Abstract
In a previous limb-girdle muscular dystrophy type 2D (LGMD2D) clinical trial, robust alpha-sarcoglycan gene expression was confirmed following intramuscular gene (SGCA) transfer. This paved the way for first-in-human isolated limb infusion (ILI) gene transfer trial to the lower limbs. Delivery of scAAVrh74.tMCK.hSGCA via an intravascular route through the femoral artery predicted improved ambulation. This method was initially chosen to avoid safety concerns required for large systemic vascular delivery viral loads. ILI methods were adopted from the extensive chemotherapy experience for treatment of malignancies confined to the extremities. Six LGMD2D subjects were enrolled in a dose-ascending open-label clinical trial. Safety of the procedure was initially assessed in the single limb of a non-ambulant affected adult at a dose of 1 × 1012 vg/kg. Subsequently, ambulatory children (aged 8–13 years) were enrolled and dosed bilaterally with either 1 × 1012 vg/kg/limb or 3 × 1012 vg/kg/limb. The six-minute walk test (6MWT) served as the primary clinical outcome; secondary outcomes included muscle strength (maximum voluntary isometric force testing) and SGCA expression at 6 months. All ambulatory participants except one had pre- and post-treatment muscle biopsies. All four subjects biopsied had confirmed SGCA gene delivery by immunofluorescence, Western blot analysis (14–25% of normal), and vector genome copies (5.4 × 103–7.7 × 104 vg/μg). Muscle strength in the knee extensors (assessed by force generation in kilograms) showed improvement in two subjects that correlated with an increase in fiber diameter post gene delivery. Six-minute walk times decreased or remained the same. Vascular delivery of AAVrh74.tMCK.hSGCA was effective at producing SGCA protein at low doses that correlated with vector copies and local functional improvement restricted to targeted muscles. Future trials will focus on systemic administration to enable targeting of proximal muscles to maximize clinical benefit.
Keywords: isolated limb infusion, gene delivery, limb-girdle muscular dystrophy, alpha-sarcoglycan
Introduction
In a proof-of-principle clinical trial, robust alpha-sarcoglycan (α-SG) gene expression was achieved in limb-girdle muscular dystrophy type 2D (LGMD2D), following intramuscular (i.m.) gene delivery mediated by adeno-associated virus (AAV) under control of a muscle-specific promoter (tMCK).1,2 The rAAV1.tMCK.hSGCA vector (3.25 × 1011 vg) was delivered to the extensor digitorum brevis muscle of LGMD2D subjects, and α-SG was expressed at 6 weeks, 3 months, and 6 months. No adverse events were encountered related to viral gene transfer, and successful, sustained gene expression was achieved without immunosuppression. The safety profile in the i.m. study negated previously expressed concerns suggesting that toxicity may be associated with SGCA gene transfer.3
Following these initial i.m. studies, strategies to achieve clinically meaningful outcomes were considered. Prolonging ambulation was targeted as an efficacy outcome that could be potentially achieved by delivery of recombinant AAV (rAAV) carrying the SGCA gene via an intravascular route. A decision was made to deliver self-complementary scAAVrh74.tMCK.hSGCA via isolated limb infusion (ILI).4,5 The decision favoring this route of delivery was based on several considerations. At the time this clinical trial was initiated, the projected rAAV viral load for systemic delivery, subsequently found successful for spinal muscular atrophy,6 had not been tested for safety in a clinical setting, and the potential limitations of cost and viral production for the LGMD population were matters of concern. ILI offered the potential for gene delivery to the lower limbs through percutaneous access to the femoral artery. The approach was first described in 1998 by Thompson et al.7 as a less invasive alternative to isolated limb perfusion (ILP), a procedure far more invasive requiring direct surgical cannulation of vessels, blood oxygenation, and cardiopulmonary bypass.8–10 Both methods had been used for the treatment of soft-tissue sarcomas and melanomas with promising results for remission and disease stabilization11–13 As a less invasive, relatively safe, and simplified procedure, muscles of the extremities most affected by LGMD2D could be targeted by ILI. In addition, for LGMD2D, a form of muscular dystrophy with minimal cardiac involvement, ILI was particularly appealing.14
For clinical translation, preclinical proof-of-principle experiments were directed at demonstrating efficacy by increasing tetanic force and resistance to eccentric contraction accompanied by robust dystrophin and utrophin expression following gene delivery through the femoral artery without high volume or high pressure in the mdx mouse.15,16 Advanced preclinical studies consisting of ILI gene transfer to the nonhuman primate (NHP) simulated a paradigm that could potentially be applied in a clinical setting for gene transfer. The vascular anatomy of the monkey provided the opportunity to explore ILI delivery of FLAG tagged transgenes to test the efficiency and safety of gene transfer to the quadriceps and gastrocnemius muscles of the NHP.16 The favorable gene expression in the rhesus macaques paved the way for a clinical trial in LGMD2D subjects. This report describes the results of an open-label, single-site, Phase I/II, first-in-human gene transfer using ILI to test safety and efficacy in a form of muscular dystrophy with minimal cardiac requirements.
Methods
Study subjects
Six total LGMD2D patients were enrolled in an ILI gene-delivery trial. All subjects enrolled in this trial had documented biallelic SGCA mutations that predicted amino-acid substitutions known to be causative for LGMD2D. All patients were put on prednisone 1mg/kg/day for 1 month starting one day prior to gene transfer. Exclusion criteria were the same as previously published.1,2 Informed consent was obtained by the Principal Investigator in compliance with 21CFR50 and the International Conference on Harmonisation guidelines before entering the trial and was signed by parents and subjects (for those aged 9–17 years). Pre–gene transfer immune studies included serum-neutralizing antibodies to AAVrh74 and interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISpot) assay for both AAV capsid proteins and the α-SG protein. Criteria excluding participation were pregnancy; active viral infections, including human immunodeficiency virus, hepatitis A, B, or C, known autoimmune disease, and presence of cardiomyopathy; diabetes; or organ system abnormalities of the bone marrow, liver, or kidney.
This was a single-site, investigator-initiated, open-label, dose-ascending, ILI trial of scAAVrh74.tMCK.hSGCA vector. The study was approved by the Recombinant DNA Advisory Committee (#1301-1200; March 25, 2013), the Food and Drug Administration (FDA; IND #15800), and the Institutional Review Board (#20131939).
The FDA mandated that the first-in-human ILI gene therapy trial be done in a single limb of a non-ambulatory adult with LGMD2D (E-01) to establish safety. The first candidate, Cohort 1A, received 1 × 1012 vg/kg scAAVrh74.tMCK.hSGCA to a single limb (right) as part of the dose-escalation clinical trial. Following subject 1, safety data were presented to the FDA (CBER), giving permission for the remaining subjects to receive scAAVrh74.tMCK.hSGCA in both limbs (the right limb initially). Ambulatory subjects with established SGCA mutations of both alleles were enrolled. Three subjects enrolled in Cohort 1B (E-02, E-03, and E-04) received bilateral infusion of 1 × 1012 vg/kg/limb. Additional safety data permitted enrollment of Cohort 2 (E-05 and E-06) receiving bilateral infusions of 3 × 1012 vg/kg/limb. Table 1 summarizes the age, sex, limb infusion (single or dual), and the dose delivered per limb, as well as the ambulatory status.
Table 1.
Description of LGMD2D cohorts at baseline
| Cohort/subject no. | Age baseline (years) | Sex | Limb infusion | Dose (vg/kg) | Baseline ambulation |
|---|---|---|---|---|---|
| 1A/E-01 | 49 | F | Single limb | 1 × 1012 right | No |
| 1B/E-02 | 9 | M | Dual limb | 1 × 1012/limb | Yes |
| 1B/E-03 | 13 | M | Dual limb | 1 × 1012/limb | Yes |
| 1B/E-04 | 8 | M | Dual limb | 1 × 1012/limb | Yes |
| 2/E-05 | 12 | M | Dual limb | 3 × 1012/limb | Yes |
| 2/E-06 | 8 | F | Dual limb | 3 × 1012/limb | Yes |
LGMD2D, limb-girdle muscular dystrophy type 2D.
Outcome measures
The primary outcome for this clinical trial was safety and tolerability of gene transfer of SGCA under control of a muscle-specific promoter, tMCK using a scAAVrh74 vector, adapting a limb-saving cancer chemotherapy method to muscular dystrophy. This was a first-in-human gene-delivery trial using ILI as the method for safe, relatively easily performed gene replacement therapy. Functional outcomes included the North Star Ambulatory Assessment Scale (NSAA), stair climbing, and the distance walked using the six-minute walk test (6MWT). Confirmatory strength testing employed force generation quantified by maximum voluntary isometric contraction testing (MVICT)17–20 of knee extensors (quadriceps muscle), a direct target of ILI through the femoral artery. Force generation required sustained contraction for 5 s. Final data were based on two trials with ≤10% error between efforts and 20 s of rest between trials.
Muscle biopsies provided the source for confirming vector delivery and SGCA protein expression as judged by immunofluorescence, quantified immunoblot analysis, and vector copy number based on previously published methods.1,2,18,19 Pre- and post-gene delivery to quadricep-muscle biopsies on day 180 were performed on four subjects (E-03, E04, E-05, and E-06). Opposite extremity biopsies were done to avoid scar tissue from the first biopsy that could impede interpretation. Subject E-01, an adult with LGMD2D, volunteered for a single-limb infusion safety study and had only a post gene-delivery muscle biopsy. Pre- and post-treatment muscle biopsies were scheduled for E-02. However, a post-treatment biopsy was deferred at parental request, given that progressive muscle weakness during follow-up resulting in loss of ambulation.
Immune studies
All subjects met the criteria for serum-binding antibody level against AAVrh74 and AAV8 no greater than 1:50 before gene transfer.6 This was determined by enzyme-linked immunosorbent assay to the viral capsid, as previously described.21 Follow-up titers post gene transfer included days 7, 14, 28, 42, 60, and 90 and every 3 months for the remainder of the trial. Pre-screening and follow-up studies to identify potential T-cell immunity were done on these same days using IFN-γ ELISpot according to previously described methods.6,21 Antigens for the ELISpot assay included three AAV capsid peptide pools and the full α-SG protein.1,2
Vector production
The rAAV, scAAVrh74.tMCK.hSGCA, was made in the Good Manufacturing Practice (GMP) production facility at the Nationwide Children's Hospital (NCH) Manufacturing Facility by triple transfection. The construct utilized a chimeric intron to promote high-level expression.22 The AAVrh74 serotype shares 93% amino-acid identity with AAV8 and is most similar to clade E virus rh10. The molecular clone was identified at the NCH from a rhesus macaque lymph node.23 The AAVrh74 vector was produced as previously described using the human α-Sg gene flanked by AAV2 inverted terminal repeat (ITR) sequences and encapsidated into AAVrh74 virions.1,2 The tMCK promoter/enhancer (GenBank Accession No. M21390) derived sequence was used to drive muscle-specific gene expression and uses the β-globin intron for high-level expression. All plasmids used in the production process were produced by Aldevron under the GMP Source (GMP-S™) quality system and infrastructure. Release testing, including the final fill product, was performed by the authors' quality assurance unit. Certificates of stability and analysis were submitted to and approved by the FDA.
ILI procedure
The proposed clinical trial is a dose-escalation study of scAAVrh74.tMCK.hSGCA delivered by modified isolated limb perfusion4 and isolated limb infusion.7 Subjects were admitted to NCH the day before the gene transfer and were NPO after midnight. An intravenous catheter with heparin lock was placed in the arm for delivery of sedation and for sodium heparin administration to maintain activated clotting time throughout the procedure. Gene transfer was performed in the cardiac catheterization suite where conscious sedation was induced by the Heart Center Anesthesia team. This avoided discomfort during the procedure (catheter placement and extremity ischemia) without complaints of pain after recovery of consciousness. A radial arterial line was placed for continuous monitoring. An appropriately sized 4–8 French sidearm sheath and dilator were placed percutaneously in the femoral vein and artery of both limbs. Once the sidearm sheaths were placed in each vessel on both sides, the patient was heparinized to keep the activated clotting time (ACT) >200 s. The ACT was followed every 30 min and heparin re-dosed as needed. A mini-Tyshak balloon (4–8 mm × 2 cm) catheter was advanced over a coronary guide wire and positioned in the femoral artery. A Rosen guidewire (0.035) was placed through the femoral venous sheath, and an 8–16 mm × 2–4 cm Tyshak II balloon catheter was placed through the venous sheath. The balloon was inflated, and a test angiogram confirmed isolation of the extremity in preparation of vector infusion with entry through the femoral artery.
For each cohort, vector was prepared by the research investigational pharmacy service, brought to the catheterization suite on ice, and allowed to warm to room temperature. With the balloons inflated, a pre-flush 2 mL/kg of lactated Ringer's solution (LRs) was infused over approximately 1 min, and then the prescribed vector-dosing regimen was infused through the femoral artery with a total of 8 mL/kg LRs over approximately 2 min. After the infusion was complete, a 10 min dwell time permitted maximum skeletal muscle transduction. Skin mottling was observed, starting at the upper thigh and progressing distally as the infusion progressed. After 10 min of isolation, the extremity was flushed using the arterial catheter with 2 mL/kg of LRs for 1 min. The arterial balloon was deflated and removed, followed by deflation and removal of the venous balloon. Direct pressure was applied to control bleeding at the site of catheter insertion. Normal color was restored to the extremity, as were pulses over the femoral artery and in the distal extremity circulation. The opposite extremity then underwent the same procedure. After both legs were infused, all equipment and syringes were properly discarded, and the patient was returned to the recovery suite where full consciousness and vital signs returned to normal before they were taken back to their hospital room.
Results
Adult non-ambulatory LGMD subject (cohort 1A)
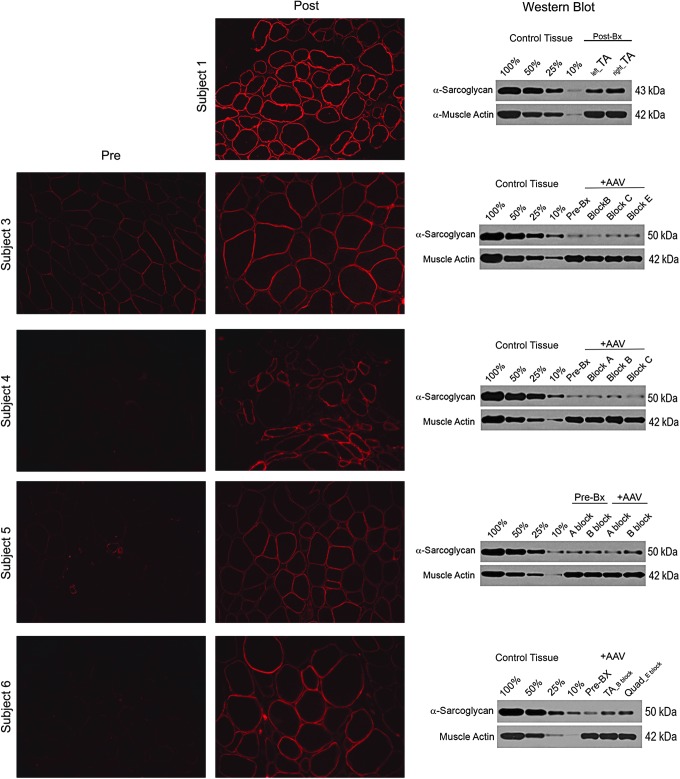
This was a pilot clinical trial that included six subjects (four males), as shown in Table 1. Safety was initially established in an adult non-ambulatory LGMD2D subject (E-01) dosed in a single limb. MVICT of knee extensors showed an increase in muscle force generation (47%) on the side of gene delivery (Table 2). Post gene transfer gene expression and vector genome copy numbers were assessed in the tibialis anterior muscle, validating increases at day 180 (Figs. 1 and 2). IF showed α-SG expression in the sarcolemma that was quantified by Western blot, revealing 35% of normal control.
Table 2.
Functional outcomes of ILI gene transfer
| Subject | MVICT | MF size, μμm (X ±SD) | Ambulatory status pre and post gene therapy | 6MWT change BL | ||
|---|---|---|---|---|---|---|
| Right | Left | Pre | Post | |||
| E-01 | Pre 2.77 | Pre 3.33 | — | Post TAa Quad not available | Non-AMB pre gene therapy; non-AMB post gene therapy | * |
| Post 4.07 | Post 3.91 | |||||
| Δ↑47% | Δ ↑17% | |||||
| E-02 | Pre 1.87 | Pre 3.44 | — | No post Bx | AMB pre gene therapy; lost AMB post gene therapy | ** |
| Post 1.46 | Post 2.48 | |||||
| Δ ↓22% | Δ↓28% | |||||
| E-03 | Pre 36.83 | Pre 36.22 | Pre 56.19 (17.43) | Post 72.30 (26.05) | AMB pre gene therapy; AMB post gene therapy | Pre 538 m |
| Post 58.41 | Post 60.92 | Post 525 m | ||||
| Δ ↑59% | Δ ↑68% | Δ ↓2% | ||||
| E-04 | Pre 11.45 | Pre 8.43 | Pre 49.85 (16.64) | Post 35.12 (27.58) | AMB pre gene therapy; AMB post gene therapy | Pre 487 m |
| Post 7.43 | Post 4.33 | Post 366 m | ||||
| Δ ↓35% | Δ ↓49% | Δ ↓24% | ||||
| E-05 | Pre 5.14 | Pre 4.96 | Pre 62.13 (24.97) | Post 67.38 (22.84) | AMB pre gene therapy; lost AMB post gene therapy | ** |
| Post 5.07 | Post 4.74 | |||||
| Δ ↓1% | Δ↓4% | |||||
| E-06 | Pre 10.61 | Pre 11.01 | Pre 46.39 (14.56) | Post 32.09 (10.63) | AMB pre gene therapy; AMB post gene therapy | Pre 571 m |
| Post 8.14 | Post 9.45 | Post 600 m | ||||
| Δ ↓23% | Δ ↓14% | Δ ↑5% | ||||
Post gene-transfer muscle biopsy done on TA.
No ambulatory change pre and post gene therapy.
Lost ambulation post gene therapy.
ILI, isolated limb infusion; MVICT, maximum voluntary isometric contraction testing (reported in kilograms); 6MWT, six-minute walk test (reported as distance walked in meters); BL, baseline; AMB, ambulation; TA, tibialis anterior; Δ, percent change in 6MWT meters from baseline to end of study (all patients completed 2 years).
Figure 1.
SGCA expression following isolated limb infusion (ILI). SGCA gene expression demonstrated by immunofluorescence following alpha-sarcoglycan (α-SG) antibody staining of muscle biopsies taken post gene transfer for subject 1 (tibialis anterior) and pre and post gene transfer for subjects 3–6 (quadriceps). Except for subject 1 where it could not be compared, membrane staining intensity post gene transfer showed increased intensity. Confirmation of gene expression by quantitative Western blots revealed an increase in α-SG protein compared to baseline as follows: subject 3: 38%; subject 4: 12.5%; subject 5: 22%; and subject 6: 172%. Comparisons as a percent of normal for each subject were as follows: subject 1: 35%; subject 3: 14%; subject 4: 16%; subject 5: 15%; and subject 6: 25%. Western blots were quantified using a four-point standard curve (10%, 25%, 50%, and 100%) generated from non-dystrophic normal control muscle samples and normalized for muscle content using muscle actin as a loading control (lower band).
Figure 2.
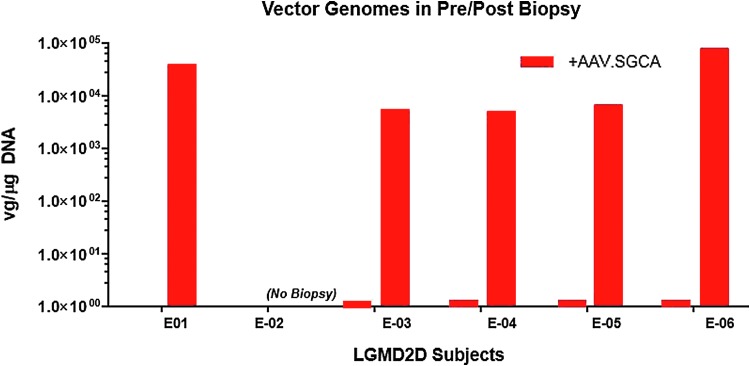
Vector genome copy number following ILI. Delivery of scAAVrh74.tMCK.SGCA vector genomes was confirmed by quantitative polymerase chain reaction using the tMCK as the target amplicon. All baseline biopsy values were below the limit of detection. All subjects demonstrated vector genome copy numbers in the range of 5.4e3 and 7.76e4 vg/μg DNA (0.03–0.54 copies/nuclei): subject 1: 3.9e4 vg/μg; subject 3: 5.4e3 vg/μg, subject 4: 5.02e3 vg/μg; subject 5: 6.48e3 vg/μg; and subject 6: 7.76e4 vg/μg.
Ambulatory LGMD2D cohort 1B (low dose) and cohort 2 (high dose)
Five LGMD2D patients (cohort 1B: subjects E-02, E-03, and E-04; cohort 2: subjects E-05 and E-06) were ambulatory at the time of screening and initiation of gene transfer (Tables 1 and 2). Muscle biopsies were performed on all subjects at day 180. Two ambulatory subjects, one in each dosing cohort (E-02, age 9 years; E-05, age 12 years) were unable to maintain independent ambulation during this 2-year clinical trial. MVICT decreased in E-02 (22% right; 28% left). E-05 had minimal change in MVICT (reduced by 1% right and 4% left) and no significant change in muscle-fiber size (pre 62.13 ± 24.97 and post 67.38 ± 22.84).
Subjects E-04 and E-06 maintained ambulation with reduced MVICT. E-04 modestly declined on 6MWT (24%), and E-06 minimally increased (5%). Subject E-03 stood out because MVICT increased in both limbs (increased 59% right and 68% left), as did his increase in quadriceps muscle-fiber size (28%). However, distance walked on the 6MWT remain unchanged (declined 2%).
For cohorts 1B and 2, muscle-biopsy results provide clear evidence that transgene was delivered to the muscles of the lower limbs through this ILI approach. SGCA gene expression pre and post treatment shows increased staining intensity by immunofluorescence confirmed by Western blot (Fig. 1). The percent α-SG increased in subjects 3–6 (14–25% of normal). The increase in vector genome copy numbers for each patient post gene transfer is shown in Fig. 2 (subject 3: 5.4e3 vg/μg; subject 4: 5.02e3 vg/μg; subject 5: 6.48e3 vg/μg; and subject 6: 7.76e4 vg/μg; 0.03–0.54 copies/nuclei).
Adverse events
Overall, for the six subjects treated, ILI-AAV gene delivery was well tolerated. Catheter placement and positioning the balloons to isolate the limbs took between 1 and 1.5 h. The procedure, done under conscious sedation, was well tolerated. There was skin mottling and dusky discoloration of the leg during gene infusion that persisted during dwell time, with rapid clearing as the limb was re-perfused. Mild soreness with applied pressure or while walking was experienced at the site of entry in the groin, reaching a peak by day 3, with bluish discoloration that persisted for several days. No hemorrhages were encountered, and no patients had deep-vein thrombosis/thrombophlebitis. Viral delivery caused no side effects, and there were no adverse events/serious adverse events (SAEs) directly related to transgene or AAV. Anti-AAVRh74 IgG antibody titers were monitored throughout the trial and peaked between 3 and 6 months before they declined slightly and maintained a plateau (Supplementary Fig. S1). An elevation of T-cell responses as measured by ELISpot assays to AAVrh74 was observed during the trial (Supplementary Fig. S2), similar to other Phase I clinical trial gene-delivery studies.6,22 T-cell response to AAVrh74 usually peaked at 1–4 weeks following gene delivery, never reaching SAE level, and there were no corresponding elevations of liver enzymes and no clinical manifestations. An ILI study may be expected to protect hepatocytes from excess exposure to viral delivery by isolation of the extremity during infusion of vector. Clinical chemistries throughout the trial remained normal.
Two of the participants in the trial, E-03 and E-06, had episodes of rhabdomyolysis/myoglobinuria reported before (by history) and during the trial (each had two occurrences). The episodes during the trial were documented by peak elevated creatine kinase levels between 59,171 IU/L (E-03) and 68,500 IU/L (E-06). Rhabdomyolysis was precipitated by excessive activity events, despite advice to the contrary (E-03 fencing competitions; E-06 participation as cheerleader). No long-term consequences were seen; patients were kept well hydrated and rested during brief hospitalizations with treatment by intravenous hydration. Sarcoglycanopathies have an increased predisposition to episodes of myoglobinuria and hyperCKemia, including LGMD2C,24 2E,25 and 2D.26–29 Subject E-02 suffered multiple limb fractures related to falls that had no relationship to transgene or AAV.
Discussion
The objective of this translational gene therapy trial was to establish if ILI would provide significant clinical improvement in LGMD2D with α-SG deficiency with potential application to other forms of muscular dystrophy. The rational for ILI gene delivery is based on targeting gene delivery to the predominant area of need; α-SG deficiency spares the heart in most cases. The disability is caused by lower-limb muscle weakness until late in the course of the disease. ILI, if effective, provides a means of gene replacement using a reduced viral load to correct the defect in the targeted extremity. Achieving clinical efficacy with ILI diminishes the cost for viral production compared to the demands for high viral load needed for systemic gene delivery.6 According to some investigators, concerns remain regarding the safety of high-dose AAV gene delivery.30 ILI offers an alternative route of gene transfer using less viral load compared to systemic delivery, especially for neuromuscular diseases starting later in childhood or early adult life where patient size increases the dosing requirements and cost.
In developing strategies for gene delivery through isolated limbs, both ILP and ILI were considered. The differences were important, considering that ILP is far more invasive as a surgical procedure compared to ILI, requiring only radiologically guided percutaneous insertion of catheters to the femoral artery and vein.7,11,12 Cardiopulmonary bypass is not necessary for ILI, and gene transfer requires only delivery of vector by manual push with a syringe, in contrast to a high-flow pump with limb recirculation when targeting neoplasia.9,31 Efficacy is somewhat less for ILI compared to ILP treatment of limb sarcoma and melanoma, but both have the potential to be effective.11,13 Considering the risk–benefit ratio for a disease with far fewer life-threatening consequences like LGMD, the choice favoring ILI was justified.
The functional outcomes for the ILI LGMD2D study were partially improved in a subset of subjects. Based on the 6MWT for five ambulatory LGMD2D subjects aged 8–13 years, two lost ambulation, one declined in distance walked (24%), and two others showed no change (decreased 2%, increased 5%). The gene-expression data for SGCA on muscle biopsy was visualized by immunofluorescence32 and confirmed by Western blot quantification and vector genome counts, providing unequivocal evidence of gene delivery through the femoral artery. The limited functional outcome improvement was likely contributed to by poor delivery to proximal muscles (hip flexors, hip abductors, and hip extensors), limiting the distance walked on the 6MWT and the ability to maintain ambulation. Importantly, subjects who improved on MVICT correlated with SGCA expression and increased fiber size. Improvement in outcomes might require higher viral doses and different catheter placement to infuse the replacement gene to more proximal muscle targets.
The current study demonstrates the challenge of transducing major muscle groups in the extremities that contribute to gait and 6MWTs. The methods used in this trial were not adequate to improve major motor functions. Apart from systemic delivery to target all muscles, options for ILI studies in the future will be to increase the dose of virus or catheterize vessels that feed more proximal muscles. The iliac artery could be a choice but presents more challenges for extremity isolation, the objective of ILI. In this study, limb delivery of scAAVrh74.tMCK. hSGCA was effective at producing SGCA protein at low doses, supporting a favorable outcome with systemic delivery at higher doses with treatment of all muscles. LGMD2D as well as the other sarcoglycanopathies represent ideal candidates to benefit from rAAV gene therapy. The cDNAs are small (∼1 kb), allowing for transfer and expression of the full-length protein based on the small size of the gene. This clinical trial provides insight and demonstrates limitations and a rationale for targeting proximal muscles to maximize clinical benefit.
Supplementary Material
Acknowledgments
The support of our Regulatory Operations and Drug Development Core at Nationwide Children's Hospital, including Chris Shilling, MS, and Patricia C. Sondergaard, PhD, was helpful and appreciated. Our GMP vector Manufacturing Core at Nationwide Children's Hospital provided scAAVrh74.tMCK.hSGCA for this clinical trial. The study was supported by NIH/NIAMS Vascular Delivery of α-sarcoglycan for LGM2D U01-AR-060911.
Author Disclosure
J.R.M. is a Principal Investigator in the Center for Gene Therapy at Nationwide Children's Hospital. L.R.R.-K. is Vice-President for Gene Therapy, Sarepta Therapeutics. No competing financial interests exist for these contributors or for the remaining authors.
Supplementary Material
References
- 1. Mendell JR, Rodino-Klapac LR, Rosales-Quintero , et al. LGMD 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 2009;66:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, et al. . Sustained alpha-sarcoglycan gene expression after gene transfer in Limb-Girdle Muscular Dystrophy, Type 2D. Ann Neurol 2010;68:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dressman D, Araishi K, Imamura M, et al. . Delivery of alpha and beta-sarcoglycan by recombinant adeno-associated virus: efficient rescue of muscle, but differential toxicity. Hum Gene Ther 2002;13:1631–1646 [DOI] [PubMed] [Google Scholar]
- 4. Seinen JM, Hoekstra HJ. Isolated limb perfusion of soft tissue sarcomas: a comprehensive review of literature. Cancer Treat Rev 2013;39:569–577 [DOI] [PubMed] [Google Scholar]
- 5. Rodino-Klapac LR, Janssen PM, Montgomery CL, et al. . A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Trans Med 2007;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mendell JR, Al-Zaidy S, Shell R, et al. . Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 2017;377:1713–1722 [DOI] [PubMed] [Google Scholar]
- 7. Thompson JF, Kam PC, Waugh RC, et al. . Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol 1998;14:238–247 [DOI] [PubMed] [Google Scholar]
- 8. Creech O, Jr, Krementz ET, Ryan RF, et al. . Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg 1958;148:616–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krementz ET, Creech O, Jr, Ryan RF, et al. . An appraisal of cancer chemotherapy by regional perfusion. Ann Surg 1962;156:417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minor DR, Allen RE, Alberts D, et al. . A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer 1985;55:2638–2644 [DOI] [PubMed] [Google Scholar]
- 11. Seinen JM, Hoekstra HJ. Isolated limb perfusion of soft tissue sarcomas: a comprehensive review of literature. Cancer Treat Rev 2013;39:569–577 [DOI] [PubMed] [Google Scholar]
- 12. Beasley GM, Caudle A, Petersen RP, et al. . A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg 2009;208:706–717 [DOI] [PubMed] [Google Scholar]
- 13. Wong J, Chen A, Fisher KJ, et al. . Isolated limb infusion in a series of over 100 infusions, a Single Center Experience. Ann Surg Oncol 2013;20:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fayssoil A, Nardi O, Annane D, et al. . Left ventricular function in alpha-sarcoglycanopathy and gamma-sarcoglycanopathy. Acta Neurol Belg 2014;114:257–259 [DOI] [PubMed] [Google Scholar]
- 15. Chicoine LG, Rodino-Klapac LR, Xu R, et al. . Vascular delivery of rAAVrh74.MCK.GALGT2 in the rhesus macaque demonstrates widespread, sustained, functional muscle transgene expression, glycosylation of α dystroglycan, and upregulation of utrophin. Mol Ther 2013;22:713–72424145553 [Google Scholar]
- 16. Rodino-Klapac LR, Montgomery CL, Bremer WG, et al. . Persistent expression of FLAG tagged micro-dystrophin in non-human primates with intramuscular and vascular delivery. Mol Ther 2010;18:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mendell JR, Kissel JT, Amato AA, King W, et al. . Randomized, double-blind, one-year trial of myoblast transfers in Duchenne muscular dystrophy. N Engl J Med 1995;333:832–838 [DOI] [PubMed] [Google Scholar]
- 18. Mendell JR, Sahenk Z, Malik V, et al. . A Phase 1/2a follistatin gene therapy trial for Becker muscular dystrophy. Mol Ther 2015;23:192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mendell JR, Sahenk Z, Al-Zaidy S, et al. . Follistatin gene therapy for sporadic inclusion body myositis improves functional outcomes. Mol Ther 2017;25:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lowes PL, Alfano L, Viollet L, et al. . Knee extensor strength exhibits potential to predict function in sporadic inclusion body myositis. Muscle Nerve 2012;45:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mendell JR, Campbell K, Rodino-Klapac L, et al. . Dystrophin immunity revealed by gene therapy in Duchenne muscular dystrophy. N Engl J Med 2010;363:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Powell SK, Rivera-Soto R, Gray SJ. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Disc Med 2015;19:45–57 [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson PR, Schnepp BC, Connell MF, et al. . Novel adeno-associated virus vector vaccine restricts replication of simian immunodeficiency virus in macaques. J Virol 2005;79:955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pena L, Kim K, Charrow J. Episodic myoglobinuria in a primary gamma-sarcoglycanopathy. Neuromuscul Disord 2010;20:337–339 [DOI] [PubMed] [Google Scholar]
- 25. Cagliani R, Comi GP, Tancredi L, et al. . Primary beta-sarcoglycanopathymanifesting as recurrent exercise-induced myoglobinuria. Neuromuscul Disord 2001;11:389–394 [DOI] [PubMed] [Google Scholar]
- 26. Mongini T, Doriguzzi C, Bosone I, et al. . Alpha-sarcoglycan deficiency featuring exercise intolerance and myoglobinuria. Neuropediatrics 2002;33:109–111 [DOI] [PubMed] [Google Scholar]
- 27. Tarnopolsky M, Hoffman E, Giri M, et al. . Alpha-sarcoglycanopathy presenting as exercise intolerance and rhabdomyolysis in two adults. Neuromuscul Disord 2015;25:952–954 [DOI] [PubMed] [Google Scholar]
- 28. Ceravolo F, Messina S, Rodolico C, et al. . Myoglobinuria as first clinical sign of a primary alpha-sarcoglycanopathy. Eur J Pediatr 2014;173:239–242 [DOI] [PubMed] [Google Scholar]
- 29. Krishnaiah B, Lee JJ, Wicklund MP, et al. . Young girl presenting with exercise-induced myoglobinuria. Muscle Nerve 2016;54:161–164 [DOI] [PubMed] [Google Scholar]
- 30. Hinderer C, Katz N, Buza EL, et al. . Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther 2018;29:285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coleman A, Augustine CK, Beasley G, et al. . Optimizing regional infusion treatment strategies for melanoma of the extremities. Expert Rev Anticancer Ther 2009;9:1599–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendell JR, Rodino-Klapac LR, Sahenk Z, et al. . Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol 2013;74:637–647 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.