Abstract
Gene therapy for Pompe disease with adeno-associated virus (AAV) vectors has advanced into early phase clinical trials; however, the paucity of cation-independent mannose-6-phosphate receptor (CI-MPR) in skeletal muscle, where it is needed to take up acid α-glucosidase (GAA), has impeded the efficacy of Pompe disease gene therapy. Long-acting selective β2 receptor agonists previously enhanced the CI-MPR expression in muscle. In this study we have evaluated the selective β2 agonist salmeterol in GAA knockout mice in combination with an AAV vector expressing human GAA specifically in the liver. Quadriceps glycogen content was significantly decreased by administration of the AAV vector with salmeterol, in comparison with the AAV vector alone (p < 0.01). Importantly, glycogen content of the quadriceps was reduced to its lowest level by the combination of AAV vector and salmeterol administration. Rotarod testing revealed significant improvement following treatment, in comparison with untreated mice, and salmeterol improved wirehang performance. Salmeterol treatment decreased abnormalities of autophagy in the quadriceps, as shown be lower LC3 and p62. Vector administration reduced the abnormal vacuolization and accumulation of nuclei in skeletal muscle. Thus, salmeterol could be further developed as adjunctive therapy to improve the efficacy of liver depot gene therapy for Pompe disease.
Keywords: Pompe disease, glycogen storage disease, adeno-associated virus vector, mannose-6-phosphate receptor, beta2 agonist
Introduction
Pompe disease (glycogen storage disease type 2, or acid maltase deficiency, MIM 232300) is caused by the deficiency of acid α-glucosidase (GAA, or acid maltase,EC 3.2.1.20) and lysosomal glycogen accumulation in muscle.1 Clinical effects include muscle weakness in all patients, and cardiomyopathy accompanied by severe hypotonia in infantile-onset Pompe disease.1 Enzyme replacement therapy (ERT) with recombinant human (rh) GAA has prolonged the survival of patients.2 However, residual muscle weakness (neck flexor weakness, dorsiflexor weakness, myopathic facies, ptosis, and strabismus) and respiratory insufficiency has been observed in multiple patients treated with ERT.3–5 Thus, ERT has not completely reversed the neuromuscular involvement of Pompe disease.
The cation-independent mannose-6-phosphate receptor (CI-MPR) is expressed at low levels in skeletal muscle, where it is needed for GAA uptake during ERT in Pompe disease.6,7 Adjunctive therapy has been developed to address this problem.8,9 We demonstrated that increased CI-MPR expression improved efficacy from ERT in GAA knockout (KO) mice, confirming the relevance of CI-MPR expression upon GAA replacement therapy in Pompe disease.9 Using GAA-KO mice, we showed that clenbuterol, a selective β2 receptor agonist, enhanced the CI-MPR expression in muscle tissues, and increased the efficacy of either ERT or gene therapy in murine Pompe disease.8–10 The underlying mechanism of clenbuterol's therapeutic action is insulin-like growth factor 1 (IGF-1) mediated muscle hypertrophy, which correlated with increased CI-MPR (also the Igf-2 receptor) expression.11 Another β2 agonist, salmeterol, increased the efficacy of an adeno-associated virus (AAV) vector expressing GAA from a ubiquitous promoter by increasing muscle strength of GAA-KO mice, although it only improved biochemical correction in the heart.12
Previous studies revealed the importance of immune tolerance induction during gene therapy in mice with Pompe disease,13–15 which has been achieved by liver-specific expression of GAA with an AAV vector as described.16–18 In contrast, constitutive expression with a ubiquitous promoter provoked immune responses in GAA-KO mice16 that might complicate gene therapy in cross-reactive immune material–negative patients with Pompe disease, who do not express any residual GAA protein.19 Liver-specific expression with an AAV vector to suppress immune responses against GAA might have advantages over other approaches involving immunosuppressive drugs that have side effects, including the risk of general immune suppression, despite the clinical efficacy of such agents in the context of ERT.20
In this study we have evaluated salmeterol in combination with an adeno-associated virus (AAV) vector expressing human GAA specifically in the liver in GAA-KO mice. The skeletal muscle response to therapy was evaluated through biochemical testing and through muscle function testing.
Materials and Methods
In vivo evaluation of AAV vector-mediated efficacy
The AAV vector was prepared as described and administered intravenously to 4 month-old GAA-KO mice with a C57BL/6 background.12,21 The liver-specific regulatory cassette in the vector contains a thyroid hormone-binding globulin promoter sequence downstream from 2 copies of a α1-microglobulin/bikunin enhancer sequence.16 Rotarod testing was performed as described.22 Wirehang testing was performed with a 0.5 cm mesh hardware cloth fixed to an 8 inch by 10 inch frame. Mice were placed on the wire mesh, which was slowly inverted 6 inches over a cage containing paper bedding. The latency, or time until the mouse fell off of the wire mesh, was recorded, and reported as holding impulse (latency recorded in seconds multiplied by body weight in grams) takes into account the generally shorter latency of heavier mice.23 GAA activity and glycogen content were analyzed as described.22 All animal procedures were done in accordance with Duke University Institutional Animal Care and Use Committee–approved guidelines.
Western blotting
Western blots on skeletal muscle was performed to quantify CI-MPR, GAA, ubiquitin-binding protein p62 (p62), and microtubulin-associated protein 1A/1B light chain 3 (LC3). Whole protein was prepared by tissue homogenization with glycerol NP-40 lysis buffer (50 mM Hepes, 0.5% NP-40, 250 mM NaCl, 10% glycerol, and 2mM EDTA) followed by microcentrifugation for 20 min at 14,000 rpm. The total amount of protein was determined by the PierceTM BCA protein assay kit (Thermo Fisher Scientific, Inc., Waltham, MA). The desired quantity of protein was mixed with SDS loading buffer followed by boiling for 5 min. Samples were electrophoresed in a 12% SDS-PAGE gel and immediately transferred to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) using transfer buffer, consisting of 3.03 g/L Tris, 14.4 g/L glycine, and 7.5% v/v methanol. After transfer the membrane was blocked in 5% skim milk in wash buffer (phosphate-buffered saline with 0.5% tween-20; PBST) to block from nonspecific binding of antibodies. The membrane was incubated overnight at 4°C with primary antibodies, anti-CI-MPR antibody, anti-GAA antibody, anti-p62 antibody, and anti-LC3 antibody from Abcam (Abcam, Cambridge, MA), and anti-GAPDH antibody (Santa Cruz, Biotechnology, Inc., Dallas, TX). After three washes with PBST buffer, the primary antibody bounded membrane was incubated with horseradish peroxidase–conjugated secondary antibodies that were chosen by the species of the primary antibody (5% w/v bovine serum albumin in PBST for 1 h at room temperature). Following three washes with PBST buffer, blots were developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc., Waltham, MA), and the images were captured with Bio-Rad imager, ChemiDocTM Imaging system (Bio-Rad Laboratories, Hercules, CA). The intensities of image signals were quantified with the software of Image LabTM software from Bio-Rad. Finally, the intensity for signals for CI-MPR, GAA, p62 and LC3 were normalized to the signal of housekeeping gene GAPDH.
Animal Studies
All animal procedures were performed under the guide lines of Duke University Institutional Animal Care and Use Committee. Four-month old GAA-KO mice24 were weighed and underwent Rotarod and wirehang testing on day 0. The GAA expression vector, AAV2/8-LSPhGAA.16 was administered through the tail vein on day 1 [1.0 × 1011 vector genomes (vg)/mouse]. Salmeterol was continuously provided from day 1 through week 18 (30 mg/L) as described to achieve muscle hypertrophy.25 Mice were weighed and underwent Rotarod and wirehang testing on day 0, week 6, week 12, and week 18. The mice were euthanized 18 weeks following AAV vector injection for collection of tissues. The collected tissues were stored at −80°C until use.
Muscle histology
Quadriceps muscle was collected from mice and immersion fixed in 10% neutral buffered formalin for 48 hours, embedded in paraffin, microtomed at 5 μm, and stained with hematoxylin and eosin. Slides were scored by a pathologist (J.E.) semiquantitatively without knowledge of treatment group allocation. A vacuolar score was assigned by multiplying a 0–4 severity × 0–4 area involved. A separate scoring of nuclear number was assigned based on 0–4 [negative (wt control), minimal (0–10% increase), mild (10–25% increase), moderate (25–50% increase) and marked (>50% increase)] in five random fields counted at 400 × .
Statistical analysis
Bar graphs in all figures were generated from gathered data with mean with standard error using GraphPad Prism5 (GraphPad Software, Inc). Multiple comparisons were performed using two-way ANOVA with Bonferroni post-hoc test.
Results
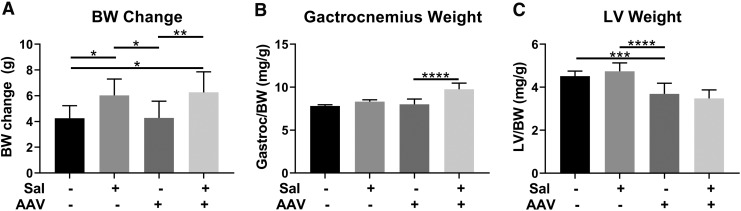
In order to gain understanding of effects of salmeterol administration upon liver depot gene therapy in Pompe disease, we evaluated the effects of oral salmeterol in GAA-KO mice injected with AAV2/8-LSPhGAA [1 × 1011 vector vg]. Salmeterol was administered continuously in drinking water starting on day 1, either with or without concurrent AAV vector administration. Body weight was increased by the administration of salmeterol, either alone or in combination with the AAV vector, in comparison with vector treatment alone (Fig. 1A). Muscle hypertrophy was demonstrated by increased weight of the gastrocnemius following salmeterol and AAV vector administration, in comparison with vector alone, suggesting that muscle benefits were greatest from the combination therapy (Fig. 1B). Finally, the left ventricle weight was significantly decreased by AAV vector administration in comparison with salmeterol alone, indicating that salmeterol was not a stand-alone treatment for heart involvement (Fig. 1C). These data suggested that salmeterol had a beneficial effect upon the effects of liver depot therapy in Pompe disease.
Figure 1.
Skeletal muscle hypertrophy and cardiomyopathy reduction following adeno-associated virus (AAV) vector and salmeterol administration. The effect of each treatment upon the change in body weight (BW) (A), gastrocnemius weight (B), and left ventricle weight/body weight (C) was evaluated. Groups were as follows: untreated controls (4 males, 4 females); salmeterol only (5 males, 5 females); AAV only (5 males, 5 females); and AAV + salmeterol (5 males, 5 females). Mean ± standard deviation shown. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 for ANOVA with Dunnett test; AAV and untreated groups were used as controls.
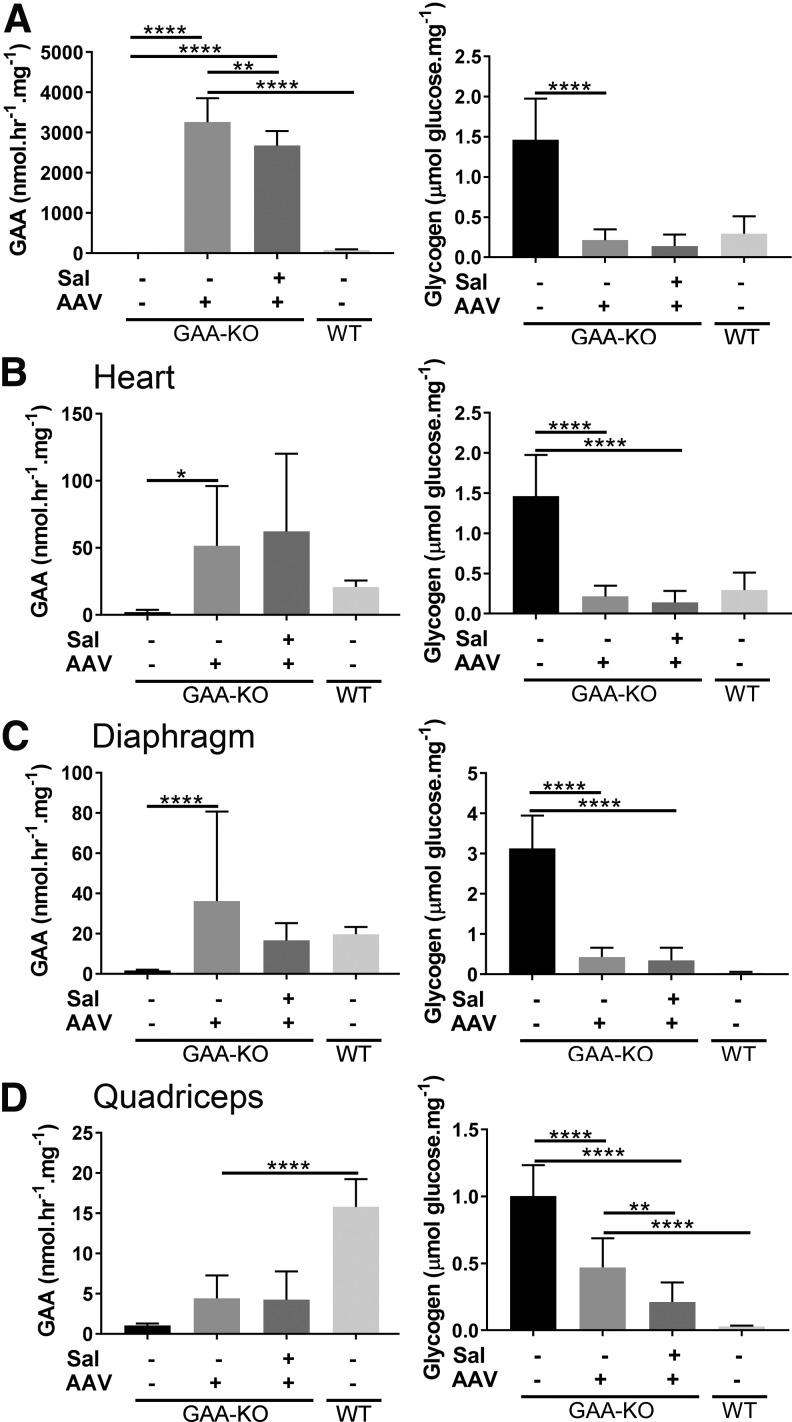
The biochemical correction of the liver and striated muscles was evaluated by analyzing GAA and glycogen content. Liver GAA activity was markedly increased by AAV vector administration, with or without salmeterol administration, in association with markedly decreased glycogen content (Fig. 2A). Similarly, heart GAA activity was markedly increased by AAV vector administration, with or without salmeterol administration, in association with >100-fold decreased glycogen content (Fig. 2B). Increased GAA activity was demonstrated in liver from salmeterol administration alone, which remains unexplained and did not significantly reduce glycogen content in liver (Supplementary Fig. S1). Glycogen content was not further reduced by the addition of salmeterol following vector administration in the liver (Fig. 2A) or in the heart (Fig. 2B). Salmeterol alone did not achieve biochemical correction in the liver or heart (Supplementary Fig. S1). Thus, the impact of salmeterol administration was minimal in liver and heart, either in combination with liver depot gene therapy or by itself.
Figure 2.
Biochemical correction of liver and heart following AAV vector and salmeterol administration. Acid α-glucosidase knockout (GAA-KO) mice were treated for AAV2/8-LSPhGAApA (AAV) either alone or in combination with salmeterol (Sal). Groups were as follows: untreated controls (GAA-KO mice: 4 males, 4 females); AAV only (GAA-KO mice: 5 males, 5 females); AAV+salmeterol (GAA-KO mice: 5 males, 5 females), and wildtype (WT; 5 males, 5 females). GAA activity and glycogen content for liver (A), heart (B), diaphragm (C), and quadriceps (D). Mean ± standard deviation is shown. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 for ANOVA with Dunnett test; AAV and untreated groups were used as controls.
The GAA activity of the diaphragm was significantly increased following vector administration, and adding salmeterol did not increase GAA further; furthermore, the glycogen content of the diaphragm was markedly reduced following vector administration and the addition of salmeterol had no additional effect upon glycogen content in the diaphragm (Fig. 2C). Similarly, the GAA activity of quadriceps was significantly increased by vector administration, and salmeterol did not increase GAA activity further (Fig. 2D). The glycogen content of the diaphragm was uniquely decreased by 50% following salmeterol treatment alone, which was not observed in other muscles (Supplementary Fig. S1).
The glycogen content of quadriceps was uniquely decreased by salmeterol administration in combination with AAV vector administration (Fig. 2D). The glycogen content of the gastrocnemius was significantly decreased following vector administration, but adding salmeterol did not further decrease glycogen content (Supplementary Fig. S2). Among the skeletal muscles, decreased glycogen content was observed following salmeterol treatment by itself only in the diaphragm (Supplementary Fig. S1). Thus, the addition of salmeterol during liver depot gene therapy decreased the glycogen content of the quadriceps to the lowest quantity observed in any group, which was a unique response among the muscles analyzed.
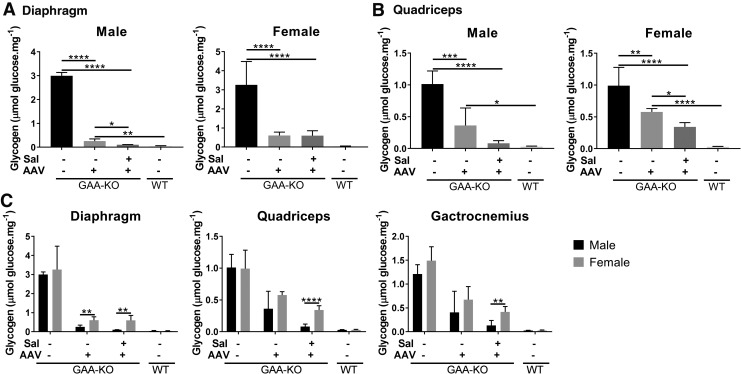
The effect of gender was evaluated, because male mice have responded more efficaciously to AAV vector–mediated gene therapy than female mice.13,22,26 GAA activity was not significantly different in muscle between male and female groups of mice (not shown). However, glycogen content is a more sensitive measure of biochemical correction in Pompe disease,27 which did reveal differences in the response to treatment with salmeterol and gene therapy. Male mice demonstrated lower glycogen content from combination treatment in the diaphragm, in comparison with gene therapy alone (Fig. 3A). While the mean glycogen content in the quadriceps was lower in male mice treated with salmeterol and gene therapy, in comparison with vector alone, this difference did not achieve statistical significance (Fig. 3B). In contrast, glycogen content in the quadriceps was significantly lower in the female mice treated with combination therapy, in comparison with gene therapy alone (Fig. 3B). Quantitative PCR revealed that transduction of the liver was consistently more efficient for male mice, in comparison with female mice (Supplementary Fig. S3), consistent with prior experiments.22 Additionally, male mice had significantly decreased glycogen content in every muscle examined following combination therapy, in comparison with female mice (Fig. 3C). Thus, male gender consistently improved the response to treatment with salmeterol plus liver gene therapy.
Figure 3.
Effect of gender on biochemical correction of skeletal muscle. Glycogen content for diaphragm (A) and quadriceps (B). Mean ± SEM is shown. Groups were as follows: untreated controls (GAA-KO mice: 4 males, 4 females); AAV only (GAA-KO mice: 5 males, 5 females); AAV+salmeterol (GAA-KO mice: 5 males, 5 females), and wildtype (WT; 5 males, 5 females). Two-way ANOVA with Bonferroni post-hoc analysis was performed; AAV and untreated groups were used as controls. Comparisons of male and female data are shown (C). Repeated t-tests were performed; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Mean ± standard deviation is shown.
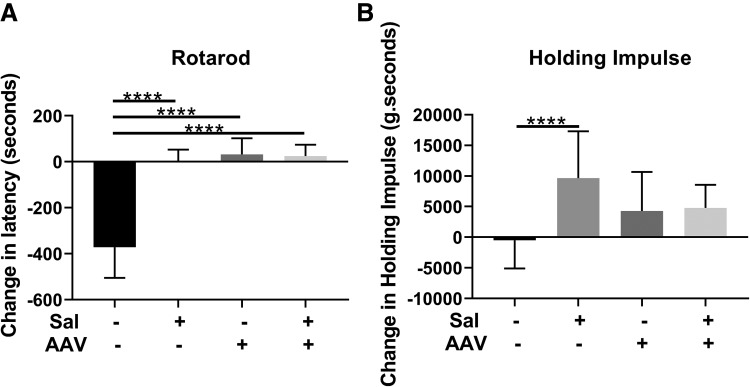
Functional testing consisting of the Rotarod test at 18 weeks following vector administration revealed that significantly increased latency in comparison with the group of untreated GAA-KO mice (Fig. 4A). Other treatment groups had a similar improvement in Rotarod performance indicating that salmeterol alone was as effective as gene therapy or gene therapy with salmeterol at improving neuromuscular function. Significant improvement in wirehang performance was observed following salmeterol treatment (Fig. 4B), indicating a beneficial effect upon muscle strength.
Figure 4.
Muscle function testing. Latency change and absolute latency in Rotarod (A) and wirehang (B) testing for each treatment group. Groups were as follows: untreated controls (4 males, 4 females), salmeterol only (5 males, 5 females), AAV only (5 males, 5 females), and AAV+salmeterol (5 males, 5 females). Mean ± standard deviation is shown. ANOVA with Dunnett test was performed and the untreated group was used as the control; ****p < 0.0001.
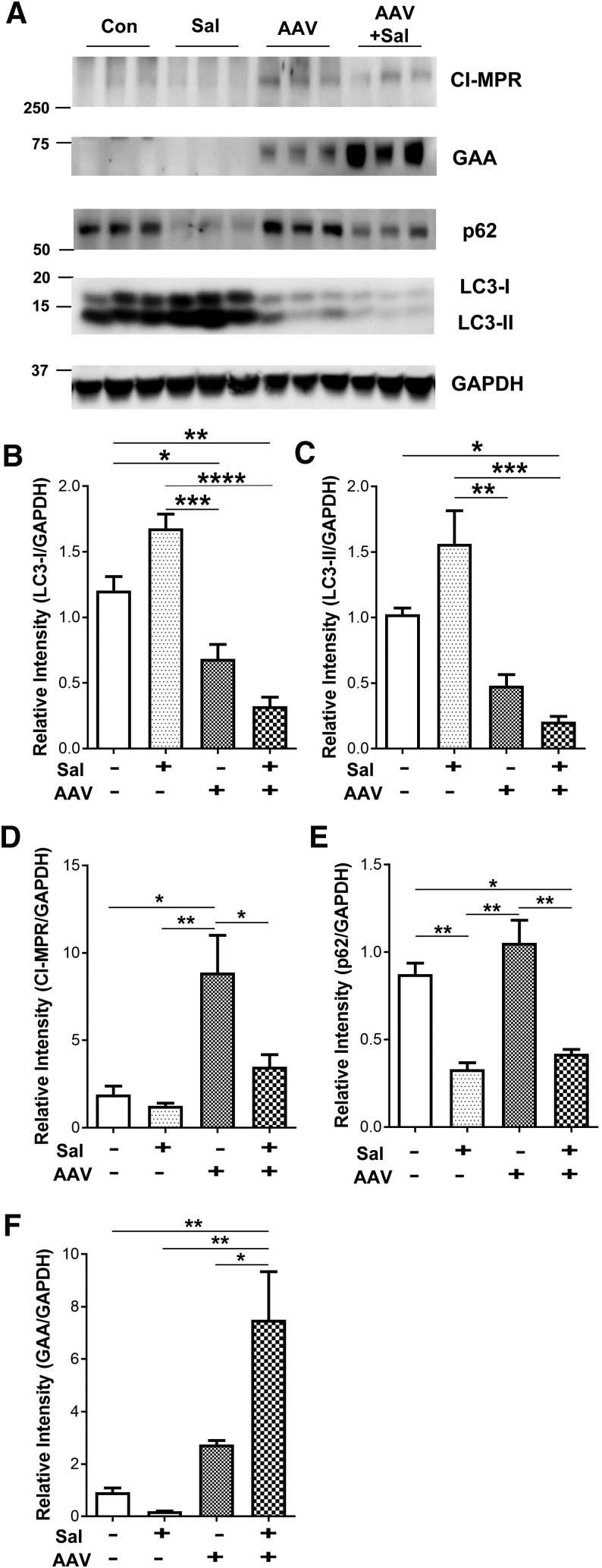
We next investigated abnormalities associated with Pompe disease in the quadriceps to better understand the benefits of salmeterol treatment. Autophagic accumulations previously described in the muscle of GAA-KO mice28 were analyzed by quantification of LC3 by Western blotting (Fig. 5A). The abnormally increased LC3 was significantly decreased in the quadriceps by the administration of vector accompanied by salmeterol or vector alone, in comparison with no treatment or with salmeterol alone (Fig. 5B and C). Lower LC3 is consistent with reversal of abnormally accumulated autophagosomes previously described in GAA-KO mice.28,29 We further investigated the expression of CI-MPR, which might increase the receptor-mediated uptake of GAA. CI-MPR was significantly increased by AAV vector administration, in comparison with the other three conditions at 18 weeks (Fig. 5D). This observation was consistent with previous studies showing that AAV vector–mediated expression of GAA increased CI-MPR expression in skeletal muscle without concurrent β2 agonist administration.12,14 We further analyzed a marker for autophagosome clearance p62, which is increased in the muscle of Pompe patients and normalized by treatment with ERT.30 Salmeterol treatment significantly decreased p62 in the quadriceps, either alone or in combination with AAV vector administration (Fig. 5E). Finally, the addition of salmeterol significantly increased the presence of mature GAA following vector administration (Fig. 5F). Overall, the presence of decreased LC3 and p62 along with increased GAA demonstrated a benefit from salmeterol administration.
Figure 5.
Effect of AAV vector and salmeterol administration upon autophagy in skeletal muscle. Western blotting and quantification for LC3-II, CI-MPR, p62, and GAA in quadriceps (A). LC3-I (B), LC3-II (C). Cation-independent mannose-6-phosphate receptor (CI-MPR) (D), p62 (E), and GAA (F) were quantified. The signals were normalized to GAPDH. Mean ± standard deviation is shown. ANOVA with Bonferroni post-hoc analysis for multiple comparisons between all groups; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
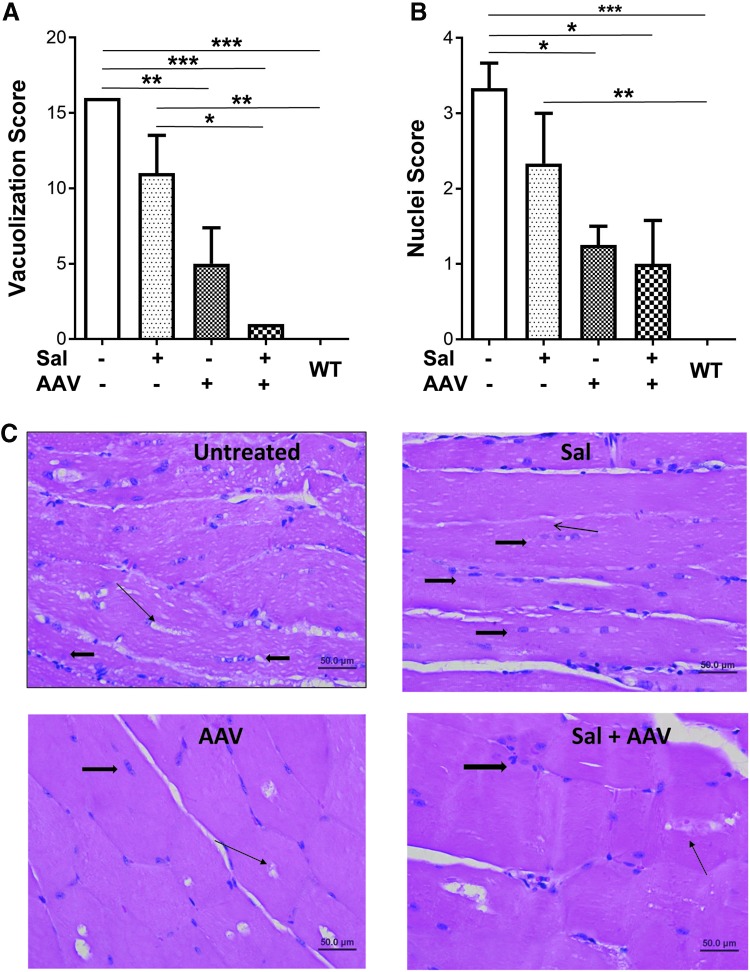
Pompe disease causes abnormal accumulations of glycogen-filled lysosomes and nuclei in skeletal muscle.31 Histology revealed that vector treatment reduced both vacuolization and accumulated nuclei in the muscle fibers (Fig. 6). These features were quantified, demonstrating significantly decreased vacuolization (Fig. 6A) and nuclei (Fig. 6B) scores in the quadriceps, in comparison with untreated or salmeterol treated mice that were not treated with liver depot gene therapy.
Figure 6.
Vector administration decreased accumulated lysosomes and nuclei. Histology revealed that abnormalities were lower following vector administration in GAA-KO mice. Vacuolization score (A) and nuclei score (B) are shown for each treatment group. Number of mice analyzed: no treatment (3 males), vector (4 males), salmeterol (3 males), salmeterol + vector (3 males), and wildtype (4 males). Mean ± standard deviation is shown. *p < 0.05, **p < 0.01, and ***p < 0.001 for ANOVA with Bonferroni post-hoc analysis for multiple comparisons between all groups. (C) Representative photomicrographs of H&E sections of quadriceps muscle from each treatment group at 400 × showing the degree of vacuolar change within myofibers (thin arrows). Thick arrows depict nuclear profiles.
Discussion
Salmeterol, a long-acting selective β2 agonist, was evaluated in combination with liver depot gene therapy in GAA-KO mice. Consistent with the ability β2 agonists to promote muscle hypertrophy, gastrocnemius and body weights were increased following salmeterol administration. The glycogen content of the quadriceps was decreased to the greatest extent following salmeterol administration to vector-injected GAA-KO mice, demonstrating the most effective biochemical correction by adding salmeterol during liver depot gene therapy. The reduction of glycogen content in skeletal muscle has previously been observed, following the addition of an adjunctive β2 agonist during gene therapy.10 Furthermore, glycogen content in the diaphragm was slightly reduced by treatment with salmeterol alone as described following clenbuterol administration.10 Functional testing with the Rotarod revealed that salmeterol treatment increased performance similarly to the administration of gene therapy. Salmeterol treatment significantly decreased the abnormalities of autophagy in muscle that are associated with Pompe disease, and the combination of salmeterol with gene therapy markedly decreased the vacuolization of muscle fibers.
The adjunctive therapy with β2 agonists is under development in Pompe disease.32,33 Salmeterol represents another option for this strategy that could be especially beneficial in the context of liver depot gene therapy. Importantly, an inhaled formulation of salmeterol has been approved for clinical use, and oral administration might be easily developed for the treatment of Pompe disease.34 A recent clinical trial revealed safety and efficacy for clenbuterol treatment in patients who were stably treated with ERT, suggesting that β2 agonists might be effective as adjunctive therapy in Pompe disease.32
Salmeterol was chosen for evaluation with liver depot gene therapy in GAA-KO mice, based upon prior evidence that β2 agonists were beneficial during GAA replacement in Pompe disease.8–10,33,35 Previously, salmeterol was evaluated with an AAV vector that expressed GAA ubiquitously with a chicken β-actin promoter and cytomegalovirus enhancer, and only the cardiac response was improved by salmeterol administration.12 That earlier study failed to demonstrate biochemical correction of skeletal muscle. The correction of skeletal muscle was currently achieved by combining salmeterol and liver depot gene therapy with an AAV vector that induced immune tolerance and continuously secreted GAA into the circulating blood, thereby achieving partial biochemical correction of skeletal muscle.27 In that prior study of salmeterol the vector ubiquitously expressing GAA did not correct skeletal muscle,12 despite the utilization of immune tolerant GAA-KO mice that express trace quantities of GAA only in liver.36 The improved biochemical correction of skeletal muscle from salmeterol administration could imply earlier upregulation of CI-MPR to increase the uptake of GAA from the blood during liver depot gene therapy, although we did not demonstrate increased CI-MPR following salmeterol treatment at the end of the current study. CI-MPR expression was not increased at the end of the study; however, this observation does not preclude an earlier increase in CI-MPR as demonstrated in short-term studies.8,9 The improved biochemical correction from salmeterol treatment during gene therapy correlated with a statistically significant reduction in LC3-II and p62, indicating that the abnormal accumulation of autophagosomes observed in Pompe disease was reduced by the addition of salmeterol. Another β2 agonist, formoterol, decreased p62 in the muscle of a rodent model for cancer-cachexia,37 very similar to the effect of salmeterol observed in the current study. Overall, the reduction in accumulated autophagosomes correlated with decreased accumulations of glycogen-filled lysosomes and nuclei in skeletal muscle, further demonstrating correction of the muscle abnormalities of Pompe disease in GAA-KO mice.31
Limitations of this study included the small group size and lack of evaluation of involvement of the nervous system or respiratory abnormalities, although significant effects were demonstrated upon muscle hypertrophy, biochemical correction, muscle function, and the accumulation of autophagosomes. The current study did not demonstrate elevated CI-MPR in skeletal muscle at 18 weeks similar to our previous study,12 which does not exclude an increased CI-MPR expression at an earlier time during β2 agonist treatment as previously observed.8,9 We detected increased GAA protein in skeletal muscle following treatment with salmeterol, which suggested that the uptake of GAA was increased by β2 agonist administration. However, the GAA activity was not increased by salmeterol treatment. Finally, glycogen content in the quadriceps was significantly decreased by salmeterol treatment with gene therapy, suggesting that biochemical correction in Pompe disease could be improved by β2 agonist therapy. A recent study of liver depot gene therapy demonstrated that adjunctive therapy might not be needed to achieve systemic correction of GAA deficiency, if GAA is secreted more efficiently from the liver.38 However, that study does not preclude that additional benefits could be achieved by adjunctive β2-agonist therapy in the context of highly secreted GAA from a liver depot.
This study demonstrated the enhancement of efficacy in skeletal muscle from an adjunctive therapy that improved the response to liver depot gene therapy. These data and the current marketing approval justify further development of salmeterol as an adjunctive therapy in combination with gene therapy for Pompe disease.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grant R01AR065873 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. GAA-KO mice were provided courtesy of Dr. Nina Raben at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Author Disclosure
D.D.K. and Duke University might benefit financially if the experimental treatments discussed here prove effective and are successful commercially. D.D.K has equity in Actus Therapeutics, which is developing gene therapy for Pompe disease. S.H., S.L., and J.I.E. have no competing financial interests.
Supplementary Material
References
- 1. Hirschhorn R, Reuser AJJ. Glyogen storage disease type II: Acid α-glucosidase (acid maltase) deficiency: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Basis for Inherited Disease, 8th ed. New York: Mcgraw-Hill, 2001:3389–3419 [Google Scholar]
- 2. Kishnani PS, Corzo D, Nicolino M, et al. . Recombinant human acid {alpha}-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology 2007;68:99–109 [DOI] [PubMed] [Google Scholar]
- 3. Nicolino M, Byrne B, Wraith JE, et al. . Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med 2009;11:210–219 [DOI] [PubMed] [Google Scholar]
- 4. Jones HN, Muller CW, Lin M, et al. . Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia 2010;25:277–283 [DOI] [PubMed] [Google Scholar]
- 5. Yanovitch TL, Banugaria SG, Proia AD, Kishnani PS. Clinical and histologic ocular findings in pompe disease. J Pediatr Ophthalmol Strabismus 2010;47:34–40 [DOI] [PubMed] [Google Scholar]
- 6. Raben N, Danon M, Gilbert AL, et al. . Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab 2003;80:159–169 [DOI] [PubMed] [Google Scholar]
- 7. Raben N, Fukuda T, Gilbert AL, et al. . Replacing acid alpha-glucosidase in Pompe disease: Recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol Ther 2005;11:48–56 [DOI] [PubMed] [Google Scholar]
- 8. Koeberl DD, Luo X, Sun B, et al. . Enhanced efficacy of enzyme replacement therapy in Pompe disease through mannose-6-phosphate receptor expression in skeletal muscle. Mol Genet Metab 2011;103:107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koeberl DD, Li S, Dai J, Thurberg BL, Bali D, Kishnani PS. Beta2 Agonists enhance the efficacy of simultaneous enzyme replacement therapy in murine Pompe disease. Mol Genet Metab 2012;105:221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li S, Sun B, Nilsson MI, et al. . Adjunctive beta2-agonists reverse neuromuscular involvement in murine Pompe disease. FASEB J 2013;27:34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsumoto T, Akutsu S, Wakana N, Morito M, Shimada A, Yamane A. The expressions of insulin-like growth factors, their receptors, and binding proteins are related to the mechanism regulating masseter muscle mass in the rat. Arch Oral Biol 2006;51:603–611 [DOI] [PubMed] [Google Scholar]
- 12. Han SO, Li S, Koeberl DD. Salmeterol enhances the cardiac response to gene therapy in Pompe disease. Mol Genet Metab 2016;118:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han SO, Li S, Brooks ED, et al. . Enhanced efficacy from gene therapy in Pompe disease using coreceptor blockade. Hum Gene Ther 2015;26:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han SO, Li S, Bird A, Koeberl D. Synergistic efficacy from gene therapy with coreceptor blockade and a beta2-agonist in murine Pompe disease. Hum Gene Ther 2015;26:743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doerfler PA, Todd AG, Clement N, et al. . Copackaged AAV9 Vectors promote simultaneous immune tolerance and phenotypic correction of Pompe disease. Hum Gene Ther 2016;27:43–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franco LM, Sun B, Yang X, et al. . Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 2005;12:876–884 [DOI] [PubMed] [Google Scholar]
- 17. Sun B, Kulis MD, Young SP, et al. . Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol Ther 2010;18:353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang P, Sun B, Osada T, et al. . Immunodominant, liver-specific expression suppresses transgene-directed immune responses in murine Pompe disease. Hum Gene Ther 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kishnani PS, Goldenberg PC, DeArmey SL, et al. . Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010;99:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banugaria SG, Prater SN, Ng YK, et al. . The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med 2011;13:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang G, Young SP, Bali D, et al. . Assessment of toxicity and biodistribution of recombinant AAV8 vector-mediated immunomodulatory gene therapy in mice with Pompe disease. Mol Ther Methods Clin Dev 2014;1:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun B, Zhang H, Franco LM, et al. . Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol.Ther. 2005;11:57–65 [DOI] [PubMed] [Google Scholar]
- 23. Hoffman E, Winder SJ. A modified wire hanging apparatus for small animal muscle function testing. Plos Curr 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raben N, Nagaraju K, Lee E, et al. . Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem. 1998;273:19086–19092 [DOI] [PubMed] [Google Scholar]
- 25. Moore NG, Pegg GG, Sillence MN. Anabolic effects of the beta 2-adrenoceptor agonist salmeterol are dependent on route of administration. Am J Physiol 1994;267: E475–484 [DOI] [PubMed] [Google Scholar]
- 26. Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood 2003;102:480–488 [DOI] [PubMed] [Google Scholar]
- 27. Han SO, Ronzitti G, Arnson B, et al. . Low-dose liver-targeted gene therapy for Pompe disease enhances therapeutic efficacy of ERT via immune tolerance induction. Mol Ther Methods Clin Dev 2017;4:126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raben N, Roberts A, Plotz PH. Role of autophagy in the pathogenesis of Pompe disease. Acta Myol 2007;26:45–48 [PMC free article] [PubMed] [Google Scholar]
- 29. Raben N, Baum R, Schreiner C, et al. . When more is less: excess and deficiency of autophagy coexist in skeletal muscle in Pompe disease. Autophagy 2009;5:111–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nascimbeni AC, Fanin M, Masiero E, Angelini C, Sandri M. The role of autophagy in the pathogenesis of glycogen storage disease type II (GSDII). Cell Death Differ 2012;19:1698–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raben N, Kanneboyina N, Lee E, et al. . Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 1998;273:19086–19092 [DOI] [PubMed] [Google Scholar]
- 32. Koeberl DD, Case LE, Smith EC, et al. . Correction of biochemical abnormalities and improved muscle function in a phase I/II clinical trial of clenbuterol in Pompe disease. Mol Ther 2018;26:2304–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koeberl DD, Austin S, Case LE, et al. . Adjunctive albuterol enhances the response to enzyme replacement therapy in late-onset Pompe disease. FASEB J 2014;28:2171–2176 [DOI] [PubMed] [Google Scholar]
- 34. Cazzola M, Matera MG. Safety of long-acting beta2-agonists in the treatment of asthma. Ther Adv Respir Dis 2007;1:35–46 [DOI] [PubMed] [Google Scholar]
- 35. Farah BL, Madden L, Li S, et al. . Adjunctive beta2-agonist treatment reduces glycogen independently of receptor-mediated acid alpha-glucosidase uptake in the limb muscles of mice with Pompe disease. FASEB J 2014;28:2272–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raben N, Lu N, Nagaraju K, et al. . Conditional tissue-specific expression of acid alpha-glucosidase (GAA) gene in the GAA knockout mice: implications for therapy. Hum Mol Genet 2001;10:2039–2047 [DOI] [PubMed] [Google Scholar]
- 37. Salazar-Degracia A, Busquets S, Argiles JM, Bargallo-Gispert N, Lopez-Soriano FJ, Barreiro E. Effects of the beta2 agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer-induced cachexia. Biochimie 2018;149:79–91 [DOI] [PubMed] [Google Scholar]
- 38. Puzzo F, Colella P, Biferi MG, et al. . Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid alpha-glucosidase. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.