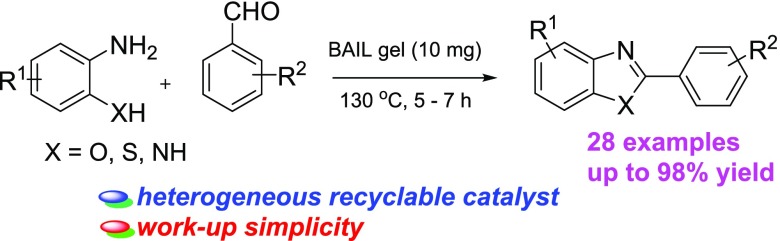
Abstract
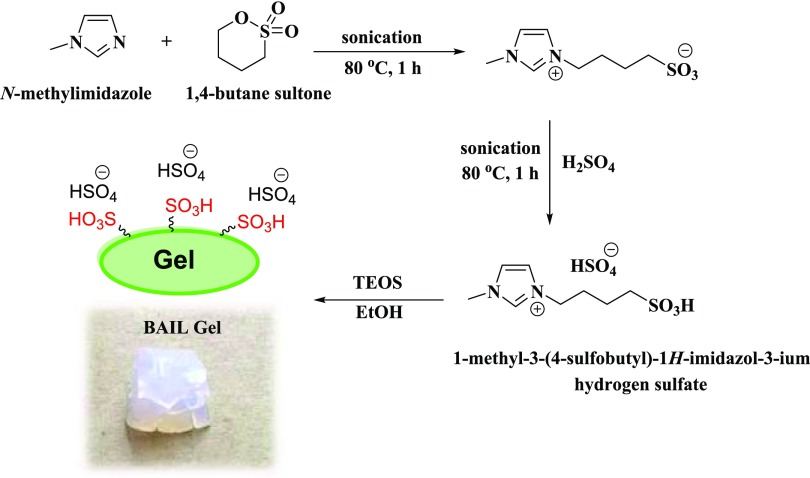
Herein, we introduce a reusable Brønsted acidic ionic liquid gel (BAIL gel) obtained by treating 1-methyl-3-(4-sulfobutyl)-1H-imidazolium hydrogen sulfate with tetraethyl orthosilicate (TEOS). The grafting of the Brønsted acidic ionic liquid to the surface of TEOS has increased the catalytic activity of the material and also simplified catalyst recovery from the reaction mixture. This reaction has a wide substrate scope, and the BAIL gel represents a new catalyst for the synthesis of benzoxazoles, benzimidazoles, and benzothiazoles. The method shows attractive characteristics such as high yields, recyclable catalyst, and work-up simplicity. More importantly, no additional additives or volatile organic solvent and inert atmosphere are required for the reaction, and the BAIL gel has shown a great promise for industrial applications. To the best of our knowledge, the synthesis of benzoxazoles, benzimidazoles, and benzothiazoles using a recyclable heterogeneous ionic liquid gel was not previously reported in the literature.
Introduction
Task-specific ionic liquids (TSILs) have attracted increasing interest of chemists because of their unique physical and chemical properties.1−4 Among these, TSILs bearing SO3H groups with a hydrogen sulfate counteranion have received special attention because of their extremely strong acidic characters.5,6 These TSILs have been used as green and useful catalysts in many organic reactions.6 However, the recovery process of ionic liquids encounters a serious problem because of the high solubility of ionic liquids in the reaction mixture.7,8 The immobilization of TSILs onto silica and magnetic nanoparticles is an excellent pathway for solving the separation process.9−12 However, tedious preparation and leaching of their constituents into the liquid increase the cost of the procedure and limit their application. Thus, ionic liquid gel materials promise a new way to further utilize ionic liquids in catalysis because of the simplicity of preparation, easy separation, and reuse without a significant loss of activity.13 Owing to their attractive properties, the ionic liquid gels have been prepared and applied as novel heterogeneous catalysts in organic synthesis.14
Aryl-substituted benzoxazoles, benzimidazoles, and benzothiazoles are also a valuable class of heterocyclic compounds that have a wide range of biological activities, including antitumor, antiviral, anti-inflammatory, antihypertensive, antihistaminic, and antimicrobial activities.15−25 Two well-known methods for the preparation of these compounds have been developed: (i) metal-catalyzed direct alkylation of benzoxazoles, benzimidazoles, and benzothiazoles through C–H activation followed by C–C bond formation;26−30 (ii) condensation–aromatization of o-aminophenol, o-phenylenediamine, and o-aminothiophenols with aldehydes using Brønsted or Lewis acid catalysts.31−38 Although the reported methods are satisfactory, synthesis of these compounds remains challenging, with procedures suffering from low yields, additional additives or volatile organic solvents, and expensive and unrecyclable catalysts.
Attracted by the unique properties of Brønsted acidic ionic liquid gels (BAIL gels) and the valuable potential applications of these heterogeneous ionic liquids in catalysis,14,39 we report herein the use of a Brønsted acidic ionic liquid gel acting as an efficient and reusable heterogeneous catalyst for the condensation–aromatization of o-aminophenols and benzaldehydes. Interestingly, the expected products were obtained in good to excellent yields, the BAIL gel was recovered easily by filtration, and leaching was negligible. To the best of our knowledge, a Brønsted acidic ionic liquid gel has never been used for the reaction.
Results and Discussion
The Brønsted acidic ionic liquid gel was prepared by following the procedure discussed in the previous literature (Scheme 1)14,39 and was characterized by various techniques including scanning electron microscopy, energy dispersive X-ray spectroscopy (EDS), Fourier transform infrared (FT-IR), thermal gravimetric analysis (TGA), and inductively coupled plasma optical emission spectrometry (ICP-OES). The analysis data were in agreement with those of the previous reports. The preparation procedure and characterization of the Brønsted acidic ionic liquid gel are presented in the Supporting Information. Thermal gravimetric analysis (TGA) of the BAIL gel displayed high thermal stability (>300 °C). EDS indicated the presence of C, O, N, Si, and S elements in the structure of the BAIL gel. The sulfur concentration was found to be 71 000 ppm in the BAIL gel as determined by ICP-OES analysis, corresponding to a BAIL loading of 1.10 mmol g–1.
Scheme 1. Synthesis of the BAIL Gel.
Initially, we screened a range of catalysts for the synthesis of benzoxazole under a solvent-free condition. Traditional catalysts including Brønsted and Lewis acids showed low catalytic activities (Table 1, entries 1–13). Interestingly, the use of the BAIL gel afforded a significant rate increase with 98% yield of 2-phenylbenzoxazole at 130 °C for 5 h (Table 1, entry 16). The reaction could proceed to 87% yield of the desired product using the BAIL (Table 1, entry 14). Next, the optimal conditions for the synthesis of benzoxazole were investigated. No product was obtained for the reaction carried out at room temperature, and the reaction hardly proceeded at the temperature range below 100 °C. Although a higher temperature (up to 130 °C) was used, 98% yield of the desired product was achieved for 5 h in the presence of a catalytic amount (1 mol %) of the BAIL gel. All attempts to reduce the amount of the BAIL gel led to significant drops in the reaction yield.
Table 1. Catalysts Screened for the Synthesis of Benzoxazolea.
| entry | catalyst | yieldb (%) |
|---|---|---|
| 1 | HCl | 48 |
| 2 | H3PO4 | 45 |
| 3 | CF3COOH | 39 |
| 4 | MsOH | 43 |
| 5 | TsOH | 51 |
| 6 | AlCl3 | 55 |
| 7 | FeCl3 | 43 |
| 8 | CuCl2 | 45 |
| 9 | HfCl4 | 39 |
| 10 | Al2O3 | 31 |
| 11 | Fe2O3 | 29 |
| 12 | MgO | 23 |
| 13 | TiO2 | 28 |
| 14 | BAIL | 87 |
| 15 | TEOS | 0 |
| 16 | BAIL gel | 98 |
Reaction conditions: benzaldehyde (1.0 mmol), 2-aminophenol (1.0 mmol).
Isolated yield.
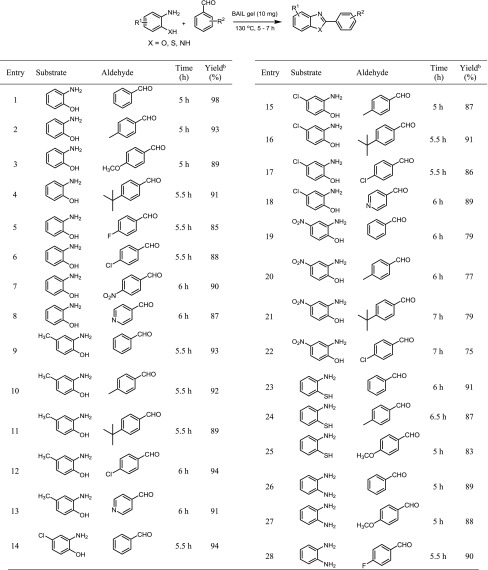
The scope of the reactants was then investigated using a wide range of aromatic aldehydes for the synthesis of benzoxazoles, benzimidazoles, and benzothiazoles. The reaction of substituted aromatic aldehydes such as electron-donating (p-Me, p-OMe, and p-tbu) and electron-withdrawing (p-NO2) substituents afforded the expected products in good to excellent yields (Table 2, entries 2–4 and 7). Halo substituents on the benzaldehyde ring (p-F, p-Cl) were also reactive, providing the desired products in 85 and 88% yields for 5.5 h (Table 2, entries 5 and 6). The reaction of pyridine-4-carbaldehyde resulted in 87% yield of the corresponding product for 6 h (Table 2, entry 8). Next, these conditions were then applied to o-aminophenol derivatives. The reactions were found to be successful for all o-aminophenol derivatives. The use of 2-amino-4-methylphenol and 2-amino-4-chlorophenol proceeded more smoothly with aromatic aldehydes, with 86–94% isolated yields (Table 2, entries 9–18). However, 2-amino-4-nitrophenol showed lower reactivity than the other substrates, with the expected products in only 75–79% yields for prolonged reaction times (Table 2, entries 19–22). To demonstrate the utility of the current method, 2-arylbenzithiazole and 2-arylbenzimidazole derivatives were synthesized under optimal conditions. We observed that the reaction of o-aminothiophenol and o-phenylenediamine with benzaldehydes provided the corresponding products in high yields (Table 2, entries 23–28).
Table 2. Synthesis of Benzoxazoles, Benzimidazoles, and Benzothiazoles Using a BAIL Gel under a Solvent-Free Conditionab.
Reaction conditions: substrate (1.0 mmol), aldehyde (1.0 mmol), BAIL gel (0.01 mmol), at 130 °C.
Isolated yield.
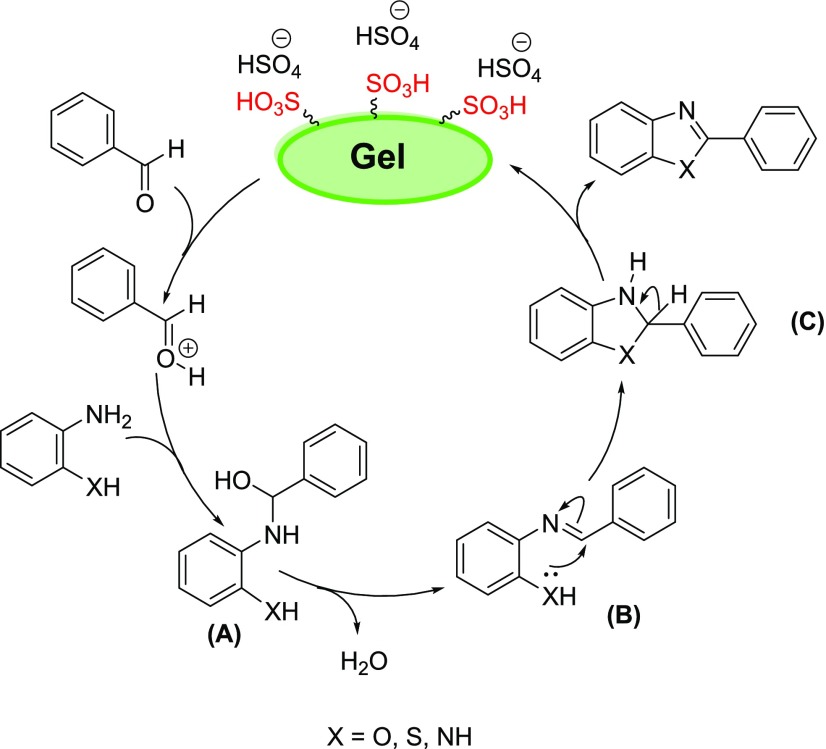
The proposed reaction mechanism was confirmed by mass spectrometry (MS) (Scheme 2). To explore the mechanism on the BAIL gel catalyst, the condensation of o-aminophenol with benzaldehyde was carried out for only 1 h under optimal conditions. We found that 2-(benzylideneamino)phenol (m/z 197) was obtained as a major precursor. As asserted in the previous literature and our experiments, the BAIL gel plays a vital role in the reaction mechanism. The first interaction would be presumably the protonation of the carbonyl oxygen from benzaldehyde by −SO3H active sites on the catalyst surface. The intermediate A was formed through the reaction of activated aldehyde with the amino group on the substrate, which was followed by dehydration to deliver the imine B. Next, the XH group in the intermediate B attacked the imine groups to generate the intermediate C. The intermediate C then subsequently underwent aromatization by oxidation with oxygen in air under the reaction conditions to form the desired product.
Scheme 2. Proposed Mechanism.
The comparison of our work with the previous literature is reported in Table 3. Although various catalysts displayed high activities, these catalysts could not be effectively recycled (Table 3, entries 1–5). The reaction of 2-aminophenol and benzaldehyde in the presence of the BAIL gel afforded the desired product in an excellent yield at 130 °C for 5 h under the solvent-free condition, and the catalyst could be recovered and reused without a significant loss in activity (Table 3, entry 6). The previous studies reported that the same reaction also afforded the target products in good yields at a lower temperature and shorter reaction time, but those methods involved the use of a volatile organic solvent and higher loading of the catalyst.
Table 3. Comparison of the Current Method with the Previous Literature for the Synthesis of Benzoxazole.
| entry | catalyst | conditions | yield (%) | recycling runa |
|---|---|---|---|---|
| 1 | samarium(III) triflate (10 mol %), EtOH–H2O (4:2 mL) | 50 °C, 2 h | 9240 | 4th (6%) |
| 2 | poly(melamine-formaldehyde) (10.0 mg), xylene (0.5 mL) | 110 °C, 10 h | 9141 | 6th (5%) |
| 3 | NiFe2–xEuxO4 (6 mol %), water (5 mL) | reflux, 50 min | 8636 | 6th (9%) |
| 4 | TiO2@ZrO2 (10 mol %), acetonitrile | 60 °C, 15 min | 9137 | 4th (17%) |
| 5 | Hf-MOF (1 mol %) | 140 °C, 6 h | 9542 | 5th (5%) |
| 6 | our work: BAIL gel (1 mol %), solvent free | 130 °C, 5 h | 98 | 5th (3%) |
The number in parenthesis is the drop in the reaction yield after the last run.
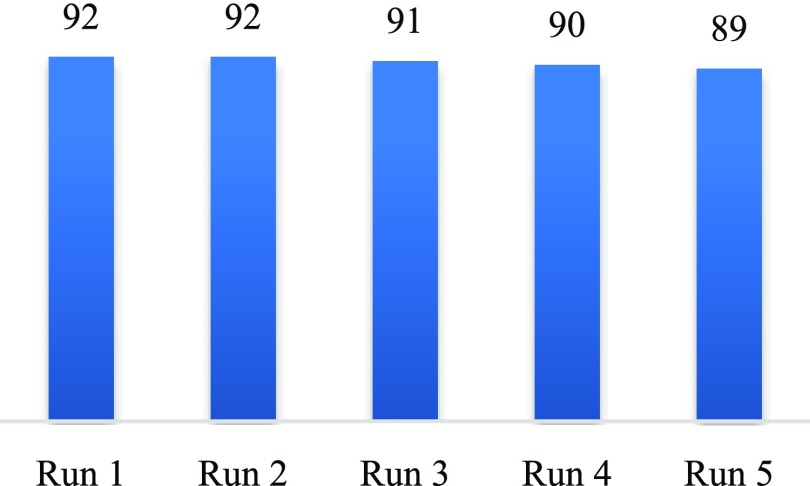
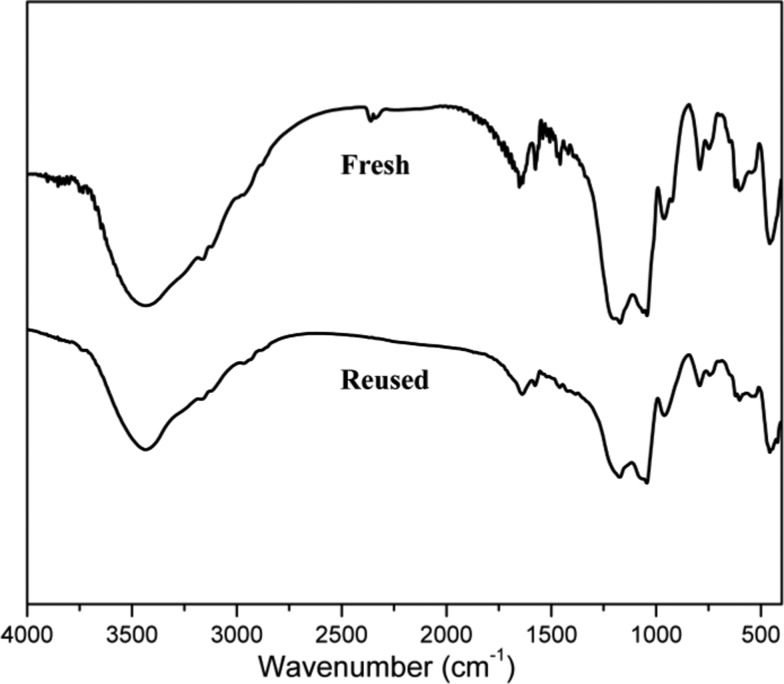
The recycling of the BAIL gel was tested in the reaction of 2-aminophenol and benzaldehyde under optimized conditions. After completion of the reaction (monitored by thin layer chromatography (TLC) or gas chromatography (GC)), the mixture was diluted with ethyl acetate and the BAIL gel was separated by filtration. The recovered catalyst was washed with hexane, ethyl acetate, and ethanol; dried in vacuum; and reused for the next cycle. Experimental results displayed that the BAIL gel catalyst could be recovered and reused many times without a significant loss in catalytic activity. Indeed, the reaction still afforded 89% yield of the desired product even after five consecutive runs (Figure 1). The FT-IR spectrum of the recovered catalyst showed no considerable change in structure (Figure 2). The leaching of the BAIL was determined by ICP-OES, and the result indicated that the amount of sulfur in ethyl acetate was less than 1 ppm, suggesting a little leaching of the BAIL from the catalyst during the reaction.
Figure 1.
Recycling test.
Figure 2.
FT-IR spectra of fresh and the fifth-time-reused BAIL gel catalyst.
Conclusions
In conclusion, we have developed a green and efficient heterogeneous Brønsted acidic ionic liquid gel for the synthesis of benzoxazoles, benzimidazoles, and benzothiazoles under the solvent-free condition for the first time. High yields of desired products were achieved in the presence of a catalytic amount of the BAIL gel (1 mol %). Moreover, the BAIL gel catalyst could be easily recovered and reused without any significant loss of catalytic activity after five runs. This association between Brønsted acidic ionic liquid and tetraethyl orthosilicate (TEOS) offers a new method for preparation of highly attractive heterogeneous catalysts with attractive characteristics such as high yields, work-up simplicity, and negligible ionic liquid leaching into the organic product phase. Furthermore, the synthesis of heteroaryl-substituted compounds using an environmentally benign acidic ionic liquid gel catalyst is an ongoing project.
Experimental Section
General Procedure for the Synthesis of Benzoxazoles
2-Aminophenol (0.119 g, 1 mmol), benzaldehyde (0.106 g, 1 mmol), and a BAIL gel (0.010 g, 1.0 mol % of BAIL) were added into a 5 mL vessel. The reaction mixture was then stirred under a solvent-free condition at 130 °C for 5 h. After completion of the reaction (monitored by TLC or GC), the mixture was dissolved in 10 mL of ethyl acetate, and the BAIL gel catalyst was separated by centrifugation. The organic layer was dried over anhydrous MgSO4 and dried in vacuum to obtain the crude product. The crude product was purified by silica gel column chromatography using acetone/petroleum ether (1:19) to afford the pure product. The purified product was confirmed by FT-IR, 1H NMR, 13C NMR, and MS. For the recycling studies, the catalyst was washed with hexane (2 × 3 mL), ethyl acetate (2 × 3 mL), and ethanol (2 mL), dried in vacuum to remove all of the volatile components, and then reused in the next run.
Acknowledgments
This research is funded by Vietnam National University, Ho Chi Minh City (VNU-HCM) under the grant number 562-2018-18-03.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02932.
Preparation and characterization of the BAIL gel; spectral data; 1H, 13C NMR, and MS spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Amarasekara A. S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. 10.1021/acs.chemrev.5b00763. [DOI] [PubMed] [Google Scholar]
- Goossens K.; Lava K.; Bielawski C. W.; Binnemans K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. 10.1021/cr400334b. [DOI] [PubMed] [Google Scholar]
- Vafaeezadeh M.; Alinezhad H. Brønsted acidic ionic liquids: Green catalysts for essential organic reactions. J. Mol. Liq. 2016, 218, 95–105. 10.1016/j.molliq.2016.02.017. [DOI] [Google Scholar]
- Egorova K. S.; Ananikov V. P. Toxicity of Ionic Liquids: Eco(cyto)activity as Complicated, but Unavoidable Parameter for Task-Specific Optimization. ChemSusChem 2014, 7, 336–360. 10.1002/cssc.201300459. [DOI] [PubMed] [Google Scholar]
- Xie L.-Y.; Peng S.; Lu L.-H.; Hu J.; Bao W.-H.; Zeng F.; Tang Z.; Xu X.; He W.-M. Brønsted Acidic Ionic Liquid-Promoted Amidation of Quinoline N-Oxides with Nitriles. ACS Sustainable Chem. Eng. 2018, 6, 7989–7994. 10.1021/acssuschemeng.8b01358. [DOI] [Google Scholar]
- Wu C.; Lu L.-H.; Peng A.-Z.; Jia G.-K.; Peng C.; Cao Z.; Tang Z.; He W.-M.; Xu X. Ultrasound-promoted Brønsted acid ionic liquid-catalyzed hydrothiocyanation of activated alkynes under minimal solvent conditions. Green Chem. 2018, 20, 3683–3688. 10.1039/C8GC00491A. [DOI] [Google Scholar]
- Zhen B.; Jiao Q.; Zhang Y.; Wu Q.; Li H. Acidic ionic liquid immobilized on magnetic mesoporous silica: Preparation and catalytic performance in esterification. Appl. Catal., A 2012, 445–446, 239–245. 10.1016/j.apcata.2012.08.023. [DOI] [Google Scholar]
- Vafaeezadeh M.; Mahmoodi Hashemi M. One pot oxidative cleavage of cyclohexene to adipic acid using silver tungstate nano-rods in a Brønsted acidic ionic liquid. RSC Adv. 2015, 5, 31298–31302. 10.1039/C5RA02339D. [DOI] [Google Scholar]
- Vafaeezadeh M.; Hashemi M. M. Efficient fatty acid esterification using silica supported Brønsted acidic ionic liquid catalyst: Experimental study and DFT modeling. Chem. Eng. J. 2014, 250, 35–41. 10.1016/j.cej.2014.04.001. [DOI] [Google Scholar]
- Karimi B.; Vafaeezadeh M. SBA-15-functionalized sulfonic acid confined acidic ionic liquid: a powerful and water-tolerant catalyst for solvent-free esterifications. Chem. Commun. 2012, 48, 3327–3329. 10.1039/c2cc17702a. [DOI] [PubMed] [Google Scholar]
- Karimi B.; Vafaeezadeh M. SBA-15 functionalized sulfonic acid containing a confined hydrophobic and acidic ionic liquid: a highly efficient catalyst for solvent-free thioacetalization of carbonyl compounds at room temperature. RSC Adv. 2013, 3, 23207–23211. 10.1039/c3ra42286k. [DOI] [Google Scholar]
- Vafaeezadeh M.; Fattahi A. A study on the catalytic activity and theoretical modeling of a novel dual acidic mesoporous silica. RSC Adv. 2014, 4, 16647–16654. 10.1039/c3ra47638c. [DOI] [Google Scholar]
- Marr P. C.; Marr A. C. Ionic liquid gel materials: applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. 10.1039/C5GC02277K. [DOI] [Google Scholar]
- Wang Y.-M.; Ulrich V.; Donnelly G. F.; Lorenzini F.; Marr A. C.; Marr P. C. A Recyclable Acidic Ionic Liquid Gel Catalyst for Dehydration: Comparison with an Analogous SILP Catalyst. ACS Sustainable Chem. Eng. 2015, 3, 792–796. 10.1021/sc5008303. [DOI] [Google Scholar]
- Gorla S. K.; Kavitha M.; Zhang M.; Chin J. E.; Liu X.; Striepen B.; Makowska-Grzyska M.; Kim Y.; Joachimiak A.; Hedstrom L.; Cuny G. D. Optimization of benzoxazole-based inhibitors of Cryptosporidium parvum inosine 5′-monophosphate dehydrogenase. J. Med. Chem. 2013, 56, 4028–4043. 10.1021/jm400241j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth K.; Garg S. K.; Kumar R.; Purohit P.; Meena V. S.; Goyal R.; Banerjee U. C.; Chakraborti A. K. 2-(2-Arylphenyl)benzoxazole As a Novel Anti-Inflammatory Scaffold: Synthesis and Biological Evaluation. ACS Med. Chem. Lett. 2014, 5, 512–516. 10.1021/ml400500e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerova M. S.; Stateva S. R.; Radonova E. M.; Kalenderska R. B.; Rusew R. I.; Nikolova R. P.; Chanev C. D.; Shivachev B. L.; Apostolova M. D.; Petrov O. I. Combretastatin A-4 analogues with benzoxazolone scaffold: Synthesis, structure and biological activity. Eur. J. Med. Chem. 2016, 120, 121–133. 10.1016/j.ejmech.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Padalkar V. S.; Borse B. N.; Gupta V. D.; Phatangare K. R.; Patil V. S.; Umape P. G.; Sekar N. Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arabian J. Chem. 2016, 9, S1125–S1130. 10.1016/j.arabjc.2011.12.006. [DOI] [Google Scholar]
- Abdelgawad M. A.; Bakr R. B.; Omar H. A. Design, synthesis and biological evaluation of some novel benzothiazole/benzoxazole and/or benzimidazole derivatives incorporating a pyrazole scaffold as antiproliferative agents. Bioorg. Chem. 2017, 74, 82–90. 10.1016/j.bioorg.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Kim D.; Won H. Y.; Hwang E. S.; Kim Y. K.; Choo H. P. Synthesis of benzoxazole derivatives as interleukin-6 antagonists. Bioorg. Med. Chem. 2017, 25, 3127–3134. 10.1016/j.bmc.2017.03.072. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Liu J.; Macho J. M.; Jiang X.; Xie D.; Jiang F.; Liu W.; Fu L. Design, synthesis and antimicrobial evaluation of novel benzoxazole derivatives. Eur. J. Med. Chem. 2017, 126, 7–14. 10.1016/j.ejmech.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Robinson E. R. T.; Walden D. M.; Fallan C.; Greenhalgh M. D.; Cheong P. H.-Y.; Smith A. D. Non-bonding 1,5-S[three dots, centered]O interactions govern chemo- and enantioselectivity in isothiourea-catalyzed annulations of benzazoles. Chem. Sci. 2016, 7, 6919–6927. 10.1039/C6SC00940A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda K.; Rajasekhar S.; Maiti B. A Decade Update on Benzoxazoles, a Privileged Scaffold in Synthetic Organic Chemistry. Synlett 2017, 28, 521–541. 10.1055/s-0036-1588671. [DOI] [Google Scholar]
- Dhopte K. B.; Zambare R. S.; Patwardhan A. V.; Nemade P. R. Role of graphene oxide as a heterogeneous acid catalyst and benign oxidant for synthesis of benzimidazoles and benzothiazoles. RSC Adv. 2016, 6, 8164–8172. 10.1039/C5RA19066E. [DOI] [Google Scholar]
- Mortimer C. G.; Wells G.; Crochard J.-P.; Stone E. L.; Bradshaw T. D.; Stevens M. F. G.; Westwell A. D. Antitumor Benzothiazoles. 26. 2-(3,4-Dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a Simple Fluorinated 2-Arylbenzothiazole, Shows Potent and Selective Inhibitory Activity against Lung, Colon, and Breast Cancer Cell Lines. J. Med. Chem. 2006, 49, 179–185. 10.1021/jm050942k. [DOI] [PubMed] [Google Scholar]
- Khalafi-Nezhad A.; Panahi F. Ruthenium-Catalyzed Synthesis of Benzoxazoles Using Acceptorless Dehydrogenative Coupling Reaction of Primary Alcohols with 2-Aminophenol under Heterogeneous Conditions. ACS Catal. 2014, 4, 1686–1692. 10.1021/cs5000872. [DOI] [Google Scholar]
- Le H. T. N.; Nguyen T. T.; Vu P. H. L.; Truong T.; Phan N. T. S. Ligand-free direct C-arylation of heterocycles with aryl halides over a metal-organic framework Cu2(BPDC)2(BPY) as an efficient and robust heterogeneous catalyst. J. Mol. Catal. A: Chem. 2014, 391, 74–82. 10.1016/j.molcata.2014.03.017. [DOI] [Google Scholar]
- Abdellaoui F.; Youssef C.; Ben Ammar H.; Roisnel T.; Soulé J.-F.; Doucet H. Palladium-Catalyzed Regioselective C–H Bond Arylations of Benzoxazoles and Benzothiazoles at the C7 Position. ACS Catal. 2016, 6, 4248–4252. 10.1021/acscatal.6b00678. [DOI] [Google Scholar]
- Yim J. C. H.; Nambo M.; Crudden C. M. Pd-Catalyzed Desulfonative Cross-Coupling of Benzylic Sulfone Derivatives with 1,3-Oxazoles. Org. Lett. 2017, 19, 3715–3718. 10.1021/acs.orglett.7b01510. [DOI] [PubMed] [Google Scholar]
- Prajapati N. P.; Vekariya R. H.; Borad M. A.; Patel H. D. Recent advances in the synthesis of 2-substituted benzothiazoles: a review. RSC Adv. 2014, 4, 60176–60208. 10.1039/C4RA07437H. [DOI] [Google Scholar]
- Azizian J.; Torabi P.; Noei J. Synthesis of benzimidazoles and benzoxazoles using TiCl3OTf in ethanol at room temperature. Tetrahedron Lett. 2016, 57, 185–188. 10.1016/j.tetlet.2015.11.092. [DOI] [Google Scholar]
- Chen W.; An W.; Wang Y.; Yu A. Mechanisms of Metal-Free Aerobic Oxidation To Prepare Benzoxazole Catalyzed by Cyanide: A Direct Cyclization or Stepwise Oxidative Dehydrogenation and Cyclization?. J. Org. Chem. 2016, 81, 10857–10862. 10.1021/acs.joc.6b01939. [DOI] [PubMed] [Google Scholar]
- Nikpassand M.; Fekri L. Z.; Farokhian P. An efficient and green synthesis of novel benzoxazole under ultrasound irradiation. Ultrason. Sonochem. 2016, 28, 341–345. 10.1016/j.ultsonch.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Chikhale R. V.; Pant A. M.; Menghani S. S.; Wadibhasme P. G.; Khedekar P. B. Facile and efficient synthesis of benzoxazole derivatives using novel catalytic activity of PEG-SO 3 H. Arabian J. Chem. 2017, 10, 715–725. 10.1016/j.arabjc.2014.06.011. [DOI] [Google Scholar]
- Naeimi H.; Rouzegar Z.; Rahmatinejad S. Catalyst-free microwave-promoted one pot synthesis of 2-aryl benzoxazoles using MnO2 nanoparticles as a convenient oxidant under mild condition. Res. Chem. Intermed. 2017, 43, 4745–4758. 10.1007/s11164-017-2909-4. [DOI] [Google Scholar]
- Ziarati A.; Sobhani-Nasab A.; Rahimi-Nasrabadi M.; Ganjali M. R.; Badiei A. Sonication method synergism with rare earth based nanocatalyst: preparation of NiFe 2– x Eu x O 4 nanostructures and its catalytic applications for the synthesis of benzimidazoles, benzoxazoles, and benzothiazoles under ultrasonic irradiation. J. Rare Earths 2017, 35, 374–381. 10.1016/S1002-0721(17)60922-0. [DOI] [Google Scholar]
- Patil M. R.; Bhanushali J. T.; Nagaraja B. M.; Keri R. S. TiO2-ZrO2 composite: Synthesis, characterization and application as a facile, expeditious and recyclable catalyst for the synthesis of 2-aryl substituted benzoxazole derivatives. C. R. Chim. 2018, 21, 399–407. 10.1016/j.crci.2016.12.008. [DOI] [Google Scholar]
- Vafaeezadeh M.; Dizicheh Z. B.; Hashemi M. M. Mesoporous silica-functionalized dual Brønsted acidic ionic liquid as an efficient catalyst for thioacetalization of carbonyl compounds in water. Catal. Commun. 2013, 41, 96–100. 10.1016/j.catcom.2013.07.004. [DOI] [Google Scholar]
- Tran P. H.; Nguyen X.-T. T.; Chau D.-K. N. A Brønsted-Acidic Ionic Liquid Gel as an Efficient and Recyclable Heterogeneous Catalyst for the Synthesis of Bis(indolyl)methanes under Solvent-Free Sonication. Asian J. Org. Chem. 2018, 7, 232–239. 10.1002/ajoc.201700596. [DOI] [Google Scholar]
- Ingle V.; Gorepatil P.; Mane Y. Samarium(III) Triflate as an Efficient and Reusable Catalyst for Facile Synthesis of Benzoxazoles and Benzothiazoles in Aqueous Medium. Synlett 2013, 24, 2241–2244. 10.1055/s-0033-1339758. [DOI] [Google Scholar]
- Yang D.; Liu P.; Zhang N.; Wei W.; Yue M.; You J.; Wang H. Mesoporous Poly(melamine-formaldehyde): A Green and Recyclable Heterogeneous Organocatalyst for the Synthesis of Benzoxazoles and Benzothiazoles Using Dioxygen as Oxidant. ChemCatChem 2014, 6, 3434–3439. 10.1002/cctc.201402628. [DOI] [Google Scholar]
- Nguyen L. H. T.; Nguyen T. T.; Nguyen H. L.; Doan T. L. H.; Tran P. H. A new superacid hafnium-based metal–organic framework as a highly active heterogeneous catalyst for the synthesis of benzoxazoles under solvent-free conditions. Catal. Sci. Technol. 2017, 7, 4346–4350. 10.1039/C7CY01668A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.