt % antimicrobial agent (sodium azide) was added. The fucoxanthin-loaded O/W emulsions were prepared by homogenizing the 10 wt % dispersed phase and the 90 wt % continuous phase at ambient temperature. Initially, coarse emulsions were homogenized by using a rotor–stator homogenizer (polytron, PT-3000 Kinematica-AG, Littace, Switzerland) at 10 000 rpm for 5 min and then passed through a high-pressure homogenizer (NanoVater, NV200, Yoshida Kikai, Japan) at a pressure range of 20–140 MPa for 0–10 passes to obtain the fine emulsions.
3.3. Characterization of Fucoxanthin-Loaded O/W Emulsions
A laser diffraction particle size analyzer (LS 13 320, Beckman Coulter, Brea, USA) was used to determine the droplet size and size distribution of the freshly prepared emulsions. This device works on the principle of laser diffraction to calculate the particle size distribution via the pattern of light scattered by the particles in the samples. It can measure particle size within the range of 0.04–2000 μm. The refractive indices for water and MCT oil were set at 1.333 and 1.450, respectively. The volume mean diameter (d4,3) was obtained for the average droplet size. All samples were measured in triplicate. The chemical stability of fucoxanthin during homogenization was calculated using eq 1, as follows
| 1 |
where C0 is the actual fucoxanthin concentration in freshly prepared emulsions, and CInitial is the fucoxanthin concentration calculated from the initial amount added.
3.4. Storage Stability of Fucoxanthin-Loaded Emulsions
The emulsion samples were stored in glass test tubes with screw caps after preparation and incubated in dark at 25 °C, for up to 15 days for observation. The d4,3 and chemical stability of fucoxanthin during storage, in the emulsions, were measured throughout the storage time. The chemical stability of fucoxanthin during storage in the emulsion samples was determined using eq 2, as follows
| 2 |
where Ct is the actual fucoxanthin concentration in the emulsions at a specific time during the storage, and C0 is the actual fucoxanthin concentration in freshly prepared emulsions.
3.5. Measurement of Fucoxanthin Concentration in Emulsions
The concentrations of fucoxanthin in the emulsions and bulk oil were quantified using HPLC (JASCO International Co., Tokyo, Japan) equipped with an AS-2055 autosampler, a PU-980 pump system, and a UV-970 UV–vis spectrophotometric detector. A C-18 reversed phase column (as stationary phase, 4.6 × 250 mm; Shimpack VP-ODS, Japan) was used with the temperature set at 25 °C. Fucoxanthin was extracted from emulsions and the dispersed phase prior to the HPLC analysis using a solvent extraction method: 200 μL of emulsion or a drop of dispersed phase (mass was analyzed) from the middle of the glass test tube was diluted to 10 mL with methanol in a volumetric flask to extract fucoxanthin and then ultrasonicated for 5 min. The samples were filtered using PTFE syringe filters (0.45 μm) and transferred to 2 mL HPLC vials; 20 μL of the filtered samples from HPLC vials were injected into the HPLC system. The mobile phase consisted of 10 wt % of Milli-Q water and 90 wt % of methanol. The mobile phase flow rate was set at 1 mL/min. UV detection of fucoxanthin was monitored at 450 nm. Fucoxanthin concentration in samples was calculated using a standard curve (R2 = 0.9995) and all of the analyses were repeated three times.
3.6. In Vitro Gastrointestinal Digestion
An in vitro gastrointestinal tract (GIT) model composed of gastric and intestinal phases was used in this study. There was a slight modification in the methodology, from previous studies, to simulate the biological fate of ingested samples.20,44−46 The samples were diluted two times with Milli-Q water in order to have 5 wt % oil before passing through the GIT model.
3.7. Gastric Phase
The simulated gastric fluid (SGF) was prepared by dissolving 7 mL of HCl (35–37%) and 2 g of sodium chloride in 1 L of Milli-Q water, and then 3.2 g of pepsin was added. HCl (1 mol/L) was used to adjust the pH of SGF to 1.2. The diluted emulsions (15 g) were mixed with SGF (15 g), and the obtained mixture contained 2.5 wt % oil. NaOH (1 mol/L) was used to adjust the pH of the samples to 2.5. The samples were maintained under continuous agitation at 250 rpm for 2 h at 37 °C.
3.8. Small Intestinal Phase
After the gastric digestion step, 30 g of the sample was adjusted to pH 7.0 immediately by using NaOH solution (1 mol/L). The simulated small intestinal fluid (SSIF) contained 1 mL of calcium chloride (110 mg/mL) dissolved in Milli-Q water and 4 mL of freshly prepared bile extract (46.87 mg/mL) dissolved in phosphate buffer (5 mM, pH 7.0). The SSIF was added into the samples and the pH was adjusted to 7.0. Subsequently, 2.5 mL of freshly prepared lipase suspension (24 mg/mL) dissolved in phosphate buffer (5 mM, pH 7.0) was incorporated into the mixture. The samples were transferred to clean beakers and incubated in a water bath with controlled temperature (37 °C) and continuous agitation at 250 rpm. During 2 h of the small intestinal digestion process, NaOH solution (1 mol/L) was manually titrated into the mixture to maintain a pH of 7.0. The pH of the samples was monitored and NaOH solution was titrated to neutralize the FFAs released during the lipid digestion.47 The volume of NaOH solution (L) was recorded throughout the digestion. The amount of FFAs released was calculated using eq 3, as follows
| 3 |
where VNaOH(t) is the volume (L) of NaOH solution (1 mol/L) titrated into the samples to neutralize the FFAs released at a certain digestion time (min), MNaOH is the molarity of NaOH solution used (mol), Moil is the molecular weight of the MCT oil (490 g/mol), and Woil is the initial mass (g) of the oil present in the reaction system.
3.9. Determination of Chemical Stability and Bioaccessibility of Fucoxanthin during Digestion
After the in vitro digestion, 10 mL of raw digesta was immediately collected and centrifuged (10 000g, MX-307 centrifuge, Tomy Digital Biology Co., Ltd., Tokyo, Japan) at an ambient temperature for 60 min. After centrifugation, samples were separated into three phases: a thin oil phase on top, a transparent micellar phase in the middle, and a sediment phase at the bottom.40,46 Fucoxanthin was assumed to be solubilized in the micellar phase. The extracted fucoxanthin from the raw digesta phase and micellar phase was collected and passed through a PTFE syringe filter (0.45 μm). Samples were added into an organic solvent (methanol) to extract fucoxanthin and, then, ultrasonicated for 5 min. The transparent phase was quantified using HPLC as described in Section 2.5. The chemical stability and bioaccessibility of fucoxanthin during digestion, and fucoxanthin retention (Rf) were calculated using eqs 4–6, respectively, as indicated below
| 4 |
| 5 |
| 6 |
where fucoxanthin concentration in the raw digesta and micellar phase are CDigesta and CMicellar, respectively. C0 is the actual fucoxanthin concentration in freshly prepared emulsions. CStep is the actual fucoxanthin concentration in the samples at every step (homogenization, storage, digestion, and bioaccessibility), and CInitial is the fucoxanthin concentration, which is calculated from the initial amount added.
3.10. Statistical Analysis
Each experiment was performed at least twice. Statistical analysis was performed using analysis of variance at a confidence level of 95%. Statistix 8.1 software (Tallahassee, USA) was used to calculate the least significant difference based on the method described in a previous report.48
Acknowledgments
The authors are thankful to BGG-Japan Co., Ltd., for kindly donating the fucoxanthin extract samples.
This study was supported by JSPS KAKENHI grant number JP17H01625.
The authors declare no competing financial interest.
References
- Kotake-Nara E.; Kushiro M.; Zhang H.; Sugawara T.; Miyashita K.; Nagao A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. 10.1093/jn/131.12.3303. [DOI] [PubMed] [Google Scholar]
- Abdul Q. A.; Choi R. J.; Jung H. A.; Choi J. S. Health benefit of fucosterol from marine algae: a review. J. Sci. Food Agric. 2016, 96, 1856–1866. 10.1002/jsfa.7489. [DOI] [PubMed] [Google Scholar]
- Peng J.; Yuan J.-P.; Wu C.-F.; Wang J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. 10.3390/md9101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Hosokawa M.; Miyashita K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. 10.3390/md11125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorofchian Moghadamtousi S.; Karimian H.; Khanabdali R.; Razavi M.; Firoozinia M.; Zandi K.; Abdul Kadir H. Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci. World J. 2014, 2014, 1. 10.1155/2014/768323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachindra N. M.; Sato E.; Maeda H.; Hosokawa M.; Niwano Y.; Kohno M.; Miyashita K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. 10.1021/jf071848a. [DOI] [PubMed] [Google Scholar]
- Awang A. N.; Ng J. L.; Matanjun P.; Sulaiman M. R.; Tan T. S.; Ooi Y. B. H. Anti-obesity property of the brown seaweed, Sargassum polycystum using an in vivo animal model. J. Appl. Phycol. 2014, 26, 1043–1048. 10.1007/s10811-013-0149-6. [DOI] [Google Scholar]
- Maeda H.; Hosokawa M.; Sashima T.; Murakami-Funayama K.; Miyashita K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. 10.3892/mmr_00000189. [DOI] [PubMed] [Google Scholar]
- Li Y.; Iwata K.; Sonoda K.; Shengquan M. Focoxanthin supplementation reduces body fat in over weight humans—A randomized, placebo controlled study—. Jpn. Pharmacol. Ther 2015, 9, 1317–1322. [Google Scholar]
- Mok I.-K.; Lee J. K.; Kim J. H.; Pan C.-H.; Kim S. M. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study. Food Chem. 2018, 258, 79–86. 10.1016/j.foodchem.2018.03.047. [DOI] [PubMed] [Google Scholar]
- Dai J.; Kim S. M.; Shin I.-S.; Kim J. D.; Lee H. Y.; Shin W. C.; Kim J.-C. Preparation and stability of fucoxanthin-loaded microemulsions. J. Ind. Eng. Chem. 2014, 20, 2103–2110. 10.1016/j.jiec.2013.09.039. [DOI] [Google Scholar]
- Zhao D.; Kim S.-M.; Pan C.-H.; Chung D. Effects of heating, aerial exposure and illumination on stability of fucoxanthin in canola oil. Food Chem. 2014, 145, 505–513. 10.1016/j.foodchem.2013.08.045. [DOI] [PubMed] [Google Scholar]
- Hii S.; Choong P.; Woo K.; Wong C. Stability studies of fucoxanthin from Sargassum binderi. Aust. J. Basic Appl. Sci. 2010, 4, 4580–4584. [Google Scholar]
- Muthuirulappan S.; Francis S. P. Anti-cancer mechanism and possibility of nano-suspension formulation for a marine algae product fucoxanthin. Asian Pac. J. Cancer Prev. 2013, 14, 2213–2216. 10.7314/apjcp.2013.14.4.2213. [DOI] [PubMed] [Google Scholar]
- McClements D. J.; Decker E. A.; Weiss J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- McClements D. J.Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, 2015. [Google Scholar]
- Tadros T.; Izquierdo P.; Esquena J.; Solans C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. 10.1016/s0001-8686(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Koo S. Y.; Mok I.-K.; Pan C.-H.; Kim S. M. Preparation of fucoxanthin-loaded nanoparticles composed of casein and chitosan with improved fucoxanthin bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. 10.1021/acs.jafc.6b04376. [DOI] [PubMed] [Google Scholar]
- Mok I.-K.; Yoon J.-R.; Pan C.-H.; Kim S. M. Development, quantification, method validation, and stability study of a novel fucoxanthin-fortified milk. J. Agric. Food Chem. 2016, 64, 6196–6202. 10.1021/acs.jafc.6b02206. [DOI] [PubMed] [Google Scholar]
- Salvia-Trujillo L.; Sun Q.; Um B. H.; Park Y.; McClements D. J. In vitro and in vivo study of fucoxanthin bioavailability from nanoemulsion-based delivery systems: Impact of lipid carrier type. J. Funct. Foods 2015, 17, 293–304. 10.1016/j.jff.2015.05.035. [DOI] [Google Scholar]
- Huang Z.; Xu L.; Zhu X.; Hu J.; Peng H.; Zeng Z.; Xiong H. Stability and Bioaccessibility of Fucoxanthin in Nanoemulsions Prepared from Pinolenic Acid-contained Structured Lipid. Int. J. Food Eng. 2017, 13, 2194. 10.1515/ijfe-2016-0273. [DOI] [Google Scholar]
- Zhao Y.; Khalid N.; Shu G.; Neves M. A.; Kobayashi I.; Nakajima M. Formulation and characterization of O/W emulsions stabilized using octenyl succinic anhydride modified kudzu starch. Carbohydr. Polym. 2017, 176, 91–98. 10.1016/j.carbpol.2017.08.064. [DOI] [PubMed] [Google Scholar]
- Bai L.; McClements D. J. Formation and stabilization of nanoemulsions using biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79. 10.1016/j.jcis.2016.06.047. [DOI] [PubMed] [Google Scholar]
- Krog N. J.; Sparso F. V.. Food Emulsifiers and Their Chemical and Physical Properties. Food emulsions, 4th ed.; Marcel Dekker, Inc.: New York, USA, 2004; Vol. 12. [Google Scholar]
- Tesch S.; Gerhards C.; Schubert H. Stabilization of emulsions by OSA starches. J. Food Eng. 2002, 54, 167–174. 10.1016/s0260-8774(01)00206-0. [DOI] [Google Scholar]
- Castellani O.; Al-Assaf S.; Axelos M.; Phillips G. O.; Anton M. Hydrocolloids with emulsifying capacity. Part 2 - Adsorption properties at the n-hexadecane-Water interface. Food Hydrocolloids 2010, 24, 121–130. 10.1016/j.foodhyd.2009.07.006. [DOI] [Google Scholar]
- Schultz S.; Wagner G.; Urban K.; Ulrich J. High-Pressure Homogenization as a Process for Emulsion Formation. Chem. Eng. Technol. 2004, 27, 361–368. 10.1002/ceat.200406111. [DOI] [Google Scholar]
- Anarjan N.; Tan C. P.; Nehdi I. A.; Ling T. C. Colloidal astaxanthin: preparation, characterisation and bioavailability evaluation. Food Chem. 2012, 135, 1303–1309. 10.1016/j.foodchem.2012.05.091. [DOI] [PubMed] [Google Scholar]
- Mao L.; Xu D.; Yang J.; Yuan F.; Gao Y.; Zhao J. Effects of small and large molecule emulsifiers on the characteristics of β-carotene nanoemulsions prepared by high pressure homogenization. Food Technol. Biotechnol. 2009, 47, 336–342. [Google Scholar]
- Shu G.; Khalid N.; Tan T. B.; Zhao Y.; Neves M. A.; Kobayashi I.; Nakajima M. Comparison of ergocalciferol nanodispersions prepared using modified lecithin and sodium caseinate: Insights of formulation, stability and bioaccessibility. J. Funct. Foods 2017, 38, 28–35. 10.1016/j.jff.2017.08.047. [DOI] [Google Scholar]
- Tan C.; Nakajima M. β-Carotene nanodispersions: preparation, characterization and stability evaluation. Food Chem. 2005, 92, 661–671. 10.1016/j.foodchem.2004.08.044. [DOI] [Google Scholar]
- Ma F.; Bell A. E.; Davis F. J. Effects of high-hydrostatic pressure and pH treatments on the emulsification properties of gum arabic. Food Chem. 2015, 184, 114–121. 10.1016/j.foodchem.2015.03.075. [DOI] [PubMed] [Google Scholar]
- Sun C.; Gunasekaran S.; Richards M. P. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocolloids 2007, 21, 555–564. 10.1016/j.foodhyd.2006.06.003. [DOI] [Google Scholar]
- McClements D. J.; Decker E. A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Ozturk B.; Argin S.; Ozilgen M.; McClements D. J. Nanoemulsion delivery systems for oil-soluble vitamins: Influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem. 2015, 187, 499–506. 10.1016/j.foodchem.2015.04.065. [DOI] [PubMed] [Google Scholar]
- Qian C.; Decker E. A.; Xiao H.; McClements D. J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. 10.1016/j.foodchem.2012.06.047. [DOI] [PubMed] [Google Scholar]
- Mu H.; Høy C.-E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. 10.1016/s0163-7827(03)00050-x. [DOI] [PubMed] [Google Scholar]
- Mun S.; Kim Y.-R.; Shin M.; McClements D. J. Control of lipid digestion and nutraceutical bioaccessibility using starch-based filled hydrogels: influence of starch and surfactant type. Food Hydrocolloids 2015, 44, 380–389. 10.1016/j.foodhyd.2014.10.013. [DOI] [Google Scholar]
- Zou L.; Zheng B.; Zhang R.; Zhang Z.; Liu W.; Liu C.; Xiao H.; McClements D. J. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82. 10.1016/j.foodres.2015.12.035. [DOI] [Google Scholar]
- Chen Z.; Shu G.; Taarji N.; Barrow C. J.; Nakajima M.; Khalid N.; Neves M. A. Gypenosides as natural emulsifiers for oil-in-water nanoemulsions loaded with astaxanthin: Insights of formulation, stability and release properties. Food Chem. 2018, 261, 322–328. 10.1016/j.foodchem.2018.04.054. [DOI] [PubMed] [Google Scholar]
- Zou L.; Liu W.; Liu C.; Xiao H.; McClements D. J. Utilizing food matrix effects to enhance nutraceutical bioavailability: increase of curcumin bioaccessibility using excipient emulsions. J. Agric. Food Chem. 2015, 63, 2052–2062. 10.1021/jf506149f. [DOI] [PubMed] [Google Scholar]
- Yi J.; Li Y.; Zhong F.; Yokoyama W. The physicochemical stability and in vitro bioaccessibility of beta-carotene in oil-in-water sodium caseinate emulsions. Food Hydrocolloids 2014, 35, 19–27. 10.1016/j.foodhyd.2013.07.025. [DOI] [Google Scholar]
- Wang P.; Liu H.-J.; Mei X.-Y.; Nakajima M.; Yin L.-J. Preliminary study into the factors modulating β-carotene micelle formation in dispersions using an in vitro digestion model. Food Hydrocolloids 2012, 26, 427–433. 10.1016/j.foodhyd.2010.11.018. [DOI] [Google Scholar]
- McClements D. J.; Li Y. Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. 10.1016/j.cis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Sarkar A.; Goh K. K. T.; Singh R. P.; Singh H. Behaviour of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocolloids 2009, 23, 1563–1569. 10.1016/j.foodhyd.2008.10.014. [DOI] [Google Scholar]
- Shu G.; Khalid N.; Tan T. B.; Zhao Y.; Neves M. A.; Kobayashi I.; Nakajima M. In vitro bioaccessibility of ergocalciferol in nanoemulsion-based delivery system: the influence of food-grade emulsifiers with different stabilising mechanisms. Int. J. Food Sci. Technol. 2018, 53, 430–440. 10.1111/ijfs.13601. [DOI] [Google Scholar]
- Li Y.; McClements D. J. New mathematical model for interpreting pH-stat digestion profiles: Impact of lipid droplet characteristics on in vitro digestibility. J. Agric. Food Chem. 2010, 58, 8085–8092. 10.1021/jf101325m. [DOI] [PubMed] [Google Scholar]
- Steel R.; Torrie J.; Dickey D.. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill Book Co.: New York, USA, 1997. [Google Scholar]