Abstract
A two-step one-pot efficient synthesis of pyrido[2,3-b]indoles via reaction between isatin, α-amino acid, and dipolarophile has been developed. The initial 1,3-dipolar cycloaddition between the reactants that is performed in the presence of either CuI or methanol results in spirooxindoles that undergo POCl3-mediated intramolecular dehydrative transformation to afford the title compounds.
Introduction
Pyrido[2,3-b]indole, commonly referred to as α-carboline, is the core unit of several natural products1 and pharmacologically active compounds endowed with cytotoxic, anticancer, antimalarial, antifungal, antibacterial, antioxidant, and anti-inflammatory activities (Figure 1).2 Given such importance, considerable efforts have been made into developing multiple strategies for the synthesis of pyrido[2,3-b]indole derivatives, which have been reviewed by Brimble et al. recently.3,4 Simultaneously, many approaches for the synthesis of neocryptolepine, an important natural compound containing this structural motif, are also reported.5 Unfortunately, most of these methods suffer from low atom economy, lack of substrate generality, use of highly functionalized starting materials, expensive transition metals, or harsh conditions, thereby underscoring the need for development of a general and efficient approach to this heterocycle.
Figure 1.
Representative natural and bioactive compounds containing pyrido[2,3-b]indole moiety.
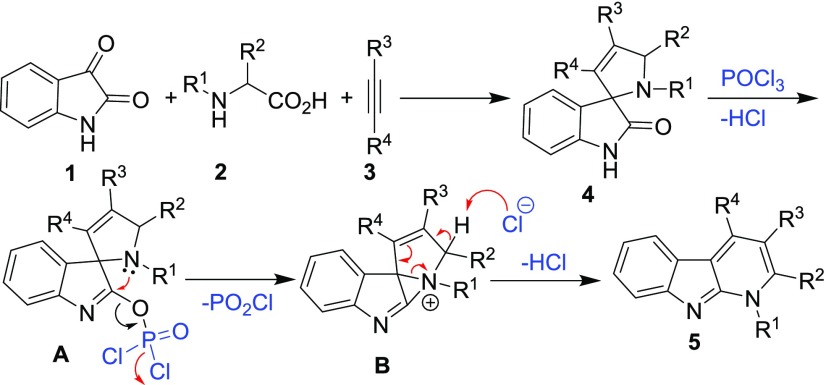
It is widely reported that a multicomponent reaction between isatin, α-amino acid, and dipolarophile offers spirooxindoles.6 The reaction proceeds via imine formation between the amino acid and isatin, followed by decarboxylation of the amino acid to produce an azomethine ylide, which then undergoes 1,3-dipolar cycloaddition with a dipolarophile to afford the product. During the studies related to the Vilsmeier–Haack reaction of spiroindolones, Black et al. reported that heating 4′,6′-dimethoxyspiro[[1,3]dithiolane-2,3′-indolin]-2′-one with POCl3 in the presence of 4,6-dimethoxy-2,3-diphenyl-1H-indole afforded biindolyl (Figure 2).7 This reaction was suggested to proceed via formation of 7,9-dimethoxy-3,6-dihydro-2H-[1,4]dithiino[2,3-b]indol-6-ylium intermediate, which underwent nucleophilic attack with 4,6-dimethoxy-2,3-diphenyl-1H-indole. Based on this report, we envisaged that spirooxindoles bearing a pyrroline ring may undergo similar ring expansion reaction with POCl3 to offer pyrido[2,3-b]indoles. Notably, the intermolecular and intramolecular aminations of amides with NH heterocycles, N-substituted anilines, or pyridine for preparing aza heterocycles using POCl3 are widely reported in the literature8 (Figure 2). Besides, it is cited that indolin-2-one furnishes a mixture of triindole and tetraindole in the presence of POCl3.9 We reasoned that treating spirooxindole with POCl3 would produce an active intermediate A, which may undergo nucleophilic displacement with the pyrroline nitrogen to form intermediate B (Scheme 1). Subsequently, an intramolecular rearrangement of intermediate B would result in 1-substituted-1H-α-carboline. To the best of our knowledge, there is a lack of report concerning synthesis of such new four-membered annulated heterocyclic system from spirooxindole, and therefore, we considered probing the envisaged reaction. Accordingly, the spirooxindole was prepared by the reaction between isatin, proline, and phenylacetylene, and treatment with POCl3 under heating resulted in the formation of pyrido[2,3-b]indole. This initial success prompted us to study the scope of this novel dehydrative transformation in detail and explore if pyrido[2,3-b]indoles could be prepared directly from isatin as a one-pot protocol. The details of the results pertaining to this study are presented herein.
Figure 2.
Previously reported strategies and the present work.
Scheme 1. Proposed Pathway for the Synthesis of Pyrido[2,3-b]indole from Spirooxindole via Intramolecular Dehydration.
Results and Discussion
Initially, a pilot reaction of isatin (1a) with proline (2a) and phenylacetylene (3a) in the presence of CuI in MeCN under heating afforded spirooxindole 4aaa.10 Subsequent reaction of 4aaa with 20 equiv of POCl3 at 105 °C for 20 h resulted in isolation of a solid product (79%) that was spectrally established to be the expected 5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole 5aaa. To improve the yield of 5aaa, optimization of the protocol by titrating the amount of POCl3, modulating the temperature, and replacing POCl3 with other dehydrating agents was performed, and the results are summarized in Table 1. Lowering the temperature to 90 °C and reducing the amount of the POCl3 gave 5aaa in a superior yield of 86%. Further lowering the temperature or the amount of POCl3, however, proved detrimental for the synthesis of 5aaa. We observed that POBr3 gave comparable yield of 5aaa, but PCl5, SOCl2, PPA, or P2O5 was ineffective for the protocol. Thus, the optimized condition for the synthesis of pyrido[2,3-b]indole from spirooxindole that worked best in our hands was reacting 4aaa with 5 equiv of POCl3 at 90 °C for 24 h.
Table 1. Results of the Optimization Study for Dehydrative Intramolecular Cyclization of Spirooxindole 4aaaa.
| entry | dehydrating agent (equiv) | temp. (°C) | time (h) | yield (%)b 5aaa |
|---|---|---|---|---|
| 1 | POCl3 (20) | 105 | 20 | 79 |
| 2 | POCl3 (20) | 90 | 24 | 85 |
| 3 | POCl3 (20) | 70 | 30 | 81 |
| 4 | POCl3 (10) | 90 | 24 | 86 |
| 5 | POCl3 (5.0) | 90 | 24 | 86 |
| 6 | POCl3 (3.0) | 90 | 24 | 65 |
| 7 | POBr3 (5.0) | 90 | 24 | 84 |
| 8 | PCl5 (5.0) | 90 | 24 | 0 |
| 9 | SOCl2 (5.0) | 90 | 24 | 0 |
| 10 | PPA (5.0) | 90 | 24 | 0 |
| 11c | P2O5 (5.0) | 90 | 24 | 0 |
All reactions were performed using 4aaa (0.5 mmol) in a sealed tube.
Isolated yield after column chromatography.
1.0 mL of toluene was used as the solvent.
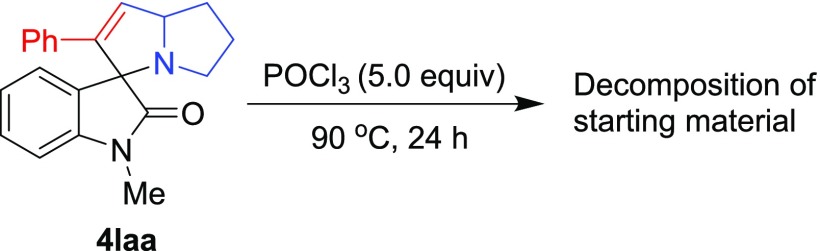
With the standardized conditions identified, the scope of the reaction was investigated with a variety of spirooxindoles 4 (Scheme 2). The first set of spirooxindoles 4aab–4aak were prepared from isatin 1a, proline 2a, and different acetylenes 3b–k (see the Supporting Information (SI) for details). It was found that all substrates smoothly afforded the corresponding products 5aab–5aak in 78–87% yields, thereby reflecting that the protocol could accommodate aryl, heteroaryl, as well as aliphatic starting materials. In addition, the structures of these products were unambiguously secured by performing X-ray crystallographic analysis on a single crystal of 5aaf. For the next set of reactions, spirooxindoles 4baa–4kaa prepared from substituted isatins 1b–k, proline 2a, and phenylacetylene 3a were used. It was pleasing to discover that all spirooxindoles underwent dehydrative transformation to afford the respective products 5baa–5kaa in excellent yields (Scheme 2). These results suggested that the electronic nature of the substitution on the isatin unit also had no impact on the outcome of the protocol. To demonstrate the essentiality of a free NH in the isatin unit for success in this transformation, 4laa was prepared and subjected to reaction with POCl3, which resulted in decomposition of the starting material (Scheme 3).
Scheme 2. Scope of Synthesis of Substituted Pyrido[2,3-b]indoles (5) from Spirooxindoles (4).
All reactions were performed using 0.5 mmol of 4 in a sealed tube.
Scheme 3. Control Reaction.
Success of the protocol with a variety of substrates motivated us to explore the possibility of a one-pot, two-step process from isatin, thereby eliminating the need for isolation of the spirooxindole. Thus, in a model reaction, 1a, 2a, and 3a were treated first with CuI in MeCN, followed by removal of the solvent and addition of POCl3 to the residue and heating the mixture for 24 h. This one-pot procedure afforded 5aaa in 70% yield, which was relatively better than the overall yield of 67% obtained in the two-step procedure (Scheme 4). Buoyed by this success, we evaluated the one-pot syntheses of 5aaj, 5aak, 5baa, and 5daa and found that, in each case, yield of the product was better than the two-step process (for comparison, refer to the SI).
Scheme 4. One-Pot Synthesis of Pyrido[2,3-b]indoles (5),
All reactions were performed using 1.0 mmol of 1, 1.0 mmol of 2a, 1.2 mmol of 3, and 5 mmol of POCl3.
8 mmol of POCl3 was used.
Aiming at broadening the scope of the protocol, we next investigated the two-step, one-pot procedure with internal alkynes instead of terminal alkynes. The reaction of alkyne 3l with 1a and 2a failed to afford the desired 5aal in a one-pot process. Hence, we considered exploring reactions with activated alkynes since they are reported to offer spirooxindoles under metal-free conditions.11 Accordingly, treating internal alkynes 3m–o with 1a and 2a in methanol under metal-free condition successfully furnished the required spirooxindoles. Evaporating of solvent and treating the crude spirooxindoles with POCl3 at 90 °C furnished 5aam, 5aan, and 5aao in 79–82% yield (Scheme 5).
Scheme 5. One-Pot Synthesis of Pyrido[2,3-b]indoles (5) from Internal Alkynes.
All reactions were performed using 1.0 mmol of 1a, 1.0 mmol of 2a, 1.0 mmol of 3, and 5–10 mmol of POCl3.
Further, we assessed the reaction of different α-amino acids (2b–f) with 1a and 3m to afford the title compounds. Notably, the selection of amino acids too was influenced by their ability to form spirooxindole.11 Reaction of 1a and 3m with l-thioproline (2b) and sarcosine (2e) produced the corresponding products 5abm and 5aem in 68 and 77% yields, respectively (Scheme 6). However, the reaction with cis-4-hydroxy-d-proline (2c) and pipecolinic acid (2d) resulted in a mixture of products, from which the desired product could not be isolated. We ascertained that reaction of 2c with 1a and 3m successfully afforded spirooxindole as reported,11a but further treatment with POCl3 produced a complex mixture of products. Likewise, the reaction of glycine (2f) also failed to furnish the desired product 5afm.
Scheme 6. Scope of the Reaction with α-Amino Acids.
All reactions were performed using 1.0 mmol of 1a, 1.0 mmol of 2a, 1.0 mmol of 3, and 5–10 mmol of POCl3.
Success with activated alkynes made it imperative to probe the reaction with activated alkenes as they too are known to afford spirooxindoles.6,12 Therefore, in a representative experiment, reaction of dimethyl maleate (3p) with 1a and 2a was performed, which resulted in isolation of 5aam in 76% yield, but 2 days were required for reaction completion (Scheme 7). It was apparent that an in situ oxidation occurred in the final step to afford the observed product. Likewise, treating 1a and 2a with diethyl maleate (3q) led to the formation of 5aan in 2 days, establishing the generality of the protocol. The scope was further investigated with 1,4-naphthoquinone (3r), benzylideneacetone (3s), and acrylonitrile (3t) and, in all cases, the expected products 5aar, 5aas, and 5aat were isolated in 70–74% yields. It is worth mentioning that the structure of 5aas was derived from the structure of the spirooxindole intermediate.12
Scheme 7. Scope of the Reaction with Activated Alkenes,
All reactions were performed using 1.0 mmol of 1a, 1.0 mmol of 2a, 1.0 mmol of 3, and 5–10 mmol of POCl3.
5aam = 5aap and 5aan = 5aaq.
Conclusions
In summary, we have developed a POCl3-mediated dehydrative transformation of spirooxindoles into pyrido[2,3-b]indoles via ring expansion and construction of the pyridine ring. The transformation could be executed from either spiroxindole or as a two-step, one-pot protocol starting from isatin. This protocol, which involves the use of readily available starting materials, is versatile and accommodates terminal and activated internal alkynes as well as alkenes. The presence of different functional groups such as ester, nitrile, and acyl groups in the title compounds provides the opportunity to expand them further for investigating different biological activities. In this context, further work is underway to develop new analogues of fused α-carbolines.
Experimental Section
General Information
Thin-layer chromatography (TLC) on precoated silica gel plates was used to monitor all reactions. After elution, the TLC plate was visualized under UV illumination at 254 nm. All melting points were recorded on a hot-stage apparatus and are uncorrected. IR spectra were recorded on an Fourier-transform infrared spectrophotometer. 1H NMR and 13C NMR spectra were recorded on Bruker 300, 400, and 500 MHz spectrometers, using tetramethylsilane as an internal standard. Peak multiplicities of NMR signals were designated as s (singlet), d (doublet), dd (doublet of doublet), dt (doublet of triplet), t (triplet), q (quartet), or m (multiplet). The electrospray ionization mass spectrometry (ESI-MS) images were recorded on an ion trap mass spectrometer. The high resolution mass spectrometry (HRMS) images were recorded as ESI-HRMS images on a quadrupole time-of-flight LC-MS/MS mass spectrometer. Commercial grade reagents and solvents were used without further purification. The title compounds obtained from 3p and 3q are 5aam and 5aan, respectively. Therefore, there are no data for 5aap and 5aaq.
General Experimental Procedure for the Synthesis of Spirooxindole 4 as Exemplified for 4aaa
In a round-bottom flask equipped with a water condenser, were added isatin 1a (0.147 g, 1.0 mmol), l-proline 2a (0.115 g, 1.0 mmol), phenylacetylene 3a (132 μL, 1.2 mmol), and CuI (0.0095 g, 5 mol %) in 5 mL of acetonitrile. The reaction mixture was refluxed under nitrogen atmosphere for 6 h. After completion of the reaction (as monitored by TLC), the reaction mixture was cooled to room temperature and passed through a bed of celite. The filtrate was concentrated under reduced pressure to obtain the crude product, which was purified by column chromatography over silica gel using hexanes/EtOAc (30:70, v/v) as eluent to obtain 0.236 g (78%) of spirooxindole 4aaa as a yellow solid.
2′-Phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aaa)
Mp 180–182 °C [Lit:10b 180 °C]; Rf = 0.42 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1721 (C=O), 3409 (N–H) cm–1. 1H NMR (300 MHz, CDCl3): δ (ppm) = 1.55–1.67 (m, 1H, CH), 1.81–1.91 (m, 2H, 2 × CH), 2.09–2.19 (m, 1H, CH), 2.68–2.81 (m, 2H, CH2), 4.75 (dt, J = 7.7, 1.7 Hz, 1H, N–CH), 6.58 (d, J = 1.9 Hz, 1H, =CH), 6.89–6.97 (m, 3H, ArH), 6.99–7.03 (m, 2H, ArH), 7.08–7.11 (m, 3H, ArH), 7.17–7.23 (m, 1H, ArH), 9.32 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.2, 31.9, 49.1, 71.6, 79.7, 111.1, 122.2, 126.3, 127.1, 127.7, 128.4, 129.7, 133.1, 133.5, 140.1, 141.9, 181.4. DEPT-135 (75 MHz, CDCl3): δ (ppm) = 27.2, 31.9, 49.1, 71.6, 111.1, 122.3, 126.3, 127.1, 127.8, 128.5, 129.8, 133.1. MS (ESI+): m/z = 303.1. ESI-HR-MS calculated for C20H18N2O [MH]+: 303.1492, found: 303.1492.
2′-(p-Tolyl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aab)
Yield: 67% (0.212 g from 0.147 g of 1a) as a yellow solid, mp 213–215 °C; Rf = 0.43 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1711 (C=O), 3490 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.55–1.65 (m, 1H, CH), 1.83–1.89 (m, 2H, 2 × CH), 2.09–2.16 (m, 1H, CH), 2.20 (s, 3H, CH3), 2.71–2.76 (m, 2H, CH2), 4.71–4.75 (m, 1H, N–CH), 6.53 (d, J = 1.8 Hz, 1H, =CH), 6.90–6.98 (m, 7H, ArH), 7.21 (dt, J = 7.6, 1.3 Hz, 1H, ArH), 9.21 (s, 1H, NH); 13C NMR (75 MHz, CDCl3): δ (ppm) = 21.2, 27.2, 31.9, 49.1, 71.6, 79.7, 111.1, 122.2, 126.2, 127.1, 127.2, 129.2, 129.7, 130.6, 132.1, 137.5, 139.9, 141.9, 181.3. MS (ESI+): m/z = 317.2. ESI-HR-MS calculated for C21H20N2O [MH]+: 317.1648, found: 317.1648.
2′-(4-(tert-Butyl)phenyl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aac)
Yield: 70% (0.251 g from 0.147 g of 1a) as a yellow solid, mp 204–207 °C; Rf = 0.40 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1712 (C=O), 3446 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.19 (s, 9H, 3 × CH3), 1.56–1.65 (m, 1H, CH), 1.85–1.91 (m, 2H, 2 × CH), 2.10–2.17 (m, 1H, CH), 2.75 (t, J = 6.9 Hz, 2H, CH2), 4.72–4.77 (m, 1H, N–CH), 6.55 (d, J = 1.9 Hz, 1H, =CH), 6.91–7.01 (m, 5H, ArH), 7.09 (s, 1H, ArH), 7.12 (s, 1H, ArH), 7.22 (dd, J = 7.4, 1.1 Hz, 1H, ArH), 9.09 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.2, 31.2, 31.9, 34.5, 49.1, 71.6, 79.5, 111.2, 122.3, 125.4, 125.9, 127.0, 127.1, 129.9, 130.2, 131.9, 139.6, 142.0, 150.7, 180.8. MS (ESI+): m/z = 359.2. ESI-HR-MS calculated for C24H26N2O [MH]+: 359.2118, found: 359.2115.
2′-(m-Tolyl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aad)
Yield: 65% (0.205 g from 0.147 g of 1a) as a yellow solid, mp 221–225 °C; Rf = 0.44 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1737 (C=O), 3415 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.48–1.58 (m, 1H, CH), 1.77–1.83 (m, 2H, 2 × CH), 2.02–2.08 (m, 1H, CH), 2.09 (s, 3H, CH3), 2.62–2.72 (m, 2H, CH2), 4.64–4.68 (m, 1H, N–CH), 6.48 (d, J = 1.9 Hz, 1H, =CH), 6.64 (d, J = 7.5 Hz, 1H, ArH), 6.83–6.92 (m, 6H, ArH), 7.14 (dt, J = 1.3 Hz, 1H, ArH), 9.03 (s, 1H, NH); 13C NMR (75 MHz, CDCl3): δ (ppm) = 21.5, 27.2, 31.9, 49.1, 71.5, 79.7, 111.0, 122.2, 123.2, 127.1, 127.3, 128.3, 128.6, 129.7, 132.9, 133.4, 137.8, 140.2, 142.0, 181.2. MS (ESI+): m/z = 317.2. ESI-HR-MS calculated for C21H20N2O [MH]+: 317.1648, found: 317.1648.
2′-(3-Fluorophenyl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aae)
Yield: 68% (0.217 g from 0.147 g of 1a) as a yellow solid, mp 190–192 °C; Rf = 0.32 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1739 (C=O), 3435 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.49–1.56 (m, 1H, CH), 1.77–1.83 (m, 2H, 2 × CH), 2.04–2.11 (m, 1H, CH), 2.62–2.72 (m, 2H, CH2), 4.67 (t, J = 7.0 Hz, 1H, N–CH), 6.53 (d, J = 1.9 Hz, 1H, =CH), 6.65–6.76 (m, 3H, ArH), 6.86–6.92 (m, 3H, ArH), 6.95–7.01 (m, 1H, ArH), 7.14–7.19 (m, 1H, ArH), 9.02 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.2, 31.8, 49.1, 71.5, 79.6, 111.3, 113.3 (d, J = 22.4 Hz), 114.7 (d, J = 21.2 Hz), 121.9, 122.4, 126.7, 126.9, 129.9, 130.1, 134.3, 135.7 (d, J = 7.7 Hz), 139.1, 142.0, 162.7 (d, J = 245.3 Hz), 181.4. MS (ESI+): m/z = 321.1. ESI-HR-MS calculated for C20H17FN2O [MH]+: 321.1398, found: 321.1394.
2′-(2-(Trifluoromethyl)phenyl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2- one (4aaf)
Yield: 65% (0.241 g from 0.147 g of 1a) as a yellow solid, mp 178–180 °C; Rf = 0.52 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1736 (C=O), 3416 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.78–1.92 (m, 3H, CH, CH2), 2.16–2.22 (m, 1H, CH), 2.69–2.73 (m, 1H, CH), 2.98–3.04 (m, 1H, CH), 4.88 (t, J = 7.3 Hz, 1H, N–CH), 6.41 (s, 1H, =CH), 6.83–6.86 (m, 2H, ArH), 7.06 (d, J = 7.6 Hz, 1H, ArH), 7.10–7.14 (m, 2H, ArH), 7.18–7.27 (m, 2H, ArH), 7.57 (d, J = 7.6 Hz, 1H, ArH), 9.58 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 26.1, 30.5, 50.5, 71.1, 81.3, 110.6, 122.1, 123.0, 125.6, 126.6, 126.7, 127.4, 128.0, 128.2, 129.7, 131.6, 133.8, 135.5, 139.3, 142.2, 181.0. MS (ESI+): m/z = 371.1. ESI-HR-MS calculated for C21H17F3N2O [MH]+: 371.1366, found: 371.1369.
2′-(Phenanthren-9-yl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aag)
Yield: 58% (0.234 g from 0.147 g of 1a) as a brown solid, mp 176–179 °C; Rf = 0.41 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1719 (C=O), 3429 (N–H) cm–1. 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 1.77–1.87 (m, 2H, 2 × CH), 1.97–2.00 (m, 1H, CH), 2.16–2.20 (m, 1H, CH), 2.56–2.60 (m, 1H, CH), 2.92–2.97 (m, 1H, CH), 4.69 (t, J = 6.5 Hz, 1H, N–CH), 6.40 (s, 1H, =CH), 6.61 (d, J = 7.6 Hz, 1H, ArH), 6.80 (t, J = 7.3 Hz, 1H, ArH), 7.04 (t, J = 7.4 Hz, 1H, ArH), 7.23 (s, 1H, ArH), 7.25 (s, 1H, ArH), 7.54–7.67 (m, 5H, ArH), 8.32–8.34 (m, 1H, ArH), 8.71–8.77 (m, 2H, ArH), 10.26 (s, 1H, NH); 13C NMR (100 MHz, CDCl3 + DMSO-d6): δ (ppm) = 27.2, 31.8, 49.1, 72.2, 82.0, 110.2, 121.1, 122.5, 122.6, 122.7, 122.8, 125.7, 126.1, 126.6, 126.7, 126.9, 127.0, 127.2, 128.5, 129.4, 130.0, 130.6, 130.8, 130.9, 136.9, 137.7, 143.0, 179.2. MS (ESI+): m/z = 403.2. ESI-HR-MS calculated for C28H22N2O [MH]+: 403.1805, found: 403.1804.
2′-(Pyridin-2-yl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aah)
Yield: 63% (0.191 g from 0.147 g of 1a) as a yellow solid, mp 222–224 °C; Rf = 0.15 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1716 (C=O), 3402 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.60–1.69 (m, 1H, CH), 1.83–1.90 (m, 2H, 2 × CH), 2.10–2.18 (m, 1H, CH), 2.74 (t, J = 6.9 Hz, 2H, CH2), 4.79–4.83 (m, 1H, N–CH), 6.81–6.93 (m, 4H, ArH), 6.99 (d, J = 1.9 Hz, 1H, =CH), 7.12–7.17 (m, 2H, ArH), 7.40–7.44 (m, 1H, ArH), 8.24 (d, J = 4.1 Hz, 1H, ArH), 9.49 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.3, 31.8, 48.8, 72.2, 78.8, 110.7, 120.3, 121.5, 122.0, 126.1, 127.6, 129.3, 135.3, 135.9, 141.0, 142.7, 149.2, 151.7, 181.7. MS (ESI+): m/z = 304.1. ESI-HR-MS calculated for C19H17N3O [MH]+: 304.1444, found: 304.1440.
2′-(Thiophen-2-yl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aai)
Yield: 59% (0.182 g from 0.147 g of 1a) as a yellow solid, mp 225–229 °C; Rf = 0.30 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1720 (C=O), 3410 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.48–1.59 (m, 1H, CH), 1.75–1.85 (m, 2H, 2 × CH), 2.01–2.09 (m, 1H, CH), 2.63–2.77 (m, 2H, CH2), 4.65 (t, J = 7.2 Hz, 1H, N–CH), 6.31 (d, J = 3.3 Hz, 1H, ArH), 6.43 (d, J = 1.4 Hz, 1H, =CH), 6.63–6.65 (m, 1H, ArH), 6.89–7.00 (m, 4H, ArH), 7.19–7.23 (m, 1H, ArH), 9.04 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.2, 31.8, 48.9, 71.8, 79.6, 111.0, 122.2, 124.1, 124.7, 126.4, 127.2, 127.3, 130.0, 131.6, 134.0, 136.1, 142.1, 180.7. MS (ESI+): m/z = 309.1. ESI-HR-MS calculated for C18H16N2OS [MH]+: 309.1056, found: 309.1054.
2′-(Cyclohex-1-en-1-yl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4aaj)
Yield: 73% (0.224 g from 0.147 g of 1a) as a yellow solid, mp 182–183 °C; Rf = 0.30 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1718 (C=O), 3359 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.39–1.63 (m, 5H, CH, 2 × CH2), 1.74–1.89 (m, 4H, 2 × CH2), 2.03–2.08 (m, 2H, 2 × CH), 2.21–2.25 (m, 1H, CH), 2.61 (t, J = 6.9 Hz, 2H, CH2), 4.59 (t, J = 7.7 Hz, 1H, N–CH), 5.00 (t, J = 4.1 Hz, 1H, =CH), 6.16 (s, 1H, =CH), 6.92 (d, J = 7.7 Hz, 1H, ArH), 6.95–7.00 (m, 2H, ArH), 7.21–7.25 (m, 1H, ArH), 8.71 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.9, 22.6, 25.7, 26.9, 27.1, 31.9, 48.9, 71.2, 78.8, 110.9, 122.1, 126.1, 126.7, 128.2, 129.4, 129.9, 130.1, 141.2, 141.7, 181.8. MS (ESI+): m/z = 307.2. ESI-HR-MS calculated for C20H22N2O [MH]+: 307.1805, found: 307.1802.
Methyl 2-Oxo-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizine]-2′-carboxylate (4aak)
Yield: 69% (0.196 g from 0.147 g of 1a) as an off-white solid, mp 179–182 °C; Rf = 0.32 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1708 (C=O), 3437 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.57–1.64 (m, 1H, CH), 1.85–1.89 (m, 2H, 2 × CH), 2.11–2.13 (m, 1H, CH), 2.72 (t, J = 6.1 Hz, 2H, CH2), 3.54 (s, 3H, CH3), 4.71 (t, J = 6.9 Hz, 1H, N–CH), 6.91–7.00 (m, 3H, ArH), 7.21–7.26 (m, 2H, =CH, ArH), 8.48 (s, 1H, NH); 13C NMR (75 MHz, CDCl3): δ (ppm) = 27.6, 31.4, 48.5, 51.8, 72.4, 110.8, 122.0, 126.0, 126.7, 129.9, 134.2, 142.2, 146.8, 162.7, 180.1. MS (ESI+): m/z = 285.1. ESI-HR-MS calculated for C16H16N2O3 [MH]+: 285.1234, found: 285.1238.
5-Methyl-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4baa)
Yield: 74% (0.234 g from 0.161 g of 1b) as an off-white solid, mp 189–193 °C; Rf = 0.35 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1723 (C=O), 3428 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.55–1.65 (m, 1H, CH), 1.85–1.88 (m, 2H, 2 × CH), 2.10–2.17 (m, 1H, CH), 2.22 (s, 3H, CH3), 2.68–2.79 (m, 2H, CH2), 4.73 (t, J = 7.3 Hz, 1H, N–CH), 6.59 (s, 1H, =CH), 6.77 (s, 1H, ArH), 6.83 (d, J = 7.8 Hz, 1H, ArH), 6.99–7.03 (m, 3H, ArH), 7.10–7.12 (m, 3H, ArH), 9.22 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.1, 27.1, 31.8, 48.8, 71.4, 79.6, 110.6, 126.2, 126.9, 127.5, 128.3, 130.0, 131.4, 132.7, 133.4, 139.4, 140.0, 181.2. MS (ESI+): m/z = 317.2. ESI-HR-MS calculated for C21H20N2O [MH]+: 317.1648, found: 317.1643.
5-Methoxy-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4caa)
Yield: 75% (0.249 g from 0.177 g of 1c) as a yellow solid, mp 206–209 °C; Rf = 0.20 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1720 (C=O), 3401 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.47–1.57 (m, 1H, CH), 1.76–1.85 (m, 2H, 2 × CH), 2.03–2.10 (m, 1H, CH), 2.65–2.71 (m, 2H, CH2), 3.63 (s, 3H, OCH3), 4.64–4.68 (m, 1H, N–CH), 6.50–6.51 (m, 2H, =CH, ArH), 6.65–6.68 (m, 1H, ArH), 6.78 (d, J = 8.4 Hz, 1H, ArH), 6.95–6.98 (m, 2H, ArH), 7.02–7.05 (m, 3H, ArH), 9.08 (s, 1H, NH); 13C NMR (125 MHz, CDCl3): δ (ppm) = 27.3, 32.0, 48.9, 55.8, 71.7, 80.1, 111.3, 113.9, 114.4, 126.3, 127.7, 128.4, 132.9, 133.4, 135.4, 140.1, 155.5, 181.3. MS (ESI+): m/z = 333.2. ESI-HR-MS calculated for C21H20N2O2 [MH]+: 333.1598, found: 333.1597.
5-Bromo-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4daa)
Yield: 74% (0.282 g from 0.226 g of 1d) as an off-white solid, mp 230–234 °C; Rf = 0.48 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1732 (C=O), 3427 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.56–1.66 (m, 1H, CH), 1.86–1.92 (m, 2H, 2 × CH), 2.11–2.18 (m, 1H, CH), 2.70–2.74 (m, 2H, CH2), 4.70–4.74 (m, 1H, N–CH), 6.59 (d, J = 1.9 Hz, 1H, =CH), 6.83 (d, J = 8.3 Hz, 1H, ArH), 6.99–7.02 (m, 2H, ArH), 7.07 (d, J = 1.8 Hz, 1H, ArH), 7.13–7.14 (m, 3H, ArH), 7.34 (dd, J = 8.3, 1.9 Hz, 1H, ArH), 9.41 (s, 1H, NH); 13C NMR (75 MHz, CDCl3 + DMSO-d6): δ (ppm) = 27.2, 31.7, 48.8, 71.5, 79.4, 112.2, 114.4, 126.1, 127.7, 128.4, 129.2, 129.6, 132.4, 133.1, 133.2, 139.6, 141.5, 180.1. MS (ESI+): m/z = 381.1. ESI-HR-MS calculated for C20H17BrN2O [MH]+: 381.0597, found: 381.0597.
5-Chloro-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4eaa)
Yield: 73% (0.246 g from 0.181 g of 1e) as an off-white solid, mp 220–224 °C; Rf = 0.47 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1731 (C=O), 3436 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.56–1.59 (m, 1H, CH), 1.84–1.94 (m, 2H, 2 × CH), 2.12–2.19 (m, 1H, CH), 2.71–2.74 (m, 2H, CH2), 4.73 (t, J = 7.2 Hz, 1H, N–CH), 6.59 (d, J = 1.7 Hz, 1H, =CH), 6.87 (d, J = 8.3 Hz, 1H, ArH), 6.94 (d, J = 1.8 Hz, 1H, ArH), 6.99–7.02 (m, 2H, ArH), 7.13–7.14 (m, 3H, ArH), 7.19 (dd, J = 8.3, 1.9 Hz, 1H, ArH), 9.29 (s, 1H, NH); 13C NMR (100 MHz, CDCl3 + DMSO-d6): δ (ppm) = 27.0, 31.5, 48.6, 71.2, 79.1, 111.5, 125.9, 126.6, 127.5, 128.2, 128.7, 129.4, 132.9, 133.2, 139.5, 141.4, 179.6. MS (ESI+): m/z = 337.1. ESI-HR-MS calculated for C20H17ClN2O [MH]+: 337.1102, found: 337.1105.
5-Fluoro-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4faa)
Yield: 72% (0.231 g from 0.165 g of 1f) as an off-white solid, mp 207–211 °C; Rf = 0.45 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1732 (C=O), 3442 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.54–1.64 (m, 1H, CH), 1.84–1.92 (m, 2H, 2 × CH), 2.12–2.19 (m, 1H, CH), 2.72–2.76 (m, 2H, CH2), 4.74 (t, J = 7.2 Hz, 1H, N–CH), 6.59 (d, J = 1.6 Hz, 1H, =CH), 6.71 (dd, J = 7.8, 2.3 Hz, 1H, ArH), 6.85–6.94 (m, 2H, ArH), 7.00–7.03 (m, 2H, ArH), 7.12–7.13 (m, 3H, ArH), 9.73 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.2, 31.9, 49.0, 71.7, 80.0, 111.7 (d, J = 7.8 Hz), 114.6 (d, J = 24.3 Hz), 116.3 (d, J = 23.5 Hz), 126.2, 127.9, 128.6, 128.8 (d, J = 7.2 Hz), 133.1, 133.3, 137.9, 139.7, 158.9 (d, J = 241.1 Hz), 181.7. MS (ESI+): m/z = 321.1. ESI-HR-MS calculated for C20H17FN2O [MH]+: 321.1398, found: 321.1394.
5-Nitro-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4gaa)
Yield: 71% (0.247 g from 0.192 g of 1g) as a yellow solid, mp 193–196 °C; Rf = 0.28 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1739 (C=O), 3435 (N–H) cm–1. 1H NMR (400 MHz, CDCl3 + DMSO-d6): δ (ppm) = 1.62–1.71 (m, 1H, CH), 1.80–1.91 (m, 2H, 2 × CH), 2.14–2.20 (m, 1H, CH), 2.67–2.71 (m, 2H, CH2), 4.68 (t, J = 7.3 Hz, 1H, N–CH), 6.63 (s, 1H, =CH), 6.97–6.98 (m, 2H, ArH), 7.06 (d, J = 8.5 Hz, 1H, ArH), 7.13–7.14 (m, 2H, ArH), 7.72–7.73 (m, 1H, ArH), 7.77 (d, J = 1.9 Hz, 1H, ArH), 8.16–8.19 (m, 1H, ArH), 11.03 (s, 1H, NH); 13C NMR (100 MHz, CDCl3 + DMSO-d6): δ (ppm) = 26.6, 30.9, 48.7, 70.9, 77.8, 110.5, 121.6, 125.6, 127.1, 127.4, 127.7, 128.5, 132.9, 134.3, 138.2, 141.9, 149.3, 179.0. MS (ESI+): m/z = 348.1. ESI-HR-MS calculated for C20H17N3O3 [MH]+: 348.1343, found: 348.1348.
2′-Phenyl-5-(trifluoromethoxy)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4haa)
Yield: 79% (0.305 g from 0.231 g of 1h) as a white solid, mp 221–223 °C; Rf = 0.59 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1732 (C=O), 3448 (N–H) cm–1. 1H NMR (500 MHz, CDCl3): δ (ppm) = 1.58–1.63 (m, 1H, CH), 1.88–1.89 (m, 2H, 2 × CH), 2.15–2.16 (m, 1H, CH), 2.67–2.75 (m, 2H, CH2), 4.74 (t, J = 6.6 Hz, 1H, N–CH), 6.57 (s, 1H, =CH), 6.86–7.12 (m, 8H, ArH), 9.41 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.1, 31.8, 49.0, 71.7, 79.9, 111.5, 120.8, 123.0, 126.3, 128.0, 128.6, 133.1, 133.6, 139.7, 140.6, 144.4, 181.5. MS (ESI+): m/z = 387.1. ESI-HR-MS calculated for C21H17F3N2O2 [MH]+: 387.1315, found: 387.1319.
7-Bromo-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4iaa)
Yield: 72% (0.275 g from 0.226 g of 1i) as a yellow solid, mp 219–222 °C; Rf = 0.35 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1732 (C=O), 3417 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.55–1.67 (m, 1H, CH), 1.84–1.89 (m, 2H, 2 × CH), 2.10–2.17 (m, 1H, CH), 2.68–2.72 (m, 2H, CH2), 4.69–4.73 (m, 1H, N–CH), 6.54 (d, J = 1.8 Hz, 1H, =CH), 6.85 (t, J = 8.00 Hz, 1H, ArH), 6.92–6.99 (m, 3H, ArH), 7.12–7.14 (m, 3H, ArH), 7.37 (d, J = 8.1, 1.0 Hz, 1H, ArH), 7.56 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.2, 31.6, 49.1, 71.5, 80.6, 103.6, 123.4, 126.0, 126.4, 127.8, 128.5, 132.4, 133.4, 133.7, 139.7, 140.9, 179.5. MS (ESI+): m/z = 381.1. ESI-HR-MS calculated for C20H17BrN2O [MH]+: 381.0597, found: 381.0598.
2′-Phenyl-7-(trifluoromethyl)-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4jaa)
Yield: 70% (0.259 g from 0.215 g of 1j) as a yellow solid, mp 242–246 °C; Rf = 0.52 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1730 (C=O), 3437 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.58–1.62 (m, 1H, CH), 1.85–1.90 (m, 2H, 2 × CH), 2.12–2.19 (m, 1H, CH), 2.67–2.70 (m, 2H, CH2), 4.74 (t, J = 7.2 Hz, 1H, N–CH), 6.56 (s, 1H, =CH), 6.95–6.96 (m, 2H, ArH), 7.04 (t, J = 7.7 Hz, 1H, ArH), 7.12–7.14 (m, 4H, ArH), 7.45 (d, J = 7.9 Hz, 1H, ArH), 7.74 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 27.0, 31.4, 49.1, 71.2, 78.0, 121.9, 126.1, 126.4, 127.8, 128.4, 128.5, 130.5, 133.2, 134.0, 138.9, 139.4, 178.7. MS (ESI+): m/z = 371.1. ESI-HR-MS calculated for C21H17F3N2O [MH]+: 371.1366, found: 371.1360.
5,7-Dimethyl-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4kaa)
Yield: 72% (0.238 g from 0.175 g of 1k) as a yellow solid, mp 229–231 °C; Rf = 0.45 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1714 (C=O), 3389 (N–H) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.56–1.65 (m, 1H, CH), 1.83–1.90 (m, 1H, CH), 2.09–2.16 (m, 1H, CH), 2.20 (s, 3H, CH3), 2.22 (s, 3H, CH3), 2.28–2.40 (m, 1H, CH), 2.67–2.79 (m, 2H, CH2), 4.71–4.75 (m, 1H, N–CH), 6.53 (d, J = 1.9 Hz, 1H, =CH), 6.63 (s, 1H, ArH), 6.86 (s, 1H, ArH), 6.98–7.00 (m, 2H, ArH), 7.08–7.11 (m, 3H, ArH), 8.32 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 16.5, 21.2, 27.1, 31.7, 49.1, 71.3, 79.9, 119.6, 125.1, 126.3, 126.5, 127.6, 128.4, 131.4, 131.7, 133.0, 133.7, 138.1, 140.1, 180.7. MS (ESI+): m/z = 331.2. ESI-HR-MS calculated for C22H22N2O [MH]+: 331.1805, found: 331.1810.
1-Methyl-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indoline-3,3′-pyrrolizin]-2-one (4laa)
Yield: 75% (0.237 g from 0.161 g of 1l) as a white solid, mp 178–181 °C; Rf = 0.45 (hexanes/EtOAc, 3:7, v/v); IR (KBr) νmax: 1725 (C=O) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.56–1.66 (m, 1H, CH), 1.82–1.89 (m, 2H, 2 × CH), 2.09–2.16 (m, 1H, CH), 2.63–2.68 (m, 1H, CH), 2.71–2.77 (m, 1H, CH), 3.23 (s, 3H, N–CH3), 4.73 (dt, J = 7.7, 1.8 Hz, 1H, N–CH), 6.51 (d, J = 1.9 Hz, 1H, =CH), 6.85 (d, J = 7.8 Hz, 1H, ArH), 6.87–6.90 (m, 2H, ArH), 6.94–6.97 (m, 1H, ArH), 7.01–7.03 (m, 1H, ArH), 7.05–7.08 (m, 3H, ArH), 7.27–7.31 (m, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 26.5, 26.9, 31.5, 49.1, 71.1, 79.0, 108.4, 122.1, 126.2, 126.4, 126.8, 127.4, 128.1, 129.6, 133.3, 133.7, 140.1, 144.5, 177.9. MS (ESI+): m/z = 317.2. ESI-HR-MS calculated for C21H20N2O [MH]+: 317.1648, found: 317.1646.
General Experimental Procedure for the Synthesis of 2,3-Dihydro-1H-indolizino[5,6-b]indoles 5 as Exemplified for 5aaa
A sealed tube charged with a mixture of spirooxindole (4aaa) (0.151 g, 0.5 mmol) and POCl3 (234 μL, 2.5 mmol) was heated at 90 °C for 24 h under stirring. On completion of the reaction (as assessed by TLC), the reaction mixture was neutralized by adding saturated NaHCO3 solution and extracted with EtOAc (50 mL × 2). The organic layers were pooled, dried over anhydrous Na2SO4, and evaporated to obtain a crude product, which was purified by column chromatography over silica gel using EtOAc/MeOH (80:20, v/v) as eluent to obtain 0.122 g (86%) of 5aaa as a yellow solid.
5-Phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5aaa)
Mp 257–260 °C; Rf = 0.45 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.43–2.50 (m, 2H, CH2), 3.37 (t, J = 7.7 Hz, 2H, CH2), 4.77 (t, J = 7.4 Hz, 2H, CH2), 6.66 (s, 1H, ArH), 6.92 (t, J = 7.2 Hz, 1H, ArH), 7.35–7.39 (m, 1H, ArH), 7.45–7.52 (m, 3H, ArH), 7.57 (d, J = 7.9 Hz, 1H, ArH), 7.62–7.64 (m, 2H, ArH), 7.74 (d, J = 8.2 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.3, 31.7, 51.0, 105.0, 118.1, 118.6, 120.7, 121.9, 123.2, 127.2, 128.7, 128.8, 129.1, 138.7, 146.1, 146.6, 152.3, 154.0. MS (ESI+): m/z = 285.1. ESI-HR-MS calculated for C20H16N2 [MH]+: 285.1386, found: 285.1387.
5-(p-Tolyl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aab)
Yield: 85% (0.127 g from 0.158 g) as a yellow solid, mp 202–204 °C; Rf = 0.41 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =2.47–2.55 (m, 5H, CH3, CH2), 3.41 (t, J = 7.6 Hz, 2H, CH2), 4.82 (t, J = 7.3 Hz, 2H, CH2), 6.71 (s, 1H, ArH), 6.99 (t, J = 7.3 Hz, 1H, ArH), 7.37 (d, J = 7.9 Hz, 2H, ArH), 7.43 (t, J = 7.2 Hz, 1H, ArH), 7.59–7.61 (m, 2H, ArH), 7.70 (d, J = 7.9 Hz, 1H, ArH), 7.81 (d, J = 8.1 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.4, 22.2, 31.6, 51.1, 105.3, 117.8, 118.6, 120.4, 121.8, 123.0, 127.0, 128.5, 129.4, 135.6, 139.1, 146.1, 146.9, 151.9, 153.4. MS (ESI+): m/z = 299.2. ESI-HR-MS calculated for C21H18N2 [MH]+: 299.1543, found: 299.1538.
5-(4-(tert-Butyl)phenyl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aac)
Yield: 87% (0.148 g from 0.179 g) as a yellow solid, mp 224–226 °C; Rf = 0.50 (EtOAc/MeOH, 9:1, v/v); 1H NMR (500 MHz, CDCl3): δ (ppm) =1.43 (s, 9H, 3 × CH3), 2.51–2.54 (m, 2H, CH2), 3.42 (t, J = 7.0 Hz, 2H, CH2), 4.89 (t, J = 6.5 Hz, 2H, CH2), 6.78 (s, 1H, ArH), 7.03 (t, J = 7.2 Hz, 1H, ArH), 7.45 (t, J = 7.2 Hz, 1H, ArH), 7.57–7.59 (m, 2H, ArH), 7.63–7.65 (m, 2H, ArH), 7.73 (d, J = 7.6 Hz, 1H, ArH), 7.83 (d, J = 7.9 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.4, 31.5, 31.7, 35.0, 51.9, 106.5, 117.4, 119.3, 120.1, 122.0, 122.6, 125.8, 127.5, 128.5, 135.3, 146.7, 147.7, 150.6, 151.4, 152.8. MS (ESI+): m/z = 341.2. ESI-HR-MS calculated for C24H24N2 [MH]+: 341.2012, found: 341.2017.
5-(m-Tolyl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aad)
Yield: 86% (0.128 g from 0.158 g) as a yellow solid, mp 188–191 °C; Rf = 0.40 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =2.47–2.56 (m, 5H, CH3, CH2), 3.42 (t, J = 7.6 Hz, 2H, CH2), 4.84 (t, J = 7.3 Hz, 2H, CH2), 6.72 (s, 1H, ArH), 7.00 (t, J = 7.4 Hz, 1H, ArH), 7.34–7.35 (m, 1H, ArH), 7.42–7.50 (m, 4H, ArH), 7.65 (d, J = 7.8 Hz, 1H, ArH), 7.82 (d, J = 8.1 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.6, 22.3, 31.6, 51.2, 105.4, 117.9, 118.7, 120.6, 121.9, 123.1, 125.7, 127.2, 128.7, 129.3, 129.8, 138.6, 146.2, 147.1, 151.9, 153.4. MS (ESI+): m/z = 299.1. ESI-HR-MS calculated for C21H18N2 [MH]+: 299.1543, found: 299.1542.
5-(3-Fluorophenyl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aae)
Yield: 87% (0.132 g from 0.160 g) as a yellow solid, mp 198–201 °C; Rf = 0.56 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =2.49–2.56 (m, 2H, CH2), 3.42 (t, J = 7.6 Hz, 2H, CH2), 4.86 (t, J = 7.2 Hz, 2H, CH2), 6.69 (s, 1H, ArH), 7.02 (t, J = 7.6 Hz, 1H, ArH), 7.21–7.24 (m, 1H, ArH), 7.37 (d, J = 9.1 Hz, 1H, ArH), 7.43–7.56 (m, 3H, ArH), 7.61 (d, J = 7.9 Hz, 1H, ArH), 7.82 (d, J = 8.0 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.3, 31.7, 51.5, 105.1, 115.9 (dd, J = 33.4, 21.0 Hz), 118.0, 119.0, 120.7, 121.8, 122.6, 124.5, 127.5, 130.5, 130.6, 140.7, 145.2, 146.5, 151.8, 153.4, 162.9 (d, J = 247.3 Hz). MS (ESI+): m/z = 303.1. ESI-HR-MS calculated for C20H15FN2 [MH]+: 303.1292, found: 303.1298.
5-(2-(Trifluoromethyl)phenyl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aaf)
Yield: 84% (0.148 g from 0.185 g) as a yellow solid, mp 208–209 °C; Rf = 0.72 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =2.49–2.60 (m, 2H, CH2), 3.37–3.52 (m, 2H, CH2), 4.85–4.89 (m, 2H, CH2), 6.71 (s, 1H, ArH), 6.79 (d, J = 7.8 Hz, 1H, ArH), 6.89–6.93 (m, 1H, ArH), 7.39–7.48 (m, 2H, ArH), 7.65–7.71 (m, 2H, ArH), 7.79 (d, J = 8.2 Hz, 1H, ArH), 7.91–7.93 (m, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.3, 31.7, 51.3, 105.4, 117.8, 119.0, 121.6, 122.4, 123.0, 125.2, 126.6 (dd, J = 10.1, 5.1 Hz), 127.4, 128.3 (d, J = 30.7 Hz), 128.9, 130.7, 132.1, 137.1, 143.5, 145.6, 152.4 (d, J = 189.2 Hz). MS (ESI+): m/z = 353.1. ESI-HR-MS calculated for C21H15F3N2 [MH]+: 353.1260, found: 353.1266.
5-(Phenanthren-9-yl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aag)
Yield: 80% (0.154 g from 0.201 g) as a yellow solid, mp 256–259 °C; Rf = 0.55 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.60–2.67 (m, 2H, CH2), 3.51–3.54 (m, 2H, CH2), 5.00 (t, J = 6.9 Hz, 2H, CH2), 6.77 (t, J = 7.8 Hz, 1H, ArH), 6.87 (d, J = 7.7 Hz, 1H, ArH), 6.93 (s, 1H, ArH), 7.36–7.41 (m, 1H, ArH), 7.43–7.47 (m, 1H, ArH), 7.67–7.74 (m, 3H, ArH), 7.78–7.85 (m, 2H, ArH), 7.90 (s, 1H, ArH), 7.94 (d, J = 7.2 Hz, 1H, ArH), 8.84–8.89 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ (ppm) = 22.3, 31.6, 51.5, 106.8, 117.3, 119.2, 122.2, 122.5, 122.6, 122.7, 123.1, 126.4, 127.0, 127.1, 127.1, 127.2, 127.3, 127.4, 129.0, 129.5, 130.5, 130.6, 131.2, 134.7, 145.3, 146.3, 150.9, 152.2. MS (ESI+): m/z = 385.2. ESI-HR-MS calculated for C28H20N2 [MH]+: 385.1699, found: 385.1694.
5-(Pyridin-2-yl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aah)
Yield: 84% (0.12 g from 0.152 g) as a white solid, mp 198–201 °C; Rf = 0.30 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, DMSO-d6): δ (ppm) =2.55–2.60 (m, 2H, CH2), 3.59 (t, J = 7.4 Hz, 2H, CH2), 4.97 (t, J = 6.8 Hz, 2H, CH2), 7.27 (t, J = 7.6 Hz, 1H, ArH), 7.62 (t, J = 7.5 Hz, 1H, ArH), 7.71–7.74 (m, 1H, ArH), 7.78 (s, 2H, ArH), 7.87 (d, J = 8.0 Hz, 1H, ArH), 8.00 (d, J = 7.8 Hz, 1H, ArH), 8.17 (t, J = 6.8 Hz, 1H, ArH), 8.94 (d, J = 4.2 Hz, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 22.3, 32.1, 55.1, 112.7, 113.4, 117.0, 119.9, 122.5, 123.9, 125.0, 125.8, 129.4, 138.4, 140.1, 143.6, 149.1, 150.6, 152.9, 154.5. MS (ESI+): m/z = 286.1. ESI-HR-MS calculated for C19H15N3 [MH]+: 286.1339, found: 286.1338.
5-(Thiophen-2-yl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aai)
Yield: 80% (0.116 g from 0.154 g) as a yellow solid, mp 201–204 °C; Rf = 0.35 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, DMSO-d6): δ (ppm) =2.39–2.47 (m, 2H, CH2), 3.42–3.46 (m, 2H, CH2), 4.68 (t, J = 7.3 Hz, 2H, CH2), 6.92 (s, 1H, ArH), 6.99 (t, J = 7.2 Hz, 1H, ArH), 7.35–7.38 (m, 2H, ArH), 7.61 (d, J = 8.1 Hz, 1H, ArH), 7.71 (d, J = 3.2 Hz, 1H, ArH), 7.91 (d, J = 5.0 Hz, 1H, ArH), 7.98 (d, J = 7.8 Hz, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 22.1, 31.7, 51.6, 105.1, 118.3, 119.0, 121.6, 123.0, 126.9, 128.7, 129.0, 129.2, 138.9, 139.5, 148.0, 152.0, 154.1. MS (ESI+): m/z = 291.1. ESI-HR-MS calculated for C18H14N2S [MH]+: 291.0950, found: 291.0953.
5-(Cyclohex-1-en-1-yl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aaj)
Yield: 81% (0.117 g from 0.153 g) as a yellow solid, mp 212–215 °C; Rf = 0.55 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =1.84 (d, J = 4.6 Hz, 2H, CH2), 1.92 (d, J = 5.1 Hz, 2H, CH2), 2.33 (s, 2H, CH2), 2.45–2.48 (m, 4H, 2 × CH2), 3.36 (t, J = 7.4 Hz, 2H, CH2), 4.78 (t, J = 7.2 Hz, 2H, CH2), 6.14 (s, 1H, =CH), 6.60 (s, 1H, ArH), 7.15 (t, J = 7.3 Hz, 1H, ArH), 7.45 (t, J = 7.4 Hz, 1H, ArH), 7.81 (d, J = 7.9 Hz, 1H, ArH), 8.04 (d, J = 7.7 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.2, 22.3, 23.0, 25.5, 28.3, 31.6, 51.2, 104.2, 117.7, 118.9, 119.9, 122.1, 123.2, 126.9, 128.8, 135.7, 146.3, 150.2, 151.6, 152.6. MS (ESI+): m/z = 289.2. ESI-HR-MS calculated for C20H20N2 [MH]+: 289.1699, found: 289.1695.
Methyl 2,3-Dihydro-1H-indolizino[5,6-b]indole-5-carboxylate (5aak)
Yield: 78% (0.104 g from 0.142 g) as a yellow solid, mp 183–186 °C; Rf = 0.30 (EtOAc/MeOH, 9:1, v/v); IR (KBr) νmax: 1729 (CO2Me) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) =2.48–2.55 (m, 2H, CH2), 3.43 (t, J = 7.6 Hz, 2H, CH2), 4.12 (s, 3H, CH3), 4.83 (t, J = 7.3 Hz, 2H, CH2), 7.22–7.26 (m, 2H, ArH), 7.56 (t, J = 7.9 Hz, 1H, ArH), 7.81 (d, J = 7.8 Hz, 1H, ArH), 8.64 (d, J = 8.0 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.2, 31.7, 51.7, 52.9, 102.9, 118.1, 119.8, 122.2, 123.8, 125.5, 129.0, 132.2, 145.8, 152.9, 155.1, 166.8. MS (ESI+): m/z = 267.1. ESI-HR-MS calculated for C16H14N2O2 [MH]+: 267.1128, found: 267.1125.
7-Methyl-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5baa)
Yield: 88% (0.131 g from 0.158 g) as a yellow solid, mp 192–194 °C; Rf = 0.41 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =2.34 (s, 3H, CH3), 2.46–2.54 (m, 2H, CH2), 3.40 (t, J = 7.7 Hz, 2H, CH2), 4.83 (t, J = 7.3 Hz, 2H, CH2), 6.69 (s, 1H, ArH), 7.28 (s, 1H, ArH), 7.41 (s, 1H, ArH), 7.54–7.59 (m, 3H, ArH), 7.67–7.71 (m, 3H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.7, 22.3, 31.7, 51.5, 105.5, 117.3, 120.4, 121.8, 122.9, 128.2, 128.7, 128.8, 128.9, 129.2, 138.6, 146.3, 146.9, 150.7, 151.2. MS (ESI+): m/z = 299.1. ESI-HR-MS calculated for C21H18N2 [MH]+: 299.1543, found: 299.1548.
7-Methoxy-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5caa)
Yield: 92% (0.145 g from 0.166 g) as a yellow solid, mp 212–214 °C; Rf = 0.38 (EtOAc/MeOH, 9:1, v/v); H NMR (400 MHz, CDCl3): δ (ppm) =2.59 (s, 2H, CH2), 3.49 (s, 2H, CH2), 3.64 (s, 3H, OCH3), 5.05 (s, 2H, CH2), 6.87 (s, 1H, ArH), 7.02 (s, 1H, ArH), 7.08 (s, 1H, ArH), 7.57–7.65 (m, 5H, ArH), 7.76 (d, J = 6.0 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.4, 31.8, 53.4, 55.7, 105.1, 108.2, 116.7, 116.9, 119.2, 121.6, 128.6, 128.9, 129.9, 137.3, 148.4, 149.1, 154.1. MS (ESI+): m/z = 315.1. ESI-HR-MS calculated for C21H18N2O [MH]+: 315.1492, found: 315.1493.
7-Bromo-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5daa)
Yield: 83% (0.151 g from 0.191 g) as a yellow solid, mp 196–197 °C; Rf = 0.51 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =2.49–2.57 (m, 2H, CH2), 3.43 (t, J = 7.7 Hz, 2H, CH2), 4.81 (t, J = 7.3 Hz, 2H, CH2), 6.74 (s, 1H, ArH), 7.50 (dd, J = 8.6, 2.0 Hz, 1H, ArH), 7.55–7.61 (m, 3H, ArH), 7.65–7.68 (m, 3H, ArH), 7.73 (d, J = 2.0 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.3, 31.8, 51.3, 105.7, 111.4, 119.5, 119.8, 124.4, 124.7, 128.5, 129.0, 129.6, 129.9, 138.1, 147.1, 147.9, 152.2, 152.3. MS (ESI+): m/z = 363.0. ESI-HR-MS calculated for C20H15BrN2 [MH]+: 363.0491, found: 363.0487.
7-Chloro-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5eaa)
Yield: 84% (0.134 g from 0.168 g) as a yellow solid, mp 238–241 °C; Rf = 0.52 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =2.49–2.57 (m, 2H, CH2), 3.44 (t, J = 7.6 Hz, 2H, CH2), 4.82 (t, J = 7.3 Hz, 2H, CH2), 6.74 (s, 1H, ArH), 7.37 (dd, J = 8.5, 2.0 Hz, 1H, ArH), 7.52–7.61 (m, 4H, ArH), 7.66–7.72 (m, 3H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.2, 31.8, 51.3, 105.6, 118.9, 121.4, 123.8, 124.1, 127.3, 128.5, 129.1, 129.5, 138.2, 147.1, 147.8, 152.1, 152.4. MS (ESI+): m/z = 319.1. ESI-HR-MS calculated for C20H15ClN2 [MH]+: 319.0997, found: 319.0998.
7-Fluoro-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5faa)
Yield: 82% (0.124 g from 0.160 mg) as a yellow solid, mp 194–196 °C; Rf = 0.58 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.50–2.58 (m, 2H, CH2), 3.45 (t, J = 7.7 Hz, 2H, CH2), 4.86 (t, J = 7.2 Hz, 2H, CH2), 6.76 (s, 1H, ArH), 7.16–7.29 (m, 1H, ArH), 7.25–7.28 (m, 1H, ArH), 7.55–7.60 (m, 3H, ArH), 7.65–7.67 (m, 2H, ArH), 7.73 (dd, J = 9.2, 4.8 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.2, 31.8, 51.6, 105.7, 107.4 (d, J = 24.8 Hz), 115.2 (d, J = 24.9 Hz), 118.1 (d, J = 9.0 Hz), 120.3, 122.7 (d, J = 10.1 Hz), 128.5, 129.0, 129.5, 138.0, 147.3, 148.1, 148.8, 151.5, 156.9 (d, J = 233.7 Hz). MS (ESI+): m/z = 303.1. ESI-HR-MS calculated for C20H15FN2 [MH]+: 303.1292, found: 303.1293.
7-Nitro-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5gaa)
Yield: 81% (0.133 g from 0.174 g) as a yellow solid, mp 202–204 °C; Rf = 0.31 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.56–2.64 (m, 2H, CH2), 3.51 (t, J = 7.7 Hz, 2H, CH2), 4.89 (t, J = 7.5 Hz, 2H, CH2), 6.93 (s, 1H, ArH), 7.60–7.76 (m, 6H, ArH), 8.32 (dd, J = 9.0, 2.3 Hz, 1H, ArH), 8.62 (d, J = 2.2 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.2, 31.9, 51.7, 107.4, 117.6, 118.9, 120.5, 122.4, 122.5, 128.5, 129.3, 130.2, 137.3, 139.9, 148.4, 149.2, 154.5, 157.7. MS (ESI+): m/z = 330.1. ESI-HR-MS calculated for C20H15N3O2 [MH]+: 330.1237, found: 330.1232.
5-Phenyl-7-(trifluoromethoxy)-2,3-dihydro-1H-indolizino[5,6-b]indole (5haa)
Yield: 84% (0.155 g from 0.193 g) as a white solid, mp 196–198 °C; Rf = 0.70 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, DMSO-d6): δ (ppm) =2.55–2.63 (m, 2H, CH2), 3.59 (t, J = 7.5 Hz, 2H, CH2), 4.97 (t, J = 6.9 Hz, 2H, CH2), 7.35 (s, 1H, ArH), 7.61 (d, J = 7.6 Hz, 1H, ArH), 7.69–7.76 (m, 6H, ArH), 7.89 (d, J = 8.8 Hz, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 22.3, 32.2, 55.2, 113.4, 114.2, 115.1, 116.2, 120.5, 120.9 (d, J = 256.4), 122.5, 128.7, 129.8, 131.2, 135.9, 138.2, 143.3, 143.6, 151.9, 154.4. MS (ESI+): m/z = 369.1. ESI-HR-MS calculated for C21H15F3N2O [MH]+: 369.1209, found: 369.1206.
9-Bromo-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5iaa)
Yield: 82% (0.149 g from 0.191 g) as a yellow solid, mp 164–167 °C; Rf = 0.54 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.48–2.56 (m, 2H, CH2), 3.44 (t, J = 7.6 Hz, 2H, CH2), 4.92 (t, J = 7.3 Hz, 2H, CH2), 6.76 (s, 1H, ArH), 6.84 (t, J = 7.8 Hz, 1H, ArH), 7.52–7.59 (m, 4H, ArH), 7.62 (dd, J = 7.7, 0.9 Hz, 1H, ArH), 7.65–7.68 (m, 2H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.3, 31.7, 51.3, 105.8, 111.5, 119.2, 120.9, 124.5, 128.5, 128.8, 129.2, 129.6, 138.2, 146.9, 147.9, 151.7, 152.2. MS (ESI+): m/z = 363.0. ESI-HR-MS calculated for C20H15BrN2 [MH]+: 363.0491, found: 363.0493.
5-Phenyl-9-(trifluoromethyl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5jaa)
Yield: 84% (0.148 g from 0.185 g) as a yellow solid, mp 182–184 °C; Rf = 0.73 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.47–2.55 (m, 2H, CH2), 3.43 (t, J = 7.6 Hz, 2H, CH2), 4.92 (t, J = 7.3 Hz, 2H, CH2), 6.78 (s, 1H, ArH), 6.99 (t, J = 7.7 Hz, 1H, ArH), 7.53–7.60 (m, 3H, ArH), 7.66–7.71 (m, 3H, ArH), 7.76 (d, J = 7.8 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.4, 31.8, 51.5, 105.9, 117.1, 118.5 (d, J = 31 Hz), 119.8, 124.0 (q, J = 5.0 Hz), 125.0, 125.4, 126.6, 128.6, 128.9, 129.3, 138.3, 147.2, 147.7, 150.7, 153.1. MS (ESI+): m/z = 353.1. ESI-HR-MS calculated for C21H15F3N2 [MH]+: 353.1260, found: 353.1255.
7,9-Dimethyl-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5kaa)
Yield: 89% (0.139 g from 0.165 g) as a yellow solid, mp 242–245 °C; Rf = 0.40 (EtOAc/MeOH, 9:1, v/v); 1H NMR (400 MHz, CDCl3): δ (ppm) =2.31 (s, 3H, CH3), 2.49–2.52 (m, 2H, CH2), 2.75 (s, 3H, CH3), 3.41 (t, J = 7.5 Hz, 2H, CH2), 4.91 (t, J = 6.3 Hz, 2H, CH2), 6.70 (s, 1H, ArH), 7.11 (s, 1H, ArH), 7.24 (s, 1H, ArH), 7.54–7.58 (m, 3H, ArH), 7.67–7.68 (m, 2H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 18.1, 21.7, 22.5, 51.9, 105.8, 119.3, 120.8, 122.4, 126.3, 128.3, 128.7, 128.8, 129.2, 129.7, 138.6, 146.3, 147.0. MS (ESI+): m/z = 313.2. ESI-HR-MS calculated for C22H20N2 [MH]+: 313.1699, found: 313.1701.
General Experimental Procedure for the One-Pot, Two-Step Synthesis of 2,3-Dihydro-1H-indolizino[5,6-b]indoles 5 as Exemplified for 5aaa
In a round-bottom flask equipped with a water condenser were added isatin 1a (0.147 g, 1.0 mmol), l-proline 2a (0.115 g, 1.0 mmol), phenylacetylene 3a (132 μL, 1.2 mmol), and CuI (0.0095 g, 5 mol %) in 5 mL of acetonitrile under nitrogen. The mixture was heated at reflux temperature for 6 h. After completion of the reaction (as monitored by TLC), the solvent was evaporated under reduced pressure to obtain a crude product. To the crude product, POCl3 (467 μL, 5.0 mmol) was added and the reaction mixture was heated to 90 °C for 24 h under stirring. On completion, the reaction mixture was neutralized by adding saturated NaHCO3 solution and extracted with EtOAc (75 mL × 2). The organic layers were collected, dried over anhydrous Na2SO4, and evaporated to afford the crude product, which was purified by column chromatography over silica gel using EtOAc/MeOH (80:20, v/v) as eluent to obtain 0.199 g (70%) of 5aaa as a yellow solid.
5-Phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5aaa)
Yield: 70% (0.199 g from 0.147 g of 1a) as a yellow solid, mp 257–260 °C.
5-(Cyclohex-1-en-1-yl)-2,3-dihydro-1H-indolizino[5,6-b]indole (5aaj)
Yield: 62% (0.179 g from 0.147 g of 1a) as a yellow solid, mp 212–215 °C.
Methyl 2,3-Dihydro-1H-indolizino[5,6-b]indole-5-carboxylate (5aak)
Yield: 58% (0.154 g from 0.147 g of 1a) as a yellow solid, mp 183–186 °C.
7-Methyl-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5baa)
Yield: 69% (0.206 g from 0.161 g of 1b) as a yellow solid, mp 192–194 °C.
7-Bromo-5-phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole (5daa)
Yield: 63% (0.228 g from 0.226 g of 1d) as a yellow solid, mp 196–197 °C.
General Experimental Procedure for the Synthesis of 1-Substituted-1H-α-carbolines 5
In a round-bottom flask equipped with a water condenser were added isatin 1a (0.147 g, 1.0 mmol), α-amino acid 2 (1.0 mmol), and dipolarophile 3 (1.0 mmol) in 5 mL of methanol, and the mixture was heated at reflux temperature. After completion (as monitored by TLC), the solvent was removed under reduced pressure to obtain the crude product, to which POCl3 (5–10 mmol) was added, and the mixture was heated at 90 °C under stirring. On completion (as assessed by TLC), the reaction mixture was neutralized by adding saturated NaHCO3 solution and extracted with EtOAc (75 mL × 2). The organic layers were combined, dried over anhydrous Na2SO4, and evaporated to furnish the crude material, which was purified by column chromatography over silica gel using EtOAc/MeOH (80:20, v/v) as eluent to afford the appropriate product 5.
Dimethyl 2,3-Dihydro-1H-indolizino[5,6-b]indole-4,5-dicarboxylate (5aam)
Yield: 82% (0.266 g) as a yellow solid, mp 229–232 °C; Rf = 0.49 (EtOAc/MeOH, 9:1, v/v); IR (KBr) νmax: 1714 (CO2Me), 1736 (CO2Me) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.47–2.55 (m, 2H, CH2), 3.79 (t, J = 7.8 Hz, 2H, CH2), 3.93 (s, 3H, CH3), 4.13 (s, 3H, CH3), 4.79 (t, J = 7.6 Hz, 2H, CH2), 7.24–7.27 (m, 1H, ArH), 7.53–7.57 (m, 1H, ArH), 7.80 (d, J = 8.1 Hz, 1H, ArH), 7.85 (d, J = 7.8 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.4, 34.3, 51.8, 52.5, 53.1, 104.2, 118.8, 121.0, 121.6, 121.9, 122.3, 129.1, 134.9, 152.1, 152.2, 154.7, 165.2, 167.6. MS (ESI+): m/z = 325.1. ESI-HR-MS calculated for C18H16N2O4 [MH]+: 325.1183, found: 325.1183.
Diethyl 2,3-Dihydro-1H-indolizino[5,6-b]indole-4,5-dicarboxylate (5aan)
Yield: 80% (0.282 g) as a yellow solid, mp 214–217 °C; Rf = 0.52 (EtOAc/MeOH, 9:1, v/v); IR (KBr) νmax: 1714 (CO2Et), 1736 (CO2Et) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 1.39–1.48 (m, 6H, 2 × CH3), 2.46–2.54 (m, 2H, CH2), 3.80 (t, J = 7.8 Hz, 2H, CH2), 4.37–4.42 (m, 2H, CH2), 4.58–4.64 (m, 2H, CH2), 4.79 (t, J = 7.5 Hz, 2H, CH2), 7.23–7.27 (m, 1H, ArH), 7.53–7.57 (m, 1H, ArH), 7.80 (d, J = 8.1 Hz, 1H, ArH), 7.89 (d, J = 7.8 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 14.2, 14.3, 21.5, 34.3, 51.7, 61.6, 62.3, 104.5, 118.8, 120.8, 121.5, 122.0, 122.5, 128.9, 135.3, 152.1, 154.7, 164.8, 167.1. MS (ESI+): m/z = 353.1. ESI-HR-MS calculated for C20H20N2O4 [MH]+: 353.1496, found: 353.1497.
Ethyl 5-Phenyl-2,3-dihydro-1H-indolizino[5,6-b]indole-4-carboxylate (5aao)
Yield: 79% (0.281 g) as a yellow solid, mp 180–184 °C; Rf = 0.38 (EtOAc/MeOH, 9:1, v/v); IR (KBr) νmax: 1700 (CO2Et) cm–1. 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 0.82 (t, J = 7.1 Hz, 3H, CH3), 2.41–2.48 (m, 2H, CH2), 3.03 (t, J = 7.6 Hz, 2H, CH2), 3.91–3.96 (m, 2H, CH2), 4.73 (t, J = 7.4 Hz, 2H, CH2), 6.77 (d, J = 7.7 Hz, 1H, ArH), 6.85 (t, J = 7.3 Hz, 1H, ArH), 7.32–7.41 (m, 3H, ArH), 7.57–7.65 (m, 4H, ArH); 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 13.7, 21.7, 33.6, 52.1, 60.7, 109.0, 118.4, 119.2, 121.6, 121.8, 124.2, 127.4, 127.8, 128.5, 128.8, 138.3, 145.4, 151.2, 151.8, 154.5, 166.2. MS (ESI+): m/z = 357.2. ESI-HR-MS calculated for C23H20N2O2 [MH]+: 357.1598, found: 357.1599.
Dimethyl 1,3-Dihydrothiazolo[3′,4′:1,6]pyrido[2,3-b]indole-4,5-dicarboxylate (5abm)
Yield: 68% (0.233 g) as a yellow solid, mp 164–166 °C; Rf = 0.51 (hexanes/EtOAc, 1:90, v/v); IR (KBr) νmax: 1703 (CO2Me), 1722 (CO2Me) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.94 (s, 3H, CH3), 4.12 (s, 3H, CH3), 4.96 (s, 2H, CH2), 5.84 (s, 2H, CH2), 7.26–7.31 (m, 1H, ArH), 7.59 (t, J = 7.4 Hz, 1H, ArH), 7.79 (d, J = 7.9 Hz, 1H, ArH), 7.88 (d, J = 7.5 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 37.8, 52.9, 53.2, 54.0, 104.9, 119.0, 121.5, 122.2, 122.3, 122.7, 129.8, 134.2, 148.2, 152.1, 154.9, 164.9, 167.0. MS (ESI+): m/z = 343.1. ESI-HR-MS calculated for C17H14N2O4S [MH]+: 343.0747, found: 343.0744.
Dimethyl 1-Methyl-1H-pyrido[2,3-b]indole-3,4-dicarboxylate (5aem)
Yield: 77% (0.229 g) as a yellow solid, mp 201–203 °C; Rf = 0.62 (EtOAc/MeOH, 9:1, v/v); IR (KBr) νmax: 1709 (CO2Me), 1739 (CO2Me) cm–1. 1H NMR (500 MHz, CDCl3): δ (ppm) = 3.93 (s, 3H, CH3), 4.15 (s, 3H, CH3), 4.31 (s, 3H, CH3), 7.26–7.29 (m, 1H, ArH), 7.59 (t, J = 7.2 Hz, 1H, ArH), 7.82 (d, J = 8.1 Hz, 1H, ArH), 7.88 (d, J = 7.8 Hz, 1H, ArH), 8.53 (s, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 40.8, 52.5, 53.2, 107.2, 118.9, 121.1, 122.0, 122.2, 123.0, 129.6, 133.4, 138.3, 153.7, 154.5, 164.2, 166.9. MS (ESI+): m/z = 299.1. ESI-HR-MS calculated for C16H14N2O4 [MH]+: 299.1026, found: 299.1024.
8,9-Dihydro-7H-benzo[g]indolo[2,3-c]pyrrolo[2,1-a]isoquinoline-10,15-dione (5aar)
Yield: 74% (0.250 g) as a dark brown solid, mp 278–282 °C; Rf = 0.29 (EtOAc/MeOH, 9:1, v/v); IR (KBr) νmax: 1653 (C=O), 1675 (C=O) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.51–2.59 (m, 2H, CH2), 3.98 (t, J = 7.9 Hz, 2H, CH2), 4.79 (t, J = 7.9 Hz, 2H, CH2), 7.33 (t, J = 8.1 Hz, 1H, ArH), 7.60 (t, J = 7.2 Hz, 1H, ArH), 7.72 (d, J = 7.9 Hz, 1H, ArH), 7.79–7.84 (m, 2H, ArH), 8.27–8.29 (m, 1H, ArH), 8.33–8.35 (m, 1H, ArH), 9.28 (d, J = 8.1 Hz, 1H, ArH); 13C NMR = the compound is too insoluble. MS (ESI+): m/z = 339.1. ESI-HR-MS calculated for C22H14N2O2 [MH]+: 339.1128, found: 339.1124.
1-(4-Phenyl-2,3-dihydro-1H-indolizino[5,6-b]indol-5-yl)ethanone (5aas)
Yield: 70% (0.228 g) as a yellow solid, mp 214–217 °C; Rf = 0.39 (EtOAc/MeOH, 9:1, v/v); IR (KBr) νmax: 1675 (COMe) cm–1. 1H NMR (500 MHz, CDCl3): δ (ppm) = 2.18 (s, 3H, COCH3), 2.47 (t, J = 7.1 Hz, 2H, CH2), 3.30 (t, J = 7.3 Hz, 2H, CH2), 4.91 (t, J = 6.9 Hz, 2H, CH2), 7.18 (t, J = 7.2 Hz, 1H, ArH), 7.35 (d, J = 6.8 Hz, 2H, ArH), 7.43–7.53 (m, 4H, ArH), 7.81 (t, J = 10.1 Hz, 2H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 22.1, 31.1, 31.8, 52.1, 114.1, 118.1, 119.6, 121.6, 128.2, 128.3, 129.0, 130.2, 135.3, 143.9, 145.3, 151.4, 154.0, 203.6. MS (ESI+): m/z = 327.1. ESI-HR-MS calculated for C22H18N2O [MH]+: 327.1492, found: 327.1493.
2,3-Dihydro-1H-indolizino[5,6-b]indole-5-carbonitrile (5aat)
Yield: 73% (0.170 g) as a red solid, mp 260–264 °C; Rf = 0.37 (EtOAc/MeOH, 9:1, v/v); IR (KBr) νmax: 2357 (CN) cm–1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.40–2.47 (m, 2H, CH2), 3.30 (t, J = 7.7 Hz, 2H, CH2), 4.68 (t, J = 7.4 Hz, 2H, CH2), 6.70 (s, 1H, ArH), 7.27–7.31 (m, 1H, ArH), 7.56–7.60 (m, 1H, ArH), 7.76 (d, J = 8.2 Hz, 1H, ArH), 8.33 (d, J = 7.8 Hz, 1H, ArH); 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.9, 31.5, 51.5, 102.8, 110.8, 116.2, 118.4, 120.3, 121.3, 122.1, 127.0, 129.9, 146.5, 151.1, 155.0. MS (ESI+): m/z = 234.1. ESI-HR-MS calculated for C15H11N3 [MH]+: 234.1026, found: 234.1028.
Acknowledgments
S.K., D.S.B., and A.G. acknowledge the financial assistance in the form of fellowship from CSIR, New Delhi. The funds for this work were provided by SERB, New Delhi, under the project EMR/2016/002162. The authors acknowledge the SAIF, CDRI, for providing the spectroscopic details. They thank Dr Tejender S. Thakur of Molecular and Structural Biology Division, CSIR-Central Drug Research Institute, for supervising the X-ray data collection and structure determination of the compound reported in this paper. The CDRI communication number for the manuscript is 9820.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00396.
Author Present Address
∥ Chemistry Department, Centre for Biomedical Research, SGPGI Campus, Lucknow (V.J. was Project fellow under the project from June-2017 to Dec-2017).
The authors declare no competing financial interest.
Supplementary Material
References
- a Cimanga K.; Bruyne T. D.; Pieters L.; Vlietinck A. J.; Turger C. A. In Vitro and in Vivo Antiplasmodial Activity of Cryptolepine and Related Alkaloids from Cryptolepis sanguinolenta. J. Nat. Prod. 1997, 60, 688–691. 10.1021/np9605246. [DOI] [PubMed] [Google Scholar]; b Kim J.-S.; Shin-ya K.; Furihata K.; Hayakawa Y.; Seto H. Structure of Mescengricin, A Novel Neuronal Cell Protecting Substance Produced by Streptomyces griseoflavus. Tetrahedron Lett. 1997, 38, 3431–3434. 10.1016/S0040-4039(97)00638-2. [DOI] [Google Scholar]; c Moquin-Pattey C.; Guyot M. Grossularine-1 and Grossularine-2, Cytotoxic α-Carbolines from the Tunicate: Dendrodoa grossularia. Tetrahedron 1989, 45, 3445–3450. 10.1016/S0040-4020(01)81023-1. [DOI] [Google Scholar]
- a Sidoryk K.; Switalska M.; Jaromin A.; Cmoch P.; Bujak I.; Kaczmarska M.; Wietrzyk J.; Dominguez E. G.; Zarnowski R.; Andes D. R.; Bankowski K.; Cybulski M.; Kaczmarek L. The Synthesis of Indolo[2,3-b]quinoline Derivatives with a Guanidine Group: Highly Selective Cytotoxic Agents. Eur. J. Med. Chem. 2015, 105, 208–219. 10.1016/j.ejmech.2015.10.022. [DOI] [PubMed] [Google Scholar]; b Saundane A. R.; Kalpana R. Synthesis and Biological Evaluation of Novel Indolo[2,3-c]isoquinoline Derivatives. Med. Chem. Res. 2015, 24, 1681–1695. 10.1007/s00044-014-1243-2. [DOI] [Google Scholar]; c Lu W.-J.; Wicht K. J.; Wang L.; Imai K.; Mei Z.-W.; Kaiser M.; Sayed I. E. T. E.; Egan T. J.; Inokuchi T. Synthesis and Antimalarial Testing of Neocryptolepine Analogues: Addition of Ester Function in SAR Study of 2,11-disubstituted Indolo[2,3-b]quinolones. Eur. J. Med. Chem. 2013, 64, 498–511. 10.1016/j.ejmech.2013.03.072. [DOI] [PubMed] [Google Scholar]; d Ueshima K.; Akihisa-Umeno H.; Nagayoshi A.; Takakura S.; Matsuo M.; Mutoh S. Implitapide, a Microsomal Triglyceride Transfer Protein Inhibitor, Reduces Progression of Atherosclerosis in Apolipoprotein E Knockout Mice Fed a Western-Type Diet: Involvement of the Inhibition of Postprandial Triglyceride Elevation. Biol. Pharm. Bull. 2005, 28, 247–252. 10.1248/bpb.28.247. [DOI] [PubMed] [Google Scholar]; e Chen Y.-L.; Hung H.-M.; Lu C.-M.; Li K.-C.; Tzeng C.-C. Synthesis and Anticancer Evaluation of Certain Indolo[2,3-b]quinoline Derivatives. Bioorg. Med. Chem. 2004, 12, 6539–6546. 10.1016/j.bmc.2004.09.025. [DOI] [PubMed] [Google Scholar]; f Hiremath S. P.; Rudresh K.; Saundane A. R. Synthesis and Biological Activities of New 5-hydrazino-10-substituted-7H-Indolo[2,3-c]isoquinolines and 1-(10-substituted-7H-indolo[2,3-c]isoquinolin-5-yl)-3,5-disubstituted Pyrazoles, -3-methyl Pyrazol-5-ones and -3,5-disubstututed Pyrazolines. Ind. J. Chem. 2002, 41B, 394–399. [Google Scholar]; g Bolton D.; Forbes I. T.; Hayward C. J.; Piper D. C.; Thomas D. R.; Thompson M.; Upton N. Synthesis and Potential Anxiolytic Activity of 4-Amino-pyrido[2,3-b]indoles. Bioorg. Med. Chem. Lett. 1993, 3, 1941–1946. 10.1016/S0960-894X(01)80991-4. [DOI] [Google Scholar]
- Wadsworth A. D.; Naysmith B. J.; Brimble M. A. A Review of the Synthesis of α-Carbolines. Eur. J. Med. Chem. 2015, 97, 816–829. 10.1016/j.ejmech.2014.11.038. [DOI] [PubMed] [Google Scholar]
- Some recent citations for the synthesis of α-crbolines; a He L.; Allwein S. P.; Dugan B.; Knouse K. W.; Ott G. R.; Zificsak C. A.; Kou K. Synthesis of α-Carboline. Org. Synth. 2016, 93, 272–285. 10.15227/orgsyn.093.0272. [DOI] [Google Scholar]; b Yu S.; Li Y.; Zhou X.; Wang H.; Kong L.; Li X. Access to Structurally Diverse Quinoline-Fused Heterocycles via Rhodium(III)-Catalyzed C-C/C-N Coupling of Bifunctional Substrates. Org. Lett. 2016, 18, 2812–2815. 10.1021/acs.orglett.6b01032. [DOI] [PubMed] [Google Scholar]; c Wang G.; You X.; Gan Y.; Liu Y. Synthesis of δ- and α-Carbolines via Nickel-Catalyzed [2 + 2 +2] Cycloaddition of Functionalized Alkyne-Nitriles with Alkynes. Org. Lett. 2017, 19, 110–113. 10.1021/acs.orglett.6b03385. [DOI] [PubMed] [Google Scholar]; d Das S. K.; Roy S.; Khatua H.; Chattopadhyay B. Ir-Catalyzed Intramolecular Transannulation/C(sp2)-H Amination of 1,2,3,4-Tetrazoles by Electrocyclization. J. Am. Chem. Soc. 2018, 140, 8429–8433. 10.1021/jacs.8b05343. [DOI] [PubMed] [Google Scholar]
- Bracca A. B. J.; Heredia D. A.; Larghi E. L.; Kaufman T. S. Neocryptolepine (Cryprotackieine), A Unique Bioactive Natural Product: Isolation, Synthesis, and Profile of Its Biological Activity. Eur. J. Org. Chem. 2014, 7979–8003. 10.1002/ejoc.201402910. [DOI] [Google Scholar]
- Borad M. A.; Bhoi M. N.; Prajapati N. P.; Patel H. D. Review of Synthesis of Spiro Heterocyclic Compounds from Isatin. Synth. Commun. 2014, 44, 897–922. 10.1080/00397911.2013.843196. [DOI] [Google Scholar]
- Black D. S.; Ivory A. J.; Kumar N. Reactivity of 3-Substituted Indolin-2-ones in Vilsmeier-type Reactions of 4,6- Dimethoxyindoles. Tetrahedron 1996, 52, 7003–7012. 10.1016/0040-4020(96)00305-5. [DOI] [Google Scholar]
- a Deng X.; Roessler A.; Brdar I.; Faessler R.; Wu J.; Sales Z. S.; Mani N. S. Direct, Metal-Free Amination of Heterocyclic Amides/Ureas with NH-Heterocycles and N-Substituted Anilines in POCl3. J. Org. Chem. 2011, 76, 8262–8269. 10.1021/jo201425q. [DOI] [PubMed] [Google Scholar]; b Kamal A.; Ramakrishna G.; Raju P.; Rao A. V. S.; Viswanath A.; Nayak V. L.; Ramakrishna S. Synthesis and Anticancer Activity of Oxindole Derived Imidazo[1,5-a]pyrazines. Eur. J. Med. Chem. 2011, 46, 2427–2435. 10.1016/j.ejmech.2011.03.027. [DOI] [PubMed] [Google Scholar]; c Schneller S. W.; Bartholomew D. G. Preparation of 5-substituted- and 3,5-disubstituted-s-triazolo[4,3-a]pyridines. J. Heterocycl. Chem. 1978, 15, 439–444. 10.1002/jhet.5570150315. [DOI] [Google Scholar]
- Wang F.; Li X.-C.; Lai W.-Y.; Chen Y.; Huang W.; Wudl F. Synthesis and Characterization of Symmetric Cyclooctatetraindoles: Exploring the Potential as Electron-Rich Skeletons with Extended π-Systems. Org. Lett. 2014, 16, 2942–2945. 10.1021/ol501083d. [DOI] [PubMed] [Google Scholar]
- a Singh S. N.; Regati S.; Paul A. K.; Layek M.; Jayaprakash S.; Reddy K. V.; Deora G. S.; Mukherjee S.; Pal M. Cu-mediated 1,3-Dipolar Cycloaddition of Azomethine Ylides with Dipolarophiles: A Faster Access to Spirooxindoles of Potential Pharmacological Interest. Tetrahedron Lett. 2013, 54, 5448–5452. 10.1016/j.tetlet.2013.07.126. [DOI] [Google Scholar]; b Pardasani R. T.; Pardasani P.; Chaturvedi V.; Yadav S. K.; Saxena A.; Sharma I. Theoretical and Synthetic Approach to Novel Spiroheterocycles Derived from Isatin Derivatives and L-Proline via 1,3-Dipolar Cycloaddition. Heteroat. Chem. 2003, 14, 36–41. 10.1002/hc.10063. [DOI] [Google Scholar]
- a Mali P. R.; Shirsat P. K.; Khomane N.; Nayak L.; Nanubolu J. B.; Meshram H. M. 1,3-Dipolar Cycloaddition Reactions for the Synthesis of Novel Oxindole Derivatives and Their Cytotoxic Properties. ACS Comb. Sci. 2017, 19, 633–639. 10.1021/acscombsci.7b00044. [DOI] [PubMed] [Google Scholar]; b Shi G.; He X.; Shang Y.; Xie M. Combinatorial Synthesis of Spiro[indoline-3,2′-pyrrole] Derivatives via a Three-component Reaction under Catalyst-free Conditions. RSC Adv. 2016, 6, 10412–10418. 10.1039/C5RA23860A. [DOI] [Google Scholar]; c Yang F.; Sun J.; Gao H.; Yan C.-G. Unprecedented Formation of Spiro[indoline-3,7′-pyrrolo[1,2-a]azepine] from Multicomponent Reaction of L-proline, Isatin and But-2-ynedioate. RSC Adv. 2015, 5, 32786–32794. 10.1039/C5RA04102C. [DOI] [Google Scholar]
- Klochkova I. N.; Shchekina M. P.; Anis’kov A. A. Synthesis of Spiropyrrolidines and Spiropyrrolizidines from Azomethine Ylides. Chem. Heterocycl. Compd. 2014, 50, 479–488. 10.1007/s10593-014-1498-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.