Abstract
Two-dimensional metal carbides or nitrides (MXenes) demonstrated wide applications in energy storage, water treatment, electromagnetic shielding, gas/biosensing, and photoelectrochemical catalysis due to their higher specific surface area and excellent conductivity. They also have the advantages of flexible and adjustable components and controllable minimum nanolayer thickness. In this study, a cube-like Co3O4 particle-modified self-assembled MXene (Ti3C2) nanocomposite has been prepared successfully by a simple solvothermal method. The Co3O4 particles are well dispersed on the surface and inner layers of the Ti3C2 sheets, which effectively prevent the restacking of Ti3C2 sheets and form an organized composite structure. The physical properties of these nanocomposites were studied by using XRD, SEM, EDX, TEM, and XPS. The performance of the obtained samples was evaluated as new nanocatalysts for degrading methylene blue and Rhodamine B in batch model experiments. The prepared Mxene-Co3O4 nanocomposites can be well regenerated and reused for eight consecutive cycles, indicating potential wide applications in wastewater treatment and composite materials.
1. Introduction
Two-dimensional materials have unique electrical, optical, and mechanical properties. MXene is a new type of two-dimensional crystalline compound with graphene-like structure and novel properties.1 It is one of the stars in the field of functional materials research in recent years. It is peeled off by MAX phase etching.2 The MAX phase is a ternary-layered carbide or nitride material that combines the properties of both metals and ceramics.3 The MAX phase has the general formula Mn+1AXn, where M is a transition metal element, A is mainly a group IIIA or IVA element, X is carbon and/or nitrogen, and n = 1, 2, or 3. So far, more than 70 MAX phases have been discovered.4 The MAX phase is a hexagonal crystal structure, the M atomic layer is closely packed, the X atom is filled in the M atom octahedron, and the M atomic layer is interspersed in the A atomic layer. It could be thought that the MAX phase is bonded by a two-dimensional layered carbide/nitride to the A atomic layer. Among them, M–A is a mixture of covalent bond/metal bond/ion bond, and M–X is a covalent bond.5 Thus, the A atomic layer is removed by etching, and a two-dimensional MXene nanosheet is obtained by liquid phase stripping.6,7 In addition, the thickness of a single MXene layer can reach 1 nm, and the area diameter can reach several tens of micrometers.8 The corresponding structures of MXene are also different from MAX phases with different n values. Nineteen kinds of MXene have been successfully prepared, and dozens of MXene have predictive stability in theory; this diversified structural composition provides a broad space for its property regulation and derivative material construction.
Moreover, the Co3O4 crystal exhibited a normal spinel structure, namely, Co2+(Co3+)2O2–4, where O2– is in a densely packed cubic structure, Co2+ is located in its tetrahedral gap, and Co3+ is located in its octahedral gap with higher crystal field stabilization energy. When the air is lower than 800 °C, the properties are very stable. At room temperature, it is not easily soluble in various concentrated acids and water but could be dissolved in a hot sulfuric acid solution at a lower rate. In addition, it is also a p-type semiconductor material. It also has a very wide range of applications, such as sensors,9 supercapacitors,10 catalysts,11−19 magnetic semiconductors,20 and rechargeable battery materials.21−23 For example, Huang et al. synthesized silver nanoparticle-modified MXene composites by self-reduction reactions with enhanced catalytic performances.11 Yang et al. used a simple pyrolysis method to prepare Co3O4 nanoparticles/nitrogen-doped carbon composites with different structures for oxygen evolution.12 Chen et al. skillfully used a multistep method to synthesize mesoporous Co3O4 and carbon nanotube composites with a layered pipe structure as a negative electrode material for lithium ion batteries.13 This unique nanostructure solved the problem of volume expansion and low electron conductivity of metal oxide anodes.24 Moreover, some similar composites exhibit excellent performance in electrochemistry and wide applications.25−31
Dyes are widely used in various industries, among which methylene blue (MB) is often used in the printing and dye industry as an important target for wastewater treatment.32−39 It is reported to be carcinogenic and mutagenic, which may be harmful to plants and animals. Rhodamine B (RhB) is also a kind of widely used dye. Therefore, the removal of MB and RhB from wastewater causes widespread concern. Herein, we proposed to synthesize Co3O4 particle-modified MXene (Mxene-Co3O4) nanocomposites by an in situ solvothermal method. The Co3O4 nanocomposites were uniformly anchored on the surface of Ti3C2 sheets, which enhanced the catalytic activity. At the same time, the close interaction between the Co3O4 component and the Ti3C2 substrate promoted the performance improvement of the catalyst. The prepared Mxene-Co3O4 nanocomposites showed good performance for catalytic degradation of methylene blue and Rhodamine B as model dyes. The present work on Mxene-Co3O4 nanocomposites had demonstrated a new clue for the research field of MXene composite catalysis.
2. Results and Discussion
2.1. Characterization of MXene-Co3O4 Nanocomposites
Herein, the targeted sheet-like nanomaterial MXene-Co3O4 was prepared by a simple solvothermal method, as shown in Figure 1. The first part is the synthesis of Mxene-Co3O4 composites. MXene was made of Ti3AlC2 type MAX ceramics and etched with a mixed solution of HCl (6 M) and LiF (2.5 M) in aqueous solution under ultrasonication. The other part is the catalytic degradation of dyes, such as methylene blue (MB) and Rhodamine B (RhB), by MXene-Co3O4 nanocomposites. In this work, we chose two catalysts, namely, MXene-Co3O4 and Co3O4, and then selected the catalyzed dyes.
Figure 1.
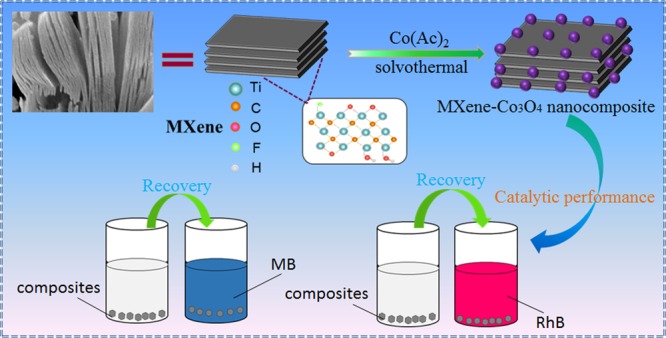
Schematic illustration of the synthesis and catalytic process of MXene-Co3O4 nanocomposites.
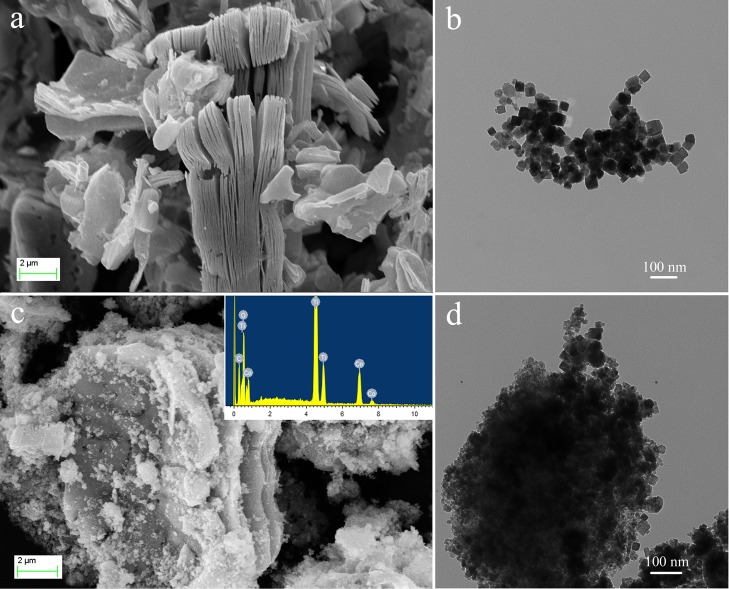
Figure 2 shows the microscopic morphology of MXene-Co3O4, Co3O4, and MXene. From Figure 2a,b, it could be seen that MXene had a well-fabricated accordion morphology. Pure Co3O4 showed a small cube type, which had an average crystallite size of 20 nm. Figure 2c,d shows the TEM and SEM images of the MXene-Co3O4 composites. It could be clearly seen that the surface of MXene was coated with a large amount of Co3O4 particles, and a small amount of Co3O4 could enter the layer of MXene, which indicated that this is beneficial to the catalytic degradation of methylene blue and Rhodamine B. It could be explained that the formed MXene-Co3O4 composite has a larger specific surface area, which was advantageous for the adsorption of dyes and could be used for catalytic experiments. Energy dispersive X-ray (EDX) spectroscopy (inset in Figure 2c) confirms that the elemental composition of the composite consists solely of carbon, titanium, cobalt, and oxygen, whose proportions were consistent to form MXene-Co3O4. In addition, Figure 3 describes the elemental mappings of the prepared MXene-Co3O4 composite. According to C, Ti, and Co images, the data further illustrated the good distribution of the Co element on the surface of MXene after the successful solvothermal process.
Figure 2.
(a) Representative SEM image of the layered MXene sample; (b) TEM image of Co3O4 sample; (c,d) SEM and TEM images of prepared MXene-Co3O4.
Figure 3.
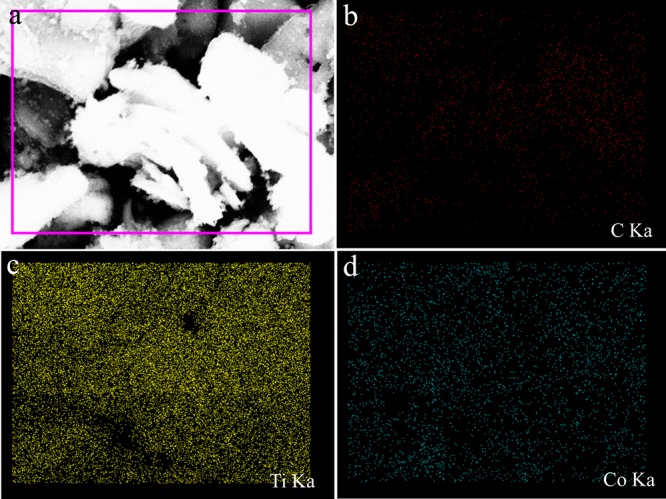
(a) Representative SEM image of the prepared MXene-Co3O4 and elemental mapping images of (b) C, (c) Ti, and (d) Co.
X-ray powder diffraction (XRD) analysis identifies the formation of MXene-Co3O4 nanocomposites, as shown in Figure 4. A set of (111), (110), (223), and (440) peaks at 17.9, 25.1, 59.8, and 64.6° appeared, respectively. The disappearance of the (006) peak from Ti3C2 MXene suggested the suppressed restacking of MXene sheets by the Co3O4 nanostructure standing on its surface (Figure 2c). The other peaks originated from MXene were quite weak even in pristine MXene, for being easily overlapped by the signals from the Co3O4 nanostructure. After the Co3O4 assembly process, three distinct characteristic peaks of Co3O4 particles could also be found in the formed composite, that is, (111), (511), and (440) crystal planes (JCPDS number 090418), indicating that a large number of Co3O4 particles were adsorbed on the surface of the MXene during the solvothermal treatment process.
Figure 4.
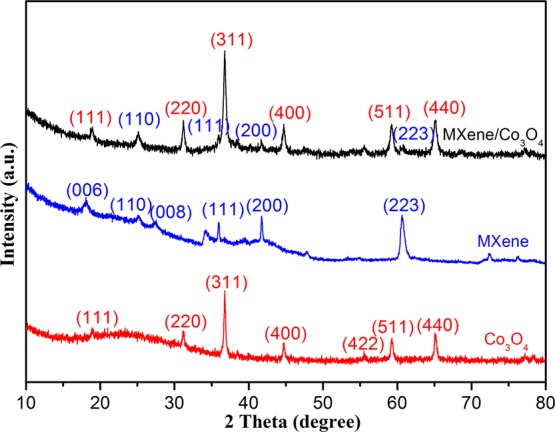
XRD curves of as-prepared MXene, MXene-Co3O4, and Co3O4.
The thermogravimetric curve showed that the mass changes with the change of temperature, as shown in Figure 5. While Co3O4 was relatively stable in the test temperature range, the weight loss was attributed to the change in Ti3C2. It could be concluded from the curve that the weight loss of as-prepared MXene-Co3O4 composites from room temperature to 200 °C was mainly due to the adsorption of water. When the temperature was gradually increased, the weight loss was mainly due to the decomposition of the oxygen-containing functional groups in which the Co3O4 and MXene complexes were bonded to each other and the decomposition of Ti3C2.
Figure 5.
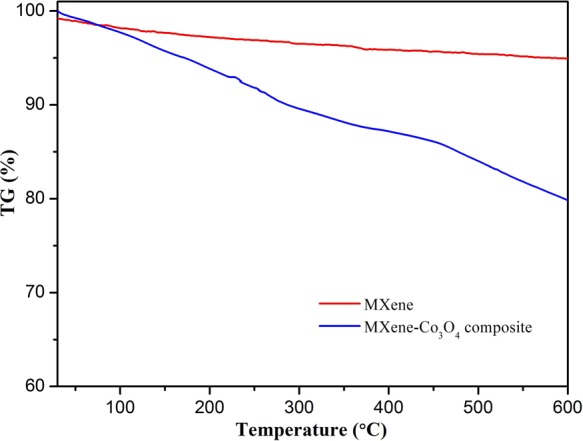
TG curves of MXene and MXene-Co3O4 nanocomposites.
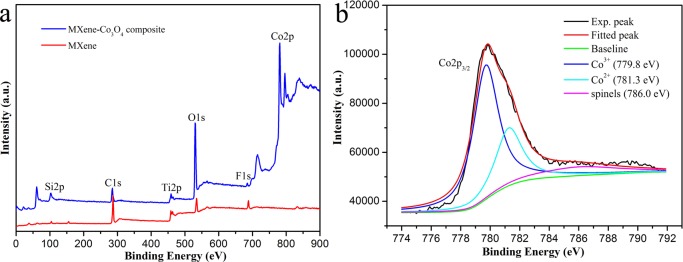
Moreover, Figure 6 shows the XPS spectra of MXene and the MXene-Co3O4 composite. Figure 6a shows the characteristic peaks in the curve of the MXene-Co3O4 nanocomposite, such as Co2p, O1s, C1s, and Ti2p. Compared with pure MXene, the peak of O1s of MXene-Co3O4 increased obviously. It could be seen that the results of XPS are consistent with the Co, C, Ti, and O elements in the elemental EDX analysis. In addition, for the Co2p XPS spectrum in Figure 6b, it showed a low energy band (Co2p3/2). This low energy band could be convolved into two peaks: 779.8 and 781.3 eV.40,41 The Co2p3/2 peak position (Figure 6b) was in accordance with the presence of Co3O4.42 The analysis results were consistent with XRD, which proved the generation of Co3O4.
Figure 6.
(a) XPS survey spectra of MXene and MXene-Co3O4 nanocomposites; (b) high-resolution scan of Co2p3/2.
2.2. Catalytic Performances of MXene-Co3O4 Nanocomposites
The catalytic performance of the obtained composites was assessed by catalytic reduction of model dyes. In the present study, the catalytic reaction was carried out in a 250 mL glass flask containing 100 mL of MB dye solution (12.5 mg/L) or 100 mL of RhB (5 mg/L) and 10 mg of catalyst in the presence of 15 mL of H2O2 (30%) at room temperature with continuous stirring. The supernatant was centrifuged at given time intervals, and the residual dye concentrations at different time intervals were investigated by UV–visible spectroscopy using different absorption wavelengths (MB 664 nm, RhB 554 nm). In this study, catalytic reductions of MB or RhB from Co3O4 particles and MXene-Co3O4 composites were also investigated. Meanwhile, the dye removal rate was calculated according to the following formula: K (%) = (A0 – AT)/A0 × 100,43 where K is defined as the dye removal rate, A0 is defined as the initial absorbance of the supernatant of the dye solution, and AT is defined as the absorbance of the supernatant of the dye solution collected at different intervals.
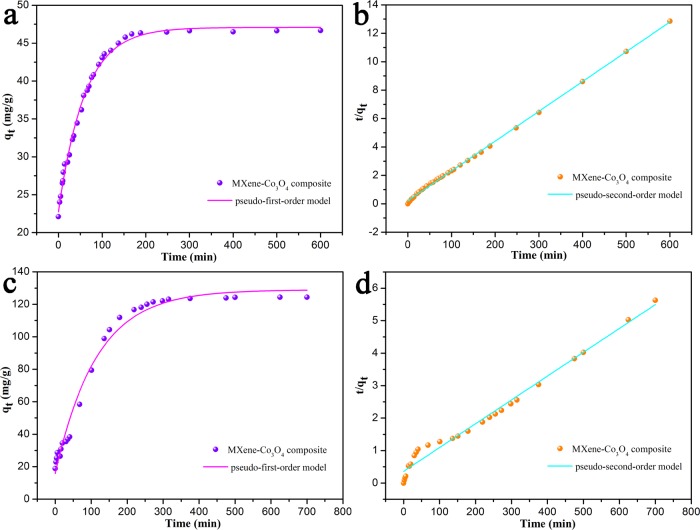
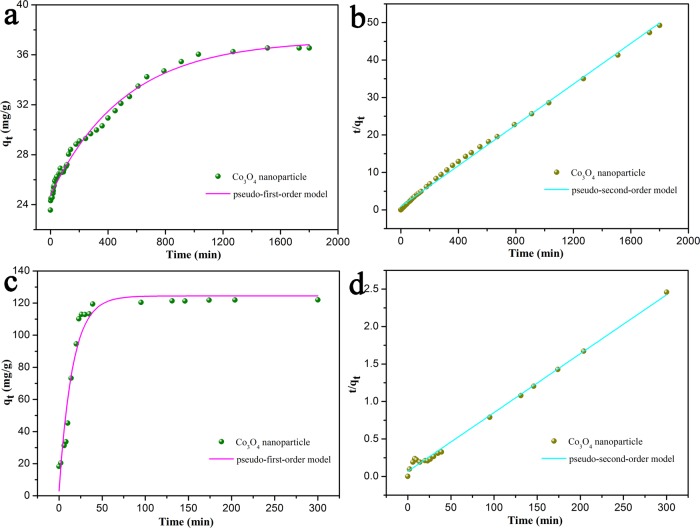
In order to compare the performance of the synthesized catalyst, we evaluated the effects of catalytic degradation of dyes (MB and RhB) using two catalysts, respectively. The adsorption kinetic experiments of the as-prepared nanocomposites were carried out using the results of RhB and MB, as shown in Figures 7 and 8. It could be seen that, for MXene-Co3O4, the removal rates of MB and RhB were stabilized in about 240 and 80 min, respectively, indicating that the prepared complex acted as an effective dye adsorbent. For Co3O4, the MB and RhB removal rates reached stable values in about 200 and 1200 min, respectively. When H2O2 was not added, it was clear that the catalyst could hardly degrade the dyes.
Figure 7.
Adsorption kinetic curves of as-prepared MXene-Co3O4 nanocomposite on RhB (a,b) and MB (c,d) at 298 K.
Figure 8.
Adsorption kinetic curves of as-prepared Co3O4 particles on RhB (a,b) and MB (c,d) at 298 K.
In addition, the adsorption kinetic process could be described by classical kinetic models as follows: The pseudo-first-order model could be represented by eq 1
| 1 |
The pseudo-second-order model could be represented by eq 2
| 2 |
where qe is the equilibrium adsorption capacity (mg/g), qt is the adsorption capacity at time t (mg/g), and k1 and k2 values are the kinetic rate constants.44−47
At the same time, Table 1 clearly shows the kinetic results of the experimental data, and the respective fitting parameters were obtained. As shown in Figure 7, by comparing the linear fitting parameters, the pseudo-first-order and pseudo-second-order kinetics of MB and RhB were catalyzed by MXene-Co3O4 and Co3O4. Based on the above data, it could be easily observed that the obtained MXene-Co3O4 composite showed the best adsorption capacity, which could be related to the synergy effect between MXene and Co3O4.
Table 1. Kinetic Parameters of the Obtained MXene-Co3O4 and Co3O4 for RhB and MB Removal at 298 Ka.
| pseudo-first-order
model |
pseudo-second-order
model |
|||||
|---|---|---|---|---|---|---|
| catalyst | qe (mg/g) | R2 | k1 (min–1) | qe (mg/g) | R2 | k2 [g/(mg·min)] |
| RhB | ||||||
| Mxene-Co3O4 | 47.076 | 0.99484 | 1.674 × 10–2 | 47.687 | 0.99910 | 1.905 × 10–4 |
| Co3O4 | 37.191 | 0.98874 | 1.940 × 10–3 | 37.037 | 0.99748 | 9.932 × 10–5 |
| MB | ||||||
| Mxene-Co3O4 | 128.91 | 0.98534 | 8.750 × 10–3 | 136.24 | 0.98391 | 6.328 × 10–2 |
| Co3O4 | 124.46 | 0.94443 | 6.354 × 10–2 | 127.23 | 0.99479 | 1.894 × 10–3 |
2.3. Reasonable Activation Mechanisms of H2O2
The discovery of antibiotics derived from natural products has aroused global attention. A promising and challenging method of antibiotic degradation is the Fenton method. This reaction can be carried out at neutral pH, which was an important advantage. A study on oxidative degradation of organic dyes with H2O2 had been reported previously.16 However, the exact mechanism for dye decomposition with H2O2 remains unclear. According to a previous study, cobalt-based Fenton/Fenton-like processes could be performed at neutral pH.18 On the one hand, it was found that homogeneous Co2+/H2O2 systems were able to degrade dye contaminants without controlling pH completely.19 On the other hand, the catalytic decomposition of organic contaminants by heterogeneous Co catalysts under the condition of adding H2O2 was also investigated. In a recent study, Co2+ adsorbed alumina was used as a heterogeneous catalyst to degrade methylene blue and methyl orange.17 In those cases, the Co-based heterogeneous catalyst could effectively degrade contaminants at neutral or even alkaline pH. Co-based heterogeneous catalysts could effectively degrade the pollutants at neutral or even basic pH.
| 3 |
| 4 |
| 5 |
In contrast, HO• produced by Co2+ mediated H2O2 activation has been recorded in only a few studies.18,21 The HO• formation mechanism by Co could be proposed as reactions 3–5. As shown in reaction 3, HO• played an important role in the Co(II)/Co(III) cycle. The ability of MXene-Co3O4 in the catalytic process of MB could be attributed to the positive synergistic effect of MXene, MB, and Co3O4 with the aid of H2O2. First, the cationic MB molecules were easily adsorbed to the surface of MXene due to electrostatic attraction, and the functional groups such as −F and −OH that were rich on the surface of MXene also endowed the excellent catalytic ability during the chemical reaction process.
This adsorption significantly improved the effective concentration of MB molecules anchored on the surface of MXene-Co3O4, leading to a high catalytic degradation rate. Second, the hydrophilicity of MXene made the MXene-Co3O4 complex well dispersed in water. Good contact between Co3O4 nanocomposites and MXene prevented Co3O4 from falling off during the catalytic process. The H2O2 was then effectively catalyzed to produce free •OH radical species and ultimately promote degradation of the MB molecules. Hence, in terms of MXene-Co3O4 composites, there was a coupling between adsorption and catalytic reactions in a single process.23 Compared to bare MXene and Co3O4, the degradations of MB and RhB were significantly increased.
Compared to other nanoparticle catalysts reported, the present prepared composite catalyst could be recovered readily from the solution.48 After reaching the adsorption equilibrium in the reaction solution, the recovered MXene-Co3O4 composite was treated by a thorough cleaning procedure to eliminate possible dye residues and regenerate the catalyst. Through repeated adsorption by using the same catalyst and fresh dye solutions, it could be used in eight consecutive cycles (Figure 9). The results showed that, after eight consecutive cycles, the MB removal rate was maintained at about 92.37%. The obtained nanocomposites performed well in terms of stability and recyclability. In addition, the reduction of MB degradation could be attributed to the loss and aggregation of the catalyst in the recycling process, as well as the adsorption of MB or intermediates. The above data indicated that the synthesized catalyst could potentially be utilized in pollutant treatment.
Figure 9.
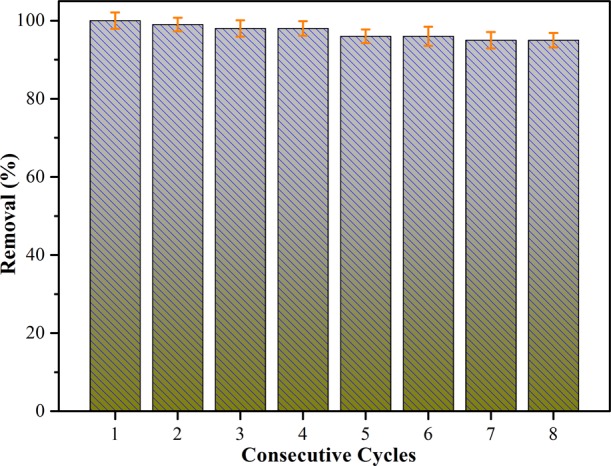
Relative regeneration studies of as-prepared MXene-Co3O4 toward MB at room temperature for different consecutive cycles.
3. Conclusions
In conclusion, we have successfully synthesized a Co3O4 nanocrystal-loaded MXene composite by simple in situ solvothermal synthesis. Due to its simple and mild reaction conditions, this simple in situ synthesis method could also be applied to the synthesis of other MXene-based transition metal oxides. The present obtained MXene-Co3O4 nanostructure has a very high MB and RhB degradation capacity and well repeatability. After eight consecutive catalytic cycles, the catalytic properties of the sample were still good, showing that the stability and repeatability of Mxene-Co3O4 nanocomposites were satisfying. The possible mechanism for adsorbing methylene blue and Rhodamine B to Mxene-Co3O4 and Co3O4 has been proposed, respectively. The research work showed that the obtained nanocomposites have good and wide applications as new catalytic composite materials.
4. Experimental Section
4.1. Materials
Ti3C2 (MXene) is obtained by etching a mixed solution of HCl (6 M) and LiF (2.5 M) using Ti3AlC2 as a raw material. Co(CH3COO)2·4H2O was provided by Aladdin Industrial Corporation, China. Hydrogen peroxide (H2O2, 30% in water) was obtained from Kermel Tianjin Chemical Reagent Co., Ltd. Methylene blue (MB) and Rhodamine B (RhB) were obtained from Hubei Mali Ltd., China, and Tianjin Kaitong Chemical Reagent Co., Ltd., respectively. Ethanol (C2H5OH) was obtained from Tianjin Kaitong Chemical Reagent Co., Ltd. Deionized (DI) water was used in all experiments.
4.2. Synthesis of the MXene-Co3O4 Nanocomposites
Original MXene (20 mg) was dissolved in 4 mL of ultrapure water, and the mixture was sonicated for 0.5 h. Then, 2 mL of 0.2 M Co(Ac)2 solution was added dropwise, 20 mL of ethanol was added, and the solution was magnetically stirred for 2 h. Then, the above reaction solution was poured into a 100 mL Teflon-lined steel autoclave. The autoclave was then heated at 120 °C for 8 h. After cooling down to room temperature and centrifugation, the final obtained precipitate was washed three times with ethanol and lyophilized to obtain the desired MXene-Co3O4 composites.
4.3. Preparation of Co3O4 Nanoparticles
The synthesis of pure Co3O4 samples was similar to the preparation of MXene-Co3O4 by reducing the addition of MXene of the same quality. Ethanol (20 mL) was added to 2 mL of 0.2 M Co(Ac)2 solution and stirred for 2 h. Then, the mixture was transferred to a 100 mL steel autoclave reactor and heated at 120 °C for 8 h.
4.4. Characterization
Thermogravimetric analysis (TGA) was tested in an argon environment by a NETZSCH STA 409 PC Luxx simultaneous thermal analyzer (Netzsch Instruments Manufacturing Co., Ltd., Germany). X-ray photoelectron spectroscopy (XPS) was measured by the Thermo Scientific ESCALab 250Xi with an Al Kα X-ray source. The adsorption experiments were monitored by using a Shimadzu UV2550 spectrophotometer. All aqueous solutions were prepared with water purified in a double-stage Millipore Milli-Q Plus purification system. The morphologies were obtained by using a transmission electron microscope (TEM) (HT7700, Hitachi High-Technologies Corporation, Japan) with an accelerating voltage of 20 kV. X-ray diffraction (XRD) analysis was recorded with an X-ray diffractometer (SMART LAB, Rigaku, Japan). The microstructures of the samples were characterized by using a field-emission scanning electron microscope (SEM) (S-4800II, Hitachi, Japan) equipped with energy dispersive X-ray spectroscopy (EDS). Absorption spectra were measured with a LabTech UV-2100 ultraviolet–visible (UV–vis) spectrophotometer.
4.5. Catalytic Test of MXene-Co3O4 Nanocomposites
In order to evaluate the catalytic performance of the obtained materials, we assessed the performance by degradation of methylene blue and Rhodamine B.49,50 The prepared Mxene-Co3O4 composites (10 mg) and 15 mL of H2O2 solution (30% in water) were added to the Rhodamine B and methylene blue solution. A small sample amount of the suspension was taken at regular intervals, and the absorbance of the sample was measured by using a UV–vis spectrophotometer. Moreover, cyclic stability of the catalyst was evaluated by eight replicate experiments. At time t (min), the dye adsorption amount qt (mg/g) per unit mass of catalyst was calculated by the following formula
| 6 |
where C0 is the initial concentration of the adsorption solution (mg/L), Ct is the concentration of the adsorption solution at time t (mg/L), m is the total amount of the sample (g), and V is the volume of the adsorption solution (L).
Acknowledgments
We greatly appreciate the financial support of the National Natural Science Foundation of China (No. 21872119), the Support Program for the Top Young Talents of Hebei Province, the China Postdoctoral Science Foundation (No. 2015M580214), the Research Program of the College Science & Technology of Hebei Province (No. ZD2018091), and the Scientific and Technological Research and Development Program of Qinhuangdao City (No. 201701B004).
The authors declare no competing financial interest.
References
- Naguib M.; Kurtoglu M.; Presser V.; Lu J.; Niu J.; Heon M.; Hultman L.; Gogotsi Y.; Barsoum M. W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. 10.1002/adma.201102306. [DOI] [PubMed] [Google Scholar]
- Li Z.; Wang L.; Sun D.; Zhang Y.; Liu B.; Hu Q.; Zhou A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, 191, 33–40. 10.1016/j.mseb.2014.10.009. [DOI] [Google Scholar]
- Khazaei M.; Ranjbar A.; Arai M.; Sasaki T.; Yunoki S. Electronic properties and applications of MXenes: a theoretical review. J. Mater. Chem. C 2017, 5, 2488–2503. 10.1039/C7TC00140A. [DOI] [Google Scholar]
- Li K.; Jiao T.; Xing R.; Zou G.; Zhou J.; Zhang L.; Peng Q. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. 10.1007/s40843-017-9196-8. [DOI] [Google Scholar]
- Naguib M.; Kurtoglu M.; Presser V.; Lu J.; Niu J.; Heon M.; Hultman L.; Gogotsi Y.; Barsoum M. W. Two-dimensional nanocrystals: two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4207–4207. 10.1002/adma.201190147. [DOI] [PubMed] [Google Scholar]
- Srivastava P.; Mishra A.; Mizuseki H.; Lee K.-R.; Singh A. K. Mechanistic insight into the chemical exfoliation and functionalization of Ti3C2 MXene. ACS Appl. Mater. Interfaces 2016, 8, 24256–24264. 10.1021/acsami.6b08413. [DOI] [PubMed] [Google Scholar]
- Mishra A.; Srivastava P.; Mizuseki H.; Lee K.-R.; Singh A. K. Isolation of pristine MXene from Nb4AlC3 MAX phase: a first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 11073–11080. 10.1039/C5CP07609A. [DOI] [PubMed] [Google Scholar]
- Naguib M.; Mochalin V. N.; Barsoum M. W.; Gogotsi Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. 10.1002/adma.201304138. [DOI] [PubMed] [Google Scholar]
- Zhang E.; Xie Y.; Ci S.; Jia J.; Wen Z. Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens. Bioelectron. 2016, 81, 46–53. 10.1016/j.bios.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Cheng G.; Kou T.; Zhang J.; Si C.; Gao H.; Zhang Z. O22-/O- functionalized oxygen-deficient Co3O4 nanorods as high performance supercapacitor electrodes and electrocatalysts towards water splitting. Nano Energy 2017, 38, 155–166. 10.1016/j.nanoen.2017.05.043. [DOI] [Google Scholar]
- Huang X.; Wang R.; Jiao T.; Zou G.; Zhan F.; Yin J.; Zhang L.; Zhou J.; Peng Q. Facile Preparation of Hierarchical AgNP-Loaded MXene/Fe3O4/Polymer Nanocomposites by Electrospinning with Enhanced Catalytic Performance for Wastewater Treatment. ACS Omega 2019, 4, 1897–1906. 10.1021/acsomega.8b03615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Chen J.; Chen Y.; Feng P.; Lai H.; Li J.; Luo X. Novel Co3O4 nanoparticles/nitrogen-doped carbon composites with extraordinary catalytic activity for oxygen evolution reaction (OER). Nano-Micro Lett. 2018, 10, 15. 10.1007/s40820-017-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M.; Yu L.; Lou X. W. Hierarchical tubular structures composed of Co3O4 hollow nanoparticles and carbon nanotubes for lithium storage. Angew. Chem., Int. Ed. 2016, 55, 5990–5993. 10.1002/anie.201600133. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Zhan Y.; Cai B.; Hao C.; Wang J.; Liu C.; Meng Z.; Yin Z.; Chen Q. Shape-controlled synthesis of Mn3O4 nanocrystals and their catalysis of the degradation of methylene blue. Nano Res. 2010, 3, 235–243. 10.1007/s12274-010-1026-0. [DOI] [Google Scholar]
- Cheng M.; Zeng G.; Huang D.; Lai C.; Liu Y.; Zhang C.; Wan J.; Hu L.; Zhou C.; Xiong W. Efficient degradation of sulfamethazine in simulated and real wastewater at slightly basic pH values using Co-SAM-SCS /H2O2 Fenton-like system. Water Res. 2018, 138, 7–18. 10.1016/j.watres.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Wang J. Degradation of sulfamethazine by gamma irradiation in the presence of hydrogen peroxide. J. Hazard. Mater. 2013, 250–251, 99–105. 10.1016/j.jhazmat.2013.01.050. [DOI] [PubMed] [Google Scholar]
- Mahamallik P.; Pal A. Photo-Fenton process in a Co(II)-adsorbed micellar soft-template on an alumina support for rapid methylene blue degradation. RSC Adv. 2016, 6, 100876–100890. 10.1039/C6RA19857K. [DOI] [Google Scholar]
- Bokare A. D.; Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. 10.1016/j.jhazmat.2014.04.054. [DOI] [PubMed] [Google Scholar]
- Ling S. K.; Wang S.; Peng Y. Oxidative degradation of dyes in water using Co2+/H2O2 and Co2+/peroxymonosulfate. J. Hazard. Mater. 2010, 178, 385–389. 10.1016/j.jhazmat.2010.01.091. [DOI] [PubMed] [Google Scholar]
- Ding D.; Wang Y.; Li X.; Qiang R.; Xu P.; Chu W.; Han X.; Du Y. Rational design of core-shell Co@C microspheres for high-performance microwave absorption. Carbon 2017, 111, 722–732. 10.1016/j.carbon.2016.10.059. [DOI] [Google Scholar]
- Yan Z.; Hu Q.; Yan G.; Li H.; Shih K.; Yang Z.; Li X.; Wang Z.; Wang J. Co3O4/Co nanoparticles enclosed graphitic carbon as anode material for high performance Li-ion batteries. Chem. Eng. J. 2017, 321, 495–501. 10.1016/j.cej.2017.03.146. [DOI] [Google Scholar]
- Huo S.; Duan P.; Jiao T.; Peng Q.; Liu M. Self-assembled luminescent quantum dots to generate full-color and white circularly polarized light. Angew. Chem., Int. Ed. 2017, 56, 12174–12178. 10.1002/anie.201706308. [DOI] [PubMed] [Google Scholar]
- Yanalak G.; Aljabour A.; Aslan E.; Ozel F.; Patir I. H.; Kus M.; Ersoz M. NiO and Co3O4 nanofiber catalysts for the hydrogen evolution reaction at liquid/liquid interfaces. Electrochim. Acta 2018, 291, 311–318. 10.1016/j.electacta.2018.08.130. [DOI] [Google Scholar]
- Lin Z.; Qiao X. Coral-like Co3O4 decorated N-doped carbon particles as active materials for oxygen reduction reaction and supercapacitor. Sci. Rep. 2018, 8, 1802. 10.1038/s41598-018-19347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.; Ma N.; Xue J.; Wang G.; Liu S.; Li H.; Guo P. Insights into the role of poly(vinylpyrrolidone) in the synthesis of palladium nanoparticles and their electrocatalytic properties. Langmuir 2019, 35, 787–795. 10.1021/acs.langmuir.8b04032. [DOI] [PubMed] [Google Scholar]
- Xing R.; Liu K.; Jiao T.; Zhang N.; Ma K.; Zhang R.; Zou Q.; Ma G.; Yan X. An Injectable Self-Assembling Collagen-Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy. Adv. Mater. 2016, 28, 3669–3676. 10.1002/adma.201600284. [DOI] [PubMed] [Google Scholar]
- Chen K.; Jiao T.; Li J.; Han D.; Wang R.; Tian G.; Peng Q.. Chiral Nanostructured Composite Films via Solvent-Tuned Self-Assembly and Their Enantioselective Performances. Langmuir, in press, 10.1021/acs.langmuir.9b00014. [DOI] [PubMed]
- Wang C.; Yin J.; Wang R.; Jiao T.; Huang H.; Zhou J.; Zhang L.; Peng Q. Facile preparation of self-assembled polydopamine-modified electrospun fibers for highly effective removal of organic dyes. Nanomaterials 2019, 9, 116. 10.3390/nano9010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R.; Wang R.; Yin J.; Jiao T.; Huang H.; Zhao X.; Zhang L.; Li Q.; Zhou J.; Peng Q. Fabrication and highly efficient dye removal characterization of beta-cyclodextrin-based composite polymer fibers by electrospinning. Nanomaterials 2019, 9, 127. 10.3390/nano9010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing R.; Wang W.; Jiao T.; Ma K.; Zhang Q.; Hong W.; Qiu H.; Zhou J.; Zhang L.; Peng Q. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustainable Chem. Eng. 2017, 5, 4948–4956. 10.1021/acssuschemeng.7b00343. [DOI] [Google Scholar]
- Liu Y.; Hou C.; Jiao T.; Song J.; Zhang X.; Xing R.; Zhou J.; Zhang L.; Peng Q. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. 10.3390/nano8010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Sun S.; Zhang L.; Yin J.; Jiao T.; Zhang L.; Xu Y.; Zhou J.; Peng Q. Facile preparation and catalytic performance characterization of AuNPs-loaded hierarchical electrospun composite fibers by solvent vapor annealing treatment. Colloids Surf., A 2019, 561, 283–291. 10.1016/j.colsurfa.2018.11.002. [DOI] [Google Scholar]
- Sun S.; Wang C.; Han S.; Jiao T.; Wang R.; Yin J.; Li Q.; Wang Y.; Geng L.; Yu X.; Peng Q. Interfacial nanostructures and acidichromism behaviors in self-assembled terpyridine derivatives Langmuir-Blodgett films. Colloids Surf., A 2019, 564, 1–9. 10.1016/j.colsurfa.2018.12.031. [DOI] [Google Scholar]
- Huang X.; Jiao T.; Liu Q.; Zhang L.; Zhou J.; Li B.; Peng Q. Hierarchical electrospun nanofibers treated by solvent vapor annealing as air filtration mat for high-efficiency PM2.5 capture. Sci. China Mater. 2019, 62, 423–436. 10.1007/s40843-018-9320-4. [DOI] [Google Scholar]
- Xu Y.; Ren B.; Wang R.; Zhang L.; Jiao T.; Liu Z. Facile preparation of rod-like MnO nanomixtures via hydrothermal approach and highly efficient removal of methylene blue for wastewater Treatment. Nanomaterials 2019, 9, 10. 10.3390/nano9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan F.; Wang R.; Yin J.; Han Z.; Zhang L.; Jiao T.; Zhou J.; Zhang L.; Peng Q. Facile solvothermal preparation of Fe3O4–Ag nanocomposite with excellent catalytic performance. RSC Adv. 2019, 9, 878–883. 10.1039/C8RA08516A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.; Gao F.; Jiao T.; Xing R.; Zhang L.; Zhang Q.; Peng Q. Selective Cu(II) ion removal from wastewater via surface charged self-assembled polystyrene-Schiff base nanocomposites. Colloids Surf., A 2018, 545, 60–67. 10.1016/j.colsurfa.2018.02.048. [DOI] [Google Scholar]
- Luo X.; Ma K.; Jiao T.; Xing R.; Zhang L.; Zhou J.; Li B. Graphene oxide-polymer composite Langmuir films constructed by interfacial thiol-ene photopolymerization. Nanoscale Res. Lett. 2017, 12, 99. 10.1186/s11671-017-1864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.; Li J.; Zhang L.; Xing R.; Jiao T.; Gao F.; Peng Q. Facile synthesis of self-assembled carbon nanotubes/dye composite films for sensitive electrochemical determination of Cd(II) ions. Nanotechnology 2018, 29, 445603. 10.1088/1361-6528/aadbf7. [DOI] [PubMed] [Google Scholar]
- Chandra V.; Park J.; Chun Y.; Lee J. W.; Hwang I.-C.; Kim K. S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. 10.1021/nn1008897. [DOI] [PubMed] [Google Scholar]
- Wang H.; Xiang X.; Li F. Facile synthesis and novel electrocatalytic performance of nanostructured Ni–Al layered double hydroxide/carbon nanotube composites. J. Mater. Chem. 2010, 20, 3944–3952. 10.1039/b924911g. [DOI] [Google Scholar]
- Li Y.; Zhao Y.; Cheng H.; Hu Y.; Shi G.; Dai L.; Qu L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. 10.1021/ja206030c. [DOI] [PubMed] [Google Scholar]
- Guo R.; Jiao T.; Li R.; Chen Y.; Guo W.; Zhang L.; Zhou J.; Zhang Q.; Peng Q. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustainable Chem. Eng. 2018, 6, 1279–1288. 10.1021/acssuschemeng.7b03635. [DOI] [Google Scholar]
- Liu K.; Yuan C.; Zou Q.; Xie Z.; Yan X. Self-Assembled Zinc/Cystine-Based Chloroplast Mimics Capable of Photoenzymatic Reactions for Sustainable Fuel Synthesis. Angew. Chem. Int. Ed. 2017, 56, 7876–7880. 10.1002/anie.201704678. [DOI] [PubMed] [Google Scholar]
- Liu K.; Xing R.; Li Y.; Zou Q.; Möhwald H.; Yan X. Mimicking primitive photobacteria: sustainable hydrogen evolution based on peptide-porphyrin co-assemblies with a self-mineralized reaction center. Angew. Chem., Int. Ed. 2016, 55, 12503–12507. 10.1002/anie.201606795. [DOI] [PubMed] [Google Scholar]
- Liu K.; Xing R.; Chen C.; Shen G.; Yan L.; Zou Q.; Ma G.; Möhwald H.; Yan X. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies. Angew. Chem., Int. Ed. 2015, 54, 500–505. 10.1002/anie.201409149. [DOI] [PubMed] [Google Scholar]
- Zou Q.; Liu K.; Abbas M.; Yan X. Peptide-Modulated Self-Assembly of Chromophores toward Biomimetic Light-Harvesting Nanoarchitectonics. Adv. Mater. 2016, 28, 1031–1043. 10.1002/adma.201502454. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhu L.; Yang X.; Shao E.; Deng X.; Liu N.; Wu M. Facile synthesis of three-dimensional Mn3O4 hierarchical microstructures and their application in the degradation of methylene blue. J. Mater. Chem. A 2015, 3, 2934–2941. 10.1039/C4TA05493H. [DOI] [Google Scholar]
- Li R.; Zhou D.; Luo J.; Xu W.; Li J.; Li S.; Cheng P.; Yuan D. The urchin-like sphere arrays Co3O4 as a bifunctional catalyst for hydrogen evolution reaction and oxygen evolution reaction. J. Power Sources 2017, 341, 250–256. 10.1016/j.jpowsour.2016.10.096. [DOI] [Google Scholar]
- Zhai T.; Wan L.; Sun S.; Chen Q.; Sun J.; Xia Q.; Xia H. Phosphate ion functionalized Co3O4 ultrathin nanosheets with greatly improved surface reactivity for high performance pseudocapacitors. Adv. Mater. 2017, 29, 1604167. 10.1002/adma.201604167. [DOI] [PubMed] [Google Scholar]