Abstract
Density functional theory calculations here elucidated that Cu38-catalyzed NO reduction by CO occurred not through NO dissociative adsorption but through NO dimerization. NO is adsorbed to two Cu atoms in a bridging manner. NO adsorption energy is much larger than that of CO. N–O bond cleavage of the adsorbed NO molecule needs a very large activation energy (ΔG°‡). On the other hand, dimerization of two NO molecules occurs on the Cu38 surface with small ΔG°‡ and very negative Gibbs reaction energy (ΔG°) to form ONNO species adsorbed to Cu38. Then, a CO molecule is adsorbed at the neighboring position to the ONNO species and reacts with the ONNO to induce N–O bond cleavage with small ΔG°‡ and very negative ΔG°, leading to the formation of N2O adsorbed on Cu38 and CO2 molecule in the gas phase. N2O dissociates from Cu38, and then it is readsorbed to Cu38 in the most stable adsorption structure. N–O bond cleavage of N2O easily occurs with small ΔG°‡ and significantly negative ΔG° to form the N2 molecule and the O atom adsorbed on Cu38. The O atom reacts with the CO molecule to afford CO2 and regenerate Cu38, which is rate-determining. N2O species was experimentally observed in Cu/γ-Al2O3-catalyzed NO reduction by CO, which is consistent with this reaction mechanism. This mechanism differs from that proposed for the Rh catalyst, which occurs via N–O bond cleavage of the NO molecule. Electronic processes in the NO dimerization and the CO oxidation with the O atom adsorbed to Cu38 are discussed in terms of the charge-transfer interaction with Cu38 and Frontier orbital energy of Cu38.
1. Introduction
The platinum group metals such as platinum, palladium, and rhodium have been exclusively used for three-way catalyst (TWC) for automobiles and electrode catalyst for fuel cells.1 For cost reduction of catalysts and resource preservation on the earth, the use of abundant metals and/or the reduction of precious metal content in catalysts must be achieved. To design such catalysts, correct understanding of catalytic function of metal cluster/particle is indispensable. However, it is not easy to elucidate experimentally the reaction mechanism and electronic process of a heterogeneous catalytic reaction by metal cluster/particle because experimental tools and analysis techniques are still limited for heterogeneous catalysts even nowadays. In this regard, theoretical investigation of reactivity and catalysis of metal cluster/particle is indispensable.
TWCs catalyze mainly three reactions—oxidation of hydrocarbon to CO2 and H2O, oxidation of CO to CO2, and NOx reduction to N2. Among these three reactions, NO reduction by CO has been investigated well both in experiments2−5 and in theoretical calculations.6−9 In these studies, the N–O bond cleavage was considered to occur on Rh,2,3,9 Pt,6,8 and Pd5 as one of the key elementary steps.
In recent years, Au nanoscale particles have attracted a lot of interest as new catalysts since the reports by Haruta and co-workers.10−12 Their reports are of considerable interest because nanoscale Au particles can be applied to catalytic reaction despite the inertness of the bulk metal. Similar catalysts, Au atom (or cluster) doped on M (M = Ni, Rh, Pd, Ag, or Ir), were applied to NO–CO reaction.13,14 In these studies, the dissociative NO adsorption was proposed as an important initial step like in the NO–CO reaction by Rh, Pt, and Pd catalysts.2,3,5,6,8,9 Also, Ag nanoscale particles were experimentally reported to be active for NO decomposition.15−18 Pioneering theoretical research reported that the NO decomposition did not occur through dissociative NO adsorption but through NO dimerization.19−21
Cu is one of the most abundant metals. Considering catalyses of nanoscale Au and Ag particles and Au-doped metal particles, nanoscale Cu cluster/particle is also expected to be useful as a catalyst. Actually, highly dispersed Cu cluster/particle on γ-Al2O3 was reported to exhibit high catalytic activity for NO decomposition and NO reduction by CO.22−26 Because N–O bond cleavage of the NO molecule is difficult to occur on the Cu surface according to theoretical calculations,24,27,28 it is likely that NO reduction by this catalyst occurs through a new reaction mechanism without N–O bond cleavage of the NO molecule like the NO reduction catalyzed by Ag nanoclusters.15−21 It is of considerable interest how and why Cu particles can easily catalyze NO reduction by CO not through N–O bond cleavage of the NO molecule. However, no information has been presented about the reaction mechanism and the full catalytic cycle of Cu-catalyzed NO reduction by CO.
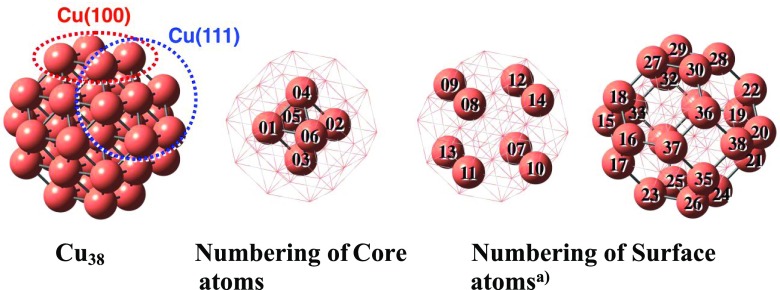
In this work, we investigated the NO reduction by CO on Cu38 cluster, using density functional theory (DFT) calculations, where the geometry of Cu38 is shown in Scheme 1 with the numbering of each Cu atom. Our purposes here are to elucidate through what reaction mechanism Cu38-catalyzed NO reduction by CO occurs, how easily the N–O bond is cleaved, and what role(s) the Cu cluster plays in this NO reduction by CO. We believe that these computational results are useful for finding a new TWC with nonprecious metals.
Scheme 1. Structure of Cu38 and Numbering of Each Atom.
Cu(07) to Cu(14) are center atoms of the (111) plane. Cu(15) to Cu(38) are corner atoms of the (111) plane and belong to the (100) plane at the same time.
2. Results and Discussion
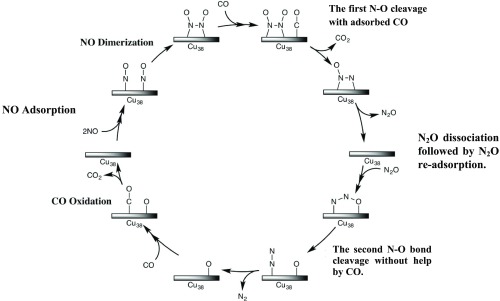
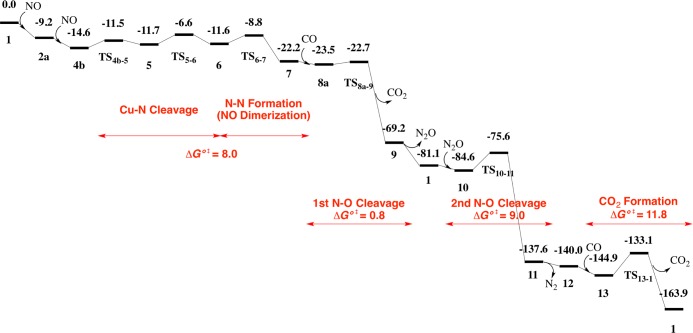
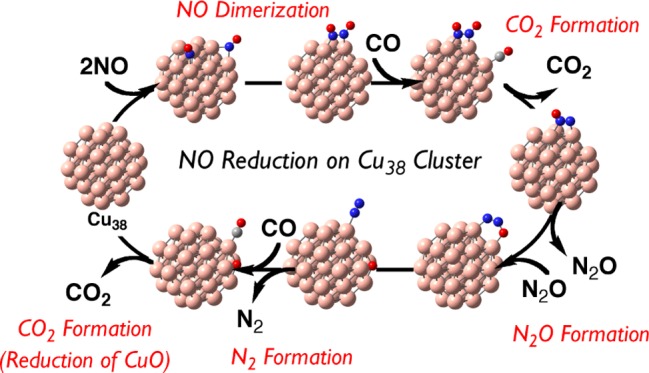
Because many elementary steps will be discussed in this catalytic reaction, we wish to briefly discuss here an overview of the catalytic cycle, as shown in Scheme 2. The first step is NO adsorption to Cu38 followed by NO dimerization on the Cu38 surface to afford the ONNO dimer. The N–O bond of the ONNO dimer reacts with CO adsorbed on the Cu38 to afford CO2 and N2O. After readsorption of N2O to Cu38, the second N–O bond cleavage occurs to afford free N2 molecule and O atom adsorbed on the Cu38 surface. The final step is adsorption of CO followed by the reaction of CO with the O atom on Cu38 to afford CO2 and regenerate the Cu38 particle. The rate-determining step is the reaction of CO with the O atom on the Cu38 surface. Besides this main reaction course, we investigated other possible reaction courses; for instance, several possible reaction courses of NO including NO dissociative adsorption (Scheme 3) and NO bond cleavage of ONNO species with and without help by the CO molecule (Scheme 4). In the next section, we will discuss each elementary step in detail.
Scheme 2. Schematic Representation of the Catalytic Cycle for NO Reduction by CO on Cu38.
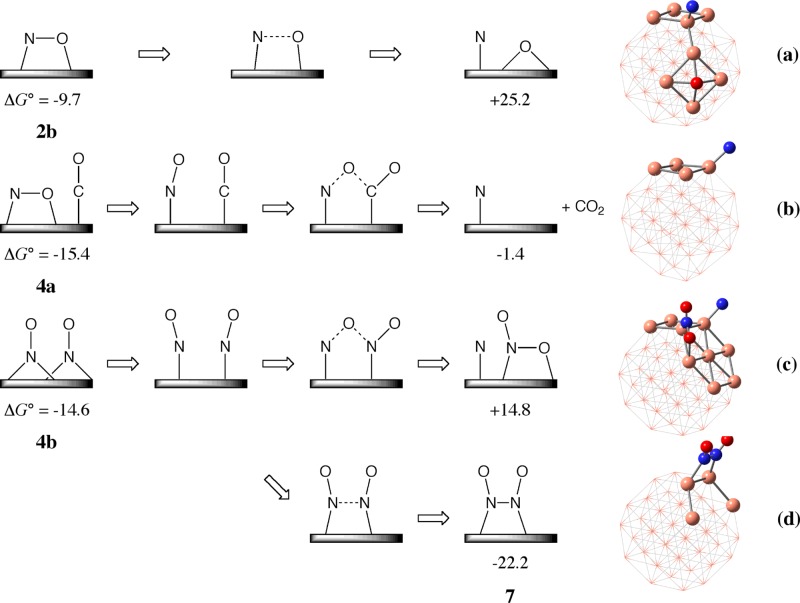
Scheme 3. Possible Reaction Pathways Starting from the NO Adsorption Structure 2b, the NO–CO Coadsorption Structure 4a, and the NO–NO Coadsorption Structure 4b.
Gibbs energies (ΔG°) relative to the sum of isolated species are presented in kcal/mol.
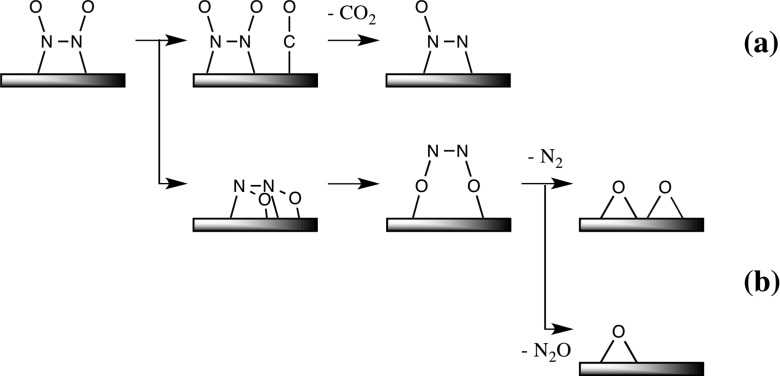
Scheme 4. Schematic Representation of N–O Bond Cleavage of ONNO Species with and without the Help of CO.
2.1. NO and CO Adsorptions on Cu38 Cluster
As the initial step of the NO reduction by CO, we investigated the most favorable adsorption position of the Cu38 surface for NO and CO molecules. For NO, we obtained many possible adsorption structures on the Cu38 surface such as end-on and side-on with 1-fold, 2-fold, 3-fold, and so on; see Figure S1 in the Supporting Information for all the calculated adsorption structures. Among them, the most stable is the side-on adsorption structure 2b in which NO is bridging Cu(22) and Cu(28) atoms, as shown in Figure 1, where the spin state is quartet and the Gibbs energy change (ΔG°) for NO adsorption is −9.7 kcal/mol (negative value means stabilization in energy). In 2b, the NO moiety has negative NBO charge (−0.53e), suggesting that charge transfer (CT) occurs from Cu38 to the vacant N–O π* orbital. The next stable structure is the end-on adsorption structure 2a in which the N atom is bridging Cu(22) and Cu(28) atoms. Its spin state is a doublet, and its ΔG° is slightly smaller (−9.2 kcal/mol) than that for the side-on adsorption 2b (Figure 1). The NBO charge (−0.48e) of the NO moiety is also moderately smaller in 2a than in 2b, suggesting that the CT from Cu38 to the NO moiety is moderately weaker in 2a than in 2b.
Figure 1.
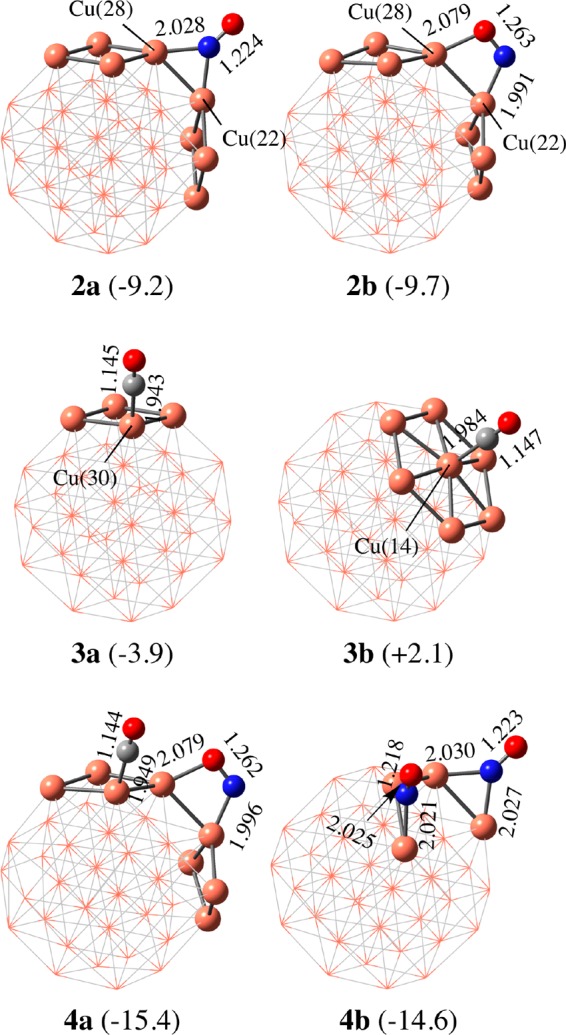
Optimized NO, CO, NO–CO, and NO–NO adsorption structures on Cu38. Distances are in Angstrom. In parentheses are Gibbs energy changes (in kcal/mol) relative to the sum of Cu38 and free gas molecule(s).
In CO, only two adsorption structures could be located; both have an end-on adsorption structure through the C atom, as shown in Figure 1. Adsorption structure 3a at the Cu(30) atom, which is equivalent to Cu(28), is the most stable, where the spin state is a singlet and the ΔG° is moderately negative (−3.9 kcal/mol). This ΔG° is much smaller than that of NO. The CO moiety has positive NBO charge (+0.18e), suggesting that CT occurs from lone-pair orbital of CO to Cu38. Another adsorption structure 3b was located at the Cu(14) atom, the center of the (111) plane, where the spin state is singlet. However, the ΔG° is positive (+2.1 kcal/mol), indicating that CO is not absorbed at this Cu atom.
On the basis of the above results, it is concluded that NO is more strongly adsorbed on the Cu38 surface than CO.
2.2. Dissociative NO Adsorption on Cu38 Cluster
In one of the plausible mechanisms, NO reduction by CO with transition-metal catalyst starts to occur by dissociative NO adsorption, as shown in Scheme 3a.4−9 Because it is likely that N–O bond cleavage starts from the side-on NO adsorbed structure 2b (Figure 1 and Scheme 3a), we investigated the energy change by elongating the N–O distance in 2b, where the geometry was optimized at each N–O distance. However, the total energy became significantly higher as the N–O distance was elongated, and the energy barrier was estimated to be very large (more than 80 kcal/mol) for the N–O bond cleavage; the potential energy change is shown in Figure S2a in the Supporting Information. In addition, the product, consisting of N and O atoms separately adsorbed on the Cu38 cluster, is much less stable than 2b by ΔG° = +34.9 kcal/mol. Although this activation barrier for the N–O bond cleavage is smaller than the N–O bond energy of free NO molecules (147.3 kcal/mol), the large ΔG° and large activation barrier clearly show that dissociative NO adsorption on the Cu38 surface is energetically difficult to occur, as reported in the previous works.24,27,28
It should be concluded that the NO reduction by CO on Cu38 occurs without NO dissociative adsorption. We will investigate the below key questions through what elementary step and how the N–O bond cleavage and the N–N bond formation occur in this catalytic reaction.
2.3. N–O Bond Cleavage of NO Molecule by Coadsorbed CO or NO vs N–N Bond Formation between Two NO Molecules
It is likely that the N–O bond cleavage occurs by the reaction with CO because thermodynamically stable CO2 is formed by this reaction (Scheme 3b). The N–O bond cleavage by the reaction with one more NO molecule is also likely to occur, as shown in Scheme 3c, where the NO2 molecule is produced. Another plausible reaction is NO dimerization affording ONNO species on Cu38 because NO dimerization in the gas phase has been well known experimentally29−31 and proposed in theoretical studies on Co and Au catalysts.32,33 For the former reaction, NO–CO coadsorption must occur at the neighboring position to each other, and for the latter two reactions, NO–NO coadsorption must occur.
The NO–CO coadsorption structure 4a was optimized, as shown in Figure 1; the ground state of 4a has doublet spin multiplicity, but other spin states such as quartet (+0.4 kcal/mol) and sextet (+5.6 kcal/mol) are not very unstable compared to 4a. Its ΔG° was calculated to be −15.4 kcal/mol relative to the isolated reactants, Cu38, NO, and CO. The coadsorption energy is close to the sum of the separate adsorption energies of NO (ΔG° = −9.7 kcal/mol) and CO (ΔG° = −3.9 kcal/mol). Another NO–CO coadsorption structure 4c is somewhat less stable than 4a, as shown in Figure S1. The N–O bond cleavage by the neighboring CO molecule is endergonic (ΔG° = +14.0 kcal/mol) relative to 4a. This result suggests that the N atom adsorbed on the Cu38 surface is not stable in energy. Thus, this pathway is ruled out hereinafter.
The ΔG° value for NO–NO coadsorption 4b was calculated to be −14.6 kcal/mol, indicating that this adsorption occurs slightly less favorably than the NO–CO coadsorption, but the energy difference between them is very small (0.8 kcal/mol); the ground state of 4b has triplet spin multiplicity, but other spin states such as singlet (+2.0 kcal/mol) and quintet (+2.6 kcal/mol) are not very unstable. The second NO molecule is adsorbed at the neighboring position to the first NO with stabilization energy (ΔG°) of −5.4 kcal/mol, which is smaller than the first one (ΔG° = −9.2 kcal/mol). Other NO–NO coadsorption structures (4d and 4e) are somewhat less stable than 4b, as shown in Figure S1. Starting from 4b, N–O bond cleavage assisted by the neighboring NO molecule occurs to afford NO2 and N atom adsorbed to Cu38 with significantly large endergonicity (ΔG° = +29.4 kcal/mol) relative to 4b, as shown in Scheme 3c, indicating that this pathway is unfavorable. On the other hand, the N–N bond formation via NO dimerization affording ONNO species on the Cu38 surface was calculated to be somewhat exergonic (ΔG° = −7.6 kcal/mol) relative to 4b; see 7 in Scheme 3d.
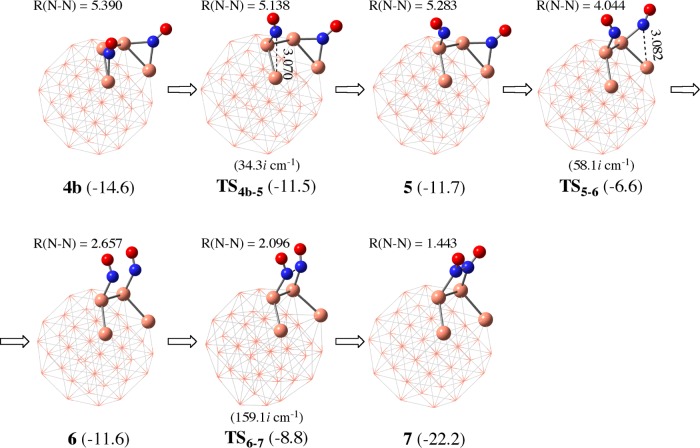
Because the NO dimerization is much more exergonic than the formations of CO2 and N atom adsorbed on Cu38, it is likely concluded that the NO dimerization is the most plausible reaction if the ΔG°‡ value is not large. Because the N–N distance between two NO molecules is too long (5.390 Å) in 4b for inducing dimerization reaction between these two NO molecules, we investigated here the reverse reaction starting from 7 to 4b for convenience and found the geometry changes connecting 4b and 7; geometry changes are shown in Figure 2 and the Gibbs energetics is shown in Figure 3. The N–N bond cleavage of the ONNO moiety occurs through transition state TS6–7 to afford intermediate 6, in which two NO molecules are adsorbed on the Cu38 surface in η1-end-on manner with the N–N distance of 2.657 Å. Starting from 6, on-top η1-end-on structure of one NO molecule changes to μ2-bridging one 5 through transition state TS5–6 (Figure 2). Then, η1-end-on structure of the other NO molecule changes to μ2-bridging structure 4b through transition state TS4b–5. When going from 4b to TS6–7, the highest energy transition state is TS5–6 and the lowest energy intermediate before TS6–7 is 4b (Figure 3). Thus, the ΔG°‡ going from 4b to 7 is 8.0 kcal/mol, indicating that the ONNO species is easily formed on the Cu38 surface with moderate ΔG°‡ and somewhat negative ΔG° value.
Figure 2.
Geometry changes in NO dimerization on Cu38. Distances are in Angstrom. In parentheses are Gibbs energy changes (in kcal/mol) relative to the sum of Cu38 and free gas molecule(s).
Figure 3.
Gibbs energy profile (in kcal/mol) of the NO reduction by CO on Cu38 cluster.
The elementary step going from 6 to 7 is crucially important because N–N bond formation occurs in this step. In TS6–7, the N–N distance (2.096 Å) is shorter than in 6 but longer than in 7. The ONN angles are 112.1 and 112.5°. The geometry of the ONNO moiety in 7 differs very much from the NO dimer experimentally observed in the gas phase.29 For instance, the N–N distance (1.443 Å) in 7 is considerably shorter than that (2.263 Å) of the free NO dimer. The long N–N distance of the free NO dimer is consistent with the small bond energy (about 2 kcal/mol).31 Because the NO dimerization process and the ONNO species are crucially important in this reaction, they will be discussed below in a different section.
2.4. N–O Bond Cleavage of ONNO Species
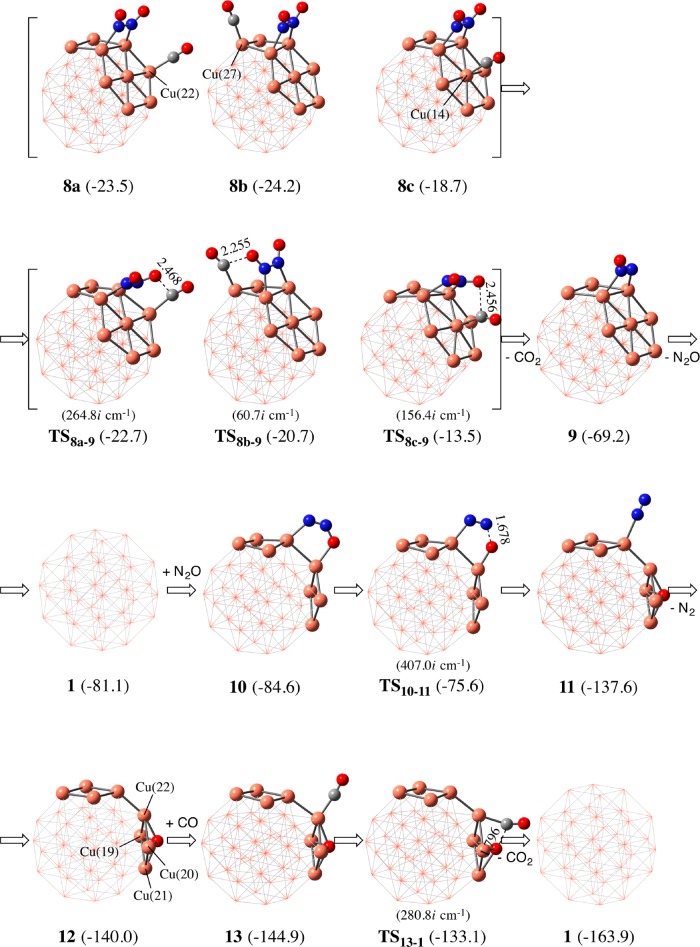
After the formation of the ONNO species on Cu38, two reaction pathways are possible. In one reaction pathway, ONNO reacts with CO, which can be viewed as oxygen atom abstraction by CO, as shown in Scheme 4a. Another is N–O bond cleavage without help by CO (Scheme 4b). In the former pathway, three CO adsorption positions were found neighboring the ONNO moiety on Cu(22), Cu(27), and Cu(14) (8a–8c), as shown in Figure 4. Among them, the CO adsorption at Cu(27) occurs to afford the most stable coadsorption species 8b, where the ΔG° is −24.2 kcal/mol relative to Cu38, 2NO, and CO molecules (Figure 3). The next stable CO adsorption occurs at Cu(22) to afford coadsorption structure 8a (ΔG° = −23.5 kcal/mol), while the difference in ΔG° between 8a and 8b is very small (0.7 kcal/mol). The CO adsorption at Cu(14), which is inside the atom of the (111) plane, affords coadsorption structure 8c, which is considerably less stable (ΔG° = −18.7 kcal/mol) than 8a and 8b. Because this CO adsorption is endergonic relative to 7 (ΔGads° = +3.5 kcal/mol), this coadsorption does not occur and can be ruled out here from the discussion. Starting from 8a, the N–O bond cleavage occurs much easier via transition state TS8a–9 (ΔG°‡ = 0.8 kcal/mol) to afford N2O-adsorbed Cu389 than that starting from 8b, which occurs via TS8b–9 (ΔG°‡ = 3.5 kcal/mol). This small ΔG°‡ for TS8a–9 than that for TS8b–9 arises from the much smaller deformation energy (Edef) of the Cu38–N2O moiety in TS8a–9 (Edef = 5.0 kcal/mol) than in TS8b–9 (Edef = 12.9 kcal/mol), where Edef is defined as destabilization energy of the Cu38–N2O moiety when going to TS8–9 from intermediate 8a or 8b. It is interesting that the strong N–O bond can be easily cleaved by the CO molecule on the Cu38 surface, the details of which will be discussed below.
Figure 4.
Geometry changes in the reaction of ONNO species with CO on Cu38, affording N2 and CO2 molecules. Distances are in Angstrom. In parentheses are Gibbs energy changes (in kcal/mol) relative to the sum of Cu38 and free gas molecule(s).
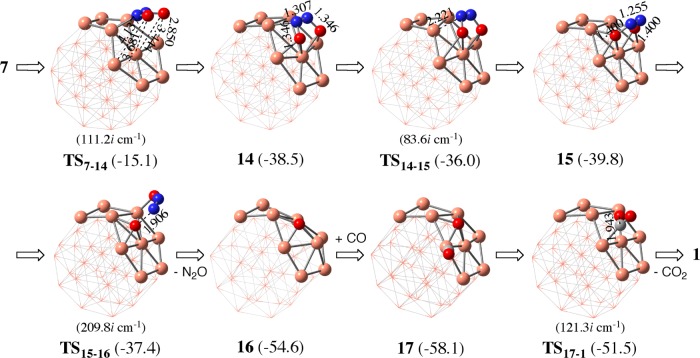
The N–O bond cleavage without CO participation is also likely to occur because the Cu atom intrinsically wants to bind with the oxygen atom. In this reaction, the ONNO-adsorbed Cu387 isomerizes to an isomer 14, in which the ONNO is parallel to the Cu38 surface, as shown in Figure 5. This isomerization occurs through transition state TS7–14 with moderate ΔG°‡ of 7.1 kcal/mol and somewhat large ΔG° of −16.3 kcal/mol relative to 7. Interestingly, 14 further isomerizes to a more stable isomer 15 through transition state TS14–15 with small ΔG°‡ (2.5 kcal/mol) and exergonicity (ΔG° = −1.3 kcal/mol) relative to 14. In 15, two oxygen atoms of the ONNO moiety interact with Cu38. Although flipping of the ONNO moiety on the same Cu atoms is another plausible isomerization course of 7 to 15, an intermediate for the flipping isomerization, in which the ONNO moiety is almost perpendicular to the (111) facet (24 in Figure S3), was calculated to be more unstable than 7 by 9.1 kcal/mol. This energy is larger than the ΔG°‡ value for TS7–14 (7.1 kcal/mol), indicating that the flipping isomerization is not easy to occur. Starting from 15, the N–O bond cleavage occurs through transition state TS15–16 with a small activation energy (ΔG°‡ = 2.4 kcal/mol) to afford 16 and a free N2O molecules with significant exergonicity (ΔG° = −14.8 kcal/mol) relative to 15. These results indicate that the N–O bond cleavage of the ONNO species easily occurs on the Cu38 surface even in the absence of CO. This reaction corresponds to NO decomposition without CO because N2O is easily converted to N2 molecule and O atom adsorbed to Cu38, as will be discussed below. However, this NO decomposition occurs less easily than the NO reduction by CO because the isomerization of 7 to 14 needs much larger ΔG°‡ (7.1 kcal/mol) than that (0.8 kcal/mol) of the reaction of 7 with CO via TS8a–9.
Figure 5.
Geometry changes in the NO bond cleavage of the ONNO species on Cu38 in the absence of CO, affording N2O and O atom adsorbed on Cu38 (from 7 to 16) and the reaction of the Cu38-adsorbed O with CO (from 16 to 1). Distances are in Angstrom. In parentheses are Gibbs energy changes (in kcal/mol) relative to the sum of Cu38 and free gas molecule(s).
In the other reaction starting from 15, two N–O bonds are simultaneously cleaved through the concerted transition state TS15–18 to afford an N2 molecule in one step, as shown in Figure S4 in the Supporting Information. The ΔG°‡ value (10.7 kcal/mol) is larger than that of the above-mentioned pathway, indicating that simultaneous cleavage of two N–O bonds is difficult to occur. In addition, we investigated the formation of anionic [ONNO]− species from 7, considering the previous report of its formation,34 but found that it was highly endergonic by 118 kcal/mol, indicating that such process is difficult to occur.
In summary, the N–O bond cleavage of the ONNO species occurs through reaction with CO adsorbed at the neighboring site of the ONNO species to afford N2O and CO2. In the absence of CO, the N–O bond cleavage of ONNO occurs after the isomerization 7 → 14 → 15 to afford N2O and O atom adsorbed to Cu38, but it needs larger ΔG°‡ value than the N–O bond cleavage of ONNO by CO.
2.5. N2 Formation from N2O through N–O Bond Cleavage
We investigated here if the N–O bond cleavage of N2O occurs through reaction with the CO molecule starting from 9 (N2O-adsorbed Cu38). Three possible CO adsorption positions were found neighboring the N2O moiety adsorbed on Cu(36), Cu(27), and Cu(14) (19a–c in Figure S5). Although the CO adsorption (19c) occurs at Cu(14) with moderate endergonicity relative to 9 (ΔGads° = +0.7 kcal/mol), the other CO adsorption (19a and 19b) occurs at Cu(36) and Cu(27) atoms with somewhat large exergonicity (ΔGads° = −8.0 and −6.0 kcal/mol, respectively). However, N2O dissociation from the Cu38 surface into the gas phase occurs more easily than these CO adsorptions because the ΔG° value is much more negative (−11.9 kcal/mol) relative to 9 than the CO adsorption. This means that N2O can be observed as an intermediate in the gas phase, which will be discussed below based on the experimental finding. Subsequently, N2O readsorption easily occurs to produce the most stable N2O adsorption structure 10, where the ΔG° is −15.4 kcal/mol relative to 9.
Starting from 10, the second N–O bond cleavage occurs through transition state TS10–11 to afford an N2 molecule and O atom adsorbed to Cu3811, as shown in Figure 4. The ΔG°‡ value (9.0 kcal/mol) is larger than that of the first N–O bond cleavage, although the exergonicity is significantly large (ΔG° = −53.0 kcal/mol) relative to 10, as shown in Figure 3. The N2 molecule dissociates from Cu38 with ΔG° = −2.4 kcal/mol relative to 11 to afford an intermediate 12, in which the O atom is bound at the hollow site of Cu(19), Cu(20), Cu(21), and Cu(22) atoms; see Figure 4. The CO molecule is adsorbed at one of these four Cu atoms neighboring the O atom with ΔGads° of −4.9 kcal/mol, which is moderately larger than that of 1 (ΔGads° = −3.9 kcal/mol). This is reasonable because the surface Cu atoms become electron deficient by the adsorbed O atom to enhance σ donation from the CO lone pair to Cu38; as mentioned above, CO interacts with Cu38 by the σ donation. The CO molecule reacts with the O atom through transition state TS13–1 to produce CO2 and regenerate Cu38 with ΔG°‡ of 11.8 kcal/mol (Figure 3).
As another possibility, we investigated N–O bond cleavage of N2O assisted by the CO molecule in 10. However, CO is adsorbed at the Cu atom neighboring the N2O to afford intermediate 20 with ΔG° = −5.9 kcal/mol relative to 10, as shown in Figure S6 in the Supporting Information. We tried to find a concerted reaction course via simultaneous N–O and Cu–O bond cleavages with CO2 formation, but could not. Instead, the N–O bond cleavage occurs without any interaction with CO at the neighboring site (Figure S6) to afford an N2 molecule and 13. This process occurs with ΔG°‡ value of about 10 kcal/mol, which is moderately larger than that for TS10–11 (ΔG°‡ = 9.0 kcal/mol). Thus, it should be concluded that the second N–O bond cleavage occurs easier without CO coordination than that by CO molecule. This is true because the adsorbed CO molecule increases the electron population of the Cu surface to enhance the bonding interaction between the O atom and the Cu38 surface.
The smaller adsorption energy of CO (13) than the activation energy for CO2 formation (TS13–1) may induce oxygen accumulation on the Cu surface. To explore this possibility, we calculated the CO2 formation process from Cu38 with two O atoms 21, as shown in Figure S7. The ΔG° for CO adsorption to 21 is −12.5 kcal/mol, which is much larger than that to 12 with one O atom (−4.9 kcal/mol), suggesting that CO adsorption energy increases as the number of O atoms increases on the Cu surface; this is not surprising because the O atom is electron-withdrawing from Cu38 to enhance CT from CO to Cu38. The ΔG°‡ for CO2 formation is calculated to be 9.4 kcal/mol, which is somewhat smaller than that from 12 with one O atom (11.8 kcal/mol). In addition, ΔG° for CO2 formation is −37.5 kcal/mol, which is considerably larger than the ΔG° for CO2 formation from 12 (−23.9 kcal/mol). These results suggest that the presence of many O atoms on the Cu surface enhances CO adsorption, decreases the ΔG°‡ value, and increases exergonicity for CO2 formation and that oxygen accumulation does not occur on the Cu surface. Actually, the X-ray diffraction (XRD) and X-ray photoelectron spectroscopy of the Cu/Al2O3 catalyst produced with 5 vol% H2/He at 773 K showed that copper oxides (CuO and Cu2O) were not detected.
Instead of CO adsorption, NO reaction with 12 affording NO2 is another possible reaction. The calculations show that the NO molecule can be adsorbed on 12 in the bridging form (23a and 23b) neighboring the O atom (see Figure S8), whereas the on-top NO adsorption structure could not be located. The ΔG° values for NO adsorption are −10.5 (23a) and +0.4 (23b) kcal/mol, indicating that NO can be adsorbed to form intermediate 23a. However, the ΔG° for NO2 formation is much endergonic relative to 23a (+39.0 kcal/mol), suggesting that ΔG°‡ for NO2 formation must be larger than it. As discussed above, on the other hand, the CO2 formation is highly exergonic. Based on these results, it is likely concluded that NO2 formation is energetically difficult but CO2 formation can occur.
The Gibbs energetics for the best reaction pathway is summarized in Figure 3, which shows that this catalytic reaction is apparently downhill with moderate ΔG°‡ and significantly large exergonicity (significantly negative ΔG°). The rate-determining step is the CO2 formation reaction between CO and the O atom adsorbed to Cu38 (ΔG°‡ = 11.8 kcal/mol), which corresponds to the reduction of Cu38O with CO. This is reasonable because Cu has strong affinity to oxygen.
2.6. Details of Electronic Process in the Formation of ONNO Species on Cu38 and Oxidation of CO with O Atom Adsorbed on Cu38
In this section, a discussion is presented on electronic processes of NO dimerization on the Cu38 surface and CO oxidation with the O atom adsorbed on Cu38 because CO oxidation is the rate-determining step and NO dimerization is a key step for NO reduction by CO on the Cu38 surface.
As discussed above, the short N–N distance (1.443 Å) and the large N–N bond energy (10.6 kcal/mol; evaluated by ΔG°(7–6)) of the ONNO species on Cu38 significantly differ from those of the NO dimer in the gas phase (2.263 Å and 2 kcal/mol, respectively).29−31 Previous theoretical works of the NO dimer35−38 in the gas phase reported that the electronic structure of the NO dimer has a multireference character because of the presence of nearly degenerated π and π* orbitals of two N–O bonds. This means that the DFT method cannot be applied to the NO dimer. Here, we investigated the NO dimer (ONNO species) on the Cu2 model system using the complete active space self-consistent field (CASSCF) method and found that the electronic structure of the NO dimer on the Cu2 cluster can be described well by the DFT method; details are presented in Pages S10–S13 in the Supporting Information. This result suggests that the DFT method can be applied to the NO dimerization on the Cu cluster/particle.
The intermediate Cu38(NO)26 has two NO molecules adsorbed to Cu38 (Figure 2), in which CT occurs from Cu38 to two NO molecules by about 0.4e, as shown in a solid green line in Figure 6. Interestingly, the CT becomes stronger, on going to TS6–7 (0.46e) and 7 (0.50e) from 6. The difference in NBO charge between free ONNO and adsorbed ONNO to Cu38 (between the solid and dashed lines) shows that the CT significantly occurs to the N atoms, where the geometry of free ONNO was taken to be the same as that of the ONNO adsorbed to Cu38 for comparison. The lowest unoccupied molecular orbital (LUMO) of free ONNO species consists of bonding overlap between π* orbitals of the NO bond; see the LUMO figure shown in Figure 6. On going from 6 to 7, the LUMO energy of the free ONNO species becomes significantly lower because the N–N distance becomes shorter (Figure 6). As a result, the CT from Cu38 to ONNO becomes stronger and simultaneously enhances the N–N bonding interaction. Also, the CT contributes to the N–O bond weakening because the LUMO is antibonding between N and O atoms. Because of this CT, the N–O bond is easily cleaved after the formation of ONNO on Cu38. Consistent with this CT, the highest energy occupied MO (φHO) localized on the Cu38 surface, which plays an important role in charge transfer to NO molecules from Cu38, becomes lower in energy on going to 7 (−6.36 eV) from 6 (−5.96 eV); see Figure S10 in the Supporting Information. This result suggests that the metal particle bearing φHO at high energy is favorable for NO dimerization, as was discussed recently.39 For instance, the NO dimerization occurs on Cu5 with the activation energy of 9.4 kcal/mol,39 which is much larger than that (2.8 kcal/mol) on the Cu38. This significant difference between Cu5 and Cu38 can be explained in terms of the lower φHO energy of Cu5 (−5.58 eV) than that of Cu38 (−4.29 eV).
Figure 6.
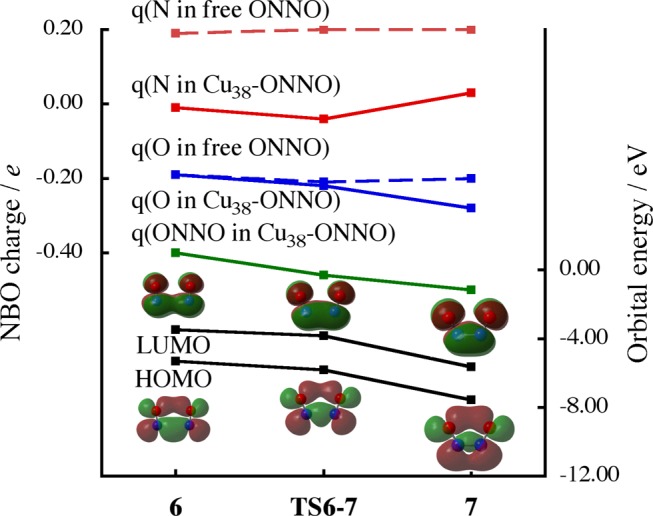
Changes of NBO charges of the ONNO moiety of Cu38-ONNO on going from 6 (two NO molecules) to 7 (ONNO species) through TS6–7 (solid line) in comparison with those of free ONNO molecules (dashed line).a Frontier orbital energies and their figures are drawn for free ONNO molecules. NBO charge and orbital energy are in e and eV, respectively. aThe geometry of free ONNO molecules is taken from 6, TS6–7, and 7.
In the CO reaction with the O atom adsorbed on Cu38, the sum of electron populations of CO and O becomes moderately more negative at TS13–1 but then changes to zero in CO2 at 1 + CO2. Though the electron population of Cu38 moderately decreases at TS13–1, it finally increases at 1 + CO2; see Table S1 in the Supporting Information. This population change is consistent with our understanding that the conversion of Cu38–O 13 to Cu38 is a two-electron reduction reaction. Consistent with this understanding, the lowest energy unoccupied MO φLU of 13 becomes the highest energy occupied MO φHO in 1; see Figure S11. Thus, the Mn–O (M = metal) species bearing φLU at low energy is favorable for the CO oxidation step.
In summary, the electronic structure of the ONNO moiety on Cu38 is quite different from that of the free NO dimer in the gas phase. Significant CT occurs from the Cu38 to the ONNO species, which contributes to the stability of the ONNO species with a short N–N bond, the large N–N bond energy, and simultaneously the weakening of the N–O bond. In the CO oxidation step, the sum of electron populations of CO and O atom decreases. It is likely concluded that the metal particle Mn bearing φHO at high energy is favorable for NO dimerization and the Mn–O species bearing φLU at low energy is favorable for CO oxidation.
2.7. Relation to Experimental Findings
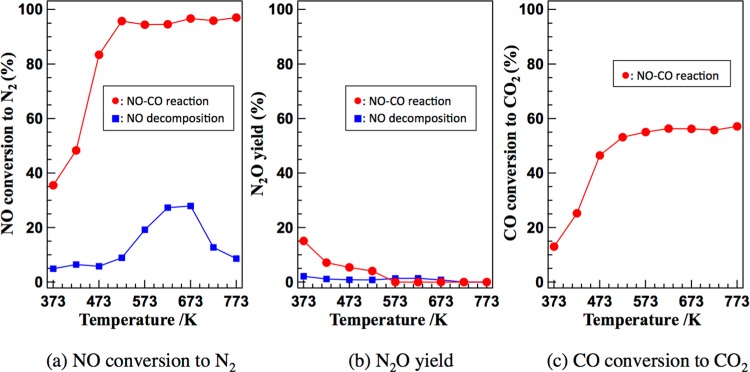
If the above reaction mechanism is correct, N2O species must be detected in the experiment. We carried out an experiment of NO reduction by CO using Cu nanoparticles supported by γ-Al2O3. Figure 7 shows the results of the reaction and NO decomposition over the 5 wt % Cu/γ-Al2O3 catalyst at 373–773 K. In the case of NO–CO reaction, NO was reduced to N2 even at low temperature (373 K), and a small amount of N2O was detected. The formation rate of N2 increased as the reaction temperature increased. On the contrary, N2O yield decreased as the reaction temperature increased, suggesting that the rate-determining step occurs after N2O formation. In our calculation, the rate-determining step is CO oxidation by O atom adsorbed on Cu38, which exists after N2O formation.
Figure 7.
Experimental results for (a) NO conversion to N2 (%), (b) N2O yield (%), and (c) CO conversion to CO2 (%) in NO–CO and NO decomposition reactions in the Cu/γ-Al2O3 catalyst.aaThe reaction time was 1 h at every temperature. The reaction temperature was increased from 373 to 573 K in a step-by-step manner. The temperature was kept constant for 1 h at every temperature. We recorded the activity (NO conversion to N2) every 15 min, and the activity was stable for 1 h.
A quite weak diffraction peak due to Cu(111) was detected around 43.4° in the XRD pattern of 5 wt % Cu/γ-Al2O3 reduced at 773 K with 5 vol % H2/He (Figure S12 in the Supporting Information). This result indicates that Cu metal nanoparticles were highly dispersed on the γ-Al2O3 surface. The XRD pattern showed a very weak and broad diffraction peak around 2θ = 43–44°, which corresponds to the peak by the Cu particle, indicating that the diameter of the Cu cluster is less than 2 nm (Figure S12). Though the size of the Cu particle employed in the experiment is not very large, it is likely that the real Cu particle used in the experiment is larger than the Cu38 employed for the calculations here, suggesting that further work is needed for investigating size effects on the NO–CO reaction by the Cu particle. The γ-Al2O3 without Cu exhibited a quite low activity (no data shown here), indicating that Cu loading was indispensable for NO reduction activity. Thus, it is likely concluded that the NO reduction by CO takes place on highly dispersed Cu metal nanoparticles. However, this result does not mean that the interface between the Cu metal nanoparticle and the Al2O3 surface does not participate in the reaction. Such NO–CO reaction on the interface was not investigated in this work, and thereby the reaction on the interface should be theoretically investigated in future because such an interface effect is important in catalysis.
The direct NO decomposition also occurred by 5 wt % Cu/γ-Al2O3, and the formation rate of N2 was increased as the reaction temperature increased up to 673 K, but then decreased. A small amount of N2O was detected together with N2. These results are also consistent with the computational result that NO decomposition can occur even in the absence of CO.
3. Conclusions
NO reduction by CO on an octahedral Cu38 cluster was theoretically investigated with the DFT method. NO is adsorbed to Cu38 in the bridging form between two Cu atoms. The side-on adsorption structure is slightly more stable than the end-on structure. In the case of CO, only the on-top end-on adsorption structure is located. The NO adsorption energy is somewhat larger than that of CO adsorption. Though dissociative NO adsorption is often discussed to occur in many TWCs, the present DFT calculation clearly shows that dissociative NO adsorption on Cu38 needs significantly large activation energy and endergonicity, suggesting such adsorption is difficult.
Then, we investigated the NO reduction by CO starting from NO–CO and NO–NO coadsorption structures on Cu38. Starting from the NO–CO coadsorption structure, N–O bond cleavage occurs through O abstraction by CO to afford CO2, and the N atom adsorbed to Cu38. However, this is significantly endergonic, suggesting that this is difficult to occur. Starting from the NO–NO coadsorption structure, the N–O bond cleavage occurs through O abstraction by another NO but it is significantly endergonic. Starting from the NO–NO coadsorption structure, on the other hand, NO dimerization occurs with a very small activation energy and significant exergonicity to produce ONNO species on Cu38. On the basis of these results, it is reasonably concluded that the N–N bond formation occurs through NO dimerization as the initial step of this catalytic NO reduction by CO.
Notably, the ONNO species on the Cu38 cluster has a significantly shorter N–N distance than that of the free ONNO species in the gas phase, which has a much longer and weaker N–N bond. The analysis of the electronic structure shows that CT significantly occurs from Cu38 to the N–N bonding orbital of the ONNO species to stabilize this ONNO species. The electronic structure of the ONNO moiety on Cu38 differs very much from that of the free NO dimer in the gas phase.
After the formation of the ONNO species on Cu38, the N–O bond cleavage easily occurs through O abstraction by the coadsorbed CO molecule at the neighboring position to produce N2O-adsorbed Cu38 and free CO2 molecule. It should be noted that a strong N–O bond can be easily cleaved on the Cu surface by CT from Cu38 to the ONNO species because the CT leads to weakening of the N–O bond. N2O dissociates from Cu38 into the gas phase with somewhat large exergonicity, and then it is adsorbed to Cu38 again in the most stable adsorption structure with moderate exergonicity. After readsorption of N2O to Cu38, the second N–O bond cleavage occurs to afford N2- and O-adsorbed Cu38 with small activation energy and significant exergonicity. The thus-formed N2 molecule easily dissociates from Cu38, and the remaining O atom on Cu38 reacts with the adsorbed CO molecule to produce CO2 molecule and regenerate Cu38; thus, the catalytic cycle is completed. The rate-determining step is the final CO2 formation because the Cu cluster has large oxygen affinity.
The formation of N2O and its dissociation from Cu38 into the gas phase are consistent with the experimental finding that N2O is observed in Cu/γ-Al2O3-catalyzed NO reduction by CO.
The reaction mechanism here completely differs from that for the Rh particle in which NO dissociative adsorption occurs as an important elementary step. This result here strongly suggests that a nonprecious metal such as Cu can be applied to the automotive deNOx catalyst through a new reaction mechanism. The role of Cu38 in this catalytic reaction is attributed to CTs from Cu38 to ONNO species, which is crucially important for formation of ONNO species and N–O bond cleavage of the ONNO species. At the end of this section, we wish to mention that Cu38 is not general but smaller than a real catalyst, as will be described in the section of models. Thus, size effects of Cu particles on NO–CO reaction must be investigated in the near future; see Figure S2b,c for preliminary computational results showing moderate size effects on NO dimerization by the Cu nanocluster.
4. Computational Details and Models
All geometries were optimized by the DFT method with the hybrid B3LYP functional.40−42 Though it is said that metal properties cannot be represented well by the hybrid functional,43 we employed the B3LYP functional because the B3LYP computation provided similar results of geometry and spin state to those by various functionals including typical generalised gradient approximation-type functionals44 after comparison with Perdew–Burke–Ernzerhof-calculated results; see Table S2 and the brief discussion below it. For the Cu atom, the LANL2DZ45 basis set was employed, where the core electrons were replaced with the effective core potentials. Because the LANL2DZ is not very large and prone to basis set superposition error (BSSE), we made the BSSE correction for typical CO and NO adsorbed structures on Cu38 using the counterpoise procedure shown in Table S3 and the explanation below it. However, the discussion and conclusion do not change by using these results with the BSSE correction. For C, N, and O atoms, Huzinaga–Dunning’s split-valence basis sets46 were employed, where one d polarization function was added. We checked in our previous work that the B3LYP/LANL2DZ calculation presented essentially the same geometry and relative energy to coupled cluster single and double substitutions with perturbative triples (CCSD(T)) calculation with triple-ζ basis sets for such diatomic systems as Cu–Cu, Cu–M, and M–M (M = Ru, Rh, Pd, Ag, Os, Ir, Pt, and Au). Also, this method was successfully applied to the geometry and electronic structure of Cu38 and M6@Cu32 core–shell cluster with the M6 core (M = Ru, Rh, Pd, Ag, Os, Ir, Pt, and Au).44 The CASSCF47 method was used for NO dimerization on Cu2 using cc-pVTZ basis sets.48 In the CASSCF calculations, 16 electrons in 14 orbitals were taken as the active space, where 2 π (in-plane and out-of-plane) and 2 π* MOs (in-plane and out-of-plane) between N and O atoms, σ-bonding and σ*-antibonding MOs between N and O atoms, and 4s–4s bonding and antibonding MOs between Cu atoms were included in the active space. Though the convergence test of the active space size was not performed in this work, the CASSCF(16, 14) presents similar energy changes to CCSD(T) calculation.39
The Gaussian0949 and SMASH50 programs were used for the DFT calculations, and the GAMESS51 program was used for the CASSCF calculations.
Octahedral-like Cu38 cluster 1 was chosen here as a model of the Cu nanocluster because the octahedral-like Cu38 was previously reported as the most stable structure of Cu38, which possesses Cu(111) planes of the face-centered cubic structure;52 the Cu(100) plane is found in Cu38, but all Cu atoms of the (100) plane are vertices, suggesting that its properties differ from those of Cu atoms of the usual (100) plane. Because the NO dimer was experimentally observed on Cu(100) and Cu(111) surfaces,53−571 was employed as a model of the Cu nanocluster catalyst for NO–CO reaction. In Cu38, 6 Cu atoms form an octahedral Cu6 core, and 32 Cu atoms form the surface of Cu38, as shown in Scheme 1. In the surface, 8 Cu atoms take center position of the (111) plane, whereas the other 24 Cu atoms belong to the (100) plane and at the same time the (111) plane. Though the XRD pattern measured in this work indicated that the size of the Cu particle was smaller than 2 nm, as was discussed above, it is likely that the real Cu particle is larger than Cu38. We wish to summarize the weak points of this model below. The calculation showed that the adsorption of NO occurs at the Cu(22) and Cu(28) atoms on the adjacent (100) planes, but such adjacent (100) planes disappear for larger Cu particles. Thus, Cu38 particles may not be in the scalable regime of realistic clusters, which is one weak point of this model. Therefore, further theoretical work must be performed on the size effect of metal cluster on NO–CO reaction in the future. In such future works, computational results on Cu38 would be valuable because comparison between Cu38 and other sizes can be made. Also, we wish to mention here that Al2O3 support was not involved in this modeling though the reaction was experimentally carried out on the Al2O3 support. In the presence of the Al2O3 support, charge transfer (CT) occurs between Al2O3 and Cu38,20,21,58 and the geometry deformation of Cu38 would be induced by the interaction with Al2O3. Besides them, we must remember that the interface between Cu38 and Al2O3 participates in the catalytic reaction. These effects could not be involved in the calculation of this work due to the heavy cost of such calculations; these important issues must be theoretically investigated in the future.
The ground state of Cu38 has triplet spin multiplicity.44 The spin multiplicities and the S2 eigenvalues for the calculated species are summarized in Table S4. The results show that the spin contamination is small for all the species.
The energetics of the reaction is discussed with the Gibbs energy. In Cu38, translational and rotational movements were not taken into account for entropy and thermal correction terms but only vibrational movements were taken because the Cu38 cluster is not a gas molecule. In free NO, CO, N2O, N2, and CO2 molecules, the usual entropy term and thermal correction by translational, rotational, and vibrational movements were employed for the Gibbs energy. All geometries were optimized without any constraint.
5. Experimental Details
γ-Al2O3 (JRC-ALO-7) was provided by the Catalysis Society of Japan. First, 5 wt % Cu/γ-Al2O3 was prepared by the impregnation method. Cu aqueous solution was prepared by dissolving Cu(NO3)2·3H2O. The support was added to the mixture of deionized water and the Cu solution, followed by evaporation. It was dried at 353 K. The resulting powder was calcined in air at 773 K for 5 h.
The NO–CO reaction was carried out in a fixed-bed flow reactor at atmospheric pressure. The Cu/γ-Al2O3 catalyst (5 wt %; 200 mg) was placed in a tubular reactor. The reaction gas (100 mL/min), consisting of 1000 ppm NO, 1000 ppm CO, and He as the balance, was introduced to the catalyst bed. Before the reaction, the catalyst was heated to 773 K in a stream of 5 vol % H2/He for 1 h. The effluent gases from the reactor were analyzed by gas chromatography (Shimadzu GC-8A, Porapak Q and MS-5A columns) equipped with a thermal conductivity detector.
Catalytic NO decomposition experiment was also performed in a fixed-bed reactor as described above. The reaction gas (100 mL/min), consisting of 1000 ppm NO and He as the balance, was introduced to the catalyst (200 mg).
Acknowledgments
This work was carried out in “Element Strategy Initiative for Catalysts and Batteries (ESICB)”, which is financially supported by the Ministry of Education, Culture, Science, Sports, and Technology (MEXT), Japan. S.S. wishes to thank the partial financial support from MEXT through JSPS KAKENHI (No. 15H03770) and the Ministry of Economy, Trade and Industry, Japan, through a project (P16010) commissioned by the New Energy and Industrial Technology Development Organization (NEDO). We wish to thank the computational center at the Institute of Molecular Science (Okazaki, Japan) for allowing the use of the computer and HPCI-Riken (Kobe, Japan) for the use supercomputer “K”.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02890.
Optimized structure and relative energy of other possible isomers of NO adsorbed structure 2 and NO–CO and NO–NO coadsorbed structure 4; potential energy curve for the N–O dissociative adsorption on Cu38; geometry and energy change for NO dimerization on Cu38 and Cu55 calculated at the PBE functional with the plane wave basis sets; other possible reaction pathways of NO–CO reaction on Cu38; natural orbitals of the NO dimer and the NO dimer on Cu2 calculated by the CASSCF method; discussion about the electronic structure of the NO dimer by the CASSCF calculations; orbital energy change for NO dimerization and CO oxidation processes on Cu38; XRD pattern of 5 wt % Cu/γ-Al2O3; geometrical parameters, NBO charges for CO oxidation processes on Cu38; energy change for NO dimerization on Cu38 calculated at the PBE functional; basis set superposition error for NO and CO adsorption on Cu38; spin multiplicity and the S2 eigenvalues for the typical calculated species; NBO charges, and spin distribution of the NO dimer on Cu2; complete ref (49) (PDF).
Cartesian coordinates and total energies of all the optimized structures in this work (XYZ).
The authors declare no competing financial interest.
Supplementary Material
References
- Cooper J.; Beecham J. A Study of Platinum Group Metals in Three-Way Autocatalysts. Platinum Met. Rev. 2013, 57, 281–288. 10.1595/147106713X671457. [DOI] [Google Scholar]
- Zhdanov V. P.; Kasemo B. Mechanism and kinetics of the NO-CO reaction on Rh. Surf. Sci. Rep. 1997, 29, 31–90. 10.1016/S0167-5729(97)00009-5. [DOI] [Google Scholar]
- Dent A. J.; Evans J.; Fiddy S. G.; Jyoti B.; Newton M. A.; Tromp M. Rhodium Dispersion during NO/CO Conversions. Angew. Chem., Int. Ed. 2007, 46, 5356–5358. 10.1002/anie.200701419. [DOI] [PubMed] [Google Scholar]
- Olsson L.; Zhdanov V. P.; Kasemo B. Role of steps in the NO–CO reaction on the (111) surface of noble metals. Surf. Sci. 2003, 529, 338–348. 10.1016/S0039-6028(03)00275-9. [DOI] [Google Scholar]
- Rainer D. R.; Koranne M.; Vesecky S. M.; Goodman D. W. CO + O2 and CO + NO Reactions over Pd/Al2O3 Catalysts. J. Phys. Chem. B 1997, 101, 10769–10774. 10.1021/jp971262z. [DOI] [Google Scholar]
- Eichler A.; Hafner J. NO Reduction by CO on the Pt(100) Surface. A Density Functional Theory Study. J. Catal. 2001, 204, 118–128. 10.1006/jcat.2001.3366. [DOI] [Google Scholar]
- Avalos L. A.; Uñac B. R.; Zaera F.; et al. Dynamic Monte Carlo Simulation of the NO + CO Reaction on Rh(111). J. Phys. Chem. B 2006, 110, 24964–24971. 10.1021/jp064967m. [DOI] [PubMed] [Google Scholar]
- Alas S. J.; Vicente L. Kinetic study of the “surface explosion” phenomenon in the NO+CO reaction on Pt(100) through dynamic Monte Carlo simulation. J. Chem. Phys. 2008, 128, 134705–134712. 10.1063/1.2885048. [DOI] [PubMed] [Google Scholar]
- Xu Q.-Q.; Yang H.-Q.; Gao C.; Hu C.-W. Theoretical study on the reaction mechanism of NO and CO catalyzed by Rh atom. Struct. Chem. 2013, 24, 13–23. 10.1007/s11224-012-0022-2. [DOI] [Google Scholar]
- Haruta M.; Kobayashi T.; Sano H.; Yamada N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 405–408. 10.1246/cl.1987.405. [DOI] [Google Scholar]
- Haruta M.; Yamada N.; Kobayashi T.; Iijima S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. 10.1016/0021-9517(89)90034-1. [DOI] [Google Scholar]
- Haruta M. Chance and Necessity: My Encounter with Gold Catalysts. Angew. Chem., Int. Ed. 2014, 53, 52–56. 10.1002/anie.201305987. [DOI] [PubMed] [Google Scholar]
- Fajín J. L. C.; Cordeiro M. N. D. S.; Gomes J. R. B. A DFT study of the NO dissociation on gold surfaces doped with transition metals. J. Chem. Phys. 2013, 138, 074701–074709. 10.1063/1.4790602. [DOI] [PubMed] [Google Scholar]
- Beniya A.; Ikuta Y.; Isomura N.; Hirata H.; Watanabe Y. Synergistic Promotion of NO-CO Reaction Cycle by Gold and Nickel Elucidated using a Well-Defined Model Bimetallic Catalyst Surface. ACS Catal. 2017, 7, 1369–1377. 10.1021/acscatal.6b02714. [DOI] [Google Scholar]
- Shimizu K.-I.; Shibata J.; Yoshida H.; Satsuma A.; Hattori T. Silver-alumina catalysts for selective reduction of NO by higher hydrocarbons: structure of active sites and reaction mechanism. Appl. Catal., B 2001, 30, 151–162. 10.1016/S0926-3373(00)00229-0. [DOI] [Google Scholar]
- Meunier F. C.; Breen J. P.; Zuzaniuk V.; Olsson M.; Ross J. R. H. Mechanistic Aspects of the Selective Reduction of NO by Propene over Alumina and Silver–Alumina Catalysts. J. Catal. 1999, 187, 493–505. 10.1006/jcat.1999.2622. [DOI] [Google Scholar]
- Bogdanchikova N.; Meunier F. C.; Avalos-Borja M.; Breen J. P.; Pestryakov A. On the nature of the silver phases of Ag/Al2O3 catalysts for reactions involving nitric oxide. Appl. Catal., B 2002, 36, 287–297. 10.1016/S0926-3373(01)00286-7. [DOI] [Google Scholar]
- Bethke K. A.; Kung H. H. Supported Ag Catalysts for the Lean Reduction of NO with C3H6. J. Catal. 1997, 172, 93–102. 10.1006/jcat.1997.1794. [DOI] [Google Scholar]
- Liu Z.-P.; Jenkins S. J.; King D. A. Why Is Silver Catalytically Active for NO Reduction? A Unique Pathway via an Inverted (NO)2 Dimer. J. Am. Chem. Soc. 2004, 126, 7336–7340. 10.1021/ja049126c. [DOI] [PubMed] [Google Scholar]
- Mazheika A. S.; Bredow T.; Ivashkevich O. A.; Matulis V. E. Theoretical Study of NO Conversion on Ag/TiO2 Systems. I. Anatase (100) Surface. J. Phys. Chem. C 2012, 116, 25262–25273. 10.1021/jp308393p. [DOI] [Google Scholar]
- Mazheika A. S.; Bredow T.; Ivashkevich O. A.; Matulis V. E. Theoretical Study of NO Conversion on Ag/TiO2 Systems. II. Rutile (110) Surface. J. Phys. Chem. C 2012, 116, 25274–25285. 10.1021/jp308812q. [DOI] [Google Scholar]
- Shimizu K.; Kawabata H.; Maeshima H.; Satsuma A.; Hattori T. Intermediates in the Selective Reduction of NO by Propene over Cu-Al2O3 Catalysts: Transient in-Situ FTIR Study. J. Phys. Chem. B 2000, 104, 2885–2893. 10.1021/jp9930705. [DOI] [Google Scholar]
- Yamamoto T.; Tanaka T.; Kuma R.; Suzuki S.; Amano F.; Shimooka Y.; Kohno Y.; Funabiki T.; Yoshida S. NO Reduction with CO in the Presence of O2 over Al2O3-Supported and Cu-based Catalysts. Phys. Chem. Chem. Phys. 2002, 4, 2449–2458. 10.1039/b201120b. [DOI] [Google Scholar]
- Yen M.-Y.; Ho J.-J. Density-functional study for the NOx (x = 1, 2) dissociation mechanism on the Cu(111) surface. Chem. Phys. 2010, 373, 300–306. 10.1016/j.chemphys.2010.06.005. [DOI] [Google Scholar]
- Kacimi M.; Ziyada M.; Liotta L. F. Cu on amorphous AlPO4: Preparation, characterization and catalytic activity in NO reduction by CO in presence of oxygen. Catal. Today 2015, 241, 151–158. 10.1016/j.cattod.2014.04.003. [DOI] [Google Scholar]
- Sakai M.; Nagai Y.; Aoki Y.; Takahashi N. Investigation into the catalytic reduction of NOx at copper–ceria interface active sites. Appl. Catal., A 2016, 510, 57–63. 10.1016/j.apcata.2015.11.007. [DOI] [Google Scholar]
- González S.; Sousa C.; Illas F. Promoter and poisoning effects on NO-catalyzed dissociation on bimetallic RhCu(111) surfaces. J. Catal. 2006, 239, 431–440. 10.1016/j.jcat.2006.02.013. [DOI] [Google Scholar]
- Fukuda R.; Takagi N.; Sakaki S.; Ehara M. Structures of bimetallic copper-ruthenium nanoparticles: incoherent interface and surface active sites for catalytic nitric oxide dissociation. J. Phys. Chem. C 2017, 121, 300–307. 10.1021/acs.jpcc.6b09280. [DOI] [Google Scholar]
- Western C. M.; Langridge-Smith P. R. R.; Howard B. J.; Novick S. E. Molecular beam electric resonance spectroscopy of the nitric oxide dimer. Mol. Phys. 1981, 44, 145. 10.1080/00268978100102341. [DOI] [Google Scholar]
- McKellar A. R. W.; Watson J. K. G.; Howard B. J. The NO dimer: 15N isotopic infrared spectra, line widths and force field. Mol. Phys. 1995, 86, 273–286. 10.1080/00268979500102011. [DOI] [Google Scholar]
- Wade E. A.; Cline J. I.; K. Lorenz T.; Hayden C.; Chandler D. W. Direct measurement of the binding energy of the NO dimer. J. Chem. Phys. 2002, 116, 4755–4757. 10.1063/1.1459702. [DOI] [Google Scholar]
- Taniike T.; Tada M.; Coquet R.; Morikawa Y.; Sasaki T.; Iwasawa Y. A new aspect of heterogeneous catalysis: Highly reactive cis-(NO)2 dimer and Eley–Rideal mechanism for NO–CO reaction on a Co-dimer/γ-alumina catalyst. Chem. Phys. Lett. 2007, 443, 66–70. 10.1016/j.cplett.2007.06.032. [DOI] [Google Scholar]
- Fajín J. L. C.; Cordeiro M. N. D. S.; Gomes J. R. B. Unraveling the mechanism of the NO reduction by CO on gold based catalysts. J. Catal. 2012, 289, 11–20. 10.1016/j.jcat.2012.01.010. [DOI] [Google Scholar]
- Tsukuda T.; Saeki M.; Zhu L.; Nagata T. Formation of N3O3– anion in (NO)n–: photoelectron spectroscopy and ab initio calculations. Chem. Phys. Lett. 1998, 295, 416–422. 10.1016/S0009-2614(98)01002-1. [DOI] [Google Scholar]
- Snis A.; Panas I. N2O2, N2O2– and N2O22–: structures, energetics and N-N bonding. Chem. Phys. 1997, 221, 1–10. 10.1016/S0301-0104(97)00165-1. [DOI] [Google Scholar]
- Sayós R.; Valero R.; Anglada J. M.; González M. Theoretical investigation of the eight low-lying electronic states of the cis- and trans-nitric oxide dimers and its isomerization using multiconfigurational second-order perturbation theory (CASPT2). J. Chem. Phys. 2000, 112, 6608–6624. 10.1063/1.481234. [DOI] [Google Scholar]
- Tobita M.; Perera S. A.; Musial M.; Bartlett R. J.; Nooijen M.; Lee J. S. Critical comparison of single-reference and multireference coupled-cluster methods: Geometry, harmonic frequencies, and excitation energies of N2O2. J. Chem. Phys. 2003, 119, 10713–10723. 10.1063/1.1619952. [DOI] [Google Scholar]
- Taguchi N.; Mochizuki Y.; Ishikawa T.; Tanaka K. Multi-reference calculations of nitric oxide dimer. Chem. Phys. Lett. 2008, 451, 31–36. 10.1016/j.cplett.2007.11.084. [DOI] [Google Scholar]
- Takagi N.; Nakagaki M.; Ishimura K.; Fukuda R.; Ehara M.; Sakaki S. Electronic Processes in NO Dimerization on Ag and Cu Clusters: DFT and MRMP2 Studies. J. Comput. Chem. 2019, 40, 181–190. 10.1002/jcc.25568. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Stroppa A.; G Kresse G. The shortcomings of semi-local and hybrid functionals: what we can learn from surface science studies. New J. Phys. 2008, 10, 063020 10.1088/1367-2630/10/6/063020. [DOI] [Google Scholar]
- Takagi N.; Ishimura K.; Matsui M.; Fukuda R.; Ehara M.; Sakaki S. Core-Shell versus Other Structures in Binary Cu38–nMn Nanoclusters (M = Ru, Rh, Pd, Ag, Os, Ir, Pt, and Au; n = 1, 2, and 6): Theoretical Insight into Determining Factors. J. Phys. Chem. C 2017, 121, 10514–10528. 10.1021/acs.jpcc.6b13086. [DOI] [Google Scholar]
- Hay P.; Wadt W. R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. 10.1063/1.448975. [DOI] [Google Scholar]
- Dunning T. H. Jr.; Hay P. J. In Modern Theoretical Chemistry; Schaefer H. F., III, Ed.; Plenum: New York, 1976; Vol. 3, pp 1–28. [Google Scholar]
- Roos B. O. The Complete Active Space Self-Consistent Field Method and its Applications in Electronic Structure Calculations. Adv. Chem. Phys. 1987, 69, 399–445. 10.1002/9780470142943.ch7. [DOI] [Google Scholar]
- Dunning T. H. Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. 10.1063/1.456153. [DOI] [Google Scholar]
- Frisch M. J.;. et al. Gaussian 09, revision D.01; Gaussian, Inc.: Wallingford, CT, 2013.
- Ishimura K.SMASH-1.1.0. http://smash-qc.sourceforge.net/ (accessed Jan, 2013).
- Schmidt M. W.; Baldridge K. K.; Boatz J. A.; Elbert S. T.; Gordon M. S.; Jensen J. H.; Koseki S.; Matsunaga N.; Nguyen K. A.; Su S.; Windus T. L.; Dupuis M.; Montgomery J. A. General Atomic and Molecular Electronic Structure System. J. Comput. Chem. 1993, 14, 1347–1363. 10.1002/jcc.540141112. [DOI] [Google Scholar]
- Itoh M.; Kumar V.; Adschiri T.; Kawazoe Y. Comprehensive study of sodium, copper, and silver clusters over a wide range of sizes 2 ≤ N ≤ 75. J. Chem. Phys. 2009, 131, 174510–174528. 10.1063/1.3187934. [DOI] [PubMed] [Google Scholar]
- Johnson D. W.; Matloob M. H.; Roberts M. W. Adsorption of nitric oxide on Cu(100) surfaces; an electron spectroscopic study. J. Chem. Soc. Chem. Commun. 1978, 40–41. 10.1039/c39780000040. [DOI] [Google Scholar]
- Matloob M. H.; Roberts M. W. Electron spectroscopic study of nitrogen species adsorbed on copper. J. Chem. Soc., Faraday Trans. 1 1977, 73, 1393–1405. 10.1039/f19777301393. [DOI] [Google Scholar]
- Johnson D. W.; Matloob M. H.; Roberts M. W. Study of the interaction of nitric oxide with Cu(100) and Cu(111) surfaces using low energy electron diffraction and electron spectroscopy. J. Chem. Soc., Faraday Trans. 1 1979, 75, 2143–2159. 10.1039/f19797502143. [DOI] [Google Scholar]
- Dhesi S. S.; Haq S.; Barrett S. D.; Liebsle F. M. LEED and STM studies of structures formed by NO dissociation on Cu(100) surfaces. Surf. Sci. 1996, 365, 602–613. 10.1016/0039-6028(96)00685-1. [DOI] [Google Scholar]
- Wee A. T. S.; Lin J.; Huan A. C. H.; Loh F. C.; Tan K. L. SIMS study of NO, CO adsorption on Cu(100) and Cu(210) surfaces. Surf. Sci. 1994, 304, 145–158. 10.1016/0039-6028(94)90760-9. [DOI] [Google Scholar]
- Matulis V. E.; Palagin D. M.; Mazheika A. S.; Ivashkevich O. A. Theoretical study of NO adsorption on neutral, anionic and cationic Ag8 clusters. Comput. Theor. Chem. 2011, 963, 422–426. 10.1016/j.comptc.2010.11.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.