Abstract

The manuscript revolves around an interesting observation of solidification of a solution of N-((1-((1-ethyl-1H-benzo[d]imidazol-2-yl)methyl)-1H-1,2,3-triazole-4-yl)methyl)aniline (A6) in the NMR tube after around 12 h. Real-time images showed fibrillar and spherulitic growth with tip branching and side branching, which is thermoreversible. The compound under investigation is unique because it is synthesized to understand the anticancer activity with two pharmacophores, benzimidazole and triazole. Click chemistry is employed for in situ generation of triazole moiety on benzimidazole. Previously, benzimidazole-based compounds have shown self-aggregation-induced gel-like behavior because of hydrogen bonding and/or π–π stacking interactions. In the present case, NMR titrations with D2O addition showed two distinct changes in the chemical shift for methylene bridges (connecting benzimidazole and triazole ring) and ortho protons of the phenyl ring (attached to triazole ring). Interestingly, a single-crystal X-ray structure shows the absence of hydrogen bonds and π–π stacking while in the presence of only two distinct close contacts, completely correlating NMR data discussed in detail. A similar “molecular origin” for self-aggregation is observed in seven other flexible but regioisomeric compounds, which were designed and synthesized for inducing hydrogen bonding through the removal of N-ethyl group and insertion of aniline and/or fluoro group.
Introduction
Discovering small organic molecules capable of forming gel is an expanding research area due to their possible applications in tissue engineering, drug delivery vehicles, and pollutant removal machineries.1−3 It has been noted that gel formation is closely related to molecular self-assembly.4 The structure of self-aggregation can be classified into primary, secondary, and tertiary structures and depends on the complexity of the structure formation, like in protein structure.1 The molecular gel is believed to be formed solely with the help of noncovalent forces. Thermoreversibility is the characteristic feature to distinguish the molecular gels from the covalent force-mediated chemical gels.5 The fiber network structure can be described by considering junctions and edges. The junctions can be further classified into two substructures, that is, transient junction and permanent junction.6,7 Further, permanent junctions include side branching and tip branching.6,8 Here, the role of nucleation of individual fibers is also critical and can occur under two conditions: (a) low super saturation, where fibers grow in one dimension with less branching; and (b) high super saturation, facilitating the growth of a densely branched morphology, also known as spherulitic growth.5 Hence, these observations give an idea about the self-assembly, which further helps in understanding the microstructure.
Triazole and benzimidazole having electron-rich and electron-poor sites in the molecular structure are good candidates for intermolecular self-assembly (Supporting Information, Figure S43). The literature reports extensively about the self-aggregation property in benzimidazole and triazole.9−11 The reason behind this is due to its multiple ways to form π–π stacking and hydrogen bonding interaction.12−14
Recently, we developed new small molecules for anticancer activity, with two pharmacophores in one compound, benzimidazole and triazole. After carrying out NMR (in DMSO-d6solvent) on 1-ethyl-2-((4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d]imidazole (A6), the NMR tube remained on the table overnight (room temperatureRT = 25 to 30 °C.). In the morning, the solution inside the NMR tube became solid (Figure 1). Proving the “‘molecular’ origin” of this gel forming ability due to self-aggregation is a subject of this work. The objective is studied with the help of (a) NMR,: effect in chemical shift due to the addition of water; (b) polarizing microscopy: growth of self-aggregation; (c) thermal behavior; (d) single single-crystal X-ray diffraction; and (f) designing of new modeled regio-isomeric compounds with increasing hydrogen bonding sites.
Figure 1.

Observation of solidification in the NMR tube. (a) N-((1-((1-ethyl-1H-benzo[d]imidazol-2-yl)methyl)-1H-1,2,3-triazole-4-yl)methyl)aniline A6. (b) NMR tube showing solidification. (c) Self-aggregated assembly under a microscope.
Results and Discussion
Design and Synthesis
Synthesis of N-((1-((1-ethyl-1H-benzo[d]imidazol-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)aniline (A6) was carried out, as shown in Scheme 1. o-Nitroaniline (1) was converted to an N-ethyl derivative using ethyl bromide as per the reported procedure. Further, compound 2 was reduced to compound 3 using iron powder. 2-Chlorobenzimidazole derivative (4) was synthesized by reacting 3 with two different reagents: 2-chloroacetyl chloride and 2-chloroacetic acid. Both these reactions gave a comparable yield. 4 was then transformed into 2-(azidomethyl)-1H-benzo[d]imidazole (5) using sodium azide in dry DMSO. The reaction condition, especially solvent selection, was optimized to get the best yields. In accordance with theory, DMSO, aprotic polar solvent, proved to be a solvent of choice, because it favors the conversion of both 4 to 5 and 5 to 6 by favoring both, SN2 reaction, and 1,3-dipolar cycloaddition reaction. Without isolating compound 5, in-situ, by adding alkyne derivatives, click chemistry was performed to obtain the final compound 6.15
Scheme 1. Synthesis of N-((1-((1-ethyl-1H-benzo[d]imidazol-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methyl)aniline and Its Derivatives.
Seven other new molecules were designed and synthesized to enhance hydrogen bonding by altering three key places: (i) changing electron-donating effect (−I effect), the presence or absence of fluoro group in the compound; (ii) changing rigidity and/or flexibility near triazole, the addition/deletion of phenyl/aniline moiety; and (iii) directly replacing hydrogen bonding sites with bulkier hydrophobic groups, the presence/absence of ethyl groups on benzimidazole moiety’s aromatic ring nitrogen. In total, eight compounds (Figures 2 and 3) with this aim were considered for the investigation, out of which five derivatives were taken from our laboratory’s previous work.15 All the compounds are completely characterized using elemental analysis and 1H- and 13C-NMR study (Supporting Information, Figures S1–S26).
Figure 2.

Schematic representation of the derivatives of benzimidazole–triazole adducts.
Figure 3.
Benzimidazole–triazole adducts considered for investigation.
Microscopic Studies
Real-time images using a polarizing optical microscopy for A6 was captured (Figure 4). Initially, the compound was dissolved in DMSO. A drop of this solution is taken on a slide, and then, a very fine small drop of water is added in the center (a detailed quantitative water addition experiment is probed using NMR experiments is discussed in the following section). Image in Figure 4a shows the clear solution of compound dissolution in DMSO. Image in Figure 4b was captured after 1 min of water addition, which showed initiation of a self-assembled structure. Image in Figure 4c was captured after 2 min, showing the mature fibrillar growth. After 5 min, image in Figure 4d clearly showed a one-dimensional fiber initially with spherulitic growth and then fibrillar growth with both tip branching and side branching. Images of fibers in Figure 4e–g were captured after 15 min clearly showed the morphology of fibers. Is this a case specific for A6 compound? No, we observed a quite similar evolution of self-assembly-driven aggregation pattern for all the other compounds (Supporting Information, Figures S34–S36).
Figure 4.
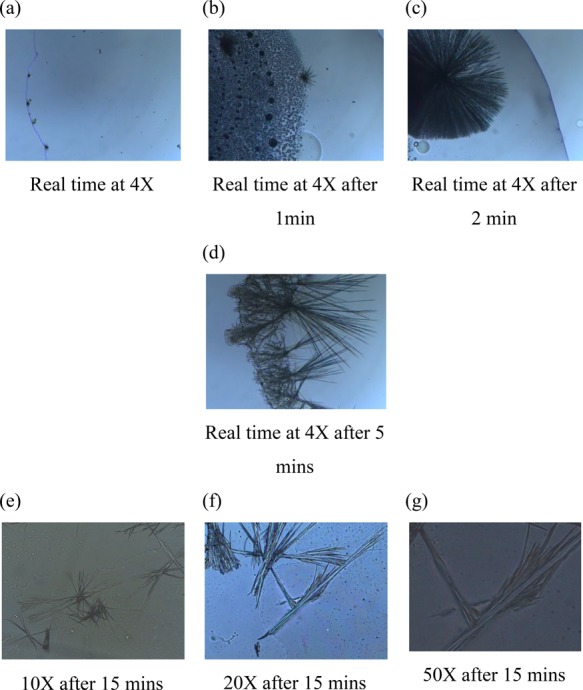
POM images of A6 (a–d) show real-time images after the water addition at various times for 4× and (e–g) show images of fibers at various magnifications after 15 min.
Thermal Studies
Thermal behavior of A6 shows a clear signature of endothermic phase transition at 125 °C in both TG-DTA and DSC studies (Figure 5a–d). On the other hand, when a gel of A6 (by the addition of water to DMSO solution of A6) was subjected for a similar study, it results in a broad signature centered at the same transition temperature. This suggests that the addition of water triggers the phase transition at a much lower temperature or lowers down the onset of this phase transition. TG-DTA for solid A6 shows the thermal stability up to 220 °C with water “absorption” or retention up to 80% until 125 °C. This prompted us to study water “sorption” for all the synthesized compounds, which is listed in Supporting Information, Figures S5 and S27–29.
Figure 5.
(a, b) TG-DTA graphs of A6 (a) solid and (b) after water addition (self-aggregation). (c, d) DSC graphs of A6 (c) solid and (d) after water addition (self-assembly).
Single-Crystal Studies
A single crystal for A6 (ethylaniline) was developed using a slow evaporation technique from ethyl acetate and petroleum ether as solvent. A6 gets crystallized in an orthorhombic crystal system with space group Pna21. Using the same solvent system, we also obtained a single crystal for A2 (ethyl phenyl), which gets crystallized in a triclinic crystal system with space group P1 (Supporting Information, Table S2).
Figure 6 shows the unit cell of A6, with four asymmetric molecules. It has a C (or cup)-shape structure, where the central (or bottom) “triazole” moiety is flanked by the two benzyl groups with an angle of 86.23 and 86.61°. The two probable reasons for the observed “C” (or cup)-shape structure are (a) the “inner” orientation of ethyl groups to minimize interaction with the surrounding solvent and (b) close contacts of aniline with the neighboring molecules. Because of the absence of this later interaction, A2 crystallizes in an L or twisted planar shape, as shown in Figure 7. A2 has only two but distinct “dimeric” close contacts, one along the a axis while the other along its exactly perpendicular c axis. The former is between the nitrogen of triazole and (neighboring molecules) hydrogens of benzylic carbon bridged between triazole and benzimidazole, whereas the latter dimeric “close contact” exists between two triazole-linked benzene rings (2 and 3 hydrogens). Overall, these two dimer-directed close contacts, as represented in Figure 7c as A–B and A′–B, lead to a cascading networked structure.
Figure 6.

Structure of A6. (Top) Single-molecule confirmation showing the angles between the parallel planes and (bottom) the molecules as organized in the unit cell. The structure has been deposited at the Cambridge Crystallographic Data Centre under CCDC 1544619.
Figure 7.
. (a) Structure of A2. (b) Single-molecule confirmation showing an interplanar angle of 86.41°, (c) the cooperative interaction between neighboring molecules, and (d) the molecular organization in the unit cell. The structure has been deposited at the Cambridge Crystallographic Data Centre under CCDC 1828854.
Both the single-crystal studies show the absence of hydrogen bonding and π–π stacking but the distinct presence of “pro-aggregation” topology in the form of close contacts in the solid state.
Nuclear Magnetic Resonance (NMR)
Initially, care is taken to correctly label the protons in all eight molecules using correlation spectroscopy (COSY) and heteronuclear single-quantum correlation spectroscopy (HSQC) experiments (Supporting Information, Figures S1–S26). Then, two different NMR experiments were planned: (i) water addition and (ii) a concentration-dependent experiment.

For water addition NMR, A6 (10 mg) was dissolved in DMSO-d6 (0.5 mL), the 1H NMR was recorded, and after this, D2O was added slowly to perform titration experiments. Each titration consists of aliquots of 10, 20, 30, 30, 30, 30, and 30 μL of D2O. We observed chemical shift perturbation in the titration experiments. All the peaks faced expected chemical shift perturbation except the 1 and 4 protons, which showed merging of the separated peaks (multiplet to two distinct doublet) (Figure 8). The reason for the chemical shift perturbation can be due to prototropic tautomerism, as proposed in Figure 9. Interestingly, for non-ethyl derivative A7, these 1,4 protons behaved exactly opposite and showed separation to the merged peaks. All four ethyl and four non-ethyl compounds show the same trend, as shown in Supporting Information.
Figure 8.
1H NMR of water addition experiment on A6 and A7 (D2O quantity for each titration is given on the right).
Figure 9.
. (a) Intramolecular hydrogen bond making 1,4 protons nonequivalent. (b) Structure of the free rotation along the CH2 linker. (c) After water addition equivalency of two N’s of benzimidazole ring. (d) After water addition non-equivalency of the ethyl derivative.
Water addition proton NMR studies for all the eight molecules showed four distinct features: (a) CH2 imidazole linker’s shifting is more prominent with ethyl derivatives as compared with non-ethyl ones; (b) triazole ring proton shifting is more for phenyl rings as compared to aniline rings, where structures are C and cross-L type; (c) shifting of ortho phenyl ring protons is more for phenyl ring derivatives; and (d) methyl protons show more upfield shift in aniline derivatives as compared to phenyl derivatives. In a concentration-dependent NMR for A4, proton 3 shifts downfield while proton 2 shifts upfield. Also, the 1,4 proton splits from triplet to two separate doublets.
As per designing of the structure, all the eight molecules are inherently flexible because of the utilization of single bond in connecting the two pharmacophores: benzimidazole and central triazole. Therefore, it is likely to adopt a number of conformations separated by low energetic barriers. This is especially true in organic solutions, for example, dimethyl sulfoxide (DMSO), where the greasy aromatic elements of the hosts are well solvated. In D2O, however, the geometries adopted will depend on the inherent bond rotational preferences of the molecules (as in DMSO) as well as aromatic clustering driven by the hydrophobic effect.
The molecular origin of these observations can be correlated to the molecular dynamics in a solution phase because of the structure. The presence of ethyl groups at the benzimidazole nitrogen and intramolecular hydrogen bonding stops the free rotation around the benzylic carbon bridged between benzimidazole and triazole. The reverse is observed in the case of non-ethyl compounds, as depicted in Figure 9. Also, downfield shifting of the second proton and upfield shifting of the third proton play a critical role in aggregation behavior. Both these factors correlate perfectly with the “close contacts” observed in the single-crystal data.
Conclusions
The present work outlines a simple synthetic strategy of designing novel anticancer drugs with more than one pharmacophore using in situ click chemistry. It is focused on revealing the molecular origin of unusual solidification of flexible regioisomeric benzimidazole-bridged triazole molecules in the NMR tube. Microscopic and NMR experiments have shown that all eight synthesized compounds exhibit self-aggregation-directed gel formation in the presence of water. The change in NMR correlates correctly to the solid-state structure observed in a single-crystal X-ray study. Interestingly, the observed aggregation is not influenced by the π–π stacking and/or hydrogen bonding as expected in benzimidazole-based compounds but marks the origin in van der Waal-type “close contacts” observed in a structural investigation. A similar aggregation in the presence of water might be of use for future formulation and/or therapeutic treatments.
Experimental Section
Materials and Methods
TLC analysis was done using precoated silica on aluminum sheets. An FT-IR (KBr pellets) spectrum was recorded in the range of 4000–400 cm–1 using a Perkin-Elmer FT-IR spectrometer. The NMR spectra were obtained by a Bruker AV-III 400 MHz spectrometer using TMS as an internal standard. The chemical shifts were reported in parts per million (ppm), coupling constants (J) were expressed in hertz (Hz), and signals were described as singlet (s), doublet (d), triplet (t), and multiplet (m). The mass spectra were recorded on Thermo Scientific DSQ-II. All chemicals and solvents were of commercial grade and were used without further purification. Single-crystal data was collected using Xcalibur (EoS, Gemini). Thermogravimetric analyses (TG-DTA) were performed using a SII TG/DTA 6300 EXSTAR analyzer under N2 atmosphere.
Acknowledgments
P.S.G. thanks the Department of Science and Technology, New Delhi (SR/S1/IC-43/2009) for financial support. I.I.S. thanks the SRF UGC-BSR fellowship. The authors also thank the research facilities of Chemistry Department (FIST and UGC CAS), Faculty of Science (DST-PURSE Single Crystal X-ray Diffraction) of The Maharaja Sayajirao University of Baroda, Vadodara.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02688.
Experimental procedures and full spectroscopic data for all new compounds and crystallographic data for A2 (CCDC 1828854) and A6 (CCDC 1544619) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Estroff L. A.; Hamilton A. D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. 10.1021/cr0302049. [DOI] [PubMed] [Google Scholar]
- a Lee K. Y.; Mooney D. J. Hydrogels for tissue engineering. Che. Rev. 2001, 101, 1869–1880. 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]; b Xing B.; Yu C. W.; Chow K. H.; Ho P. L.; Fu D.; Xu B. Hydrophobic interaction and hydrogen bonding cooperatively confer a vancomycin hydrogel: a potential candidate for biomaterials. J. Am. Chem. Soc. 2002, 124, 14846–14847. 10.1021/ja028539f. [DOI] [PubMed] [Google Scholar]
- a Skilling K. J.; Citossi F.; Bradshaw T. D.; Ashford M.; Kellam B.; Marlow M. Insights into low molecular mass organic gelators: a focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. 10.1039/C3SM52244J. [DOI] [PubMed] [Google Scholar]; b Miao R.; Peng J.; Fang Y. Molecular gels as intermediates in the synthesis of porous materials and fluorescent films: concepts and applications. Langmuir 2017, 33, 10419–10428. 10.1021/acs.langmuir.6b04655. [DOI] [PubMed] [Google Scholar]
- Babu S. S.; Praveen V. K.; Ajayaghosh A. Functional π-gelators and their applications. Chem. Rev. 2014, 114, 1973–2129. 10.1021/cr400195e. [DOI] [PubMed] [Google Scholar]
- Wang R. Y.; Liu X. Y.; Narayanan J.; Xiong J. Y.; Li J. L. Architecture of fiber network: from understanding to engineering of molecular gels. J. Phys. Chem. B 2006, 110, 25797–25802. 10.1021/jp065101j. [DOI] [PubMed] [Google Scholar]
- Wang R.; Liu X.-Y.; Xiong J.; Li J. Real-time observation of fiber network formation in molecular organogel: supersaturation-dependent microstructure and its related rheological property. J. Phys. Chem. B 2006, 110, 7275–7280. 10.1021/jp054531r. [DOI] [PubMed] [Google Scholar]
- a Geiger C.; Stanescu M.; Chen L.; Whitten D. G. Organogels resulting from competing self-assembly units in the gelator: structure, dynamics, and photophysical behavior of gels formed from cholesterol–Stilbene and cholesterol–Squaraine gelators. Langmuir 1999, 15, 2241–2245. 10.1021/la981386i. [DOI] [Google Scholar]; b Terech P.; Allegraud J. J.; Garner C. M. Thermoreversible gelation of organic liquids by arylcyclohexanol derivatives: a structural study. Langmuir 1998, 14, 3991–3998. 10.1021/la980160c. [DOI] [Google Scholar]
- a Lescanne M.; Colin A.; Mondain-Monval O.; Fages F.; Pozzo J.-L. Structural aspects of the gelation process observed with low molecular mass organogelators. Langmuir 2003, 19, 2013–2020. 10.1021/la026660u. [DOI] [Google Scholar]; b Lescanne M.; Grondin P.; d’Aléo A.; Fages F.; Pozzo J.-L.; Monval O. M.; Reinheimer P.; Colin A. Thixotropic organogels based on a simple N-hydroxyalkyl amide: Rheological and aging properties. Langmuir 2004, 20, 3032–3041. 10.1021/la035219g. [DOI] [PubMed] [Google Scholar]
- a Shen X.; Jiao T.; Zhang Q.; Guo H.; Lv Y.; Zhou J.; Gao F. Nanostructures and self-assembly of organogels via benzimidazole/benzothiazole imide derivatives with different alkyl substituent chains. J. Nanomater. 2013, 2013, 1–8. 10.1155/2013/409087. [DOI] [Google Scholar]; b Yim S.-L.; Chow H.-F.; Chan M.-C. Transition from low molecular weight non-gelating oligo (amide-triazole)s to a restorable, halide-responsive poly (amide-triazole) supramolecular gel. Chem. Commun. 2014, 50, 3064–3066. 10.1039/C3CC49323G. [DOI] [PubMed] [Google Scholar]
- Sambanthamoorthy K.; Gokhale A. A.; Lao W.; Parashar V.; Neiditch M. B.; Semmelhack M. F.; Lee I.; Waters C. M. Identification of a Novel Benzimidazole that Inhibits Bacterial Biofilm Formation in a Broad-Spectrum Manner. Antimicrob. Agents Chemother. 2011, 55, 4369–4378. 10.1128/AAC.00583-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H. C.; Zick P. L.; Roberts W. R.; Geiger D. K. Synthesis and characterization of a novel long-alkyl-chain ester-substituted benzimidazole gelator and its octan-1-ol solvate. Acta Crystallogr., Sect. C: Struct. Chem. 2017, 73, 350–356. 10.1107/S2053229617004314. [DOI] [PubMed] [Google Scholar]
- Liu M.; Kira A.; Nakahara H. Silver (I) ion induced monolayer formation of 2-substituted benzimidazoles at the air/water interface. Langmuir 1997, 13, 4807–4809. 10.1021/la9703252. [DOI] [Google Scholar]
- Liu M.; Cai J. Silver (I) ion induced reverse U-shape monolayers of poly (methylenebis (benzimidazoles)) at the air/water interface. Langmuir 2000, 16, 2899–2901. 10.1021/la9913203. [DOI] [Google Scholar]
- Guo P.; Liu M. Fabrication of Chiral Langmuir-Schaefer Films of Achiral Amphiphilic Schiff Base Derivatives through an Interfacial Organization. Langmuir 2005, 21, 3410–3412. 10.1021/la047089x. [DOI] [PubMed] [Google Scholar]
- Sahay I. I.; Ghalsasi P. S. Synthesis of new 1,2,3-triazole linked benzimidazole molecules as anti-proliferative agents. Synth. Commun. 2017, 47, 825–834. 10.1080/00397911.2017.1289412. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








