Abstract
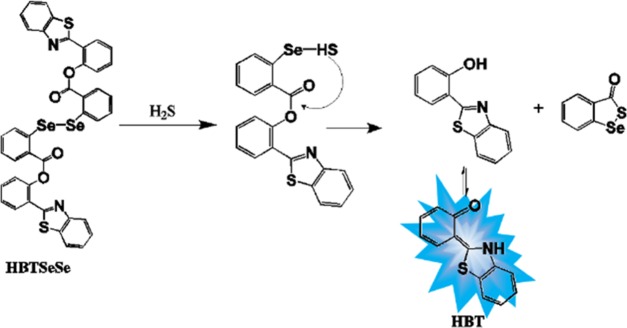
A novel fluorescence probe, HBTSeSe, was designed and synthesized for the detection of H2S with a double-switch mechanism of a broken diselenide bond followed by thiolysis of ether. Then, 2-(2′-hydroxyphenyl)benzothiazole (HBT) was released as fluorophore, which has large Stokes shift based on the excited state intramolecular proton transfer process. The probe responded selectively and rapidly to H2S, with the fluorescence increased by 47-fold immediately after the addition of H2S. HBTSeSe was able to detect H2S in the cytoplasm, specifically in cell imaging experiments. The results also showed that H2S was produced in the immune response of RAW264.7 cells activated by phorbol-12-myristate-13-acetate.
Introduction
Hydrogen sulfide (H2S) has long been recognized as an odorous and toxic compound.1,2 However, it has recently been found that H2S can be produced in cells. Intracellular H2S is produced from cysteine (Cys) and cysteine derivatives through enzymatic pathways, where cystathionine γ-lyase and cystathionine β-synthase are usually involved.3 H2S participates in cellular signal transduction as well as antioxidation, thus contributing to the regulation of energy production, apoptosis, and redox homeostasis of cells.4 On the other hand, aberrant production of H2S is proved to be connected with a variety of diseases such as diabetes, Down’s syndrome, and Alzheimer’s disease.5−7 Therefore, it is important to monitor the endogenous H2S concentration. However, in biological systems, the physiological features of H2S include low concentration, high reactivity, and short lifetime. Therefore, it is still a huge challenge to determine the intracellular concentration of H2S.
Several methods have been used to detect H2S.8−11 Tang et al.12 developed a chemoselective-reduction-based fluorescence probe for the detection of hydrogen sulfide in living cells, and the effect of pH on the probe was also investigated; Wang et al.31 developed a new metal-oxide sensor for detecting hydrogen sulfide gas at room temperature; Long et al.14 used silver nanoparticles to trace H2S by resonance light scattering technique. However, fluorescent probes represent powerful tools for detecting H2S in living cells due to latter’s sensitivity and noninvasive property.33 A variety of fluorescent probes have been developed based on the selective reaction of H2S with azides,28,29 azamacrocyclic Cu(II) complexes,15 and H2S-specific electrophiles.16 Organic compounds containing selenium have been developed for many years, and some work in fluorescence probe field has been reported.13,17 Peng et al.22 reported a unique phenyl diselenide-ester compound, which has the ability to be highly selective and rapid for H2S detection based on the double-switch recognition mechanism. Designing fluorescent probes for detecting H2S based on nucleophilic attack reaction between H2S and Se–Se bond has been a sophisticated strategy.19 However, the nucleophilic reaction rate between H2S and diselenides would be 105 times faster than that between H2S and disulfides.20,22 It can be speculated that diselenide groups represent ideal candidates for recognition center for the design of fluorescence probes that could respond to H2S rapidly. Since the pKa value of H2S (ca. 7.0) was much lower than that of other cellular thiols such as cysteine and glutathione (GSH) (≥8.5), the nucleophilic reaction between H2S and Se–Se bond was considered to be superior then that of thiols.23 To minimize the possible interference from thiols, a further switch that utilized the reactivity of the second sulfhydryl group of H2S for liberating the fluorescence molecule was adopted. Excited state intramolecular proton transfer (ESIPT) is a phototautomerization occurring in the electronic excited state of a molecule, where a heterocyclic ring is formed by the intramolecular hydrogen bond between a hydroxyl group and a neighboring proton acceptor.27,32 Moreover, 2-(2′-hydroxyphenyl)benzothiazole (HBT) is a typical ESIPT chromophore, which has attracted much attention in the fluorescence probe field by taking advantage of a large Stokes shift.30 These probes were used to capture and visualize H2S in living cells and have greatly facilitated the study of H2S biology. However, intracellular production and metabolization of H2S are very fast and the self-absorption phenomenon would have an effect on the fluorescence signal, indicating the demand for selective, rapid, and large Stokes shift fluorescence probes for the real-time detection of H2S.
Herein, we report the design, synthesis, and biological applications of a fluorescence probe with selective and rapid response toward H2S, and a large Stokes shift is achieved. The probe, named HBTSeSe, was designed by using a diselenide group for recognizing and capturing H2S and an ESIPT mechanism for switching the fluorescence, so it has a large Stokes shift.18 It should be noted that the reaction between HBTSeSe and biothiols cannot turn the fluorescence on; therefore, the selectivity of HBTSeSe is found to be excellent. Besides, the reaction of a nucleophilic attack by H2S at selenium is both kinetically much faster and thermodynamically more favorable.20 A 47-fold enhancement in the fluorescence can be observed immediately after the addition of H2S to the probe solutions. The results also showed that the probe can detect endogenous H2S in the cytoplasm in the immune response of RAW264.7 cells.
Results and Discussion
Design and Synthesis of HBTSeSe
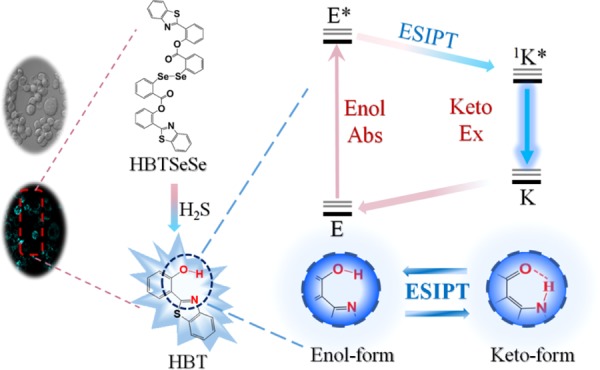
The design of HBTSeSe relied on the fast nucleophilic attack on selenium by H2S. In this regard, an ester group was introduced in the ortho position of selenium (Scheme 1). For comprehending the fluorescence mechanism, ESIPT with a large Stokes shift was prepared. Furthermore, HBT was selected as the signal transducer for preparing HBTSeSe. By this design, HBTSeSe was expected to respond to H2S selectively and rapidly and produce a strong ESIPT fluorescence in response to H2S due to the production of HBT. The ESIPT chromophores (HBT) with an intramolecular hydrogen bond existed in the cis enol form at the ground state. Under photoexcitation, the singlet excited state of the enol form is populated. It is worth noting that no geometry relaxation occurs during the excitation, which is consistent with the Franck–Condon principle. Then, an ultrafast ESIPT process occurs, and the cis-keto form at the singlet excited state is formed. The structure of cis-ketones is more stable due to the existence of intramolecular hydrogen bonds. Since the ESIPT is much faster than the fluorescence process (radiative decay), the observed fluorescence of HBT originated from the keto tautomer.21 However, the ESIPT fluorescence will be inhibited in HBTSeSe because the phenolic hydroxyl of the HBT unit was esterified. HBTSeSe was synthesized via a 5-step route as demonstrated in Scheme S1. The synthetic procedure is described in the Supporting information in detail. The overall yield of HBTSeSe was 12.8%.
Scheme 1. Detection Mechanism of HBTSeSe toward H2S Based on the Double-Switch Recognition Mechanism and ESIPT Fluorescence Emission Property.
Fluorescence Response of HBTSeSe to H2S
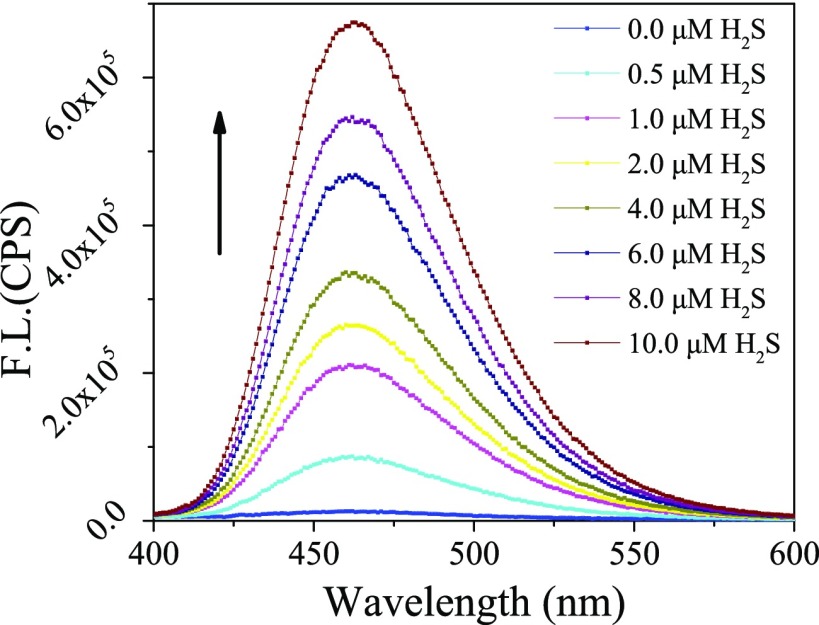
The fluorescence response of the HBTSeSe toward H2S was investigated under simulated physiological pH (phosphate-buffered saline, PBS, pH 7.4 and 100 mM). As expected, HBTSeSe exhibited negligible fluorescence due to the inhibition of ESIPT process. However, the fluorescence at 460 nm increased when H2S was added to PBS containing 10 μM HBTSeSe. In the presence of 10 μM H2S, the fluorescence intensity immediately increased by 47-fold (Figure 1). It was found that the fluorescence intensity was linear to H2S concentration in the range of 0–10 μM. The regression equation was y = 64 174x + 65 300, with a linear coefficient of 0.98 (Figure S2). The high-resolution mass spectra (HRMS) analysis of the assay solution containing HBTSeSe and H2S revealed a new peak of m/z 228.0473, clearly indicating the formation of free HBT (Figure S7). These results demonstrated that the fluorescence intensity of HBTSeSe could be used to quantify the H2S concentrations.
Figure 1.
Emission spectra (λex = 380 nm) of HBTSeSe (10 μM) in the presence of different concentrations of H2S in aqueous (dimethyl sulfoxide (DMSO)/PBS = 1:1, v/v) under ambient conditions.
Selectivity of HBTSeSe
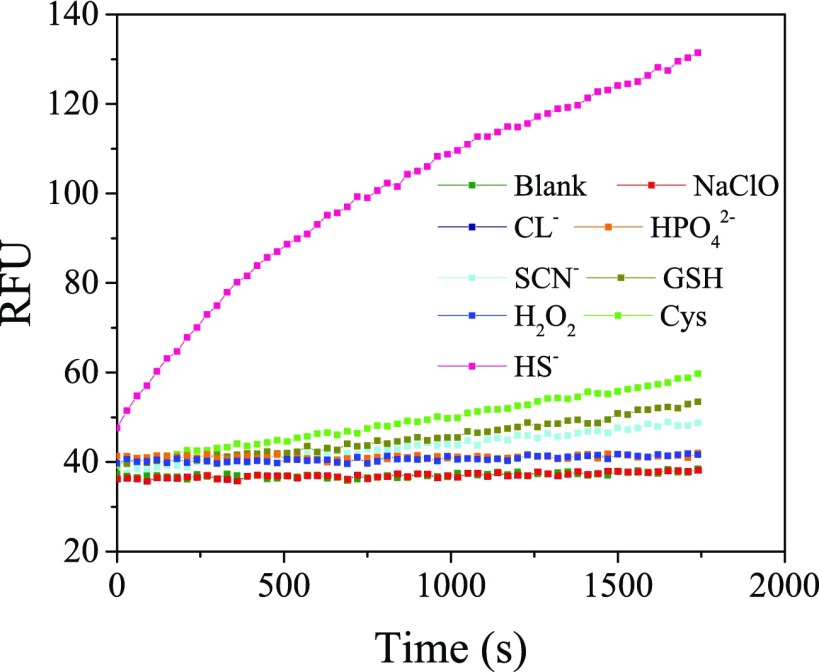
Differentiation of H2S from the coexisting species is important for the application of HBTSeSe in complex biological samples. Therefore, a variety of biologically relevant species including thiols (Cys, GSH), anions (SCN–, HPO42–, Cl–), and oxidant (H2O2) were used to evaluate the selective fluorescence response of HBTSeSe to H2S. As shown in Figure 2, the fluorescence intensity almost remains same when 1000 times HPO42–, 1000 times Cl–, or 100 times H2O2 was added in the probe solution, respectively. And a small increase fluorescence intensity is obtained when 1000 times Cys, 100 times GSH, or 100 times SCN– was added in the probe solution, respectively. However, there is significant fluorescence enhancement immediately after the probe reacted with 10 times H2S. Therefore, compared with H2S, the coexisting substances had no significant effect on the fluorescence response of HBTSeSe. It was demonstrated that HBTSeSe has high selectivity to H2S. These results suggest that the design strategy employing a double switch to improve the selectivity of HBTSeSe in this study is feasible and effective.
Figure 2.
Time-dependent fluorescent spectra of 10 μM HBTSeSe at 460 nm incubated by different species (100 mM Cys, 10 mM GSH, 10 mM KSCN, 100 mM Na2HPO4, 100 mM KCl, 10 mM H2O2, 100 μM Na2S) in aqueous (DMSO:PBS = 1:1, v/v).
Kinetic Assay of Reaction between HBTSeSe and H2S
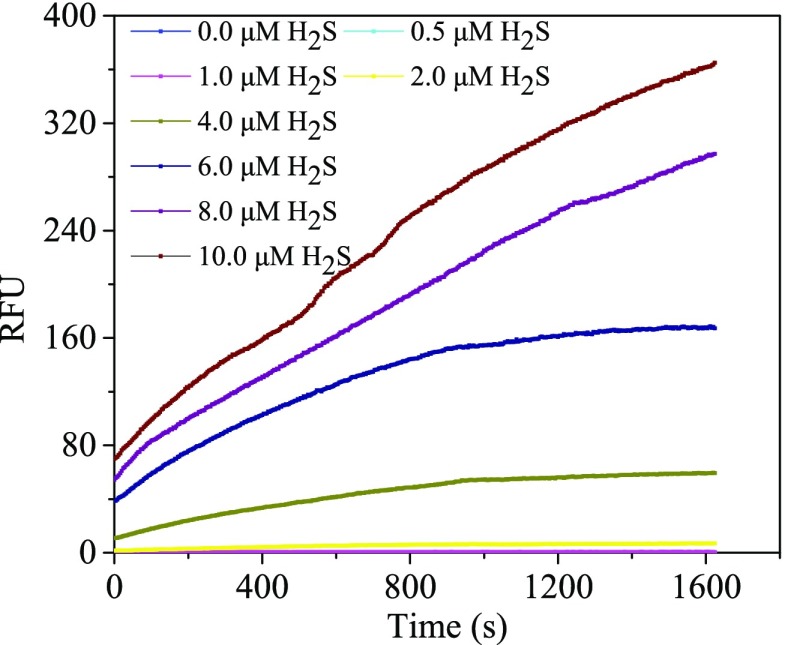
Kinetic assays were carried out to investigate the fluorescence changes along with the reaction time. As shown in Figure 3, in the absence of H2S, the weak fluorescence of HBTSeSe remained stable under conditions of light and air in a 30 min assay. After the addition of H2S, the fluorescence intensity increased consistently with increasing concentration of H2S. Moreover, there is a sharp enhancement in the fluorescence immediately after the addition of H2S into the probe solution. It can be speculated that HBTSeSe has a fast-speed response to H2S during the initial time. Overall, an ESIPT fluorescence probe with selectivity and rapid response to H2S has been successfully achieved.
Figure 3.
Kinetics of HBTSeSe reaction with different ratios of H2S at 37 °C (DMSO:PBS = 1:1, v/v).
Detection of Exogenous H2S Using HBTSeSe
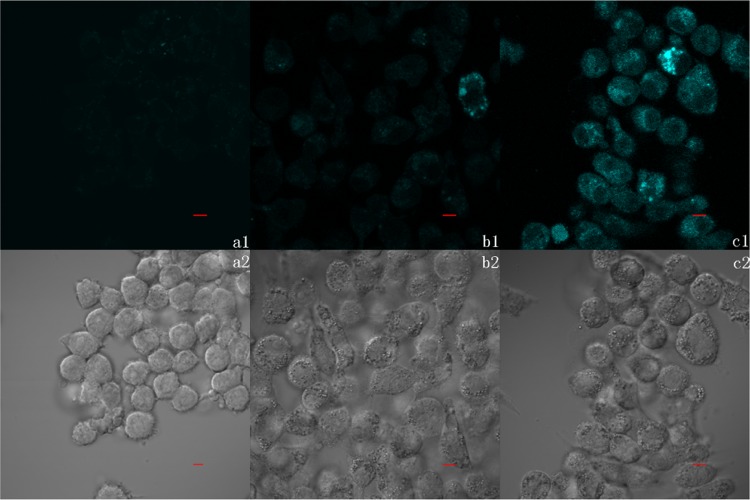
HBTSeSe probe was successfully applied to the detection of exogenous H2S in living cells. As shown in Figure 4, RAW264.7 macrophage cells incubated with HBTSeSe presented minimal fluorescence signal compared with those incubated with HBTSeSe and different concentrations of exogenous H2S. It has been reported that H2S can move freely across lipid membranes, as its solubility is five times more in lipophilic solvents than that in water.24 Therefore, a bright cyan fluorescence signal was observed when the cells were treated with different concentrations of Na2S in the culture media. The above observations are in accordance with the results obtained in the solution. These results demonstrated that the HBTSeSe probe is cell permeable and suitable for H2S detection in living cells.
Figure 4.
(a–c) Confocal fluorescence images of living RAW264.7 cells. (a1, a2) Cells were induced by HBTSeSe (10 μM) for 20 min and subsequently 30 min without Na2S. (b1, b2) Cells were induced by HBTSeSe (10 μM) for 20 min and subsequently induced by Na2S (50 μM) for 30 min. (c1, c2) Cells were induced by HBTSeSe (10 μM) for 20 min and subsequently induced by Na2S (100 μM) for 30 min (λex = 405 nm, scale bar = 5 μM). All images were collected at the same microscopy settings.
Detection of Endogenous H2S Using HBTSeSe in the Immune Response of RAW264.7 Cells
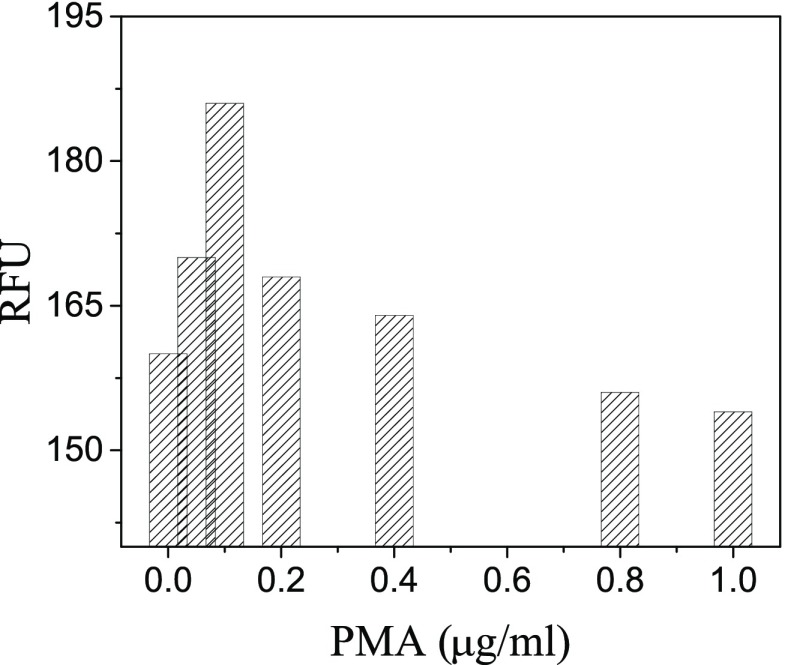
Phagocytes can produce reactive oxygen species (ROS) via nicotinamide adenine dinucleotide phosphate oxidase for killing pathogens. In vitro, the process producing ROS can be induced by phorbol-12-myristate-13-acetate (PMA). Therefore, the PMA stimulation has been employed as an effective means for decreasing H2S concentrations in cells.25 However, it was reported that H2S played important roles in immune response of cells.26 For understanding the regulation mechanism of H2S in the immune response process, we monitored the intracellular level of H2S in RAW264.7 macrophages that were stimulated using different concentrations of PMA. The fluorescence intensity analysis is shown in Figure 5. It was shown that the endogenous H2S concentration gradually increased when the PMA concentration was varied from 0 to 0.1 μg/mL. However, there is a decrease in the endogenous H2S concentration as the PMA concentration further increased. The confocal fluorescence images are shown in Figure S9.
Figure 5.
Relative fluorescent intensity of the cell incubated by 0.0, 0.05, 0.1, 0.2, 0.4, 0.8, and 1.0 μg/mL PMA.
Subcellular Location of HBTSeSe
To illustrate the intracellular distribution of HBTSeSe, the cells RAW264.7 were counter-stained with HBTSeSe and commercial nuclear dyes STYO-59. The confocal microscopy images reveal the cyan fluorescence signals from HBTSeSe and the red fluorescence signals from STYO-59 (Figure 6). Moreover, the cytoplasm was dyed by HBTSeSe; concomitantly, the nucleus was dyed by STYO-59. The result demonstrates that the cell is alive when it was stained by HBTSeSe. Therefore, the probe has low cytocoxicity and can be used in living cells.
Figure 6.

(a–d) Confocal fluorescence images of RAW264.7 treated with HBTSeSe and nuclear dye SYTO-59: (a) original cell; (b) dyed by HBTSeSe; (c) dyed by SYTO-59; and (d) co-staining image (λex = 405 nm for HBTSeSe, λex = 635 nm for SYTO-59, scale bar = 5 μm).
Conclusions
In summary, a novel fluorescence probe HBTSeSe was designed and synthesized. The design strategy was based on nucleophilic attack of H2S on the Se–Se bond, followed by thiolysis of ether to release the HBT fluorophore. The HBT has the ESIPT property, and a large Stokes shift is achieved. HBTSeSe has the advantage of rapid response and high selectivity to H2S detection with a 47-fold fluorescence enhancement based on the double-switch recognition mechanism and ESIPT property. It also can be known that the low PMA concentration (<0.8 μg/mL) could promote the H2S production in RAW264.7. In addition, the application of the HBTSeSe probe for imaging intracellular H2S has been well demonstrated, which shows its potential applications in biological system.
Experimental Section
Materials and Equipment
All reagents are obtained from commercial suppliers of analytical reagent and used without further treatment except as otherwise noted. Na2S was used as the donor of H2S. 1H NMR spectra were recorded on a Bruker 400MHz spectrometer (Bruker, Germany). 1H NMR chemical shifts are expressed in ppm with tetramethylsilane as the internal standard. The UV–vis absorption spectra were recorded by a lambda 35 (Perkin-Elmer) spectrometer at room temperature. The sample was placed in 1 cm quartz. Fluorescence spectra were recorded by Fluoromax-4 (Horiba-Jobin Yvon, France) spectrometer at room temperature. A high-resolution mass spectra (HRMS) were obtained by using the UFLC/MS spectrometry system (Agilent 6540UHD Accurate Mass Q-TOF LC/MS) at DICP research facilities center.
Synthetic Route
The synthetic route of the probe is shown in Scheme S1 and detailed description is as follows. All synthesis reactions are carried out in round-bottom flasks with magnetic stirring.
Synthesis of 2-Aminobenzoic Acid (Compound 1)
Two hundred milliliters of methanol and 100.0 mL of tetrahydrofuran were add in a round-bottom flask with three necks. Next, 100.0 mL of aqueous water with LiOH (9.12 g, 0.38 mol) was added. Then, methylanthranilate (30.11 g, 0.20 mol) was added. The temperature was maintained at 41 °C. The reaction was monitored by thin layer chromatography until an equilibrium was achieved. Then, the solvent was evaporated under reduced pressure. The residue was dissolved in 30.0 mL ether and extracted by 50.0 mL water three times. The water phase was mixed. The pH of the water phase was adjusted by 1 M hydrochloric acid (HCl) to pH = 4.0. Compound 1 as a pale yellow solid (26.32 g, 96%) was acquired through filtration and dried.
Synthesis of 2-Carboxybenzenediazonium (Compound 2)
One hundred milliliters of H2O was poured into a round-bottom flask with three necks. Next, 36% (w/w) hydrochloric acid (38.0 mL) was slowly added. Then, anthranilic acid (18.0 g, 0.13 mol) was added, and the temperature was maintained at 5 °C. Thirty milliliters of aqueous water containing sodium nitrite (8.99 g, 0.13 mol) was distributed in the flask. Compound 2 was obtained. The product was used next without further purification.
Synthesis of 2,2′-Diselanediyldibenzoic Acid (Compound 3)
H2O (35.0 mL) and Se powder (5.10 g, 0.065 mol) were added in a round-bottom flask with three necks under nitrogen atmosphere. Aqueous waters containing sodium borohydride (4.45 g, 0.13 mol) was distributed in the flask. Then, another part of Se powder (5.10 g, 0.065 mol) was added. To make the mixture alkaline, sodium hydroxide aqueous (25.0 mL) with the concentration of 10 M was added.34 The temperature of the mixture was kept under 5 °C. Compound 2 was slowly distributed in the vessel with temperature under 10 °C. Then, the mixture was heated at 60 °C for 3 h and then stirred at room temperature for 3 h. The mixture was adjusted to pH = 3–4 by 1 M HCl. Compound 3 as obtained as a brown powder (9.86 g, 38%) after filtration and washed with water to neutralize the mixture.
Synthesis of HBTSeSe
This step has been reported by Mlochowski et al.35 Compound 3 (2.50 g, 6.2 mmol) and benzene (33.0 mL) were added in a round-bottom flask with three necks. Then, the mixture was heated to 85 °C, and thionyl chloride (1.85 g, 15.7 mmol) was slowly distributed in the flask. The solution was heated until the reaction reaches an equilibrium. Without further purification, triethylamine (0.90 g, 9.0 mmol), dichloromethane (17.0 mL), and 2-(2-hydroxyphenyl)benzothiazole (HBT) (2.54 g, 11.2 mmol) were added in a round-bottom flask with three necks. The reaction continued for 7 h under room temperature. HBTSeSe (1.57 g, 43%) was obtained as a gray solid by flash chromatography column.
General Details for UV–vis and Fluorescence Measurement
H2S and the probe HBTSeSe are hydrophilic and hydrophobic compounds, respectively. It was reported that the probe could sufficiently contact with H2S in the solution of DMSO:PBS = 1:1.36 In this study, dimethyl sulfoxide (DMSO) and PBS aqueous (pH 7.4, 100 mM) were pre-mixed in a 1 cm quartz cuvette with the volume ratio of 1:1. Then 10 μM probe and 0, 0.5, 1, 2, 4, 6, 8 and 10 μM H2S were added with ratio of 0, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0, respectively. And another quartz cuvette only contains 1:1 (v/v) of DMSO/water as the control.
To test the selectivity of HBTSeSe, 20 μL DMSO with 100 μM HBTSeSe was added in a 96-well ELISA plate. Then, 160 μL aqueous solution of 1:1 (v/v) = of DMSO/PBS (pH 7.4, 100 mM) was added. Then, 20 μL aqueous solution containing 100 mM cysteine (Cys), 10 mM glutathione (GSH), 10 mM KSCN, 100 mM Na2HPO4, 100 mM KCl, 10 mM H2O2, and 100 μM Na2S were added, respectively. Thermo Scientific Varioskan Flash was used to measure the fluorescence intensity at 460 nm with a 5 nm excite slit and a 5 nm emission slit.
To test the kinetic of HBTSeSe reaction with different ratios of H2S, different concentrations of H2S (0, 0.5, 1, 2, 4, 6, 8, and 10 μM H2S) were added in the 10 μM probe solution. Then, the fluorescence intensity at 460 nm was measured by Thermo Scientific Varioskan Flash with a 5 nm excite slit and a 5 nm emission slit.
Cell Imaging Experiments
The rat macrophages, RAW264.7, was cultured by biology group in Dalian Institute of Chemical Physics. All the RAW264.7 cells were cultured according to the definition of American Type Tissue Culture Collection (ATTC). The cells were cultured in a MCO-15AC (Sanyo, Japan) incubator in the RPMI 1640 medium at 37 °C with the condition of CO2/air = 5:95. The normal medium is acquired after 20% fetal bovine serum (Invitrogen), NaHCO3 (2 g/L), and 1% antibiotic (penicillin/streptomycin, 100 U/mL) were added in the RPMI 1640 medium. Na2S was as the H2S donor to provide H2S. The working concentration of the cell density was 106 cell/mL.
To image exogenous H2S, RAW264.7 was cultured in the normal medium as the control. Other cells were cultured in the media that have another 50 μM Na2S and 100 μM Na2S, respectively. To image endogenous H2S, the cells were induced with different concentrations of PMA (0.0, 0.05, 0.1, 0.2, 0.4, 0.8, and 1.0 μg/mL) for 30 min. Then, the images were patterned onto the normal medium containing 10 μM probe and cultured at 37 °C for 20 min. After the end of culture, the culture medium was removed and washed with PBS solution (pH 7.4, 100 mM) and characterized by an Olympus Fluoview FV1000 laser scanning confocal microscopy. Stock solutions of the cell nucleus dye SYTO-59 (0.1 μM) were prepared in DMSO. RAW264.7 was cultured for 20 min on a normal medium containing 10 μM probe and then stained for 20 min by STYO-59. In the process, 100 μM Na2S was added into the medium. The cell was excited by mercury lamp 405 and 635 nm and then imaged by Olympus Fluoview FV1000 laser scanning confocal microscopy.
Acknowledgments
This work was supported by China National Natural Science Foundation (Nos 31270620 and 21673237) and Dalian Scientific and Technological Innovation Foundation (No. 2018J12SN072). Thanks are given to Prof. Dr Guo, Prof. Dr Li, and Prof. Dr Zhao for their guidance, help, and constructive suggestions.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00934.
Full experimental details and characterization of investigated materials (synthetic route, absorption spectrum, linear relationship, limit of detection, NMR data, HRMS data, pH trituration experiment, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, confocal fluorescence images) (PDF)
Author Contributions
∥ H.G. and A.Z. contributed equally to this work.
Author Contributions
All authors have given approval to the final version of the paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Kolanowski J. L.; Liu F.; New E. J. Fluorescent probes for the simultaneous detection of multiple analytes in biology. Chem. Soc. Rev. 2018, 47, 195–208. 10.1039/c7cs00528h. [DOI] [PubMed] [Google Scholar]
- Csaba S. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discovery 2007, 6, 917–935. 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Filipovic M. R.; Zivanovic J.; Alvarez B.; Banerjee R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. 10.1021/acs.chemrev.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.; Li P.; Song P.; Wang B.; Zhao J.; Han K. An ICT-based strategy to a colorimetric and ratiometric fluorescence probe for hydrogen sulfide in living cells. Chem. Commun. 2012, 48, 2852–2854. 10.1039/c2cc17658k. [DOI] [PubMed] [Google Scholar]
- Kamoun P.; Chabli A.; Lallouchi K.; Chadefaux-Vekemans B.; et al. Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Genet., Part A 2003, 116, 310–311. 10.1002/ajmg.a.10847. [DOI] [PubMed] [Google Scholar]
- Eto K.; Asada T.; Arima K.; Makifuchi T.; Kimura H. Brain hydrogen sulfide is severely decreased in alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 293, 1485–1488. 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- Gao M.; Wang R.; Yu F.; You J.; Chen L. Imaging and evaluation of sulfane sulfur in acute brain ischemia using a mitochondria-targeted near-infrared fluorescent probe. J. Mater. Chem. B 2018, 6, 2608–2619. 10.1039/C7TB03200E. [DOI] [PubMed] [Google Scholar]
- Jiao X.; Li Y.; Niu J.; Xie X.; Tang B.; et al. Small-molecule fluorescent probes for imaging and detection of reactive oxygen, nitrogen, and sulfur species in biological systems. Anal. Chem. 2018, 90, 533–555. 10.1021/acs.analchem.7b04234. [DOI] [PubMed] [Google Scholar]
- Kowada T.; Maeda H.; Kikuchi K. Bodipy-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. 10.1039/C5CS00030K. [DOI] [PubMed] [Google Scholar]
- Cao J.; Lopez R.; Thacker J. M.; Moon J. Y.; Jiang C.; Morris S. N. S.; et al. Chemiluminescent probes for imaging H2S in living animals. Chem. Sci. 2017, 6, 1979–1985. 10.1039/C4SC03516J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A.; Das S.; Sarkar H. S.; Kundu S.; Sahoo P. Consumption of H2S from Our Daily Diet: Determination by a Simple Chemosensing Method. ACS Omega 2018, 3, 11617–11623. 10.1021/acsomega.8b01751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.; Lv C.; Tang X. Chemoselective reduction-based fluorescence probe for detection of hydrogen sulfide in living cells. Anal. Bioanal. Chem. 2012, 404, 1919–1923. 10.1007/s00216-012-6292-0. [DOI] [PubMed] [Google Scholar]
- Kuang Y.; Chen S.; Long Y. Highly sensitive and selective determination of hydrogen sulfide by resonance light scattering technique based on silver nanoparticles. Anal. Bioanal. Chem. 2018, 409, 4001–4008. 10.1007/s00216-017-0343-5. [DOI] [PubMed] [Google Scholar]
- Kuang Y.; Chen S.; Long Y. Highly sensitive and selective determination of hydrogen sulfide by resonance light scattering technique based on silver nanoparticles. Anal. Bioanal. Chem. 2017, 409, 4001–4008. 10.1007/s00216-017-0343-5. [DOI] [PubMed] [Google Scholar]
- Lippert A. R.; New E. J.; Chang C. J. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- Yu F.; Li P.; Li G.; Zhao G.; Han K. A near-IR reversible fluorescent probe modulated by selenium for monitoring peroxynitrite and imaging in living cells. J. Am. Chem. Soc. 2011, 133, 11030–11033. 10.1021/ja202582x. [DOI] [PubMed] [Google Scholar]
- Pijeau S.; Foster D.; Hohenstein E. G. Excited-state dynamics of 2-(2′-hydroxyphenyl)benzothiazole: ultrafast proton transfer and internal conversion. J. Phys. Chem. A 2017, 121, 4595–4605. 10.1021/acs.jpca.7b01215. [DOI] [PubMed] [Google Scholar]
- Bachrach S. M.; Demoin D. W.; Luk M.; J V. M. Jr. Nucleophilic attack at selenium in diselenides and selenosulfides. a computational study. J. Phys. Chem. A 2004, 108, 4040–4046. 10.1021/jp037972o. [DOI] [Google Scholar]
- Steinmann D.; Nauser T.; Koppenol W. H. Selenium and sulfur in exchange reactions: a comparative study. J. Org. Chem. 2010, 75, 6696–6699. 10.1021/jo1011569. [DOI] [PubMed] [Google Scholar]
- Peng B.; Zhang C.; Marutani E.; Pacheco A.; Chen W.; Ichinose F.; et al. Trapping hydrogen sulfide (H2S) with diselenides: the application in the design of fluorescent probes. Org. Lett. 2015, 17, 1541–1544. 10.1021/acs.orglett.5b00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Zhu H.; Li M.; Gu X. A novel fluorescent probe for imaging endogenous hydrogen sulphide via the CSE enzymatic pathway. Chem. Commun. 2015, 51, 13135–13137. 10.1039/C5CC03927D. [DOI] [PubMed] [Google Scholar]
- Peng B.; Zhang C.; Marutani E.; Pacheco A.; Chen W.; Ichinose F.; Xian M. Trapping Hydrogen Sulfide (H2S) with Diselenides: The Application in the Design of Fluorescent Probes. Org. Lett. 2015, 17, 1541–1544. 10.1021/acs.orglett.5b00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuffi D.; Alvarez C.; Laspina N.; Gotor C.; Lamattina L.; Garcia-Mata C. Hydrogen sulfide generated by l-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol. 2014, 166, 2065–2076. 10.1104/pp.114.245373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna A.; Sepe F.; Lanza I.; Capasso D.; Zappavigna R.; Capasso S.; Caraglia G.; Ingrosso M. Hydrogen sulfide reduces cell adhesion and relevant inflammatory triggering by preventing ADAM17-dependent TNF-α activation. J. Cell. Biochem. 2013, 114, 1536–1548. 10.1002/jcb.24495. [DOI] [PubMed] [Google Scholar]
- Yang R.; Qu R.; Zhou C.; Konkel Y.; Shi J. E.; Liu S.; Chen Y.; Liu C.; Liu S.; Chen D.; Zandi Y.; Chen E.; Zhou W.; Shi Y. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity 2015, 43, 251–263. 10.1016/j.immuni.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.; Chen Y.; Sum N. T.; Lo S. P.; Yang L. W. Ligand exchanged photoluminescent gold quantum dots functionalized with leading peptides for nuclear targeting and intracellular imaging. Chem. Commun. 2008, 39, 4762–4764. 10.1039/b808207c. [DOI] [PubMed] [Google Scholar]
- Zhao G. J.; Han K. Hydrogen Bonding in the Electronic Excited State. Acc. Chem. Res. 2012, 45, 404–413. 10.1021/ar200135h. [DOI] [PubMed] [Google Scholar]
- Wan Q.; Song Y.; Li Z.; Gao X.; Ma H. In vivo monitoring of hydrogen sulfide using a cresyl violet-based ratiometric fluorescence probe. Chem. Commun. 2013, 49, 502–504. 10.1039/C2CC37725J. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Guo W. A new fluorescent probe for gasotransmitter H2S: High sensitivity, excellent selectivity, and a significant fluorescence off-on response. Chem. Commun. 2014, 50, 4214–4217. 10.1039/C3CC49605H. [DOI] [PubMed] [Google Scholar]
- Xu P.; Gao T.; Liu M.; Zhang H.; Zeng W. A novel excited-state intramolecular proton transfer (ESIPT) dye with unique near-IR keto emission and its application in detection of hydrogen sulfide. Analyst 2015, 140, 1814–1816. 10.1039/C4AN02285H. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Maity A.; Sui X.; Pu H.; Mao S.; Singh N. K.; Chen J. In Operando Impedance Spectroscopic Analysis on NiO–WO3 Nanorod Heterojunction Random Networks for Room-Temperature H2S Detection. ACS Omega 2018, 3, 18685–18693. 10.1021/acsomega.8b01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P.; Han K. Unraveling the Detailed Mechanism of Excited-State Proton Transfer. Acc. Chem. Res. 2018, 51, 1681–1690. 10.1021/acs.accounts.8b00172. [DOI] [PubMed] [Google Scholar]
- Gao M.; Wang R.; Yu F.; Chen L. Evaluation of sulfane sulfur bioeffects via a mitochondria-targeting selenium-containing near-infrared fluorescent probe. Biomaterials 2018, 160, 1–14. 10.1016/j.biomaterials.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Wójtowicz H.; Chojnacka M.; Młochowski J.; Palus J.; Syper L.; Hudecova D.; Uher M.; Piasecki E.; Rybka M. Functionalized alkyl and aryl diselenides as antimicrobial and antiviral agents: Synthesis and properties. Farmaco 2003, 58, 1235–1242. 10.1016/j.farmac.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Mlochowski J.; Ryszard J.; Gryglewskib; Anna D.; Inglot A. J.; Leszek J.; Krystian K. Synthesis and Properties of 2-Carboxyalkyl-l,2-benzisoselenazol-3(2H)-ones and Related Organoselenium Compounds as Nitric Oxide Synthase Inhibitors and Cytokine Inducers. Liebigs. Ann. 1996, 3, 1751–1755. [Google Scholar]
- Zhang J.; Ji X.; Zhou J.; Chen Z.; Dong X.; Zhao W. Pyridinium substituted BODIPY as NIR fluorescent probe for simultaneous sensing of hydrogen sulphide/glutathione and cysteine/homocysteine. Sens. Actuators, B 2018, 257, 1076–1082. 10.1016/j.snb.2017.10.133. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.