Abstract
Anti-transgene immune responses elicited after intramuscular (i.m.) delivery of recombinant adeno-associated virus (rAAV) have been shown to hamper long-term transgene expression in large-animal models of rAAV-mediated gene transfer. To overcome this hurdle, an alternative mode of delivery of rAAV vectors in nonhuman primate muscles has been described: the locoregional (LR) intravenous route of administration. Using this injection mode, persistent inducible transgene expression for at least 1 year under the control of the tetracycline-inducible Tet-On system was previously reported in cynomolgus monkeys, with no immunity against the rtTA transgene product. The present study shows the long-term follow-up of these animals. It is reported that LR delivery of a rAAV2/1 vector allows long-term inducible expression up to at least 5 years post gene transfer, with no any detectable host immune response against the transactivator rtTA, despite its immunogenicity following i.m. gene transfer. This study shows for the first time a long-term regulation of muscle gene expression using a Tet-On-inducible system in a large-animal model. Moreover, these findings further confirm that the rAAV LR delivery route is efficient and immunologically safe, allowing long-term skeletal muscle gene transfer.
Keywords: AAV, gene transfer, locoregional delivery, nonhuman primate, immunogenicity, long-term follow-up
Introduction
Over the last decade, recombinant adeno-associated virus (rAAV)-based gene therapies have shown great promises for the treatment of genetic disorders, with numerous studies evolving from proof-of- concepts to clinical trials or even Marketing Authorization Application (MAA) in Europe or Biologic License Application (BLA) in the United States.1–4 Nevertheless, immune responses directed against the vector capsid or the transgene product remain potential limiting factors to long-term gene transfer efficiency, even though their impact on transgene expression can be difficult to understand.5–7 For several years, the authors have been taking a particular interest in the impact of anti-transgene immune responses triggered after muscle-directed rAAV-mediated gene transfer.8–10 Skeletal muscle is the target tissue of choice for gene therapy of numerous neuromuscular diseases and metabolic disorders.11 In the context of rAAV-mediated gene transfer, muscle-directed gene therapy strategies have first relied on the direct intramuscular (i.m.) delivery of the viral vector. Indeed, this administration route is noninvasive and easily transposable into clinic, and long-term transgene expression has been obtained upon i.m. delivery of rAAV in large-animal models as well as in humans.8,11–14 However, in large-animal models, numerous cases of immune responses directed against the transgene product have been reported, all leading to the loss of transgene expression that can be attributed to the activation of transgene-specific CD8+ T lymphocytes.8,15–17 In clinical trials, the limited number of available data seems to indicate that the impact of anti-transgene immune responses on rAAV-mediated gene transfer efficiency is not as predictive as animal models led us to expect.13,18 Indeed, their occurrence seems not to be systematically correlated to the loss of transgene expression, although consensual conclusions are hard to draw considering the wide diversity of clinical settings (pathological state and premedication of the patients, immunosuppressive regimens administered before or during clinical trials, genetics and vector dosing).19 In addition, in the majority of trials, patients with null mutations and/or a high risk of transgene rejection were generally excluded.
Several key features of rAAV-mediated gene transfer, likely to influence the occurrence of immune responses directed against the transgene product, have now been identified, including the vector serotype, the dose, the genome conformation, the immunogenicity of the transgene product, and the route of vector administration.20 In an effort to circumvent the risks of anti-transgene immune responses following rAAV i.m. delivery and to allow the transduction of larger muscle territories upon a single injection of a rAAV vector, an alternative mode of muscle-directed vector administration has been developed: the locoregional (LR) intravenous (i.v.) route.10,21–24 The LR administration route has shown in large-animal models (dogs and nonhuman primates [NHP]) that on top of being well tolerated, it allows a more diffused transgene expression in all muscles of the perfused limb (when compared to i.m. administration), thanks to an increased extravasation of vector particles into the muscles. A persisting gene transfer (for at least 1 year post perfusion) after LR delivery was also previously reported of an rAAV2/1 vector expressing an inducible erythropoietin (Epo) reporter gene under the control of the Tet-On system that is based on the expression of the rtTA transactivator. This persisting gene regulation was observed in the absence of any immunosuppressive regimen in all three cynomolgus monkeys, whereas transgene expression did not exceed 3 months in i.m.-injected NHPs.10 Additionally, although anti-AAV2/1 directed antibodies were observed in these LR-injected animals, no humoral response directed toward the transgene product or cellular infiltrates were detected in muscle biopsies.
The present study documents the long-term follow-up of these three LR-perfused NHPs over a period of 5 years. Vector biodistribution and level of transgene expression were characterized, as well as the state of anti-transgene immune responses during this extended period. In all animals, a persistent and stable Tet-On inducible transgene expression was observed in the muscles of the perfused limb for at least 5 years post rAAV2/1 LR injection. This persisting transgene expression was not associated with any humoral or cellular responses directed against the rtTA transgene product, neither in the periphery nor in the perfused muscles with no cellular infiltrates. To the authors' knowledge, this is the first time that the Tet-On inducible system was proven to be efficient over a long period of time in a large-animal model of muscle gene transfer. In previous studies, the Tet-On rtTA-based system was systematically rejected by the host immune system when the vector was administered to the muscle or systemically, with a rapid loss of inducible gene expression.8,25–27 The present findings strongly suggest that LR delivery of rAAV vectors is efficient and immunologically safe in contrast to i.m. injection and confirm the critical impact of vector mode of delivery on host immunity.
Methods
A large part of this work was performed under the control of a quality management system that is approved by Lloyd's Register Quality Assurance to meet the requirements of International Management System Standards ISO 9001:2008. It has been implemented to cover all activities in the laboratory, including research experiments and production of research-grade viral vectors.
Vector production
Recombinant AAV2/ vector was produced, as described in Toromanoff et al.10
Animal care and welfare
NHPs (Macaca fascicularis) were maintained for experimentation after approval of the Institutional Animal Care and Use Committee of the University of Nantes and under the supervision of a doctor of veterinary medicine. Special attention was paid to the health and welfare of the animals. Blood samples were collected regularly to follow biochemical and hematological parameters, conforming with physiological guidelines. More precisely, primates were anesthetized with an i.m. injection of 20 μg/kg medetomidine (Domitor®; Pfizer) associated with 8 mg/kg of ketamine (Imalgène®; Rhone Merieux). For each Epo protein expression induction, 20, 10, and 10 mg/kg doxycycline (ELERTE) were administrated per os on days 1, 2, and 3, respectively. At the end of the protocol, euthanasia was performed with i.v. injection of sodium pentobarbital (Dolethal®; Vétoquinol) after 0.1 mg/kg morphine-induced analgesia.
Analysis of secreted erythropoietin
As described in previous studies,8,10,25 serum cynomolgus Epo protein levels were measured by enzyme-linked immunosorbent assay (ELISA; Quantikine IVD kit; R&D Systems) according to the manufacturer's procedures. Physiological variation levels of serum Epo protein were obtained from titration of a total of 182 serum samples obtained from 32 different NHPs and were calculated as follows: mean of Epo protein level +2 standard deviations (SD).
Quantitative real-time polymerase chain reaction analysis
Total genomic DNA from total tissues was extracted using the Gentra Puregene kit (Qiagen) and Tissue Lyser II (Qiagen) according to the manufacturer's procedures. Total RNA was obtained from lysis of tissues by Tissue Lyser II and using TRIzol® (Thermo Fisher Scientific) and chloroform-based (Sigma–Aldrich) extraction. Total RNA was then treated with RNAse-free DNAse I from the TURBO DNA-Free™ Kit (Thermo Fisher Scientific) to eliminate DNA contamination. RNA (500 ng) was reverse transcribed using the M-MLV Reverse-Transcriptase Kit (Thermo Fisher Scientific). Vector genome DNA and transgene cDNA were measured through quantification of bovine growth hormone polyadenylation signal (BGH-pA) localized downstream of the rtTA sequence25 using a StepOne Plus instrument (Thermo Fisher Scientific). The primers and TaqMan probe used were: forward primer 5′-TCTAGTTGCCAGCCATCTGTTGT-3′; reverse primer 5′-TGGGAGTGGCACCTTCCA-3′ and BGH-pA probe 5′ (6 FAM)-TCCCCCGTGCCTTCCTTGACC-3′ TAMRA. The BGH-pA quantitative polymerase chain reaction (qPCR) was done using the following program: initial denaturation 20 s at 95°C followed by 45 cycles of 1 s at 95°C and 20 s at 60°C.
For vector genome quantification, data were normalized by quantifying the endogenous NHP ɛ -globin DNA using the following primers: forward primer 5′-ACATAGCTTGCTTCAGAACGGT-3′; reverse primer 5′-AGTGTCTTCATCCTGCCCTAAA-3′ and ɛ-globin probe 5′ (6 FAM)-TGCAGGCTGCCTGGCAGAAGC-3′ TAMRA. The ɛ-globin qPCR was performed with the following program: initial denaturation 20 s at 95°C followed by 45 cycles of 3 s at 90°C and 30 s at 60°C. For each sample, Ct values were compared to those obtained with plasmid (containing either the BGH-pA sequence or the ɛ-globin sequence) standard dilutions.
The reverse-transcribed mRNA measurement was normalized by quantifying the endogenous NHP HPRT1 reverse-transcribed mRNA using the following primers to target the HPRT1 sequence: forward primer 5′-GCTTTCCTTGGTCAGGCAGTA-3′; reverse primer 5′-TGGAGTCCTTTTCACCAGCA-3′; and HPRT1 probe 5′ (6 FAM) AATCCAAAGATGGTCAAGGTCGCAA-3′ TAMRA. The HPRT1 qPCR was done using the following program: initial denaturation 20 s at 95°C followed by 40 cycles of 3 s at 95°C and 30 s at 62°C. The Ct results obtained for the transgene and transcripts were normalized with HPRT values using relative quantity (RQ) = 2–ΔCt.
Follow-up of anti-TetR humoral immune responses
Detection of serum anti-rtTA antibodies was conducted using ELISA, as previously described.28 Briefly, Nunc MaxiSorp P96 plates (Sigma–Aldrich) were coated with recombinant rtTA protein (5 μg/mL; Proteogenix). After washing steps and saturation, sera were added in each well at various dilutions (twofold dilutions from 1/10 to 1/20,480) and incubated for 2 h at 37°C. Following incubation for 1 h at 37°C of horseradish peroxidase (HRP)-conjugated anti-rhesus immunoglobulin G (IgG; Cliniscience), revelation was performed using 2.2-3,3′,5,5′-tetramethylbenzidine (TMB; BD OptEIA, BD Biosciences). Absorbances of duplicate samples were read at 450 nm with a correction at 570 nm on a Multiskan Go reader (Thermo Fisher Scientific). Threshold of positivity was determined using 21 negative sera obtained from naïve NHPs as the mean of the optical density for each dilution +2 SD. IgG titers for experimental animals were defined as the last serum dilution whose optical density remained above the threshold.
Follow-up of anti-TetR cellular immune responses
The anti-TetR Immune cellular response was evaluated with an interferon gamma (IFN-γ) enzyme-linked immunospot (ELISpot) assay, as previously described.28 Briefly, 2E5 thawed peripheral blood mononuclear cells (PBMCs) were stimulated with five rtTA peptides pools covering the rtTA sequence (overlapping peptide library 15 per 10 mers; Sigma–Aldrich) into human anti-IFN-γ (MabTech) precoated polyvinylidene difluoride membrane MultiScreen® high-throughput filter plates (Millipore). Positive and negative controls were obtained using Concanavalin A (Sigma–Aldrich) or medium alone, respectively. After incubation with a biotinylated anti-IFN-γ antibody (clone 7-B6-1; MabTech) and ExtrAvidin® alkaline phosphatase (Sigma–Aldrich), the enzymatic reaction was revealed using NBT/BCIP (Thermo Fisher Scientific). Spot number was determined using an ELISpot reader ELR07 (AID) and analyzed with the AID ELISpot Reader Software v7.0. Responses were considered positive when the number of spot-forming colonies per million cells was >50 and at least threefold higher than the negative control (C–). The rtTA-immunized macaque was obtained by the administration of the same vector rAAV1-rtTA/Epo combined with two intradermal injections of rtTA-pulsed dendritic cells, as published by Moreau et al.28
Follow-up of anti-AAV2/1 humoral immune responses
Detection of serum anti-AAV2/1 antibodies was conducted using ELISA. Nunc MaxiSorp P96 plates (Sigma–Aldrich) were coated with 1E9 vg/well of rAAV2/1 rtTA-Epo. Following saturation, sera were added in wells at various dilutions (twofold dilutions from 1/10 to 1/40,960) and incubated for 2 h. A biotinylated anti human IgG (Jackson Immunoresearch) was incubated for 1 h at 37°C followed by a HRP-conjugated Streptavidin (Vector). Finally, revelation was performed using TMB (BD OptEIA; BD Biosciences), and absorbance of duplicate samples were read at 450 nm with a correction at 570 nm on a Multiskan Go reader (Thermo Fisher Scientific). As for rtTA, threshold of positivity was determined using 14 negative sera obtained from naïve NHPs as the mean of optical density for each dilution +2 SD. IgG titers for experimental animals were defined as the last sera dilution whose optical density remained above the threshold curve.
Follow-up of anti-AAV1 neutralizing factors
Two hours prior to rAAV transduction, a permissive cell line was infected with wild-type adenovirus serotype 5. During this incubation time, a rAAV2/1 expressing the reporter gene LacZ (encoding beta-galactosidase) was incubated with diluted serum, and the mix was added to the cell line. Twenty-four hours later, the cells were fixed with 0.5% of glutaraldehyde (Sigma–Aldrich) and stained with X-gal solution (Promega). The transduction was determined by light microscopy. The titer of neutralizing factors was defined as the last dilution that inhibits the transduction as compared to the transduction control (without serum).
Histopathological analysis
Muscles were sampled at euthanasia >5 years post injection. Hematoxylin-phloxine-saffron (HPS) staining was performed as per standard histological protocols using formalin-fixed paraffin-embedded muscle sections. The slides were observed using a NanoZoomer Slide Scanner (Hamamatsu).
Results
Persisting expression of an inducible transgene 5 years after locoregional delivery of rAAV2/1
Three NHPs (Mac 19, 20, and 21) were LR perfused with 1E11 vg/kg of rAAV2/1 rtTA/Epo. Doxycycline was then administered periodically to induce the rtTA-mediated transactivation of the Tet-O-CMV promoter and subsequently the expression of the Epo protein.8,10,24,25 Epo protein secretion in the serum was measured by ELISA during the 21 days after every doxycycline administration cycle. Previous results showed a persisting Epo transgene expression, with a secretion peak of recombinant Epo protein in the serum following every doxycycline administration, during 1 year after LR delivery of rAAV2/1, without any immune suppression.10 Moreover, although the rtTA transactivator was permanently expressed (under the control of the Desmin promoter), no immune response was detected against this immunogenic foreign protein in any of the three LR-injected NHPs, as opposed to i.m.-injected NHPs.10
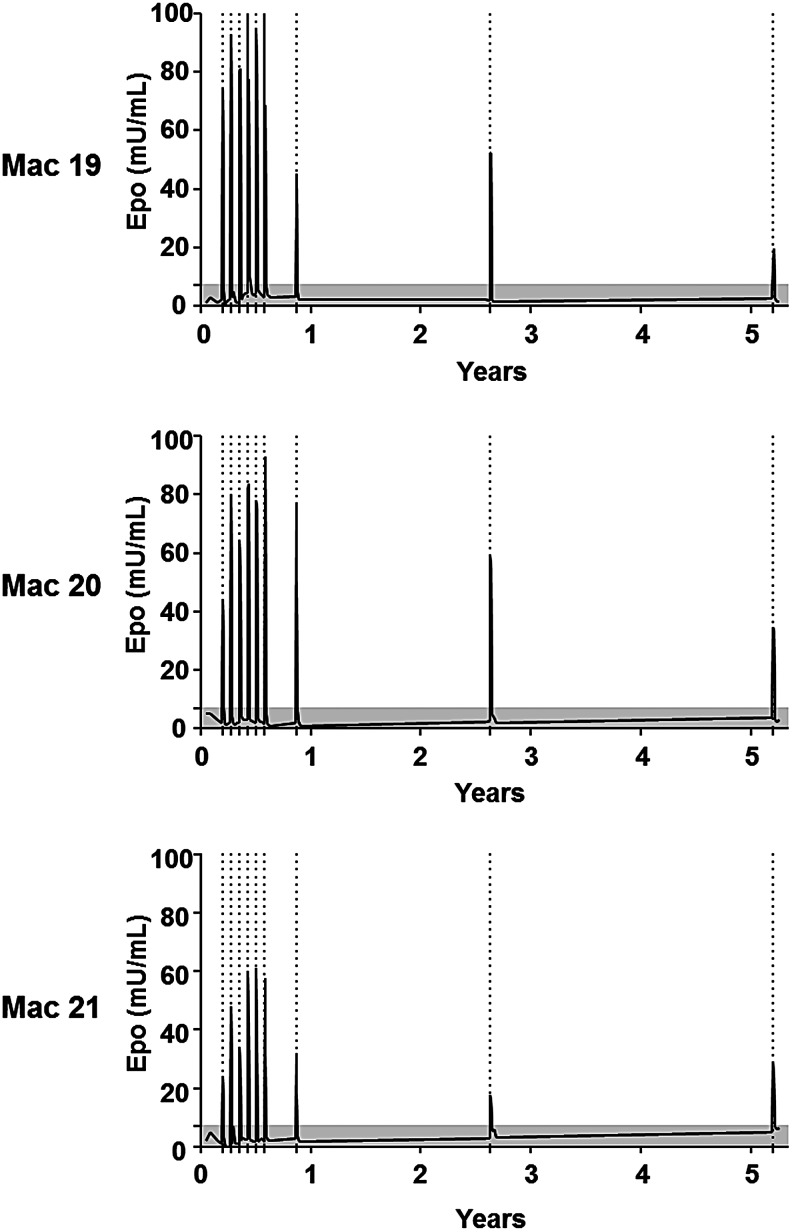
Here, the follow-up of the three LR-perfused animals was extended over a period of 5 years (Table 1 and Fig. 1). Interestingly, all three animals still exhibited a peak of Epo protein secretion in the serum after each doxycycline induction, which was systematically correlated with an increase of the levels of circulating reticulocytes (Supplementary Fig. S1). However, a slight decrease of Epo protein levels in the serum was noticed after the 7th month post perfusion: Epo peak expression was 1.3- to 2.6-fold lower than after the first six inductions. This level then stabilized until the end of the protocol. Nonetheless, despite this decrease, each Epo protein peak was higher than physiologic variations of intrinsic Epo protein (Fig. 1). These results demonstrate for the first time a persistent inducible transgene expression up to 5 years after LR delivery of AAV in NHPs.
Table 1.
Summary of long-term transgene expression and host immunity at 5 years post perfusion
| NHP | Mode of vector delivery | Transgene expression | Anti-AAV1 Ab | Anti-AAV1 NF | Anti-rtTA Ab | rtTA directed IFN-γ cellular response | Muscle infiltrates |
|---|---|---|---|---|---|---|---|
| Mac 19 | LR | Persisting | + | + | – | – | – |
| Mac 20 | LR | Persisting | + | + | – | – | – |
| Mac 21 | LR | Persisting | + | + | – | – | – |
NHP, nonhuman primate; AAV, adeno-associated virus; Ab, antibodies, IFN-γ, interferon gamma.
Figure 1.
Long-term dox-mediated transgene regulation. Long-term follow-up (5 years) of serum erythropoietin (Epo) level in three macaques (Mac 19, Mac 20, and Mac 21) injected using the locoregional (LR) intravenous route of administration.10 Dox administrations are indicated by dotted lines. The gray area illustrates the physiologic basal levels of Epo (established as the mean ± 2 SD of 182 measures of Epo in serum samples obtained from 32 cynomolgus macaques).
Most vector genomes and transgene transcripts are localized in perfused muscles 5 years after locoregional delivery
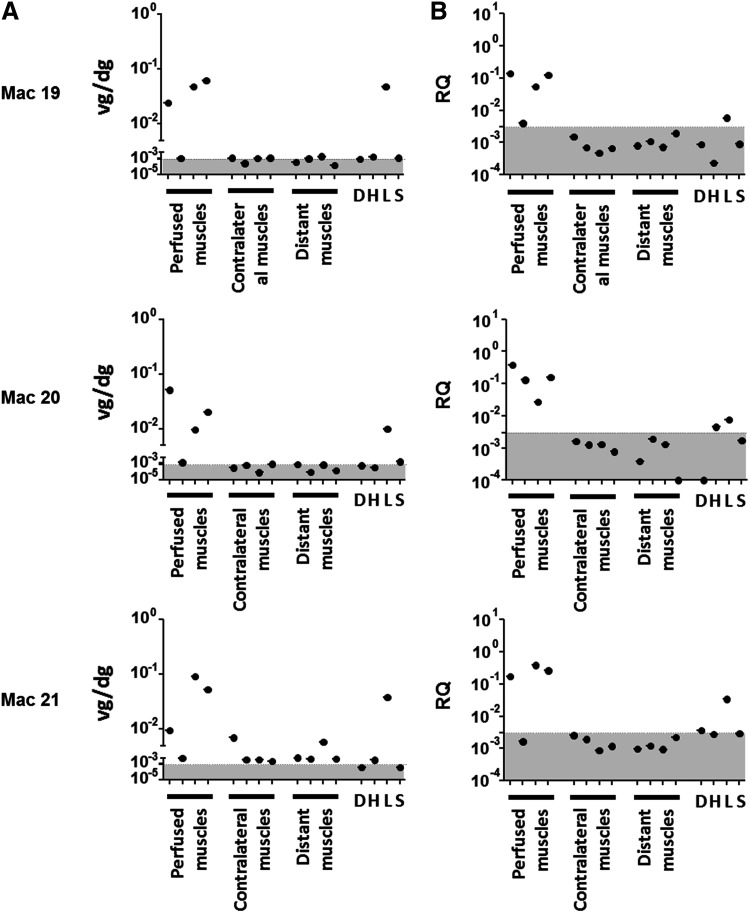
Vector biodistribution was evaluated by measuring viral vector genomes and constitutively expressed rtTA transcripts through qPCR and reverse transcriptase quantitative PCR, respectively. Quantifications were performed in four muscles of the perfused limb (biceps femoris, tibialis anterior, gastrocnemius medialis, and gastrocnemius lateralis), in the same muscles of the contralateral limb, in distant muscles (biceps brachialis and brachioradialis), and in various organs (the diaphragm, heart, liver, and spleen). At 5 years post infusion, most of the vector genomes (up to 0.08 vg/dg) were detected in perfused muscles and the liver (Fig. 2A). Only Mac 21 had detectable, though rare (<0.007 vg/dg), copies of vector genomes in contralateral or distant muscles.
Figure 2.
Detection of viral genomes and transgene transcripts. Tissue samples were collected during necropsy. For each panel, perfused muscles correspond to biceps femoris, tibialis anterior, gastrocnemius medialis, and gastrocnemius lateralis of the injected limb; contralateral muscles correspond to the same muscles of the non-injected limb; and distant muscles correspond to biceps brachialis and brachioradialis of the two anterior limbs. D, H, L, and S indicate the diaphragm, heart, liver, and spleen, respectively. (A) Viral genomes were determined by quantitative polymerase chain reaction PCR (qPCR) and are expressed as viral genome per diploid genome (vg/dg). (B) Transcripts were detected by reverse transcription qPCR (RT-qPCR) and are expressed as relative quantity (RQ) to an endogenous gene. The gray areas correspond to the limit of quantification of PCR detection: 0.000809 vg/dg for (A) and RQ of 0.00304 for (B).
Related to these results, rtTA transcripts were mainly detected in perfused muscles with RQ comprises between 0.0264 and 0.3843. No or few transcripts were detected in other tissues (contralateral and distant muscles, heart, diaphragm, liver, and spleen) with RQ values always below the limit of PCR quantification (Fig. 2B). rtTA transcripts were also detected in the liver of the three NHPs, though to a lesser degree than in perfused muscles (RQ = 0.0058, 0.0076, and 0.0340 for Mac 19, Mac 20, and Mac 21, respectively). Overall, these data indicate that the transgene is essentially found and expressed in the muscles of the perfused limb, with low to no expression at distant sites, except in the liver.
Monitoring humoral and cellular immune responses
A slight decrease in Epo protein secretion was observed 7 months post LR infusion of rAAV2/1 vectors. To investigate whether this could be attributed to a transient immune response targeting transduced cells, anti-transgene humoral and cellular responses were monitored in the periphery. First, the kinetics of serum anti-rtTA humoral immune were investigated using ELISA at 15 days, 3 months, 10 months, 2.5 years, and 5 years post gene transfer. As shown in Tables 1 and 2, no anti-rtTA IgG antibodies could be detected at any time during the protocol. In the same manner, anti-rtTA T-cell responses were assessed at 5 years post injection on PBMCs stimulated with rtTA peptide pools through an IFN-γ ELISpot assay (Table 1 and Fig. 3). No IFN-γ secretion was detected at 5 years post perfusion in contrast to an rtTA-immunized macaque. Overall, these results indicate that LR administration did not induce any detectable anti-transgene immune response, at least not at the time points analyzed in this study.
Table 2.
Kinetic of humoral response (anti-rtTA, anti-AAV1 responses, and anti-AAV1 neutralizing factors
| Before injection | 15 days post injection | 3 months post injection | 10 months post injection | 2.5 years post injection | 5 years post injection | ||
|---|---|---|---|---|---|---|---|
| Mac 19 | Anti-AAV1 Ab | Negative | 1/40 | 1/10,240 | 1/10,240 | 1/5,120 | 1/5,120 |
| Anti-AAV1 NF | Negative | ND | 1/5,000 | ND | ND | 1/1,000 | |
| Anti-rtTA Ab | Negative | Negative | Negative | Negative | Negative | Negative | |
| Mac 20 | Anti-AAV1 Ab | Negative | 1/80 | 1/5,120 | 1/1,280 | 1/320 | 1/80 |
| Anti-AAV1 NF | Negative | ND | 1/1,000 | ND | ND | 1/100 | |
| Anti-rtTA Ab | Negative | Negative | Negative | Negative | Negative | Negative | |
| Mac 21 | Anti-AAV1 Ab | Negative | 1/80 | 1/10,240 | 1/10,240 | 1/5,120 | 1/1,280 |
| Anti-AAV1 NF | Negative | ND | 1/5,000 | ND | ND | 1/1,000 | |
| Anti-rtTA Ab | Negative | Negative | Negative | Negative | Negative | Negative |
ND, not determined; NF, neutralizing factors.
Figure 3.
No detection of anti-rtTA interferon gamma (IFN-γ) T-cell response at 5 years post injection. Peripheral blood mononuclear cells (PBMCs) were collected at euthanasia (5 years post injection), and secretion of IFN-γ by rtTA-stimulated PBMCs was evaluated by enzyme-linked immunospot assay. Cells were stimulated using an overlapping 15 per 10 amino acids peptide library covering the rtTA protein and divided in five pools (p1–p5). Negative controls (C–consisted of PBMCs cultured with medium alone, and positive control (C+) consisted of mitogenic Concanavalin A (Con A) stimulation. IFN-γ was measured as spot-forming cells (SFC). The threshold of positivity (dotted line) consisted of SFC >50 spots and three times the number of SFC obtained with negative control (C–). For positive responses, statistical analysis was performed using a DFR test. ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05. DFR, distribution free resampling.
To complete the assessment of vector immunogenicity, AAV2/1 humoral response was evaluated by determining anti-AAV2/1 IgG antibody titers in the serum using ELISA at various time points over 5 years (Tables 1 and 2). For all three NHPs, serum anti-AAV2/1 antibodies occurred from day 15 post perfusion onward, peaking between 3 and 10 months, with titers ranging from 1/5,120 to 1/10,240. Afterwards, anti-AAV2/1 IgG antibody amounts tended to decrease slowly over time but remained relatively high for two NHPs at 5 years post perfusion (1/5,120 and 1/1,280 for Mac 19 and Mac 21, respectively).
Anti-AAV2/1 IgG antibodies were associated with the induction of anti-AAV2/1 neutralizing factors in the serum. Neutralizing factors were detected from month 3 post perfusion onward, with titers ranging from 1/1,000 to 1/5,000. As for anti-AAV2/1 IgG antibodies, neutralizing factor titers decreased over time but remained relatively high at 5 years post perfusion (1/100 and 1/1,000; Tables 1 and 2). These results indicate that LR administration induced a long-term anti-AAV2/1 humoral response, which tends to decrease gradually over time.
Absence of infiltrating immune cells in muscles 5 years after locoregional delivery
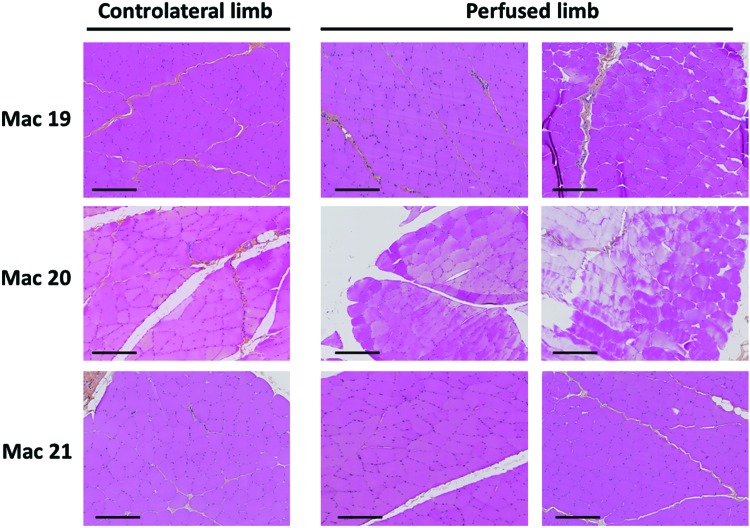
To deepen the analysis of anti-transgene cellular response after LR delivery, the potential infiltration of immune cells was investigated in perfused muscles. HPS staining was performed on four muscle sections from the injected limb (gastrocnemius lateralis, tibialis anterior, gastrocnemius medialis, and biceps femoris) and one muscle section from the contralateral limb (tibialis anterior) obtained at sacrifice 5 years after vector delivery (Table 1 and Fig. 4 for representative sections). No infiltrated immune cells were detected in any of the muscle sections analyzed 5 years post LR infusion.
Figure 4.
No detection of muscle lesions at 5 years post injection. Perfused (tibialis anterior and gastrocnemius lateralis) and contralateral (tibialis anterior) muscles were collected at euthanasia (5 years post injection). Hematoxylin-phloxine-saffron staining was performed on formalin-fixed paraffin-embedded muscle sections. Pictures are representative of the whole section and show no infiltration of immune cells or lesions in perfused muscles. Scale bar = 200 μm. Color images are available online.
Discussion
Gene therapy using rAAV has grown by leaps and bounds over the past decade, culminating with the first EMA-approved product, Glybera, delivered in 2012 to treat lipoprotein lipase deficiency.1,3 Since a second rAAV gene therapy product was approved at the end of 2017 by the Food and Drug Administration for genetic retinal dystrophy.3,4 Preclinical studies in large-animal models have largely contributed to these successful developments, although few have provided insights into the long-term efficiency and stability of rAAV-mediated gene transfer. The highest number of long-term reports (between 2.5 and 6 years post injection) has been provided for gene transfer studies targeting the relatively immune-privileged retina and the central nervous system in NHP and canine models.29–31 With regard to systemic, i.m., or intrahepatic routes, there are now also numerous promising long-term reports (between 2 and 8 years post injection) in NHP32 and canine models for diabetes,33 metabolic diseases,34 hemophilia,35,36 or muscular dystrophies.37,38 Moreover, the field now has the benefit of up to 10 years of hindsight in humans with persisting gene transfer following one single injection.39–42 All these studies highlight the tremendous achievements of rAAV-based gene therapy.
With regard to rAAV-mediated gene transfer to the skeletal muscle, i.m. strategies have shown long-term efficient gene transfer where host effector immunity is not triggered. This has been achieved in large-animal models,8,10,33 reaching 5 and 10 years in Phase I/II clinical trials for alpha-anti-trypsin deficiency43 and hemophilia B,40 respectively. However, the i.m. route generally requires multiple sites of injection to achieve sufficient amounts of gene expression with a wider distribution, which in turn could increase the risk of gene transfer immunogenicity, as described in multiple studies.8,10–13,15,25,44–46 There is clear evidence that the vector dose per i.m. injection site is a major determinant of unwanted immune responses to the transgene, as shown for instance for factor IX (FIX) in hemophilia B dogs.47
The LR mode of delivery has been described as a promising alternative to overcome limited gene transfer efficiency following i.m. vector delivery as well as the associated that may potentially arise immunogenicity towards the transgene product. Indeed, it has been shown that LR-based gene transfer allows persisting gene transfer in larger muscle territories of the perfused limb using NHP or canine models.10,22–24,38,48
The present study followed up three NHPs over what is believed to be the longest follow-up period (a 5-year time course), subsequent to LR perfusion of an AAV vector. Inducible Epo transgene expression over time was measured in serum by ELISA and revealed a systematic peak of expression after each doxycycline induction phase, showing for the first time the ability of rAAV2/1-LR administration to sustain long-term Tet-On-mediated inducible transgene expression in a NHP model, not just for one year but for at least 5 years, in the absence of any immunosuppressive treatment.
Biodistribution analyses at 5 years post perfusion showed that most vector genomes are confined to the perfused muscles and the liver. This confirms previous results wherein most transgene copies were found in the injected limb after LR delivery of rAAV2/1 or rAAV2/8 in NHPs24 and rAAV8 in a golden retriever model of Duchenne muscular dystrophy.23,38 While significant biodistribution of the vector to the liver was initially shown to be a particular feature of serotype 8,49 it is now confirmed that other AAV serotypes, including AAV1, are able to home partially to the liver, even following i.m. injection.24,50
In parallel, rtTA transcript quantification showed that vector copies in perfused muscles are the main source of transgene expression. However, rtTA transcripts were also detected in the liver of the three NHPs. This event parallels the leak of the desmin promoter in the liver initially described by Toromanoff et al. in NHPs10 and could be explained by the transduction of hepatic stellate cells by rAAV2/1 where desmin protein can be expressed.10,51
The long-term follow-up of the animals highlighted that at 7 months post injection, the level of Epo protein secretion in the serum decreased to approximatively half of the initial level for each NHP, and remained stable until the end of the protocol, a phenomenon already observed in previous studies.8,24,52–54 Moderate or partial silencing of the transgene, as described by Suzuki et al. for adenoviral vectors,55 might account for this partial loss. Nonetheless, the decrease of Epo inducible expression in the model did not affect the subsequent rate of reticulocytes in the blood, which remained constant after each doxycycline induction step (Supplementary Fig. S1).
Another possible explanation for the decrease in Epo expression is a partial and transitory immune-mediated destruction of the genetically modified myofibers. To understand this event further, capsid and transgene-directed immune responses were explored. With regard to anti-transgene immune responses, no transgene-directed antibodies could be detected at any time during the protocol, even at early time points. Nonetheless, these results are not in line with previous LR studies in Duchenne and hemophilia canine models where transgene-directed IgG antibodies were detected.22,38,48 However, in these studies, rAAV2 or rAAV8 serotypes were used instead of rAAV1, and vectors were administered at higher doses, ranging from 1E12 to 1E13 vg/kg, instead of 1E11 vg/kg as used in the present study. Interestingly, persisting μ-dystrophin or FIX transgene expression was observed, suggesting a particular humoral immunity. With regard to anti-transgene cellular response, rtTA-directed IFN-γ secreting cells were not detected at 5 years post perfusion. Unfortunately, PBMCs were only collected at only the end of the protocol and not at intermediate time points, as such a transitory anti-transgene cellular immune response cannot be excluded. On the other hand, this result is consistent with the previous LR studies in dogs.22,23,48
With regard to anti-capsid immunity, as expected, circulating IgG antibodies as well as neutralizing factors could be detected in all NHPs during the entire protocol. Neutralizing factors and anti-capsid antibody titers reached high levels during the first year and, despite an important decrease, remained high, even at 5 years post injection. This indicates long-term capsid immunization after rAAV2/1 delivery in line with levels reported by others following i.v. administration of rAAV8 in dogs.32 Importantly, these events seem to have no effect on the persistence of transgene expression, consistent with previous observations in preclinical and clinical trials.13 This study did not check for an anti-capsid cellular response, but the long-term persistence of transgene expression indicates that even if a cellular anti-capsid response did occur, it did not completely abrogate transgene expression.
In addition, no infiltrated cells were detected in the muscle sections at 5 years post infusion, and furthermore, muscles did not show any evidence of past immune cell infiltration (observation of an EMA-certified expert in anatomy and pathology. In contrast to these findings, in situ infiltrated cells have been detected at 4 weeks and 6 months in the muscles of dogs in LR studies by Arruda et al. and Haurigot et al., respectively.22,48 The presence of cell infiltrates does not systematically indicate an effector immune response. Indeed, regulatory T cells (Tregs) and/or exhausted/anergic T cells were shown to play a potential role in the modulation of anti-transgene or capsid immune responses following AAV-mediated gene transfer.43,56–60 The involvement of Tregs in muscle local tolerance was reported in two Phase I/II clinical trials for alpha anti-tryspin and lipoprotein lipase deficiencies and was associated with persisting gene expression.43,57,60 In addition with regards to the transgene product, liver-directed gene transfer was shown to be associated with tolerance61–63 via the induction of specific Tregs.64 However, our observations argue in favor of no cellular infiltration after LR injection or rAAV2/1 in this particular model of rAAV mediated inducible gene transfer.
Overall, the model suggests that LR delivery does not elicit anti-transgene immune rejection with neither humoral nor cellular immunity being provoked. Furthermore, even at relatively low doses of vectors (1E11 vg/kg), in this study, i.m. injection of the same vector from various serotypes induced a transgene-directed immune response in the majority of animals.8,10,24,25,28 This study confirms that the TetR-regulatable system is able to support long-term inducible transgene expression where the immune system is not mobilized against the rtTA transactivator following their i.m. or LR vector administration.
Following LR delivery, no adverse events were detected at either the clinical or biochemical level (Supplementary Table S1), whether at early10,24 or late time points. The results, along with previous studies in large-animal models,21–23,38 confirm the safety of this procedure of injection, which has already been validated for the perfusion of leg muscles in dystrophic patients.65
Past and present studies involving LR administration of rAAV2/1 without immunosuppression demonstrated that the LR mode of delivery allowed a diffused transduction of large muscle territories within the perfused limb, with minor diffusion to other organs (except for the liver). The expression of an inducible transgene product was found stable in the long term, ranging over 5 years, without any detectable deleterious effect of immune responses or toxicity associated with the expression of a foreign and highly immunogenic protein (rtTA transactivator). Whether these observations remain true across various AAV serotypes, transgene products, and high vector doses remains to be investigated. Interestingly and despite the induction of a humoral response against the transgene product, data from other studies have also shown safe and long-term gene transfer following LR delivery.22,38,48 Altogether, this study along with previous reports strongly support that the LR route, similar to the standard i.v. route currently used in clinics, is safe and efficient. We therefore recommend its potential use as an alternative to i.m. injection for muscle delivery, in particular in the context of metabolic diseases requiring the secretion of a therapeutic factor. Moreover, this study further confirms the critical impact of the vector mode of delivery on host immunity.
Supplementary Material
Acknowledgments
The authors thank the Oniris Boisbonne Centre (Nantes, France) for NHPs housing and care, the Center for Production of Vector (CPV—vector core from the University Hospital of Nantes/French Institute of Health [INSERM]/University of Nantes) for providing the rAAV2/1 vector stock, the Cellular and Tissular Imaging Core Facility of University of Nantes (MicroPICell) for HPS staining and allowing us access to the Hamamatsu NanoZoomer slide scanner, and the technical core facility of University Hospital of Nantes for blood reticulocytes titration. The authors also thank Therese Cronin, PhD, for English editing of this manuscript.
The study was supported by the INSERM, the CHU de Nantes, the “Fondation pour la Therapie Genique en Pays de Loire,” the AFM-Telethon (Association Française contre les Myopathies), the National Research Agency (ANR-09-BLAN-0265 GENETOL program), the “Region Pays de La Loire” in the context of IMBIO-DC consortium, and the IHU-Cesti project that received French government financial support managed by the National Research Agency (ANR-10-IBHU-005 program). The IHU-Cesti project is also supported by Nantes Metropole and Region Pays de la Loire.
Author Disclosure
No competing financial interests exist.
Supplementary Material
References
- 1. Büning H. Gene therapy enters the pharma market: the short story of a long journey. EMBO Mol Med 2013;5:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burnett JR, Hooper AJ. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr Opin Mol Ther 2009;11:681–691 [PubMed] [Google Scholar]
- 3. Ginn SL, Amaya AK, Alexander IE, et al. . Gene therapy clinical trials worldwide to 2017: an update. J Gene Med 2018;20:e3015. [DOI] [PubMed] [Google Scholar]
- 4. Voretigene neparvovec-rzyl (Luxturna) for inherited retinal dystrophy. Med Lett Drugs Ther 2018;60:53–55 [PubMed] [Google Scholar]
- 5. Ertl HC, High KA. The impact of AAV capsid-specific T-cell responses on design and outcome of clinical gene transfer trials with recombinant adeno-associated viral vectors: an evolving controversy. Hum Gene Ther 2017;28:328–337 [DOI] [PubMed] [Google Scholar]
- 6. Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 7. Mingozzi F, Meulenberg JJ, Hui DJ, et al. . AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood 2009;114:2077–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Favre D, Blouin V, Provost N, et al. . Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol 2002;76:11605–11611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gernoux G, Guilbaud M, Dubreil L, et al. . Early interaction of adeno-associated virus serotype 8 vector with the host immune system following intramuscular delivery results in weak but detectable lymphocyte and dendritic cell transduction. Hum Gene Ther 2015;26:1–13 [DOI] [PubMed] [Google Scholar]
- 10. Toromanoff A, Adjali O, Larcher T, et al. . Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther J Am Soc Gene Ther 2010;18:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boisgerault F, Mingozzi F. The skeletal muscle environment and its role in immunity and tolerance to AAV vector-mediated gene transfer. Curr Gene Ther 2015;15:381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brantly ML, Chulay JD, Wang L, et al. . Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A 2009;106:16363–16368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flotte TR, Trapnell BC, Humphries M, et al. . Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum Gene Ther 2011;22:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang H, Pierce GF, Ozelo MC, et al. . Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther 2006;14:452–455 [DOI] [PubMed] [Google Scholar]
- 15. Ross CJD, Twisk J, Bakker AC, et al. . Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther 2006;17:487–499 [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, Allen JM, Riddell SR, et al. . Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther 2007;18:18–26 [DOI] [PubMed] [Google Scholar]
- 17. Yuasa K, Yoshimura M, Urasawa N, et al. . Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther 2007;14:1249–1260 [DOI] [PubMed] [Google Scholar]
- 18. Mendell JR, Campbell K, Rodino-Klapac L, et al. . Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 2010;363:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basner-Tschakarjan E, Mingozzi F. Cell-mediated immunity to AAV vectors, evolving concepts and potential solutions. Front Immunol 2014;5:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther J Am Soc Gene Ther 2011;19:16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arruda VR, Stedman HH, Haurigot V, et al. . Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood 2010;115:4678–4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haurigot V, Mingozzi F, Buchlis G, et al. . Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther J Am Soc Gene Ther 2010;18:1318–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Guiner C, Montus M, Servais L, et al. . Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol Ther J Am Soc Gene Ther 2014;22:1923–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toromanoff A, Chérel Y, Guilbaud M, et al. . Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol Ther J Am Soc Gene Ther 2008;16:1291–1299 [DOI] [PubMed] [Google Scholar]
- 25. Chenuaud P, Larcher T, Rabinowitz JE, et al. . Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol Ther J Am Soc Gene Ther 2004;9:410–418 [DOI] [PubMed] [Google Scholar]
- 26. Latta-Mahieu M, Rolland M, Caillet C, et al. . Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum Gene Ther 2002;13:1611–1620 [DOI] [PubMed] [Google Scholar]
- 27. Aurisicchio L, Tomassi AD, Monica NL, et al. . Regulated and liver-specific tamarin alpha interferon gene delivery by a helper-dependent adenoviral vector. J Virol 2005;79:6772–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moreau A, Vandamme C, Segovia M, et al. . Generation and in vivo evaluation of IL10-treated dendritic cells in a nonhuman primate model of AAV-based gene transfer. Mol Ther Methods Clin Dev 2014;1:14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Acland GM, Aguirre GD, Bennett J, et al. . Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther J Am Soc Gene Ther 2005;12:1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bankiewicz KS, Forsayeth J, Eberling JL, et al. . Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther J Am Soc Gene Ther 2006;14:564–570 [DOI] [PubMed] [Google Scholar]
- 31. Stieger K, Schroeder J, Provost N, et al. . Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther J Am Soc Gene Ther 2009;17:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nathwani AC, Rosales C, McIntosh J, et al. . Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther J Am Soc Gene Ther 2011;19:876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaén ML, Vilà L, Elias I, et al. . Long-term efficacy and safety of insulin and glucokinase gene therapy for diabetes: 8-year follow-up in dogs. Mol Ther Methods Clin Dev 2017;6:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee YM, Conlon TJ, Specht A, et al. . Long-term safety and efficacy of AAV gene therapy in the canine model of glycogen storage disease type Ia. J Inherit Metab Dis 2018;1–8 [DOI] [PubMed] [Google Scholar]
- 35. Niemeyer GP, Herzog RW, Mount J, et al. . Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood 2009;113:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sabatino DE, Lange AM, Altynova ES, et al. . Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol Ther J Am Soc Gene Ther 2011;19:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elverman M, Goddard MA, Mack D, et al. . Long-term effects of systemic gene therapy in a canine model of myotubular myopathy. Muscle Nerve 2017;56:943–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Guiner C, Servais L, Montus M, et al. . Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat Commun 2017;8:16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bennett J, Wellman J, Marshall KA, et al. . Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on Phase 1 trial. Lancet 2016;388:661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buchlis G, Podsakoff GM, Radu A, et al. . Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood 2012;119:3038–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le Meur G, Lebranchu P, Billaud F, et al. . Safety and long-term efficacy of AAV4 gene therapy in patients with RPE65 Leber congenital amaurosis. Mol Ther 2018;26:256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marks WJ, Baumann TL, Bartus RT. Long-term safety of patients with Parkinson's disease receiving rAAV2-neurturin (CERE-120) gene transfer. Hum Gene Ther 2015;27:522–527 [DOI] [PubMed] [Google Scholar]
- 43. Mueller C, Gernoux G, Gruntman AM, et al. . 5 Year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol Ther 2017;25:1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chenuaud P, Larcher T, Rabinowitz JE, et al. . Autoimmune anemia in macaques following erythropoietin gene therapy. Blood 2004;103:3303–3304 [DOI] [PubMed] [Google Scholar]
- 45. Gao G, Lebherz C, Weiner DJ, et al. . Erythropoietin gene therapy leads to autoimmune anemia in macaques. Blood 2004;103:3300–3302 [DOI] [PubMed] [Google Scholar]
- 46. Herzog RW, Mount JD, Arruda VR, et al. . Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther 2001;4:192–200 [DOI] [PubMed] [Google Scholar]
- 47. Arruda VR, Schuettrumpf J, Herzog RW, et al. . Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood 2004;103:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arruda VR, Stedman HH, Nichols TC, et al. . Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood 2005;105:3458–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asokan A, Johnson JS, Li C, et al. . Bioluminescent virion shells: New tools for quantitation of AAV vector dynamics in cells and live animals. Gene Ther 2008;15:1618–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rip J, Nierman MC, Sierts JA, et al. . Gene therapy for lipoprotein lipase deficiency: working toward clinical application. Hum Gene Ther 2005;16:1276–1286 [DOI] [PubMed] [Google Scholar]
- 51. Zhang K, Zhang Y-Q, Ai W-B, et al. . Hes1, an important gene for activation of hepatic stellate cells, is regulated by Notch1 and TGF-β/BMP signaling. World J Gastroenterol 2015;21:878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Léger A, Le Guiner C, Nickerson ML, et al. . Adeno-associated viral vector-mediated transgene expression is independent of DNA methylation in primate liver and skeletal muscle. PLoS One 2011;6:e20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Penaud-Budloo M, Le Guiner C, Nowrouzi A, et al. . Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol 2008;82:7875–7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rivera VM, Gao G, Grant RL, et al. . Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 2005;105:1424–1430 [DOI] [PubMed] [Google Scholar]
- 55. Suzuki M, Bertin TK, Rogers GL, et al. . Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs. adeno-associated viral vectors in vivo. Mol Ther J Am Soc Gene Ther 2013;21:796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gernoux G, Wilson JM, Mueller C. Regulatory and exhausted T cell responses to AAV capsid. Hum Gene Ther 2017;28:338–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mueller C, Chulay JD, Trapnell BC, et al. . Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J Clin Invest 2013;123:5310–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cramer ML, Shao G, Rodino-Klapac LR, et al. . Induction of T-cell infiltration and programmed death ligand 2 expression by adeno-associated virus in rhesus macaque skeletal muscle and modulation by prednisone. Hum Gene Ther 2017;28:493–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Velazquez VM, Bowen DG, Walker CM. Silencing of T lymphocytes by antigen-driven programmed death in recombinant adeno-associated virus vector–mediated gene therapy. Blood 2009;113:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ferreira V, Twisk J, Kwikkers K, et al. . Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPLS447X) in a Phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther 2013;25:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, et al. . Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 2007;110:2334–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mingozzi F, Liu Y-L, Dobrzynski E, et al. . Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 2003;111:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mount JD, Herzog RW, Tillson DM, et al. . Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood 2002;99:2670–2676 [DOI] [PubMed] [Google Scholar]
- 64. Dobrzynski E, Mingozzi F, Liu Y-L, et al. . Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood 2004;104:969–977 [DOI] [PubMed] [Google Scholar]
- 65. Fan Z, Kocis K, Valley R, et al. . Safety and feasibility of high-pressure transvenous limb perfusion with 0.9% saline in human muscular dystrophy. Mol Ther J Am Soc Gene Ther 2012;20:456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.