Abstract
The development of antifouling membranes plays a vital role in the widespread application of membrane technology, and the hybridization strategy has attracted a significant amount of attention for antifouling applications. In this work, TA/PEI@TiO2 hierarchical hybrid nanoparticles (TPTi HHNs) are first synthesized through a simple strategy combining the multiple catechol chemistries of phenolic tannic acid (TA) with the biomimetic mineralization chemistry of titania. The TPTi HHNs are used as nanofillers to prepare PVDF/TPTi hybrid membranes. The TPTi HHNs endow the membrane with higher porosity, hierarchical roughness, greater hydrophilicity, and underwater superoleophobicity. Upon TPTi HHN loading, the PVDF/TPTi hybrid membranes exhibit enhanced antifouling performance. The flux recovery ratio can reach 92% when utilized to separate oil-in-water emulsion. Even being applied to the three-cycle filtration of oil-in-water emulsion with much higher concentration, the PVDF/TPTi membrane can still maintain a high flux recovery ratio about 85%. This study will provide a facial polyphenol-based platform to fabricate antifouling hybrid nanofillers and antifouling hybrid membranes with promising applications in oil/water separation.
1. Introduction
In recent years, the massive discharge of oily wastewater has run counter to the concept of sustainable use of resources.1 Emulsified oil from oily wastewater greatly restricted the efficient treatment of oily wastewater via traditional oil/water separation techniques due to their small oil droplet size (less than 20 μm), strong system stability, high concentration, and wide scope.2,3 From the aspects of wastewater reuse, environmental protection, and sustainable development, it is very necessary to search for simple, efficient, low-energy-consumption and eco-friendly solutions for oily wastewater treatment. Ultrafiltration membrane separation technology for oily wastewater treatment shows priorities in all of the above requirements, especially in its effective removal of emulsified oil from oily wastewater.4 However, owing to the inherent oleophilic properties of the common polymers for preparing ultrafiltration membranes, such as poly(vinylidene fluoride) (PVDF), polysulfone (PSf), polyethersulfone (PES), and so on, the conventional ultrafiltration membranes generally have high affinity with oil foulants. Thus, oil spreading and adsorbing easily take place on the surfaces of traditional membrane materials and cause serious membrane fouling.5 Therefore, the development of antifouling ultrafiltration membranes has become a prerequisite for the application of membrane technology in oily wastewater treatment.
Hybrid membranes derived from polymer matrixes and nanomaterial fillers (nanofillers) have drawn considerable attention for membrane material engineering because of their exceptional membrane hydrophilicity, antifouling ability, permeability, mechanical properties, and so on.6 For developing antifouling ultrafiltration membranes, the design of nanomaterial fillers can provide more diversity to refine membrane properties for antifouling purpose. Various nanofillers with inherent hydrophilicity and dimension variety have been introduced into the polymeric membrane matrix, generally including inorganic nanofillers, organic nanofillers, and inorganic/organic hybrid nanofillers. Inorganic nanofillers such as TiO2 nanoparticles/nanotubes,7−9 ZrO2 nanoparticles,10,11 ZnO nanoparticles,12 SiO2 nanoparticles,13 Mg(OH)2 nanorods,14 carbon nanotubes,15,16 graphene oxide nanosheets,17 and MOF nanoparticles18−20 were directly blended into membrane matrixes and proved to be effective in improving the membrane hydrophilicity and antifouling ability. However, the direct blending of inorganic nanofillers usually suffers from two major issues of agglomeration and poor compatibility between inorganic nanofillers and membrane-forming polymers, which are harmful to membrane properties and performance.21 Organic nanofillers such as polydopamine nanoparticles22 were also added into membrane casting solution of hybrid membranes. The compatibility between the membrane-forming polymers and the organic nanofillers was enhanced, and the agglomeration was limited. Recently, inorganic/organic hybrid nanofillers (IOH-nanofillers) have attracted considerable attention and were believed to combine the merits of both inorganic nanofillers and organic nanofillers. For the design of IOH-nanofillers, popular approaches force decorating hydrophilic polymers onto the surface of inorganic nanoparticles.23,24 Our previous work also synthesized nanoparticles with a zwitterionic shell layer on a TiO2 nanoparticle core as IOH-nanofillers for antifouling hybrid membranes.25 However, in this strategy, the hydrophilic merit of inorganic moieties could not be profoundly exploited with the inorganic cores embedded in organic shells. For exerting fully the role of both inorganic nanofillers and organic nanofillers, hierarchical hybrid nanoparticles (HHNs) with inorganic nanoparticles decorated on organic nanoparticles will become another promising class of IOH-nanofillers for preparing hybrid membranes. The unique structures of HHNs can not only better combine the excellent hydrophilic property of inorganic moieties and the interfacial compatibility of organic moieties but also provide hydrophilic hierarchical structures to hybrid membranes for enhanced antifouling properties. However, HHNs are rarely used as IOH-nanofillers for preparing antifouling hybrid membranes. The complex synthesis process can be one of the main roadblocks. The breakthrough will possibly rely on the facial, low cost, and scalable synthesis of HHNs through an advanced chemical process.
Catechol chemistry has attracted widespread interest and is available to engineer functional materials using polyphenols as starting materials.26 Tannic acid (TA) is a natural dendritic polyphenol that contains five digalloyl ester groups covalently attached to a central glucose core.27 TA can serve as a versatile building block for preparing various nanomaterials with a rich and complex spectrum of physical and chemical properties because of their important chemical versatility including self-polymerization, formation of an electrostatic interaction polyplex, and metal ion complexation.28 As a catechol derivative, TA can be oxidized and further oligomerized in mildly alkaline solutions to form nanoparticles or thin films with adhesive capacity in a manner that is similar to polydopamine formation.29 As an anionic polyelectrolyte, TA can form polyelectrolyte complexes with cationic polyelectrolytes via strong electrostatic interactions.30 As a phenolic ligand, TA can chelate metals where it acts as a polydentate ligand for the coordination of metallic oxide nanoparticles.31−33 These unique features render TA a powerful tool for functional nanomaterial engineering. It should be mentioned that the TA chemistry, different from dopamine or other chemistries, possesses more synthetic routes, faster deposition/assembly rate, lower cost, more eco-friendly features, and greater availability.9 Thus, TA chemistry may offer a simple and effective strategy in the preparation of HHNs as IOH-nanofillers for hybrid membranes.
In this study, taking advantage of the facile and versatile TA chemistry, a novel hierarchical hybrid nanoparticle was synthesized and mixed with PVDF to fabricate hybrid membranes for antifouling purposes. First, the TA/PEI nanoconjugates (TA/PEI NCs) formed rapidly via the strong electrostatic complex between anionic TA and cationic PEI and then covalently cross-linked into TA/PEI nanoparticles (TA/PEI NPs) in alkaline solutions. Then, the TiIV–TA coordination favored the biomimetic mineralization of TiO2 nanoparticles on the TA/PEI NPs, and thus TA/PEI@TiO2 hierarchical hybrid nanoparticles (TPTi HHNs) were obtained. The obtained TPTi HHNs were incorporated into PVDF as IOH-nanofillers to prepare PVDF/TPTi hybrid membranes via the phase inversion method. The effects of TPTi HHNs on membrane morphology, chemical composition, surface hydrophilicity, surface energy oleophobicity, and permeation performance were evaluated. In addition, the nanoenhanced antifouling property of the PVDF/TPTi hybrid membranes was assessed and discussed in terms of their resistance to oil fouling with time for oily wastewater separation.
2. Results and Discussion
2.1. Formation Mechanism of TPTi HHNs
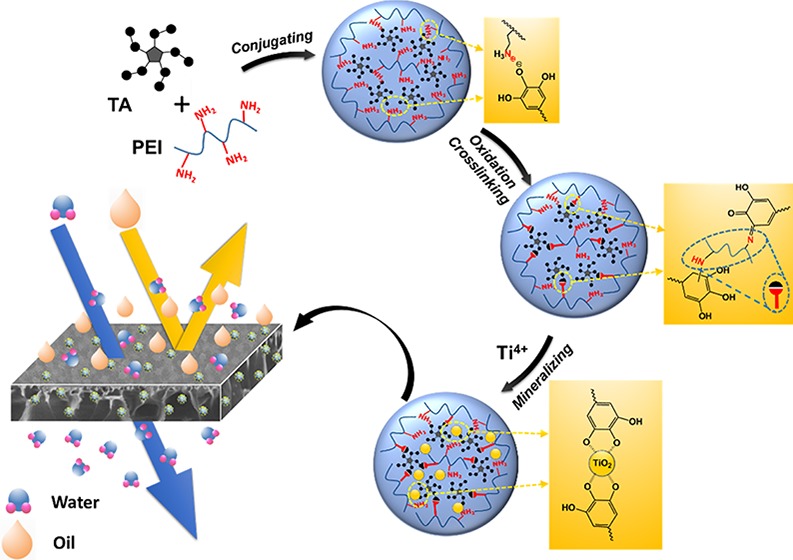
Figure 1a describes the fabrication mechanism of the TPTi HHNs. Firstly, due to the strong electrostatic interaction, anionic TA and cationic polymer PEI dispersed in aqueous solution could quickly form the polyelectrolyte TA/PEI nanoconjugates when they are mixed together.34 With the addition of sodium hydroxide, the solution environment was changed to alkaline, which could trigger the oxidation and self-polymerization reaction of TA and form a large amount of quinone groups. PEI had a higher cation density, and the reactive secondary amino group of PEI could easily undergo Michael addition or Schiff base reaction with the quinone group to form the cross-linked TA/PEI nanoparticles.35−37 The large number of phenolic hydroxyl groups led to TA–metal chelating features and promoted chelating sites for Ti4+ ions. This metal coordination was beneficial to the biomimetic mineralization of titania. The amino group in PEI adsorbed and concentrated with the negatively charged Ti-BALDH, and hydrogen bonding between Ti–OH of Ti-BALDH and the lone electron of nitrogen in PEI favored the nucleophilic substitution of the Ti–O bonds with adjacent Ti-BALDH molecules.38 Subsequent polycondensation of the titanium precursor led to the in situ formation of TiO2 nanoparticles. The complexation of Ti4+ ions with TA molecules contributed to the acceleration of the polycondensation and the anchorage of TiO2 nanoparticles on the TA/PEI nanoparticles. The TPTi HHNs were thus synthesized and could be directly introduced into the casting solution to fabricate hybrid membranes.
Figure 1.
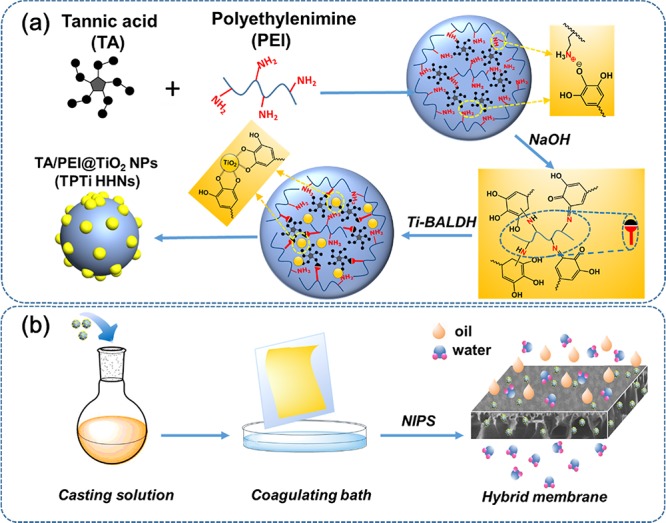
(a) Formation mechanism of TPTi HHNs and (b) preparation process of PVDF/TPTi hybrid membranes.
2.2. Characterization of TPTi HHNs
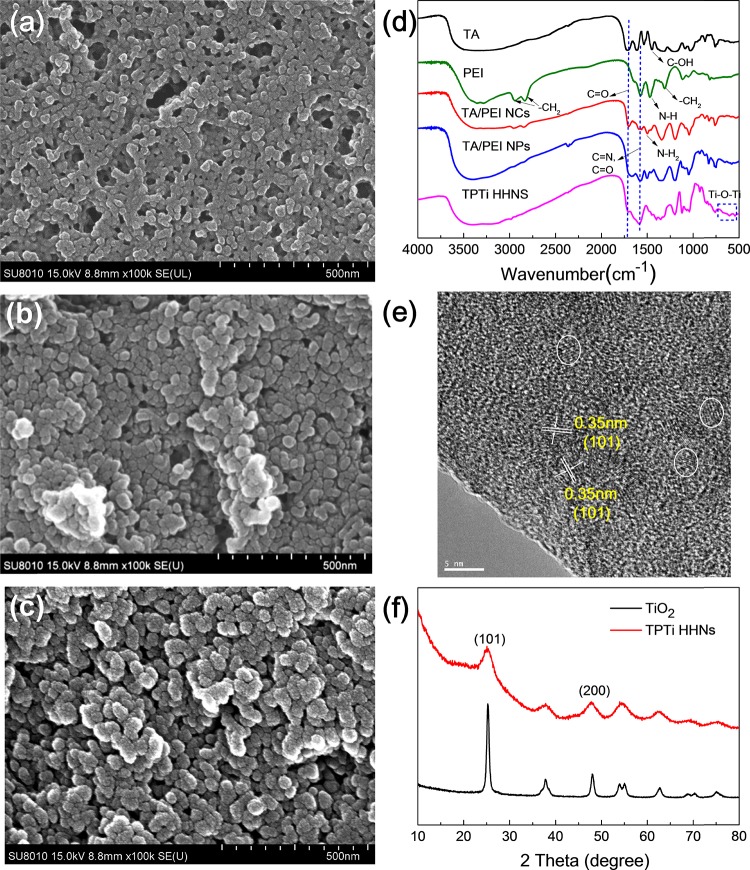
Field emission scanning electron microscopy (SEM) was used to present the surface morphology of the synthesized nanoparticles. As shown in Figure 2a, the TA/PEI nanoconjugates formed by electrostatic interaction had a relatively smooth surface, and its particle size was around 20 ± 11 nm. With the oxidation and self-polymerization reaction of tannic acid and the Michael addition reaction with PEI, the particle size of TA/PEI nanoparticles becomes larger (Figure 2b), reaching about 43 ± 13 nm. Figure 2c shows the synthesized TPTi HHNs, and the overall particle size was around 64 ± 20 nm. It could be seen that the surface of the particle had a large number of punctiform spheres, which proved to be TiO2 nanoparticles, so the TPTi HHNs became rough and hierarchical compared with the forward TA/PEI nanoconjugates and TA/PEI nanoparticles.
Figure 2.
SEM images of (a) TA/PEI nanoconjugates (TA/PEI NCs), (b) TA/PEI nanoparticles (TA/PEI NPs), and (c) TPTi HHNs. (d) FTIR spectra for TA, PEI, TA/PEI NCs, TA/PEI NPs, and TPTi HHNs. (e) TEM image of TPTi HHNs. (f) XRD patterns of TPTi HHN and commercial powder of TiO2 nanoparticles.
The successful synthesis of TPTi HHNs was confirmed by Fourier transform infrared spectroscopy (FTIR) of TA, PEI, TA/PEI nanoconjugates, TA/PEI nanoparticles, and TPTi HHNs, which are presented in Figure 2d. The FTIR spectra of TA molecules presented a C=O peak at 1702 cm–1 alongside some characteristic peaks of phenolic OH peaks between 3600 and 3000 cm–1.39 The peak at 1447 cm–1 was attributed to symmetrical stretching vibrations of the carboxyl groups. Besides, the N–H stretching from −NH2 groups was at 3200–3400 cm–1, the C–H stretching from −CH2 groups was at 2936 and 2833 cm–1, the N–H bending from −NH2 and −NH groups was at 1581 cm–1, and the in-plane bending vibration peak of −CH2 was at 1466 cm–1; all of the above peaks were derived from PEI. Similarly, the CH2 and C=O peaks could be both found in TA/PEI nanoconjugates and TA/PEI nanoparticles. The peaks at 1504 and 1581 cm–1 could be ascribed to NH2 and N–H, all of them also appearing in both TA/PEI NPs and PTA/PEI NPs. The smaller peaks of phenolic OH peaks indicated the electrostatic interaction between TA and PEI. It could be seen that new peaks arise at 1665 cm–1 due to C=N (Schiff base reaction) and C=O (quinone) in TA/PEI nanoparticles.40 The NH2 complex or pyrogallol–Ti4+ coordination appeared around 1330–1550 cm–1, and Ti–O–Ti stretching vibration appeared around 500–700 cm–1 in TPTi HHNs.41 The above infrared spectrum results were consistent with the reaction mechanism.
The high-resolution transmission electronic microscopy (TEM) image of TPTi HHNs (Figure 2e) showed ultrasmall nanocrystals (3–5 nm) with a spacing of 0.35 nm corresponding to d101 of the anatase TiO2 crystal phase. The crystal phase of TiO2 nanoparticles on TPTi HHNs produced by biomimetic mineralization was further confirmed by XRD diffraction, and the result is shown in Figure 2f. The preferred orientation corresponding to the (101) plane was observed for TPTi HHNs and commercial powder of TiO2 NPs; all of the peaks in the X-ray diffraction (XRD) spectrograph could be indexed to the anatase phase of TiO2, and the result is consistent with the TEM image.
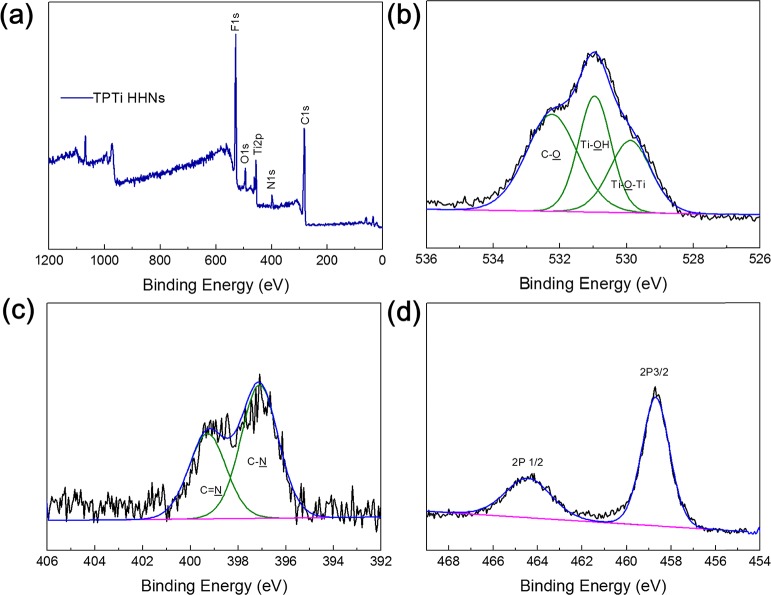
To further explore the formation mechanism of TPTi HHNs, X-ray photoelectron spectroscopy (XPS) was carried out on TPTi HHNs. Figure 3a shows the XPS wide-scan spectra of TPTi HHNs; the main peaks exhibited by the particles at 284.6, 401, and 531.3 eV were from C 1s, N 1s, and O 1s, respectively. The appearance of the Ti 2p peak at 415 eV meant that there was a titanium component. Figure 3b shows the high-resolution XPS spectra for the O 1s region of which the major peaks of Ti–O–Ti, Ti–OH, and C–O (from TA) are observed in the spectra at their typical positions of 529.8, 531.4, and 532.9 eV, respectively. Moreover, Figure 3c shows the high-resolution XPS spectrum of the N 1s region of TPTi HHNs, and the existence of the C=N bond proved that a Schiff base reaction of amino groups and quinone groups occurred, which was in accordance with the FTIR results. From the Ti 2p core-level photoelectron spectrum (Figure 3d), Ti 2p3/2 and Ti 2p1/2 peaks were observed at binding energies of 459.4 and 465.1 eV, respectively, and were assigned to Ti4+ species in the TPTi HHNs.42 The XPS results demonstrated that TPTi HHNs were successfully synthesized, which was consistent with previous analysis.
Figure 3.
(a) XPS wide-scan spectra and the XPS spectrum of the (b) O 1s, (c) N 1s, and (d) Ti 2p regions of the TPTi HHNs.
2.3. Characterization of PVDF/TPTi Hybrid Membranes
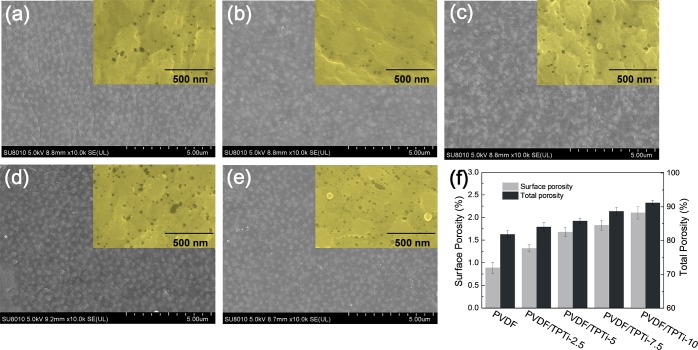
PVDF/TPTi hybrid membranes were fabricated incorporating TPTi HHNs into the PVDF matrix via the phase inversion method. The structures of the PVDF membrane and PVDF/TPTi hybrid membranes were studied using SEM. As seen in Figure 4, all membranes showed a typical porous structure with a uniform pore distribution and a pore size of approximately 40 nm. Figure 4a shows the SEM images of the PVDF membrane, which was relatively clean and smooth. After the TPTi HHNs were added to the casting solution, the particles could be clearly seen on the surface of the PVDF/TPTi hybrid membranes. The number of particles on the surface of PVDF/TPTi hybrid membranes also increased with the TPTi HHN loading increasing. Using the image processing tool (ImageJ) on the SEM images of the membrane top surface,43 the surface porosity data of the membrane was obtained (Figure 4f). The total porosity (gravimetric method) of different membranes is shown in Figure 4f. The total porosity and surface porosity of the membranes were increased with increasing TPTi HHN loading. This suggests that the TPTi HHNs in membrane casting solution could promote the exchange rate of solvent and water during the phase inversion and promote pore forming.44
Figure 4.
SEM images of the surface morphologies of PVDF/TPTi hybrid membranes with different TPTi HHN loadings: (a) PVDF; (b) PVDF/TPTi-2.5; (c) PVDF/TPTi-5; (d) PVDF/TPTi-7.5; and (e) PVDF/TPTi-10. (f) Surface porosity and total porosity data of the PVDF/TPTi hybrid membranes. The insets in the pictures show the enlarged SEM images of membranes.
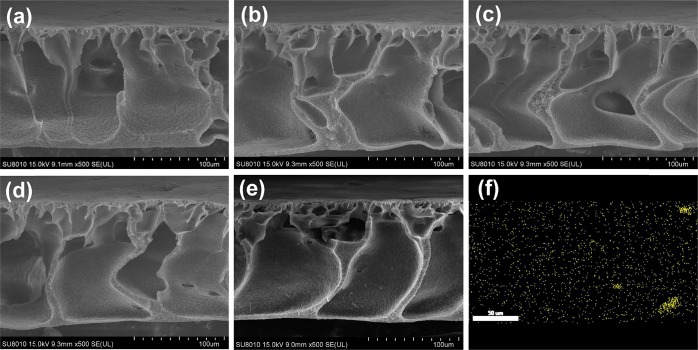
Figure 5 shows the cross-sectional SEM images of the pure PVDF membrane and PVDF/TPTi hybrid membranes doped with different contents of TPTi HHNs. The cross-sectional image of membranes showed a typical asymmetrical structure including a dense skin layer supported by large cavity structures in the sublayer. Compared with the pure PVDF membrane, with the TPTi HHN loading increasing, the volume of the cavity in the sublayer in the PVDF/TPTi hybrid membranes gradually increased. During the phase inversion process, the pore former PEG could be dissolved and diffuse in water (nonsolvent) and form the growth point of the pores. The further diffusion of water (nonsolvent) into the channels inside the membrane favored the growth of large cavities in the membrane matrix in the subsequent process. Ti element analysis with the cross-sectional EDX mapping image of PVDF/TPTi-10 (Figure 5f) demonstrated that the TPTi HHNs were successfully loaded onto the PVDF/TPTi-10 hybrid membrane with homogeneous distribution. The evenly distributed TPTi HHNs have greater potential to further improve the permeability and hydrophilicity of PVDF/TPTi hybrid membranes. In addition, the hybrid membranes exhibited excellent mechanical properties, despite the large cavity structures in the sublayer (Figure S1).
Figure 5.
Cross-sectional SEM images of the membranes with different TPTi HHN loadings: (a) PVDF; (b) PVDF/TPTi-2.5; (c) PVDF/TPTi-5; (d) PVDF/TPTi-7.5; and (e) PVDF/TPTi-10. (f) Ti element analysis with the cross-sectional EDX mapping image of the PVDF/TPTi-10 membrane.
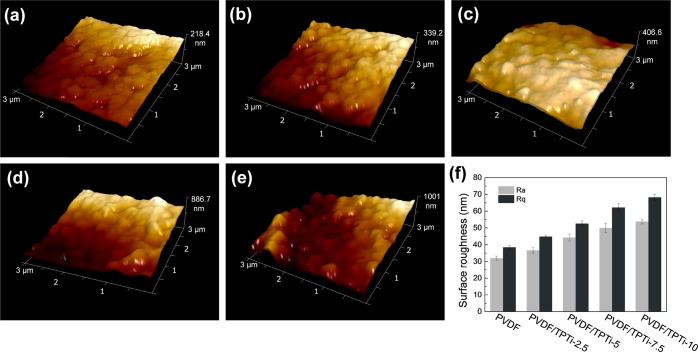
To describe the changes in topography and roughness of membranes, AFM imaging was performed at multiple locations across the sample. Figure 6 shows tapping mode AFM images of the PVDF membrane and hybrid membranes loaded with TPTi HHNs from 2.5 to 10% at a scan size of 3 × 3 μm2. The PVDF membrane had a mean surface roughness (Ra) of 32.1 nm, and the Ra of PVDF/TPTi-2.5, 5, 7.5, and 10 membranes were 36. 7, 44. 3, 50. 1, and 53. 8 nm, respectively. Similar to the mean surface roughness data, the data of the root-mean-square roughness showed an increasing trend as the dose of TPTi HHNs increases. The Rq of the PVDF/TPTi-10 membrane reached 68.2 nm. This was mainly attributed to the migration of TPTi HHNs to the membrane surface during the phase separation process, which was in good agreement with SEM results. Moreover, the effect of porosity on membrane roughness was also negligible. The changes in the surface roughness of membranes showed a positive correlation with changes in the surface porosity (Figure 4f). The morphology and roughness of membrane surface were of great importance for the antifouling performance of the membrane. The TPTi HHNs induced hierarchical bulge structures on the surface of PVDF/TPTi membranes, which would play an indispensable role in resisting oil fouling.
Figure 6.
AFM images of the membranes with different TPTi HHN loadings: (a) PVDF; (b) PVDF/TPTi-2.5; (c) PVDF/TPTi-5; (d) PVDF/TPTi-7.5; and (e) PVDF/TPTi-10. (f) Surface roughness of the PVDF/TPTi hybrid membranes.
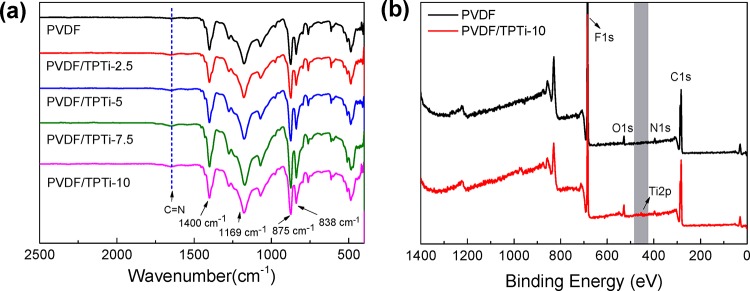
FTIR and XPS were used to verify the surface chemical composition of the PVDF membrane and PVDF/TPTi hybrid membranes. The FTIR spectra results of membranes are presented in Figure 4a. The peaks at 1400, 1178 and 878, and 838 cm–1 were assignable to −CH2, −CF2, and vibration of the crystal phase absorption peak, respectively. These peaks were characteristic peaks of PVDF. A new peak was observed at 1660 cm–1 in the spectra of the PVDF/TPTi hybrid membranes, which was attributed to the C=N/C=O stretching vibrations of PTA/PEI@TiO2 TPTi HHNs. The result was consistent with the FTIR spectra image of TPTi HHNs. With the increase in the loading of TPTi HHNs, the intensity of the C=N/C=O stretching vibrations peak was also increased, indicating that the TPTi HHNs were indeed introduced into the membrane system. According to the XPS wide spectra in Figure 7b, the PVDF membrane exhibited some peaks at 284.6, 531.2, and 684.0 eV, and these peaks were attributed to C 1s, O 1s, and F 1s, respectively. To compare, the PVDF/TPTi hybrid membranes displayed new peaks of N 1s and Ti 2p, which were located at 401.2 and 455.9 eV, demonstrating that there were TPTi HHNs distributed on the surface of the membrane, which corresponded to the SEM results.
Figure 7.
(a) FTIR spectra for the PVDF membrane and PVDF/TPTi hybrid membranes loaded with TPTi HHNs from 2.5 to 10%, and (b) XPS wide-scan spectra of the PVDF membrane and the PVDF/TPTi-10 membrane.
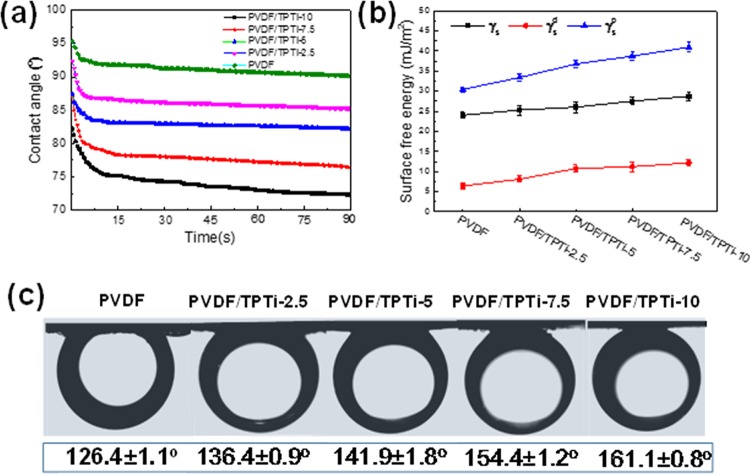
To demonstrate the wettability of the membranes, time-dependent water contact angles of the PVDF membrane and PVDF/TPTi hybrid membranes were investigated (Figure 8). The water contact angle of the pristine PVDF membrane was about 95°, and little change was observed for the water contact angle after 90 s, demonstrating the high hydrophobicity. PVDF/TPTi hybrid membranes exhibited smaller original water contact angles, with the decrease rate gradually improving as the loading of nanoparticles increased. The contact angle of PVDF/TPTi-10 was only 72° after 90 s, which was 20° smaller than that of the PVDF membrane, indicating that the hydrophilic feature of TPTi HHNs could endow the hybrid membranes with better hydrophilicity. The Ti–OH groups located on TPTi HHNs were beneficial to attracting water molecules to form hydration microdomains on membrane surfaces, which was better able to enhance hydrophilicity. The increased surface energy of PVDF/TPTi hybrid membranes from 30.4 (PVDF membrane) to 40. 9 mJ/m2 could also be attributed mainly to the strengthened interaction between water molecules and membrane surfaces. The higher surface energy and hydration structures promised to improve the antifouling tendency of PVDF/TPTi hybrid membranes. The underwater oil contact angles of the PVDF membrane and the PVDF/TPTi hybrid membranes are shown in Figure 8c. The underwater oil contact angles increased from 126.4° for the PVDF membrane to 161.8° for the PVDF/TPTi-10 membrane. The TPTi-induced hydration microdomains on membrane surfaces could prevent the oil droplet from spreading on the membrane surfaces. Therefore, the introduction of TPTi HHNs endowed the membrane with a superoleophobic ability to repel oil droplets.
Figure 8.
(a) Water contact angle decaying with drop age, and (b) surface energy and (c) underwater oil contact angles of the PVDF membrane and PVDF/TPTi hybrid membranes loaded with TPTi HHNs from 2.5 to 10%.
2.4. Filtration and Antifouling Performance of PVDF/TPTi Hybrid Membranes
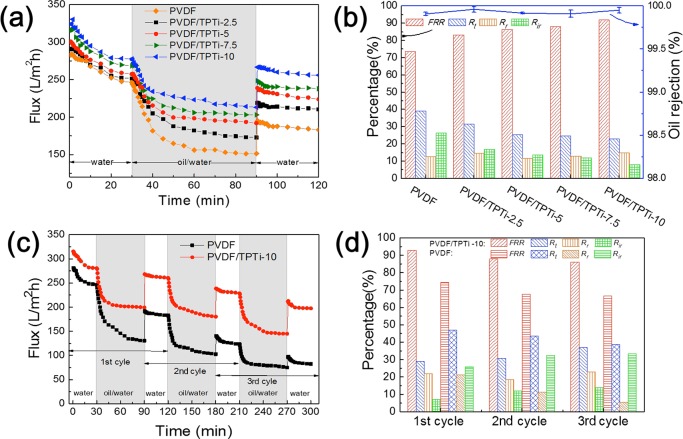
Considering oils are the common contaminant in wastewater, emulsified hexadecane was chosen to investigate the anti-oil-fouling performance of the membranes. Figure 9a depicts the time-dependent fluxes of the PVDF membrane and the PVDF/TPTi hybrid membranes during oil/water emulsion filtration. Three steps to form the filtration recycles are as follows: (1) 30 min of deionized water permeation, (2) the filtration of emulsified hexadecane (1 h), and (3) hydraulic cleaning for a short period of time and testing the permeation again with pure water for 30 min. It could clearly be seen that with the addition of TPTi HHNs, the flux of the PVDF/TPTi hybrid membranes increased from 248.8 L/m2 h for the PVDF membrane to 278.3 L/m2 h for the PVDF/TPTi-10 membrane. This result was because the membrane formation process can benefit from the introduction of HHNs by increasing the connectivity between the holes and the number of surface pores for greater hydrophilicity permeation flux.45 During emulsion filtration (Figure 9a), the flux sharply reduced in the beginning because of the oil deposition and spreading. However, by comparing with the PVDF membrane, the flux decline rates of PVDF/TPTi hybrid membranes were significantly reduced, which was primarily because the underwater oleophobicity of the hybrid membrane was improved. After being subjected to hydraulic cleaning, the membranes exhibited different extents of flux recovery upon TPTi HHN loading. Because the membranes had submicron pore size, all membranes exhibit more than 99.9% rejection of emulsified oil. The initial oil/water emulsion was milky with numerous micrometer and submicrometer oil droplets, and the permeate became transparent after filtration. No droplets could be observed in the corresponding optical microscopy images after the separation, which further confirmed the high separation efficiency of the membrane (Figure S2).
Figure 9.
Time-dependent fluctuation of pure water flux and oil/water emulsion filtration flux in three cycles and corresponding antifouling parameters: (a, b) PVDF membrane and PVDF/TPTi hybrid membranes with different TPTi HHN loadings for 1 g/L oil/water emulsion filtration and (c, d) PVDF/DA membrane and PVDF/TPTi-10 membrane for three-cycle filtration of 5 g/L oil/water emulsion.
The calculated FRR, Rt, Rr, and Rir were used as the main parameters to investigate the anti-oil-fouling performance of the membrane. The results are shown in Figure 9. Obviously, by comparing with the PVDF membrane, all hybrid membranes exhibited lower Rir and higher FRR. Among the hybrid membranes, the PVDF/TPTi-10 membrane had the best antifouling performance with FRR as high as 92% and Rir as low as 8%. Nevertheless, the FRR and Rir of the PVDF membrane were 73 and 27%. The total flux decline ratio of the PVDF/TPTi-10 membrane was 26% as compared to the 39% Rt of the PVDF membranes. This result illustrated that the PVDF/TPTi hybrid membranes have a good anti-oil-fouling performance. To provide insight into the mechanism of the oil-in-water emulsion separation, a schematic illustration of the proposed antifouling mechanism is provided in Figure 10. The oil droplets were rejected by the submicrometer porous skin layer. The Ti–OH groups located on TPTi HHNs were beneficial to attracting water molecules to construct a stable hydrated sheath to prevent oil adhesion.46,47 In addition, the TPTi HHNs favored the formation of hydration microdomains around the hierarchical bulge structures on the surface of PVDF/TPTi membranes, which made the oil surface adhesion occur in a thermodynamic inverse state and further reduced the contact/fouling sites among oil droplets and the membrane surface.48 The anti-oil-fouling performance of PVDF/TPTi hybrid membranes was due to the synergistic effect of the above factors.
Figure 10.
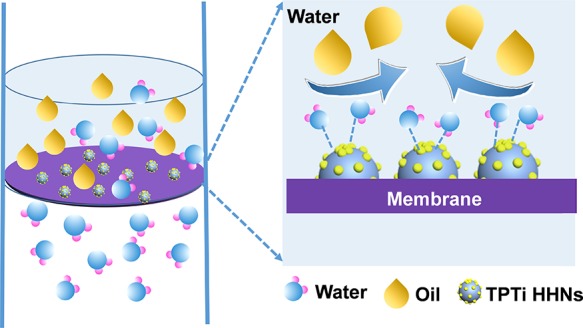
Schematic illustration of the antifouling mechanism of hybrid membranes during oil/water separation.
To investigate the durability and reusability of the membrane with good anti-oil-fouling performance, we used 5 g/L emulsified hexadecane as a model foulant and implemented a three-cycle filtration test, the results of which are shown in Figure 9c,d. After three cycles of testing, the flux recovery of the PVDF membrane was as low as 66%, indicating that the performance of the PVDF membrane was significantly influenced by oil fouling. In contrast, the PVDF/TPTi-10 membrane can reach a flux recovery rate of 86%. The results of three-cycle fouling demonstrated the excellent stability and durability of the PVDF/TPTi membranes.
3. Conclusions
In this study, TPTi HHNs were synthesized and introduced into a PVDF casting solution to prepare antifouling PVDF/TPTi hybrid membranes by means of a phase inversion method. The incorporation of hydrophilic TPTi HHNs into the PVDF matrix endowed the PVDF/TPTi hybrid membranes with higher hydrophilicity and underwater superoleophobicity, thus improving the antifouling performance of the membranes. In general, when the content of TPTi HHNs is 10 wt %, the PVDF/TPTi-10 hybrid membrane has superior performance: a pure water flux as high as 278.3 L/m2 h, a rejection of emulsified oil of more than 99.9%, and upon separating the 1 g/L emulsion, a flux recovery ratio up to 92%. Moreover, good and reliable stability and durability of the antifouling property of PVDF/TPTi membrane (flux recovery ratio higher than 85%) was also observed for long-term operation with emulsified hexadecane (5 g/L) after three cycles. Therefore, the detailed results highlighted that this work provided a candidate strategy for the design of an antifouling hybrid membrane based on hierarchical hybrid nanoparticles.
4. Experimental
4.1. Materials
PVDF (FR-904) was supplied by Shanghai 3F New Material Co. Ltd. TA, PEI (MW = 600), and titanium (IV) bis-(ammonium lactate) dihydroxide (Ti-BALDH, 50 wt% aqueous solution) were supplied by Sigma-Aldrich Co. and used as received. 1-Methyl-2-pyrrolidone (NMP) was supplied by Aladdin. n-Hexadecane (HD) was obtained from J&K Scientific Ltd. Sodium dodecyl sulfate (SDS) was purchased from Sigma-Aldrich. Deionized (DI) water (≤5 μS cm–1) was produced using a two-stage reverse osmosis system.
4.2. Preparation of TA/PEI@TiO2 Hierarchical Hybrid Nanoparticles (TPTi HHNs)
TA (0.2 g) and PEI (50 mg) were each dissolved in water (50 mL). Then, the two solutions were mixed together, and a milky dispersion of TA/PEI nanoconjugates (TA/PEI NCs) was obtained. Afterward, 0.8 mL of NaOH (1 mol/L) was added to the TA/PEI NC dispersion. The dispersion was further stirred at room temperature for 3 h at 300 rpm, and a dispersion of TA/PEI nanoparticles (TA/PEI NPs) was obtained. Finally, Ti-BALDH (0.5 mL) was added to the TA/PEI NP dispersion. After further stirring for another 2 h, the dispersion of TPTi HHNs was thus obtained. Figure 1 presents a schematic diagram of the preparation procedure of TPTi HHNs. The prepared TPTi HHNs were characterized by FTIR, SEM, and X-ray diffraction (XPert Pro).
4.3. Fabrication of Hybrid Membranes
The nascent PVDF membrane and hybrid membranes were fabricated in a coagulation bath by nonsolvent-induced phase inversion (NIPS):49 First, given precise amounts of TPTi HHNs based on the weight of polymer and PEG were dispersed in NMP to prepare a homogeneous mixture under ultrasound treatment for 30 min. After sonication, PVDF was led into the solution by mechanical stirring at 60 °C for 6 h. To guarantee that the bubbles in the casting solution were sufficiently removed, the solution was left to settle at 25 °C for more than 8 h. The casting solutions were cast onto glass plates using a casting knife with a gap height of 200 μm and immediately immersed in a deionized water coagulation bath at 25 °C. Finally, the obtained membranes were washed repeatedly with deionized water and soaked in deionized water. Different membranes were made by changing the loading of the TPTi HHNs. The proportion of various components constituting the casting solution is described in Table 1. The resulting hybrid membranes with TPTi HHN loading (based on the weight of polymer) amount of 2.5, 5, 7.5, and 10 wt % were named PVDF/TPTi-2.5, PVDF/TPTi-5, PVDF/TPTi-7.5, and PVDF/TPTi-10, respectively. The pristine membrane was named PVDF.
Table 1. Proportion of Various Components Constituting the Casting Solution.
| casting
solution compositions (wt %) |
||||
|---|---|---|---|---|
| membranes | PVDF | PEG | TPTi HHNs | NMP |
| PVDF | 1 | 0.8 | 0 | 8.20 |
| PVDF/TPTi-2.5 | 1 | 0.8 | 0.025 | 8.175 |
| PVDF/TPTi-5 | 1 | 0.8 | 0.05 | 8.15 |
| PVDF/TPTi-7.5 | 1 | 0.8 | 0.075 | 8.125 |
| PVDF/TPTi-10 | 1 | 0.8 | 0.1 | 8.1 |
4.4. Membrane Characterization
The obtained hybrid membranes were characterized by field emission scanning electron microscopy (FESEM, HitachiS-4700) to ascertain cross-sectional and surface morphology. Atomic force microscopy (AFM, Dimension Icon) was carried out to characterize the surface roughness of hybrid membranes (scan area range of 3 μm × 3 μm). The chemical structures of the PVDF membrane and PVDF/TPTi HHNs were characterized by Fourier transform infrared spectroscopy (FTIR, Nicolet iS50) and X-ray photoelectron spectroscopy (XPS, KRATOS AXIS ULTRA). The static water contact angles of the membrane surface were determined using a JC2000C contact angle goniometer. The reported contact angle values and standard deviations were based on at least five measurements of each membrane. The total surface tension (γs) of membrane surfaces and the polar (γsp) and dispersive (γs) components were quantified using the Owens, Wendt, Rabel, and Kaelble method employing two probe liquids (water and diiodomethane) with known surface tension component parameters.50
| 1 |
| 2 |
where θ refers to the contact angle of water and diiodomethane and γl, γld, and γl represent total, polar, and dispersive surface energy of the test liquid, respectively. The total porosities of the hybrid membranes were measured via gravimetric method by the following formula
| 3 |
where ε (%) is the porosity of the membrane, Ww (g) is the wet sample weight Wd (g) is the dry sample weight, ρw (g/cm3) is the density of pure water, A (cm2) is the area of membrane in the wet state, and δ0 (cm) is the thickness of the membrane in the wet state.
4.5. Filtration Performance of the Hybrid Membranes
The dead-end filtration cell (Amicon UFSC05001 Millipore Co., effective area of 13.4 cm2) was used to test the permeation of the prepared membranes at a transmembrane pressure of 0.05 MPa. Before the test, all samples were filtered with deionized water for at least 30 min (0.05 MPa) for compaction and stabilization. The pure water flux J1 (L/(m2 h)) is defined by the following equations
| 4 |
where V (L) is the volume of permeated water, A (m2) is the membrane effective area, and Δt (h) is the permeation time.
4.6. Oil-in-Water Emulsion Preparation and Separation Experiment
Hexadecane and water were mixed in a ratio of 1:99 (v/v) with the addition of 1 g/L SDS. The mixed solution was ultrasonicated for 30 min to obtain an oil-in-water emulsion with a drop size of about 300 nm (Figure S3). Dynamic light scattering with Zetasizer Nano ZS90 (Malvern, UK) was used to characterize the droplet sizes and size distributions of oil-in-water emulsions. To obtain the J0 (L/(m2h)) of membranes, the deionized water was poured into the dead-end filtration cell and tested for 30 min, followed by oil-in-water emulsion for the next 60 min, and the flux of oil-in-water emulsions (J1) was recorded, Then, the membranes were cleaned with DI water for 30 min, and lastly the flux of the cleaned membrane (J2) was measured again for another 30 min with DI water.
The flux recovery ratio (FRR), the total flux decline ratio (Rt), reversible flux decline ratio (Rr), and irreversible flux decline ratio (Rir) of membranes were calculated using eqs 5, 6, 7, and 8 to evaluate their antifouling properties:51
| 5 |
| 6 |
| 7 |
| 8 |
The separation efficiency (R, %) of the hybrid membranes was calculated using the equation
| 9 |
where Cp (g/L) is the oil concentration of the permeating solute and Cf (g/L) is the oil concentration of the feed solute. The concentrations of oil-in-water emulsion were quantified using TOC (TOC-LCPH).
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant no. 21706230), the Zhejiang Provincial Natural Science Foundation of China under grant no. LQ17B060002, and the National Key Research and Development Program of China (grant nos. 2016YFC0401508 and 2017YFD0400604).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b03408.
Stress–strain curve of membranes, photographs and microscopy images of oil-in-water emulsion, and particle size distribution of 1 g/L emulsified oil (PDF)
Author Contributions
⊥ X.Z. and N.J. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Gupta R. K.; Dunderdale G. J.; England M. W.; Hozumi A. Oil/water separation techniques: a review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. 10.1039/C7TA02070H. [DOI] [Google Scholar]
- Zhu Y.; Xie W.; Zhang F.; Xing T.; Jin J. Superhydrophilic in-situ-cross-linked zwitterionic polyelectrolyte/PVDF-blend membrane for highly efficient oil/water emulsion separation. ACS Appl. Mater. Interfaces 2017, 9, 9603–9613. 10.1021/acsami.6b15682. [DOI] [PubMed] [Google Scholar]
- Lee C. H.; Tiwari B.; Zhang D.; Yap Y. K. Water purification: oil–water separation by nanotechnology and environmental concerns. Environ. Sci.: Nano 2017, 4, 514–525. 10.1039/C6EN00505E. [DOI] [Google Scholar]
- Fane A. G.; Wang R.; Hu M. X. Synthetic membranes for water purification: status and future. Angew. Chem. Int. Ed. 2015, 54, 3368–3386. 10.1002/anie.201409783. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Liu Y.; He M.; Su Y.; Zhao X.; Elimelech M.; Jiang Z. Antifouling membranes for sustainable water purification: strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. 10.1039/C5CS00579E. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Ying Y.; Japip S.; Jiang S.-D.; Chung T.-S.; Zhang S.; Zhao D. Advanced porous materials in mixed matrix membranes. Adv. Mater. 2018, 1802401. 10.1002/adma.201802401. [DOI] [PubMed] [Google Scholar]
- Low Z.-X.; Wang Z.; Leong S.; Razmjou A.; Dumée L. F.; Zhang X.; Wang H. Enhancement of the Antifouling Properties and Filtration Performance of Poly(ethersulfone) Ultrafiltration Membranes by Incorporation of Nanoporous Titania Nanoparticles. Ind. Eng. Chem. Res. 2015, 54, 11188–11198. 10.1021/acs.iecr.5b03147. [DOI] [Google Scholar]
- Zhang J.; Wang Z.; Wang Q.; Pan C.; Wu Z. Comparison of antifouling behaviours of modified PVDF membranes by TiO2 sols with different nanoparticle size: Implications of casting solution stability. J. Membr. Sci. 2017, 525, 378–386. 10.1016/j.memsci.2016.12.021. [DOI] [Google Scholar]
- Alsohaimi I. H.; Kumar M.; Algamdi M. S.; Khan M. A.; Nolan K.; Lawler J. Antifouling hybrid ultrafiltration membranes with high selectivity fabricated from polysulfone and sulfonic acid functionalized TiO2 nanotubes. Chem. Eng. J. 2017, 316, 573–583. 10.1016/j.cej.2017.02.001. [DOI] [Google Scholar]
- Pang R.; Li X.; Li J.; Lu Z.; Sun X.; Wang L. Preparation and characterization of ZrO2/PES hybrid ultrafiltration membrane with uniform ZrO2 nanoparticles. Desalination 2014, 332, 60–66. 10.1016/j.desal.2013.10.024. [DOI] [Google Scholar]
- Lv Y.; Yang H.-C.; Liang H.-Q.; Wan L.-S.; Xu Z.-K. Novel nanofiltration membrane with ultrathin zirconia film as selective layer. J. Membr. Sci. 2016, 500, 265–271. 10.1016/j.memsci.2015.11.046. [DOI] [Google Scholar]
- Raturi P.; Yadav K.; Singh J. P. ZnO-nanowires-coated smart surface mesh with reversible wettability for efficient on-demand oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 6007–6013. 10.1021/acsami.6b14448. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Lü Z.; Wei C.; Yu S.; Liu M.; Gao C. Separation and antifouling properties of hydrolyzed PAN hybrid membranes prepared via in-situ sol-gel SiO2 nanoparticles growth. J. Membr. Sci. 2018, 545, 250–258. 10.1016/j.memsci.2017.09.081. [DOI] [Google Scholar]
- Han S.; Mao L.; Wu T.; Wang H. Homogeneous polyethersulfone hybrid membranes prepared with in-suit synthesized magnesium hydroxide nanoparticles by phase inversion method. J. Membr. Sci. 2016, 516, 47–55. 10.1016/j.memsci.2016.05.040. [DOI] [Google Scholar]
- Zhang X.; Lang W.-Z.; Yan X.; Lou Z.-Y.; Chen X.-F. Influences of the structure parameters of multi-walled carbon nanotubes(MWNTs) on PVDF/PFSA/O-MWNTs hollow fiber ultrafiltration membranes. J. Membr. Sci. 2016, 499, 179–190. 10.1016/j.memsci.2015.10.034. [DOI] [Google Scholar]
- Kang B.; Li Y. D.; Liang J.; Yan X.; Chen J.; Lang W. Z. Novel PVDF hollow fiber ultrafiltration membranes with antibacterial and antifouling properties by embedding N-halamine functionalized multi-walled carbon nanotubes (MWNTs). RSC Adv. 2016, 6, 1710–1721. 10.1039/C5RA24804C. [DOI] [Google Scholar]
- Miao W.; Li Z.-K.; Yan X.; Guo Y.-J.; Lang W.-Z. Improved ultrafiltration performance and chlorine resistance of PVDF hollow fiber membranes via doping with sulfonated graphene oxide. Chem. Eng. J. 2017, 317, 901–912. 10.1016/j.cej.2017.02.121. [DOI] [Google Scholar]
- Li X.; Liu Y.; Wang J.; Gascon J.; Li J.; Van der Bruggen B. Metal-organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. 10.1039/C7CS00575J. [DOI] [PubMed] [Google Scholar]
- Lin R.; Ge L.; Hou L.; Strounina E.; Rudolph V.; Zhu Z. Mixed matrix membranes with strengthened MOFs/polymer interfacial interaction and improved membrane performance. ACS Appl. Mater. Interfaces 2014, 6, 5609–5618. 10.1021/am500081e. [DOI] [PubMed] [Google Scholar]
- Sun H.; Tang B.; Wu P. Development of hybrid ultrafiltration membranes with improved water separation properties using modified superhydrophilic metal-organic framework nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 21473–21484. 10.1021/acsami.7b05504. [DOI] [PubMed] [Google Scholar]
- Li X.; Sotto A.; Li J.; Van der Bruggen B. Progress and perspectives for synthesis of sustainable antifouling composite membranes containing in situ generated nanoparticles. J. Membr. Sci. 2017, 524, 502–528. 10.1016/j.memsci.2016.11.040. [DOI] [Google Scholar]
- Jiang J.-H.; Zhu L.-P.; Zhang H.-T.; Zhu B.-K.; Xu Y.-Y. Improved hydrodynamic permeability and antifouling properties of poly(vinylidene fluoride) membranes using polydopamine nanoparticles as additives. J. Membr. Sci. 2014, 457, 73–81. 10.1016/j.memsci.2014.01.043. [DOI] [Google Scholar]
- Jo Y. J.; Choi E. Y.; Choi N. W.; Kim C. K. Antibacterial and Hydrophilic Characteristics of Poly(ether sulfone) Composite Membranes Containing Zinc Oxide Nanoparticles Grafted with Hydrophilic Polymers. Ind. Eng. Chem. Res. 2016, 55, 7801–7809. 10.1021/acs.iecr.6b01510. [DOI] [Google Scholar]
- Liu Y.; Wei R.; Lin O.; Zhang W.; Du Y.; Wang C.; Zhang C. Enhanced hydrophilic and antipollution properties of PES membrane by anchoring SiO2/HPAN nanomaterial. ACS Sustainable Chem. Eng. 2017, 5, 7812–7823. 10.1021/acssuschemeng.7b01304. [DOI] [Google Scholar]
- Zhao X.; Su Y.; Liu Y.; Zhang R.; Jiang Z. Multiple antifouling capacities of hybrid membranes derived from multifunctional titania nanoparticles. J. Membr. Sci. 2015, 495, 226–234. 10.1016/j.memsci.2015.08.026. [DOI] [Google Scholar]
- Xu L. Q.; Neoh K.-G.; Kang E.-T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018, 87, 165–196. 10.1016/j.progpolymsci.2018.08.005. [DOI] [Google Scholar]
- Ejima H.; Richardson J. J.; Liang K.; Best J. P.; van Koeverden M. P.; Such G. K.; Cui J.; Caruso F. One-Step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- Rahim M. A.; Bjornmalm M.; Suma T.; Faria M.; Ju Y.; Kempe K.; Mullner M.; Ejima H.; Stickland A. D.; Caruso F. Metal-Phenolic Supramolecular Gelation. Angew. Chem. Int. Ed. 2016, 55, 13803–13807. 10.1002/anie.201608413. [DOI] [PubMed] [Google Scholar]
- Yang H.-C.; Waldman R. Z.; Wu M.-B.; Hou J.; Chen L.; Darling S. B.; Xu Z.-K. Dopamine: just the right medicine for membranes. Adv. Funct. Mater. 2018, 28, 1705327. 10.1002/adfm.201705327. [DOI] [Google Scholar]
- Wu J.; Wang Z.; Yan W.; Wang Y.; Wang J.; Wang S. Improving the hydrophilicity and fouling resistance of RO membranes by surface immobilization of PVP based on a metal-polyphenol precursor layer. J. Membr. Sci. 2015, 496, 58–69. 10.1016/j.memsci.2015.08.044. [DOI] [Google Scholar]
- Zhao X.; Jia N.; Cheng L.; Liu L.; Gao C. Metal-polyphenol coordination networks: Towards engineering of antifouling hybrid membranes via in situ assembly. J. Membr. Sci. 2018, 563, 435–446. 10.1016/j.memsci.2018.06.014. [DOI] [Google Scholar]
- Kim H. J.; Kim D.-G.; Yoon H.; Choi Y.-S.; Yoon J.; Lee J.-C. Polyphenol/Fe-III Complex Coated Membranes Having Multifunctional Properties Prepared by a One-Step Fast Assembly. Adv. Mater. Interfaces 2015, 2, 1500298. 10.1002/admi.201500298. [DOI] [Google Scholar]
- Guo J.; Ping Y.; Ejima H.; Alt K.; Meissner M.; Richardson J. J.; Yan Y.; Peter K.; von Elverfeldt D.; Hagemeyer C. E.; Caruso F. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. 10.1002/anie.201311136. [DOI] [PubMed] [Google Scholar]
- Sahiner N.; Sagbas S.; Sahiner M.; Demirci S. Degradable tannic acid/polyethyleneimine polyplex particles with highly antioxidant and antimicrobial effects. Polym. Degrad. Stab. 2016, 133, 152–161. 10.1016/j.polymdegradstab.2016.08.012. [DOI] [Google Scholar]
- Xue S.; Li C.; Li J.; Zhu H.; Guo Y. A catechol-based biomimetic strategy combined with surface mineralization to enhance hydrophilicity and anti-fouling property of PTFE flat membrane. J. Membr. Sci. 2017, 524, 409–418. 10.1016/j.memsci.2016.11.075. [DOI] [Google Scholar]
- Lim M.-Y.; Choi Y.-S.; Kim J.; Kim K.; Shin H.; Kim J.-J.; Shin D. M.; Lee J.-C. Cross-linked graphene oxide membrane having high ion selectivity and antibacterial activity prepared using tannic acid-functionalized graphene oxide and polyethyleneimine. J. Membr. Sci. 2017, 521, 1–9. 10.1016/j.memsci.2016.08.067. [DOI] [Google Scholar]
- Liu C.; Wu L.; Zhang C.; Chen W.; Luo S. Surface hydrophilic modification of PVDF membranes by trace amounts of tannin and polyethyleneimine. Appl. Surf. Sci. 2018, 457, 695–704. 10.1016/j.apsusc.2018.06.131. [DOI] [Google Scholar]
- Tong Z.; Jiang Y.; Yang D.; Shi J.; Zhang S.; Liu C.; Jiang Z. Biomimetic and bioinspired synthesis of titania and titania-based materials. RSC Adv. 2014, 4, 12388. 10.1039/c3ra47336h. [DOI] [Google Scholar]
- Fan H.; Wang L.; Feng X.; Bu Y.; Wu D.; Jin Z. Supramolecular hydrogel formation based on tannic acid. Macromolecules 2017, 50, 666–676. 10.1021/acs.macromol.6b02106. [DOI] [Google Scholar]
- Yin J.; Zhu G.; Deng B. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. 10.1016/j.desal.2015.11.001. [DOI] [Google Scholar]
- Liu Y.; Su Y.; Guan J.; Cao J.; Zhang R.; He M.; Gao K.; Zhou L.; Jiang Z. 2D Heterostructure membranes with sunlight-driven self-cleaning ability for highly efficient oil-water separation. Adv. Funct. Mater. 2018, 28, 1706545. 10.1002/adfm.201706545. [DOI] [Google Scholar]
- Shao L.; Wang Z. X.; Zhang Y. L.; Jiang Z. X.; Liu Y. Y. A facile strategy to enhance PVDF ultrafiltration membrane performance via self-polymerized polydopamine followed by hydrolysis of ammonium fluotitanate. J. Membr. Sci. 2014, 461, 10–21. 10.1016/j.memsci.2014.03.006. [DOI] [Google Scholar]
- Cui Z.; Li X.; Zhang Y.; Wang Z.; Gugliuzza A.; Militano F.; Drioli E.; Macedonio F. Testing of three different PVDF membranes in membrane assisted-crystallization process: Influence of membrane structural-properties on process performance. Desalination 2018, 440, 68–77. 10.1016/j.desal.2017.12.038. [DOI] [Google Scholar]
- Wu H.; Liu Y.; Mao L.; Jiang C.; Ang J.; Lu X. Doping polysulfone ultrafiltration membrane with TiO2 -PDA nanohybrid for simultaneous self-cleaning and self-protection. J. Membr. Sci. 2017, 532, 20–29. 10.1016/j.memsci.2017.03.010. [DOI] [Google Scholar]
- Ko T.; Kim K.; Kim S.-K.; Lee J.-C. Organic/inorganic composite membranes comprising of sulfonated Poly(arylene ether sulfone) and core–shell silica particles having acidic and basic polymer shells. Polymer 2015, 71, 70–81. 10.1016/j.polymer.2015.06.055. [DOI] [Google Scholar]
- Qin A.; Li X.; Zhao X.; Liu D.; He C. Engineering a highly hydrophilic PVDF membrane via binding TiO2 nanoparticles and a PVA Layer onto a membrane surface. ACS Appl. Mater. Interfaces 2015, 7, 8427–8436. 10.1021/acsami.5b00978. [DOI] [PubMed] [Google Scholar]
- Shi H.; He Y.; Pan Y.; Di H.; Zeng G.; Zhang L.; Zhang C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. 10.1016/j.memsci.2016.01.053. [DOI] [Google Scholar]
- Peng Y.; Guo Z. Recent advances in biomimetic thin membranes applied in emulsified oil/water separation. J. Mater. Chem. A 2016, 4, 15749–15770. 10.1039/C6TA06922C. [DOI] [Google Scholar]
- Luo F.; Zhao Q.; Xie R.; Cao Y.; Ju X.-J.; Wang W.; Liu Z.; Chu L.-Y. Effects of fabrication conditions on the microstructures and performances of smart gating membranes with in situ assembled nanogels as gates. J. Membr. Sci. 2016, 519, 32–44. 10.1016/j.memsci.2016.07.045. [DOI] [Google Scholar]
- Owens D. K.; Wendt R. C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. 10.1002/app.1969.070130815. [DOI] [Google Scholar]
- Zhao X.; Jia N.; Cheng L.; Liu L.; Gao C. Dopamine-induced biomimetic mineralization for in situ developing antifouling hybrid membrane. J. Membr. Sci. 2018, 560, 47–57. 10.1016/j.memsci.2018.05.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.