Abstract
Reading is a complex cognitive ability, which relies on visual and language processing as well as on executive functions (EFs). Recent studies have demonstrated that increased reading ability in children aged 7–17 years is related to greater activation of cognitive control regions during verb generation, a task which merges linguistic and cognitive control ability. The aim of the current study is to determine the relationships between neural circuits specifically related to EF and reading ability. We focused on functional connectivity between the dorsolateral prefrontal cortex (DLPFC), a region involved in EF and is part of the frontoparietal network during a verb generation task, and reading ability in seventeen 8–12-year-old typical readers. Results show positive functional connectivity between the left and right DLPFCs and regions related to cognitive control and visual processing while generating verbs. Increased reading ability was positively correlated with greater functional connectivity between the left and right DLPFCs and right-lateralized visual processing regions. The current study highlights the importance of neural circuits related to EF during both verb generation and reading and points to the role of the right occipital cortex in generating verbs as well as automatic word recognition in typical readers.
Keywords: children, executive functions, functional connectivity, reading, verb generation
Introduction
What are executive functions?
What are the capabilities that we use every day to read and process information? Executive functions (EFs) are higher-order cognitive abilities, including inhibition, working memory, shifting, and fluency (Anderson, 2002). These abilities develop throughout the lifetime, reaching full maturity during the mid-20s (Romine and Reynolds, 2005). EFs are the guiding mechanisms behind successful reading (Kendeou et al., 2015), whereas executive dysfunction, together with other linguistic components such as phonemic awareness (Pugh et al., 2013), is a predominant component in reading failure (Horowitz-Kraus, 2014, 2016). It is therefore crucial to explore the neurobiology behind EFs to discover the underpinnings of reading ability.
Verbal fluency: an aspect of EFs
Verbal fluency is an information-processing component of EF, which incorporates the integration of frontal systems for effective capability (Baldo et al., 2001). A task that assesses verbal fluency is a verb generation task, where one generates verbs in response to auditory or visual cues (Horowitz-Kraus et al., 2014). Verbal fluency has been shown to be positively correlated with reading ability (Horowitz-Kraus et al., 2014; Snowling et al., 1997), demonstrating shared reliance on linguistic, cognitive control, and visual processes.
Verb generation has been shown to activate frontal and language areas throughout development (i.e., 5–18 years of age) (Holland et al., 2001, 2007; Karunanayaka et al., 2010, 2011; Thompson-Schill et al., 1998), specifically the left inferior frontal gyrus (Herholz et al., 1996; Thompson-Schill et al., 1998) and the middle temporal gyrus and paracingulate gyri (Herholz et al., 1996). Additionally, as verbal fluency relies on semantics, right hemisphere activation can also be seen in adults (Bowden and Beeman, 1998).
Verb generation and reading
In a recent study, functional magnetic resonance imaging (fMRI) data from the verb generation task were collected from 16 typically developing children at ages 7, 11, and 17 years and correlated with reading scores at 17 years of age (Horowitz-Kraus et al., 2014). Lateralization indices were calculated in key language, reading, and EF-related regions in all age groups (Horowitz-Kraus et al., 2014). Typical development was associated with increasingly left-lateralized patterns in language regions and more profound left-lateralized activation for reading (BA 37) and EF-related (BA 9,10) regions when correlating with reading scores. That study reported of a positive correlation of reading and activation of the fusiform gyrus (BA 37) and suggested that the fusiform gyrus plays an important role also when generating words (Horowitz-Kraus et al., 2014).
The need to visualize the appropriate verb to the presented noun results in an involvement of this visual processing region (Frings et al., 2006; Horowitz-Kraus et al., 2014; Pang et al., 2011) as one needs to visually attend to the given stimuli. The need to perform this task in a fluent manner involves EF regions such as the dorsolateral prefrontal cortex (DLPFC) (Holland et al., 2001). The DLPFC plays a critical role in cognitive control (MacDonald et al., 2000) and is a core region in the frontoparietal network in particular (Dosenbach et al., 2008). The frontoparietal network is part of the dual-network model for cognitive control (Dosenbach et al., 2008), which is critical in both reading and verbal fluency in typical reading development (Horowitz-Kraus et al., 2014).
Several critical EFs were related to the DLPFC such as working memory through maintaining and manipulating information (Baker et al., 1996; MacDonald et al., 2000), an important cognitive ability, which is important for reading as well as for verbal fluency (Horowitz-Kraus et al., 2014). However, the relationships between neural circuits supporting cognitive control, language, and visual processing during verb generation and reading ability are yet to be known.
The importance of revealing such connections precedes previous studies demonstrating an increased functional connection between the fusiform gyrus and another cognitive control region (anterior cingulate cortex) (Horowitz-Kraus and Holland, 2015), which is also part of the dual-networks model for cognitive control (Dosenbach et al., 2008). The authors suggested that following an EF-based reading training, greater functional connections were formed between the visual and the EF system focusing on the cingulo-opercular network, whereas in the current study, we would like to examine if better reading can be related to increased functional connections with key regions in the frontoparietal network as well.
In the current study, we aimed to define functional connections between the left and right DLPFCs and the whole brain in relation to reading ability in typically developing children. Since both better reading and verb generation rely on EF and visual processing (Horowitz-Kraus et al., 2014; McCandliss et al., 2003), we hypothesized that greater functional connectivity of the DLPFC with regions related to visual processing will be correlated with greater reading scores.
Materials and Methods
Participants
Seventeen children (9 boys and 8 girls), ages 144.353 (±18.231) months, were included in this study, all right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971) and native English speakers. All participants (N = 17) completed the verb generation task during fMRI.
The study was approved by the Cincinnati Children's Hospital Medical Center Institutional Review Board. Informed consent was obtained from the child's parent or guardian and assent was obtained from all participants. Exclusion criteria were previous neurological illness, learning disability, head trauma with loss of consciousness, current or past use of psychostimulant medication, pregnancy, birth at 37 weeks gestational age or earlier, or abnormal findings at a routine neurological examination performed by an experienced pediatric neurologist. All participants were part of a parent study investigating normal language development in children and were considered healthy based on neurological, psychological, and structural measures (Holland et al., 2007; Szaflarski et al., 2012).
All participants were prescreened for any conditions that would prevent an MRI scan from being acquired safely. Intelligence was measured using the age-appropriate Wechsler Intelligence Scale for Children–Third Edition (WISC-III, ages 6–16) (Wechsler, 1991). Verbal ability was assessed using the Peabody Picture Vocabulary Test (Dunn and Dunn, 1997). EFs were also assessed using the Behavior Rating Inventory of Executive Function parent report questionnaire (Gioia and Isquith, 2011).
Reading was assessed using the Woodcock Johnson-III letter–word and passage comprehension reading subtests (Woodcock et al., 2001). In the letter–word task, participants were instructed to read a list of words written in English as accurately as possible, increasing in degree of difficulty. In the passage comprehension task, participants were required to complete a missing sentence. Both tasks were stopped by the administrator after six reading errors in a row.
fMRI verb generation paradigm
The fMRI paradigm consisted of a covert verb generation task as previously detailed (Holland et al., 2001, 2007) using a 30-sec on–off block design. All stimuli were presented using MacStim (White Ant Software, Melbourne, Australia). Stimuli were presented at a rate of one noun every 5 sec for 6 stimuli during each 30-sec epoch. During the active epochs, participants were asked to think of appropriate verbs such as throw or kick for aurally presented concrete nouns such as ball. Typically, developing children can think of two or three verbs associated with each noun during each response interval. They are instructed not to move the lips or mouth while covertly generating the verb responses.
During the control epochs, participants were asked to bilaterally tap their fingers when they heard a modulated tone. The bilateral finger-tapping task was chosen as the control for verb generation, as done in previous studies (Holland et al., 2001; Szaflarski et al., 2012). To compare the control task with auditory stimulation and response initiation on the verb generation task, participants were instructed to sequentially tap the fingers against the thumbs on both hands simultaneously when they hear a tone, in a self-paced manner. Participants were asked to stop tapping after touching each finger with the thumb twice. This control task provided participants with a task to shift their attention away from generating verbs during the control epoch, and it is used to provide an indirect measure of participants' compliance inside the scanner.
Neuroimaging data acquisition
An MRI-compatible audiovisual system was used for presentation of stimuli (Avotec, SS3150/SS7100) while children were scanned using a Philips Achieva 3T scanner (Philips Medical Systems, Best, The Netherlands). Details of the techniques used to obtain fMRI data from younger children, as well as the success rates, are given in a study by Byars and colleagues (2002). Echo-planar imaging-fMRI scan parameters were TR/TE = 3000/38 ms, 125 kHz, FOV = 25.6 × 25.6 cm, matrix = 64 × 64, flip angle of 8°, and slice thickness = 5 mm. Twenty-four slices were acquired, covering the entire cerebrum. One hundred ten whole-brain volumes were acquired (the first 10 were discarded during postprocessing to ensure image contrast at relaxation equilibrium) for a total scan time of 5 min 30 sec.
Techniques detailed elsewhere (Byars et al., 2002) were used to acclimate participants to the MRI procedure and render them comfortable inside the scanner. Soft head restraints were used to minimize head motion. In addition to fMRI scans, whole-brain T1-weighted magnetization prepared rapid gradient-echo scans were acquired for anatomical coregistration.
Data analysis
All datasets were preprocessed in SPM12 (Welcome Department of Cognitive Neurology, London). Slice-time correction, realignment to the image of the session for motion correction using three translational and three rotational parameters, coregistration of the anatomical image to the mean aligned functional image, normalization of all images to the Montreal Neurological Institute template 152 linear (suited for children above age 5), and spatial smoothing with an 8-mm full-width at half-maximum Gaussian kernel were performed on data as part of the preprocessing.
Functional connectivity analysis and regression
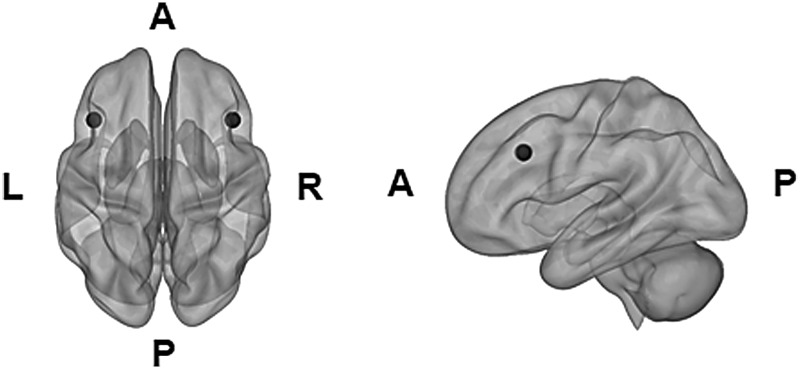
Preprocessed data were then fed into CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) for the functional connectivity analysis. A seed-to-voxel functional connectivity analysis was performed, using the left and right DLPFCs as seeds (see Fig. 1 for seeds). The left and right DLPFC regions were determined using the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002) implemented in CONN. To define functional connections between the left and right DLPFCs and all voxels in the brain during the verb generation task, a whole-brain seed-to-voxel analysis was conducted without a covariate, while equally weighting the left and right DLPDC seeds.
FIG. 1.
Mask: Left and right DLPFCs. Mask for left and right DLPFCs is noted in a 10-mm sphere in black (AAL atlas). AAL, automated anatomical labeling; DLPFC, dorsolateral prefrontal cortex.
Then, to answer our research question and to determine any correlations between the functional connectivity of the left and right DLPFCs and reading ability with all voxels in the brain, Pearson correlations were performed on the functional connectivity of these regions and the scaled scores from the letter–word reading task. Data were corrected for multiple comparisons using family discovery rate (FDR) correction (p < 0.05).
Results
Behavioral measures
Neuropsychological testing
Neuropsychological test results verified that the participants had normal to above average verbal and nonverbal general ability scores. Results also pointed at average–above average reading, reading comprehension, and EF abilities. See Table 1 for these results.
Table 1.
Verbal and Reading Abilities for Children Participating in the Study (N = 17)
| Mean | SD | |
|---|---|---|
| IQ (NEPSYa), standard score (average range: 10 ± 3) | 11.18 | 3.4 |
| Verbal IQ (PPVT) (scaled score, average range: 100 ± 15) | 113.29 | 14.42 |
| Word reading ability, letter–word, WJ (scaled score, average range: 100 ± 15) | 112.35 | 11.59 |
| Reading comprehension, WJ (scaled score, average range: 100 ± 15) | 106.71 | 7.40 |
| Executive function abilities (T score, average range: 50 ± 10) | 47.65 | 9.19 |
(Korkman et al., 1998)
NEPSY, a developmental neuropsychological assessment; PPVT, peabody picture vocabulary test; WJ, Woodcock-Johnson.
Neuroimaging data
Number of generated verbs
The average number of verbs generated post-test was 12.4/25 ± 3.6.
A whole-brain seed-to-voxel functional connectivity analysis
To verify the involvement of the DLPDC in the verb generation task, as was previously observed (Karunanayaka et al., 2010, 2011), the preprocessed data were fed into a general linear model, contrasting the verb condition with the control finger tapping condition (see Supplementary Fig. S1 and Supplementary Table S1 for these results).
A whole-brain seed-to-voxel functional connectivity analysis
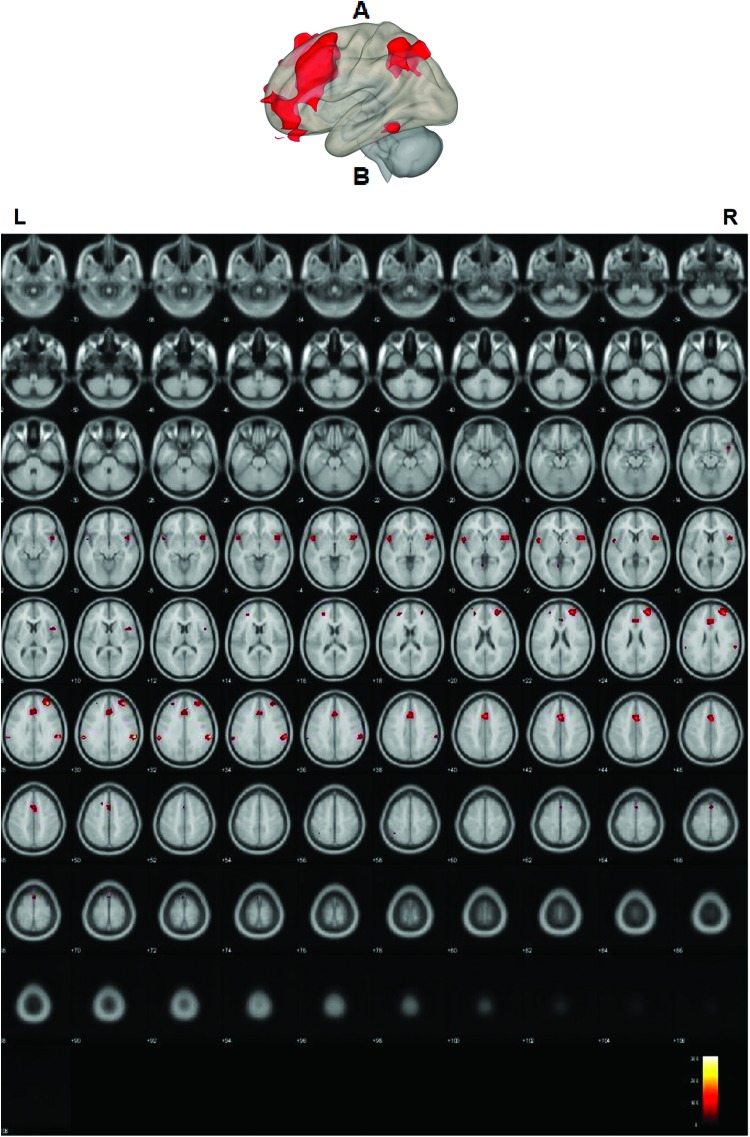
Left and right DLPFCs were defined as seeds for this analysis, and seed-to-voxel analysis was applied on the data. Results revealed significant positive functional connectivity (T(16) = 3.69; p < 0.05, r = 0.25, FDR corrected) between the left and right DLPFCs and clusters related to visual processing (i.e., superior occipital cortex), language (i.e., inferior temporal gyrus), and cognitive control (i.e., middle frontal gyrus and right superior frontal gyrus). See Figure 2 and Table 2 for these results.
FIG. 2.
Sagittal and axial views of the whole-brain seed-to-voxel analysis for the verb generation fMRI task. A whole-brain seed-to-voxel analysis during the verb generation task. The clusters functionally connected with the left and right DLPFCs (T(16) = 3.69; p < 0.05, r = 0.25, FDR corrected). A: A glass brain sagittal view, with regions in red representing areas functionally connected with the seeds, and B: axial slices Z = −72 to 108, two slides are incremental. Hotter color is represented in red. Neurological orientation (L = left, R = right). FDR, family discovery rate; fMRI, functional magnetic resonance imaging. Color images are available online.
Table 2.
Whole-Brain Seed-to-Voxel Analysis (T(16) = 3.69, r = 0.25) and Regression Analysis of Magnetic Resonance Imaging Data from the Verb Generation Task with Letter–Word Reading Standard Scores (T(15) = 3.73, r = 0.02) (p < 0.05, Family Discovery Rate Corrected)
| Cluster centroid | |||||
|---|---|---|---|---|---|
| Condition | Region | Cluster size | X | Y | Z |
| Whole brain | R middle frontal gyrus | 7890 | 44 | 22 | 32 |
| L middle frontal gyrus | 6388 | −40 | 20 | 32 | |
| Left superior occipital cortex | 2074 | −26 | −74 | 58 | |
| Right superior occipital cortex | 1853 | 38 | −60 | 58 | |
| Right superior frontal gyrus | 1284 | 2 | 28 | 54 | |
| Right inferior temporal gyrus | 674 | 68 | −26 | −28 | |
| Left inferior temporal gyrus | 335 | −56 | −52 | −18 | |
| Left frontal pole | 231 | −18 | 42 | −24 | |
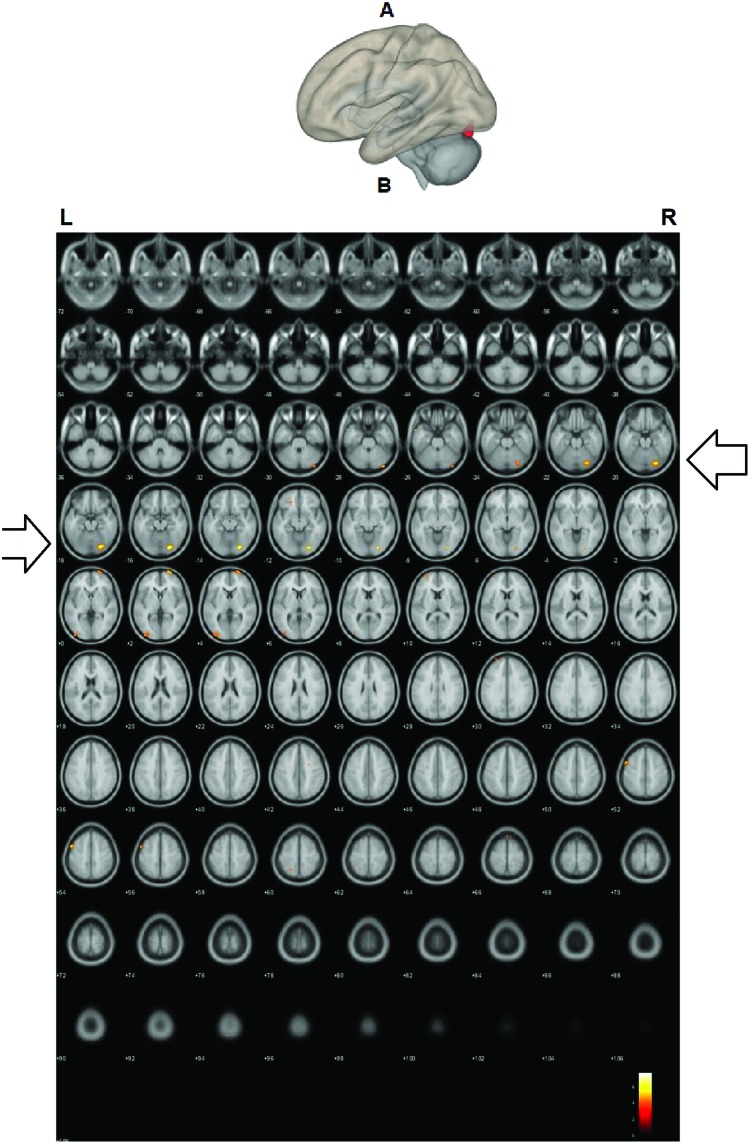
| Correlation with reading ability (WJ, letter–word) | Right occipital fusiform gyrus | 265 | 28 | −80 | −16 |
Correlation of seed-to-voxel functional connectivity and word reading ability (letter–word, Woodcock-Johnson)
The correlation analysis revealed a significant positive correlation (T(15) = 3.73; p < 0.05, r = 0.02, FDR-corrected) between the left and right DLPFCs and a cluster in the visual processing region (i.e., right fusiform gyrus) and the letter–word reading measure. Increased functional connectivity between the left and right DLPFCs and the right fusiform gyrus was related to better reading scores (Figure 3 and Table 2).
FIG. 3.
Correlation analysis between seed–voxel functional connectivity during the fMRI task and word reading scores from the letter–word test [from the WJ battery (Woodcock et al., 2007)]. A correlation analysis between the seed-to-voxel functional connectivity and letter–word reading scores from WJ. The clusters functionally connected with the left and right DLPFCs were correlated with word reading scores (T(15) = 3.73; p < 0.05, r = 0.02 FDR corrected). A: A glass brain sagittal view, regions functionally connected with the seeds are colored in red, and B: axial slices Z = −72 to 108, two slides are incremental. Hotter color is represented in red. Neurological orientation (L = left, R = right). WJ, Woodcock-Johnson. Color images are available online.
Discussion
The aim of the current study was to determine the role of the left and right DLPFCs in both verb generation and reading ability in typically developing children. Due to previous findings pointing at the central role of this region in both verbal fluency and reading (Horowitz-Kraus et al., 2014), and a previous finding pointing at the involvement of visual processing in both abilities, we hypothesized that positive functional connectivity between these regions will be related to better reading ability.
Indeed, the current study highlights the importance of a major cognitive control region, the DLPFC, in 8–12-year-old children during reading and its connectivity to visual regions during a verb generation task, a task that assesses semantic verbal fluency (Piatt et al., 1999). Previous studies have found activation in occipital regions during the verb generation task, hypothesized to be due to visual imagery that is involved during the task (Karunanayaka et al., 2011), and also in frontal regions (Holland et al., 2001, 2007; Karunanayaka et al., 2010, 2011). However, this study is the first to explore the whole-brain seed-to-voxel connectivity of the DLPFC, highlighting its connections to visual and occipital regions, aiding in fundamental knowledge of underpinnings of reading ability in children.
Functional connectivity between the DLPFC and visual processing during word generation is also related to successful reading
The current study finds a functional connection between the DLPFC and the right fusiform gyrus, an occipital region. Right hemisphere activation was previously related to semantic abilities (Bowden and Beeman, 1998), so involvement of the right fusiform gyrus could represent the role of semantics in verb generation. An additional meta-analysis demonstrated involvement of the right occipital cortex for pictures remembered versus forgotten (Kim, 2011), emphasizing the role of installing and retrieving the pictorial representation of words, both verbally and while encountering written language, both critical for verb generation and reading.
This novel finding of the importance of the right hemisphere in verb generation as well as in reading solidifies previous research on the reliance of the right hemisphere during reading comprehension (Horowitz-Kraus et al., 2015a) and in semantics (Roman et al., 1987; Wapner et al., 1981). Previous studies examining the role of the two EF networks, frontoparietal and cingulo-opercular networks, found more critical involvement of the cingulo-opercular network related to monitoring and responding to feedback, and all demand slow in-depth processing of the stimulus and response (Petersen and Posner, 2012) in reading remediation in children with reading difficulties (Horowitz-Kraus et al., 2015b).
The frontoparietal network, of which the dorsal lateral prefrontal cortex is a major hub (Dosenbach et al., 2008), is involved in adaptive control and in fast processing of stimuli (Petersen and Posner, 2012) and has been proposed to be involved in maintenance of tasks (MacDonald et al., 2000), which is critical during reading. Since fast processing of stimuli is important both during an automatic reading (Frith, 1985) and during fast generation of verbs, it is not surprising that the components of this network participate in both abilities.
Interestingly, to process both the written material (reading) and to generate verbs, visual processing regions should be engaged. It may be that the visual representation must be maintained for the stimulus to be processed accurately. It might be that children with reading difficulties, who share challenges maintaining the visual stimulation while reading (i.e., showing decreased activation in the visual cortices while reading), will show a similar connectivity pattern as reported in the current study. A future study should confirm this point.
Study's limitations
There are several limitations for the current study. In this study, we focused on one region associated with cognitive control, the DLPFC, instead of incorporating a whole network approach. The reason for doing so is evidence of late maturation between networks and physiologically far connections (Fair et al., 2012) in this age group, which may impair our ability to look at functional connections between frontal and occipital networks. Future studies should examine functional connectivity of cognitive control networks (i.e., frontoparietal and cingulo-opercular networks) and their relationship with occipital areas to discover the comprehensive role of network activation during word generation.
Second, the number of participants was relatively small (i.e., 17), which may affect the strength of our analysis. Further studies should include diverse populations speaking languages different than English and a higher number of participants.
Conclusions
This study fills an important gap in the literature about the neurobiological infrastructure for reading development in children, highlighting the importance of cognitive control in verb generation and its relationship with reading. Word generation is a basic communication skill, and children who show early language delays show reading disabilities later in life during reading acquisition (Scarborough and Dobrich, 1990). The current study gives the opportunity for earlier intervention and assessment during word generation development.
As cognitive control abilities are impaired in reading disabilities (Reiter et al., 2005), this finding about semantic fluency in typical readers could help in assessing these abilities in young children as a possible marker for future reading difficulties, as well as creating better interventions in those with reading disabilities and those at risk of developing reading difficulties, to improve their reading even before it is officially acquired.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Child Health and Human Development (R01 HD086011; PI: T.H.-K.) and a grant from the U.S. National Institutes of Health (NIH grant R01-HD38578; PI: Holland).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- Anderson P. 2002. Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8:71–82 [DOI] [PubMed] [Google Scholar]
- Baker S, Frith C, Frackowiak S, Dolan RJ. 1996. Active representation of shape and spatial location in man. Cerebral Cortex 6:612–619 [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. 2001. Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc 7:586–596 [DOI] [PubMed] [Google Scholar]
- Bowden EM, Beeman MJ. 1998. Getting the right idea: semantic activation in the right hemisphere may help solve insight problems. Psychol Sci 9:435–440 [Google Scholar]
- Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, Schmithorst VJ, Plante E. 2002. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol 17:885–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. 2008. A dual-networks architecture of top-down control. Trends Cogn Sci 12:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. 1997. PPVT-III: Peabody Picture Vocabulary Test. Circle Pines, MN: American Guidance Service [Google Scholar]
- Fair DM, Dosenbach NU, Petersen SE, Schlaggar BL. 2012. Resting state studies on the development of control systems. In: Posner MI. (eds.) Cognitive Neuroscience of Attention. New York: Guilford Press; 291–311 [Google Scholar]
- Frings M, Dimitrova A, Schorn CF, Elles H-G, Hein-Kropp C, Gizewski ER, et al. . 2006. Cerebellar involvement in verb generation: an fMRI study. Neurosci Lett 409:19–23 [DOI] [PubMed] [Google Scholar]
- Frith U. 1985. Beneath the Surface of Developmental Dyslexia. London: Erlbaum [Google Scholar]
- Gioia GA, Isquith PK. 2011. Behavior Rating Inventory for Executive Functions. Encyclopedia of Clinical Neuropsychology. Lutz, FL: Springer: 372–376 [Google Scholar]
- Herholz K, Thiel A, Wienhard K, Pietrzyk U, Von Stockhausen H-M, Karbe H, et al. . 1996. Individual functional anatomy of verb generation. Neuroimage 3:185–194 [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr. 2001. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage 14:837–843 [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema J-M, Karunanayaka PR, et al. . 2007. Functional MRI of language lateralization during development in children. Int J Audiol 46:533–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T. 2014. Pinpointing the deficit in executive functions in adolescents with dyslexia performing the Wisconsin card sorting test: an ERP study. J Learn Disabil 47:208–223 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T. 2016. The role of executive functions in the reading process. In: Khateb B-KA. (eds). Reading Fluency: Current Insights from Neuro-Cognitive Research and Intervention Studies. I. Netherlands, Springer [Google Scholar]
- Horowitz-Kraus T, Grainger M, DiFrancesco M, Vannest J, Holland SK, Consortium CA. 2015a. Right is not always wrong: DTI and fMRI evidence for the reliance of reading comprehension on language-comprehension networks in the right hemisphere. Brain Imaging Behav 9:19–31 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Holland SK. 2015. Greater functional connectivity between reading and error-detection regions following training with the reading acceleration program in children with reading difficulties. Ann Dyslexia 65:1–23 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Toro-Serey C, DiFrancesco M. 2015b. Increased resting-state functional connectivity in the cingulo-opercular cognitive-control network after intervention in children with reading difficulties. PLoS One 10:e0133762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Vannest JJ, Gozdas E, Holland SK. 2014. Greater utilization of neural-circuits related to executive functions is associated with better reading: a longitudinal fMRI study using the verb generation task. Front Hum Neurosci 8:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanayaka P, Schmithorst VJ, Vannest J, Szaflarski JP, Plante E, Holland SK. 2010. A group independent component analysis of covert verb generation in children: a functional magnetic resonance imaging study. Neuroimage 51:472–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanayaka P, Schmithorst VJ, Vannest J, Szaflarski JP, Plante E, Holland SK. 2011. A linear structural equation model for covert verb generation based on independent component analysis of fMRI data from children and adolescents. Front Syst Neurosci 5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendeou P, Papadopoulos TC, Spanoudis G. 2015. Reading Comprehension and Pass Theory. Cognition, Intelligence, and Achievement. San Diego, CA: Elsevier; pp. 117–136 [Google Scholar]
- Kim H. 2011. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studie. NeuroImage 54:2446–2461 [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. 1998. NEPSY: A Developmental Neuropsychological Assessment: Manual. San Antonio, TX: Harcourt Assessment Inc [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. 2000. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288:1835–1838 [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. 2003. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci 7:293–299 [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Pang EW, Wang F, Malone M, Kadis DS, Donner EJ. 2011. Localization of Broca's area using verb generation tasks in the MEG: validation against fMRI. Neurosci Lett 490:215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. 2012. The attention system of the human brain: 20 years after. Annu Rev Neurosci 35:73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Tröster AI. 1999. Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia 37:1499–1503 [DOI] [PubMed] [Google Scholar]
- Pugh KR, Landi N, Preston JL, Mencl WE, Austin AC, Sibley D, et al. . 2013. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang 125:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter A, Tucha O, Lange KW. 2005. Executive functions in children with dyslexia. Dyslexia 11:116–131 [DOI] [PubMed] [Google Scholar]
- Roman M, Brownell HH, Potter HH, Seibold MS, Gardner H. 1987. Script knowledge in right hemisphere-damaged and in normal elderly adults. Brain Lang 31:151–170 [DOI] [PubMed] [Google Scholar]
- Romine CB, Reynolds CR. 2005. A model of the development of frontal lobe functioning: findings from a meta-analysis. Appl Neuropsychol 12:190–201 [DOI] [PubMed] [Google Scholar]
- Scarborough HS, Dobrich W. 1990. Development of children with early language delay. J Speech Lang Hear Res 33:70–83 [DOI] [PubMed] [Google Scholar]
- Snowling M, Nation K, Moxham P, Gallagher A, Frith U. 1997. Phonological processing skills of dyslexic students in higher education: a preliminary report. J Res Reading 20:31–41 [Google Scholar]
- Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, et al. . 2012. Left-handedness and language lateralization in children. Brain Res 1433:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, Esposito MD', Kan IP, Knight RT. 1998. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A 95:15855–15860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. . 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- Wapner W, Hamby S, Gardner H. 1981. The role of the right hemisphere in the apprehension of complex linguistic materials. Brain Lang 14:15–33 [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1991. Manual for the Wechsler Intelligence Scale for Children-(WISC-III). San Antonio, TX: The Psychological Corporation [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141 [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Mather N, McGrew KS, Wendling BJ. 2001. Woodcock-Johnson III Tests of Cognitive Abilities. iverside Publishing Company [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. 2007. Woodcock-Johnson Tests of Achievement, Three. Rolling Meadows, IL, Riverside Publishing [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.