Abstract
Objective
Retinal prosthetic implants restore partial vision to patients blind due to outer retinal degeneration, using a camera-guided multielectrode array (MEA) that electrically stimulates surviving retinal neurons. Commercial epi-retinal prostheses use millisecond-scale charge-balanced, symmetric, cathodic-first biphasic pulses to depolarize retinal ganglion cells (RGCs) and bipolar cells (BCs), frequently creating oblong perceptions of light related to axonal activation of RGCs. Stimulation strategies that avoid axonal stimulation and decrease the threshold of targeted neurons may significantly improve prosthetic vision in terms of spatial resolution and power efficiency.
Approach
We developed a virus-transduced genetically encoded calcium indicator (GECI) GCaMP6f and microscopy platform for calcium imaging to record the neural activity from RGCs at single-cell resolution in wholemount retinas. Multiple stimulation paradigms were applied through a microelectrode array (MEA) with transparent indium tin oxide electrodes. The evoked neuronal activities were converted to corresponding 2-D calcium imaging transient pattern and spatial threshold map to identify the ideal focal response which corresponds to optimal percept in patient.
Main results
The proposed optical system with GCaMP6f is capable of recording from population of mouse RGCs in real time during electrical stimulation with precise location information relative to the stimulation sites. Optimal duration and phase order of pulse were identified to avoid axonal stimulation and selectively activate targeted RGC somas, without requiring a significant increase in stimulation charge. Additionally, we show that reduced stimulus threshold can be achieved with the special design of asymmetric anodic-first pulse.
Significance
Our findings support the possibility of manipulating the responses of RGCs through varying the stimulation waveform. Focal response can be achieved with relative short duration (≤120 μs) pulses, and can be improved by reversing the standard phase order. The RGCs threshold can be significantly reduced by 33.3%-50% in terms of charge through applying hyperpolarizing pre-pulses with a 20:1 ratio (pre-pulse:stimulus pulse). The results support the future retinal prosthesis design that potentially forms more ideal shape perception with higher spatial resolution and power efficiency.
1. Introduction
Retinitis pigmentosa (RP) and dry age-related macular degeneration (AMD) (1) are prevalent degenerative diseases of retina that lead to significant visual impairment or blindness. Prior clinical testing demonstrated that applying electrical stimulation to a degenerated retina can elicit visual percepts (2). Based on these findings, several retinal prostheses have been developed, and three systems now have regulatory approval. Long-term human testing studies of patients with these devices show improved object discrimination and localization, mobility, and, in some patients, the ability to recognize simple patterns such as letters (3, 4). The best reported visual acuity for any retinal prosthesis is 20/546 (5). With the Argus II epiretinal implants, although subjects are capable of reading only large letters and at a rate slower than normal and performing the most basic of activities of daily living, the improvements significantly increase the quality of life in these RP subjects who had demonstrated minimal or no light perception prior to implementation (6).
One of the most critical challenges of retinal implants is to achieve ideal shape perception. That is, each electrode should activate only nearby retinal cells, thus forming a single small, round visual percept. By combining multiple percepts each generated by a different electrode, perception of complex shapes such as a letter is possible. However, clinical studies of patients with Argus II epiretinal implants reported that most percepts evoked by single electrodes were elongated and aligned with the expected pathway of the axons of retinal ganglion cells (RGCs), suggesting the activation of axon bundles (7). Some reports of complex shape perception exist, but, in general, patients do not accurately report the perception of shape when multiple electrodes are used in a pattern. Spatial responses consistent with axonal stimulation have also been measured using calcium imaging techniques and in vitro rodent retina (8). Stimulation strategies that avoid axonal stimulation and decrease the threshold of targeted neurons may significantly improve prosthetic vision in terms of spatial resolution and power efficiency.
RGCs are a main target of retinal prosthetic research, since visual percepts may reflect the activation pattern of RGCs. RGCs can be activated directly upon sufficient depolarization of the RGC membrane or indirectly via synaptic transmission from activated bipolar cells (BC) (9–12). Direct stimulation has the advantage of precise control of retinal output and superior temporal resolution. Advantages of indirect stimulation include the retention of the neural processing that occurs at the inner plexiform layer and avoiding direct activation of axons of passage. Studies have shown the BCs tend to respond preferentially to stimuli having longer pulse width (25 Hz sinusoidal) (13), and, indeed, highly localized responses of RGCs result from indirect stimulation achieved with 25-ms duration pulses (14). However, RGC responses to continuous indirect stimulation undergo desensitization – arguing against this approach. It is well documented that, depending on the stimulation frequency, responsiveness of RGCs progressively decreases with the repeated indirect stimulation (15, 16). This desensitization in the cellular response is observed in multiple animal models through electrical recording and is believed to be one of the causes of percept fading reported by patients (17, 18). Therefore, direct stimulation of RGC cell bodies, which are more (vs. axons) easily excited by short duration pulse (≤150 μs) (11) and are less prone to desensitization when directly activated, is worth further investigation, to increase temporal resolution of prosthetic vision and alleviate phosphene fading in response to continuous stimulation.
We developed a virus-transduced genetically encoded calcium indicator (GECI) GCaMP6f and performed calcium imaging to record the neural activity from RGCs at single-cell resolution in wholemount retinas while applying stimulation through a microelectrode array (MEA) with transparent indium tin oxide electrodes, using methods similar to those used in our prior studies (14, 19). The results suggest that the electrical stimulation thresholds and spatial response of RGCs can be manipulated through modifying the duration, phase, and profile of pulses to confine excitation of RGCs near the active electrode and to achieve reduction of RGC threshold.
2. Methods
2.1. Overview
Adult mice (C57BL6/J) received an intravitreal injection of an adeno-associated virus (AAV) vector encoding a GECI (AAV2-CAG-GCaMP6f) two to four weeks prior to be being sacrificed for calcium imaging experiments. Based on the established in vitro calcium imaging and electrophysiological mouse animal model, we tested different stimulation paradigms to identify patterns that avoid axonal stimulation, and to manipulate the thresholds in response to continuous stimulation. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and the Institutional Biosafety Committee (IBC) at the University of Southern California and University of Michigan, Ann Arbor.
2.2. Animal
For preliminary studies, wildtype (WT) C57BL6/J mice (aged between 1-2 months at the time of virus injection, and 2-3 months at the time of calcium imaging) purchased from Jackson Lab were used to perform calcium imaging experiments, aimed at identifying stimulation parameters that selectively activate RGCs near the stimulating electrode. We then performed studies of retinal degeneration (RD) B6.CXB1-Pde6brd10/J mice (aged between 2-3 months at the time of virus injection, and 3-4 months at the time of calcium imaging). The selected strain line has mutations in phosphodiesterase (PDE) that are associated with some types of RP and night blindness. Although similar to rd1, the rd10 phenotype has a later onset and delayed retinal degeneration which provide a better experimental model for RP with full development of retina and extra time window for viral vector expression in our study (20).
2.3. Virus-transduced Calcium Indicator
Recombinant AAV2-CAG-GCaMP6f was constructed with a pGFP plasmid where the original GFP sequence was removed and replaced with the GECI GCaMP6f, following a procedure similar to that used for another plasmid used in our lab with GCaMP5G (8). Before GCaMP6f cDNA, the CMV enhancer, chicken β-actin promoter (CBA promoter), exon, and intron were collectively used to form a ubiquitously strong CAG promoter. To enhance protein translation, woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) was placed downstream of the transgene. The entire cassette was flanked by AAV2 inverted terminal repeats. Recombinant AAV vector was produced at the University of Florida Vector Core by the two-plasmid co-transfection method (8). Final concentration of AAV2-CAG-GCaMP6f was 2.6 × 1012 vector genomes per milliliter (vg/ml). To limit the viral expression and cytomorbidity, viral stock was diluted to 1.04 × 1012 vg/ml with balanced salt solution before injection. With this scale of concentration, a previous study with older-generation AAV2-CAG-GCaMP5G showed that the same type of virus has high specificity with rodent RGCs after intravitreal injection (~85%) (8).
2.4. Intravitreal AAV Injection
Animals were anesthetized with intraperitoneal injection of a mixture of ketamine (80mg/kg) and xylazine (10mg/kg). The pupil was dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride. Topical tetracaine hydrochloride was applied for local corneal anesthesia. For intravitreal injection, a pilot hole near the cornea (0.5-1 mm posterior to corneal limbus) was first made using a 30-gauge needle through sclera, choroid, and retina. A 5 μL microliter syringe (Hamilton Robotics, Reno, NV) with a blunt 32-gauge needle was inserted through the pilot hole and 1μL of AAV2-CAG-GCaMP6f was slowly injected into the vitreous on top of retina over a roughly 30-seccond period. After injection, the needle remained in place for another 30 seconds and was withdrawn slowly (to prevent leakage). Antibiotic eye ointment was applied at the end to help the wound seal and prevent infection.
2.5. Calcium Imaging
Virus-transduced mice were euthanized at 3-4 weeks post injection, which was determined by a customized fundus imaging system to be the best time window for optimal GCaMP6f expression in RGCs (21). After anesthesia with ketamine/xylazine, the mice were rapidly decapitated and the treated eye was enucleated and hemisected with Vannas spring scissors. To flatten the retina while still attached the posterior eyecup, 4 cuts were made, from periphery to center, to create quadrants of near-equal size. Vitreous was gently peeled from the retina surface with fine forceps to allow for a tight interface between the retina and MEA. After removal from the eye cup, the retina was mounted on a porous membrane (cat. No. JVWP01300; Millipore) held by titanium ring and placed on the transparent MEA chamber with ganglion cell side facing down. The wholemount retina was incubated and imaged using a customized microscopic platform (Olympus, Center Valley, PA) as illustrated in Figure 1. Fluorescence excitation was provided by a super bright cool white light-emitting diode (LED) via the inverted optical path. Excitation and emission light were filtered through a commercial filter set (49002 - ET - EGFP (FITC/Cy2), Chroma Technology Corp, Bellows Falls, VT) for GCaMP6f. Images were viewed through an Olympus (Center Valley, PA) UPLFLN 0.3-numerical aperture (NA) ×10 objective and captured by an electron-multiplied charge-coupled device (EMCCD) camera. (iXon 897, Andor Technology, Belfast, Northern Ireland) at 10Hz (75% exposure duty cycle). For superfusion, the bicarbonate-buffered Ames’ Medium (Sigma-Aldrich, St. Louis, MO) was used in all procedures. Media was supplemented with penicillin-streptomycin to prevent bacterial growth, equilibrated with 5% CO2 - 95% O2 gas, and adjusted to pH 7.4 and 280 mOsm. During the course of each experiment, the retina was continuously superfused at a flow rate of 4-5 ml/min and a temperature of 33 °C.
Figure 1:
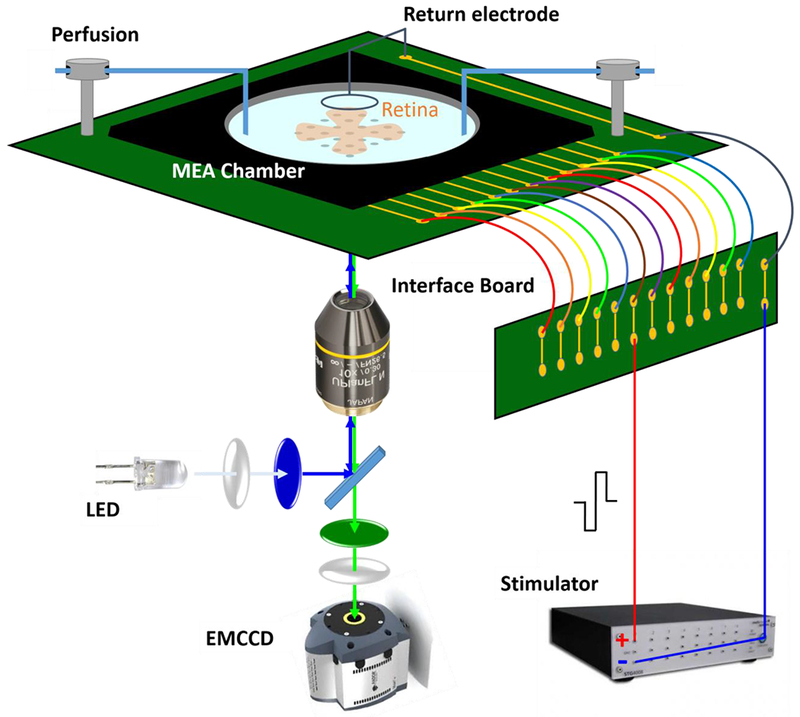
Schematic of the electrical stimulation and calcium imaging microscopic platform. Recording chamber contains a transparent microelectrode array that delivers current-controlled electrical stimulation. A platinum wire encircling the top of the recording chamber served as the current return electrode. A customized interface board set was used to hold the recording chamber and relay the stimulation signals to designated electrode. An inverted microscope equipped with an EMCCD camera and FITC filter set is located below the recording chamber for imaging acquisition. The system is not drawn to scale.
2.6. Electrical Stimulation
Transparent MEAs were fabricated in the W. M. Keck Photonics Laboratory at USC, a class 100 cleanroom. Arrays were patterned by selective etching of indium tin oxide (ITO) on no. 1 cover glass substrates (Vaculayer, Mississauga, Ontario, Canada). A dual-insulation layer, including SU-8 epoxy photoresist and silicon nitride, was formed atop the ITO. The insulation layer was removed to create 200-μm diameter disk electrodes, in a 6×10 pattern with 500-μm electrode pitch. These dimensions are within the range of microelectrode arrays used in present-day retinal prostheses.
Electrical stimuli consisted of charge balanced, biphasic, current pulse waveform. Current stimuli were generated from a computer-controlled stimulus generator (STG-4008 – 1.6mA, Multi Channel Systems, Reutlingen, Germany) and fed through a custom capacitative isolation circuitry to prevent DC leak current. A custom interface circuit board was used to relay the signal to the designated electrode (Figure 1). A platinum wire encircling the top of the recording chamber served as the current return electrode (submerged in the perfusion solution).
Three types of rectangular pulses, including symmetric cathodic-first, symmetric anodic-first, and asymmetric anodic-first were applied to the retina, with individual pulse durations from 40 μs to 4 ms. The asymmetric biphasic pulse consisted of an anodic first phase of relatively long duration and low amplitude and a cathodic second phase of short duration and high amplitude. Table 1 provides the pulse width selection of cathodic phase for each waveforms. The ratio is computed by dividing the longer phase width by the shorter phase width. For each waveform and duration, stimulation protocols were delivered as a repetitive pulse train at 120Hz with current amplitude progressively increasing from subthreshold to suprathreshold to evoke a burst of spikes and generate a detectable calcium transient (8). A total of 10 amplitudes were used, each lasting 5 seconds with 20-second intervals between to allow the calcium transient to return back to baseline. For each type of pulse, we repeated the monotonically increasing protocol more than one time to make sure the response is consistent and reproducible. No stimuli were delivered during the first and last 5 seconds of each protocol. The overall stimulation protocol is shown in Figure 2.
Table 1:
Electrical stimulation waveform parameters.
| Symmetric | Asymmetric (anodic-first) | |||||
|---|---|---|---|---|---|---|
| Cathodic-first | Anodic-first | 20 time ratio | 10 time ratio | 5 time ratio | 2 time ratio | |
| 40 μs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 80 μs | ✓ | ✓ | ✓ | |||
| 120 μs | ✓ | ✓ | ||||
| 0.5 ms | ✓ | ✓ | ||||
| 1 ms | ✓ | |||||
| 4 ms | ✓ | |||||
Figure 2:
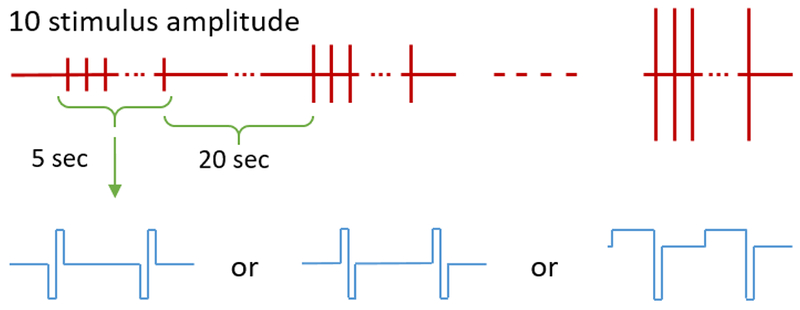
Stimulation protocol. Stimuli were delivered 10 times for 5-second durations with monotonically increasing amplitudes and 20-second resting intervals between. Each stimulus was a burst of rectangular pulses with symmetric cathodic-first, symmetric anodic-first, or asymmetric anodic-first pulse paradigms designed to evoke a burst of spikes and generate a detectable calcium transient.
2.7. Spatial Threshold Mapping and Analysis
For each stimulation paradigm, the fluorescence images near an active electrode are recorded at 10 fps. Images during stimulation (2-3 seconds after pulse train initiation) were extracted and the baseline image was subtracted (baseline obtained 1-2 second before stimulation initiation), so the calcium transient of responsive RGCs can be detected. For each pixel, the obtained transient (ΔF) was further normalized with respect to baseline (F) to calculate the normalized 2-D calcium imaging transient pattern (ΔF/F). With proper threshold selection to remove noise (> 15%), processed spatial and temporal information for individual stimuli in the region of interest (ROI) forms the 2-D spatial threshold maps, which show the current amplitude (equivalent to delivered charge when multiplied with pulse duration) required for each pixel in the image to reach 15% ΔF/F. Pixels that did not reach this level at any point during the analysis time were set to zero.
The 2-D spatial threshold maps were used to assess whether soma or axons were activated. Activated pixels were associated with somatic activation and axonal activation respectively, depending on the relative distance to the center of active electrode and the known axon path. We assigned the activated pixels directly above the active electrode to the somatic activation region whereas those occurring outside two times the radius of electrode where assigned to the axonal activation region. For those activated pixels located between, we did not assign them to either group, given the uncertainty of classification.
The average threshold Ithresh within each region, for each pulse width (tPW), was fitted using a decaying exponential model in (equation (1)) with decay time constant τ to establish the strength-duration curves for targeted regions (22–24). Average threshold Ithresh was calculated using all activated pixels in a region.
| (1) |
Parameters of fits were optimized to minimize the sum of squared error. A pixel based analysis uses samples that are not independent, since multiple adjacent pixels may represent the same cell. We also analyzed threshold of the somatic and axonal regions by estimating cell threshold, although cells overlap complicated this approach. Threshold was computed for cells according to calcium transient within the defined cell boundary, typically a circle between 10-20 microns in diameter. Activated pixels at the mean thresholds were summed for the somatic and axonal regions to allow computation of the relative percentage of active pixels in each region for varying pulse shapes.
We performed the two-dimensional correlation analysis of response patterns (normalized 2-D calcium imaging transient pattern (ΔF/F)) elicited by different stimulation waveforms). Response patterns evoked by symmetric cathodic-first pulses at a single amplitude served as baseline data (array A). Baseline response patterns were chosen that were suprathreshold and with focal pattern, but not at saturation (i.e. not all cells responded at the chosen current amplitude, but more cells were activated if amplitude was increased). The baseline response pattern was correlated with response patterns evoked by asymmetric anodic-first pulses with varying amplitudes (Bk, k represents the index of array with different amplitudes) (Figure 3). The correlation coefficient rk can be computed using equation (2):
| (2) |
where m, n denote the index of element in array, and denote the mean of elements respectively. The correlation coefficient served as a measure of response similarity and allowed assessment of relative efficiency of pulse shapes.
Figure 3:
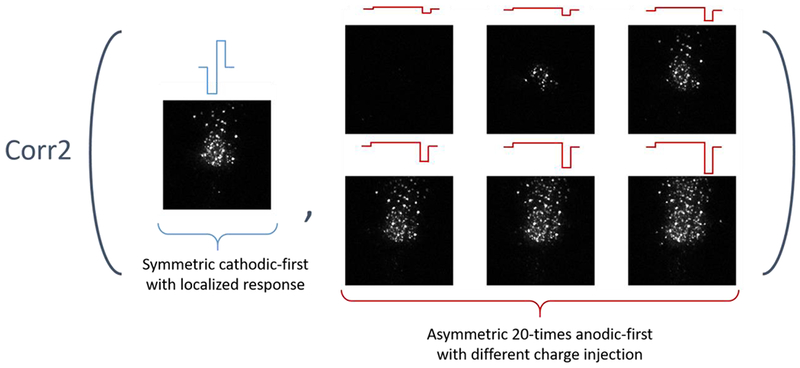
Illustration of correlation tests between responses. Example of correlations of response patterns between 20 times ratio asymmetric anodic-first pulses with different delivered charges and control symmetric cathodic-first pulses with at a suprathreshold (in this case 60 μA, 40 μs /phase) that generally leads to focal response.
All post-processing steps and analysis of calcium imaging were performed using MATLAB (The Mathworks, Natick, MA).
3. Results
3.1. GCaMP Expression Profile
Representative retinal whole mounts for WT and RD mice are shown in Figure 4. For WT retina, the GCaMP expression, evident as green fluorescence, is generally abundant across the four quadrants. Typically, the baseline fluorescence intensity within ganglion cells bodies was greater than that in their axons, so calcium transient dynamics of the somas can be observed through the transparent superficial nerve fiber layer. The brightest region in the middle of retina represents the convergence of axon bundles to form the optic nerve. Due to the dominating fluorescence of converging axons, individual RGCs could not be resolved so data was not collected from the middle region. Compared with WT retina, the RD retina has fewer RGCs with GCaMP expression and exhibits a less uniform distribution of transfected cells. Patchy dark regions, with few fluorescent RGCs, were evident. (Figure 4 (B)). The decreased fluorescence might be attributed to deterioration of RGC layer in the RD model. Experiments also showed that RGC responses were confined to the lighter regions. Using retina within the optimum time frame of 3-4 weeks post injection resulted in fluorescence predominantly localized to cell cytoplasm without major overexpression and cytomorbidity issues.
Figure 4:
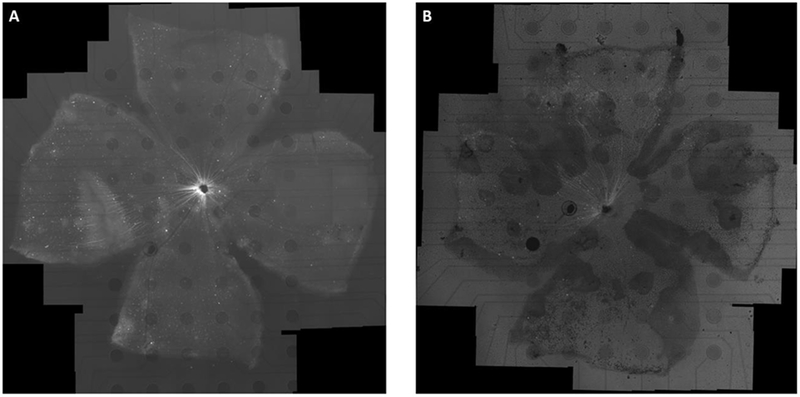
Retinal whole mounts of a WT (A) and RD (B) adult mice transduced with AAV2-CAG-GCaMP6f (3 weeks post injection). The retina was mounted on a 6 × 10 rectangular MEA with a homemade retaining ring and imaged with an inverted fluorescence microscope. The baseline fluorescent image shows that GCaMP6f indicators are well expressed throughout the ganglion cell layer (GCL) for WT and most regions (with lighter but frosted texture background) for RD. The mosaic was created by stitching together 101 and 78 × 10 images respectively. The dark circles are 200μm-diameter indium tin oxide (ITO) electrodes.
3.2. Calcium Transient
To maximize the number of observable neurons, we selected for analysis regions of interest with a relatively high density of GCaMP-expressing cells where there was also an active electrode, since expression was highly non-uniform across the retina. The averaged baseline, post-stimulation, and normalized fluorescent difference 2-D calcium images are shown in Figure 5. The responding neurons, as well as responsive area, can be highlighted by the normalized fluorescent difference (ΔF/F) in the 2-D imaging mapping. Some RGCs that appear very bright in the baseline are non-responsive (no change in fluorescence detected) so are eliminated by the normalization process.
Figure 5:
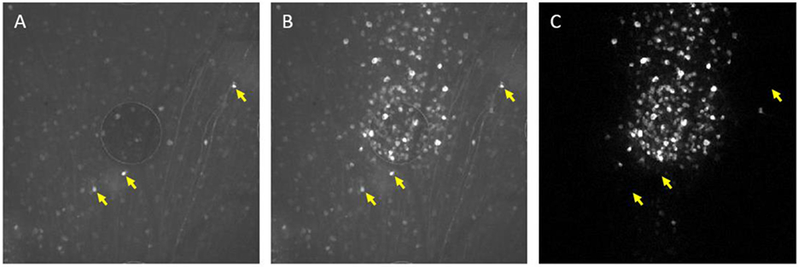
Electrical stimulation activates RGCs as revealed through large changes in GCaMP6f fluorescence intensity in region of high-density expression, in vitro. (A) Before Stimulation, cells exhibit baseline fluorescence (F). (B) Raw calcium image in response to external stimuli from the ITO electrode in the middle of the image. (C) Normalized image of fluorescent difference (ΔF/F) highlights the responding cells in the field of view. The yellow arrows indicate that some RGC with very bright baseline fluorescence did not generate detectable calcium transients. Background subtraction eliminated the non-responding cells (as shown in (C)).
Normalized changes in fluorescence in response to stimulation for two randomly selected RGC expressing GCaMP6f are shown in Figure 6. The initiation of each stimulus is indicated by the red arrow. Increasing stimulus amplitude leads to a larger calcium transient, which corresponds to stronger RGC activation. In addition, the chosen 20 seconds resting interval between stimuli is sufficient for the calcium fluorescence to return back to baseline for the next stimulus, which is important for capturing time-invariant results and preventing electroporation of cells.
Figure 6:
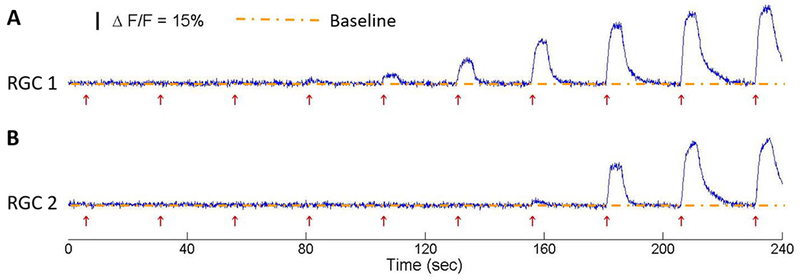
Normalized changes in fluorescence (ΔF/F) for two RGCs expressing GCaMP6f in response to the 120 Hz 40-μs duration biphasic symmetric pulse train stimuli with different stimuli amplitudes, from subthreshold to suprathreshold. Each stimulus was sustained for 5 seconds followed by a 20-second inter-stimulus interval, to allow the calcium level to return back to baseline. The red arrows indicate the onset of each stimulation pulse train.
3.3. Duration Manipulation
Figure 7 shows a set of representative spatial threshold maps for pulse widths ranging from 0.04 to 4 ms (40 μs, 80 μs, 120 μs, 0.5 ms, 1 ms, 4 ms) with symmetric cathodic-first waveforms at 120 Hz (minimum temporal resolution for STG-4008 MCS system is 20 μs), for WT and RD retinas respectively. Based on the geometrical distance to the electrode, the responses were classified as somatic or axonal activations and the areas were illustrated using the blue contours (location of electrode) and green contours (two times the radius of electrode), respectively. For easier comparison, all ROIs have first been re-oriented with the optic disc at the bottom of the image and axons of passage vertically oriented in the image. The corresponding threshold for each pixel (corresponding current amplitude) is illustrated with color map, where lower and higher thresholds are represented with more reddish and whitish colors, respectively, as shown in the scale color bar. All pulses ≤ 120-μs duration show preferential activation of somas vs. axons, whereas pulses ≥ 0.5 ms activate axons and somas at approximately the same level of stimulus. The stripe-shaped black shadow inside the activation patterns resulted from blood vessel occlusion at the top of the retinal ganglion cell layer. Similar findings for varying pulse durations were also shown using an RD model, although the number of responsive cells within the ROI is much less (Figure 7(B)).
Figure 7:
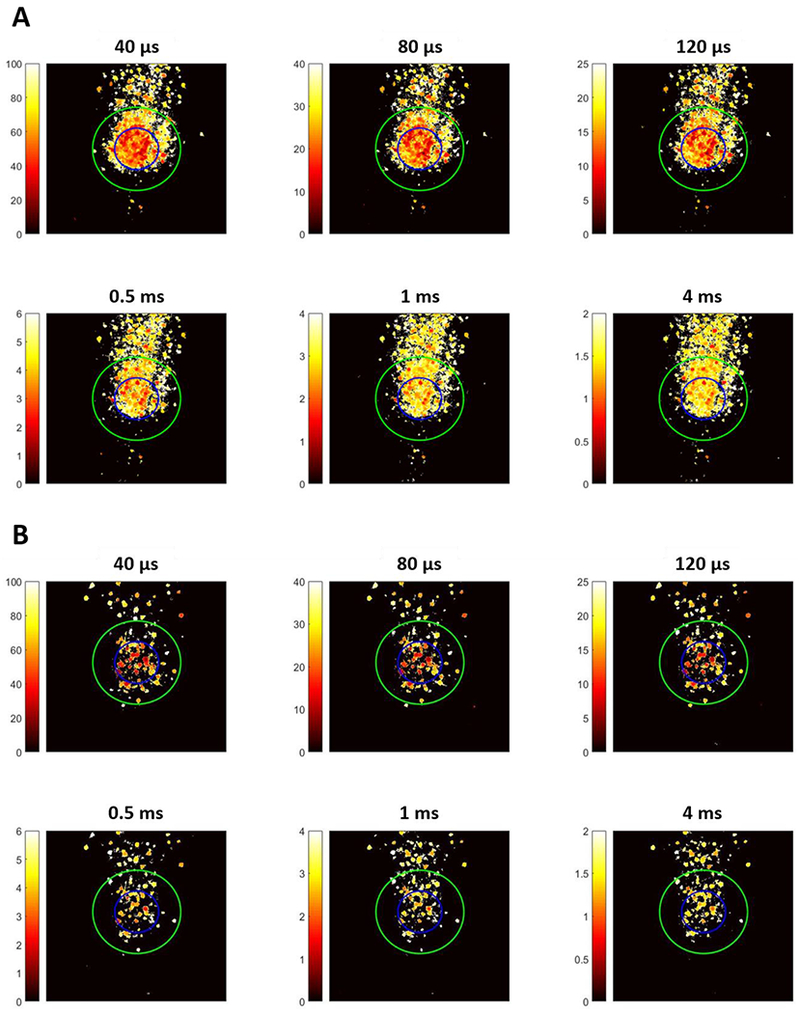
2-D spatial threshold maps for symmetric cathodic-first pulses with different duration in WT (A) and RD (B) retina. The color bar shows thresholds in terms of current amplitude (μA). The blue and green contours represent the location of electrode and two times the radius of electrode respectively, defining the somatic activation region (inside the blue circle) axonal activation region (outside the green circle).
The strength duration curves analyzed in pixel basis (as shown in Figure 8) for somatic and axonal responses from multiple ROIs (20 regions, 5 WT animals and 15 regions, 5 RD animals) were also fitted using a decaying exponential model for all pulse durations of symmetric cathodic-first pulses in Table 1. For both WT and RD retinas, we observe that the largest percentage difference in average soma and axon thresholds occurred for short duration pulses (≤ 120 μs, especially 40 μs), offering a strategy for selective activation of soma, which is important for the retinal prosthesis owing to limited resolution scale of current amplitude settings. Similar result and trend of strength duration curves were also obtained with the cell-based approach (not shown).
Figure 8:
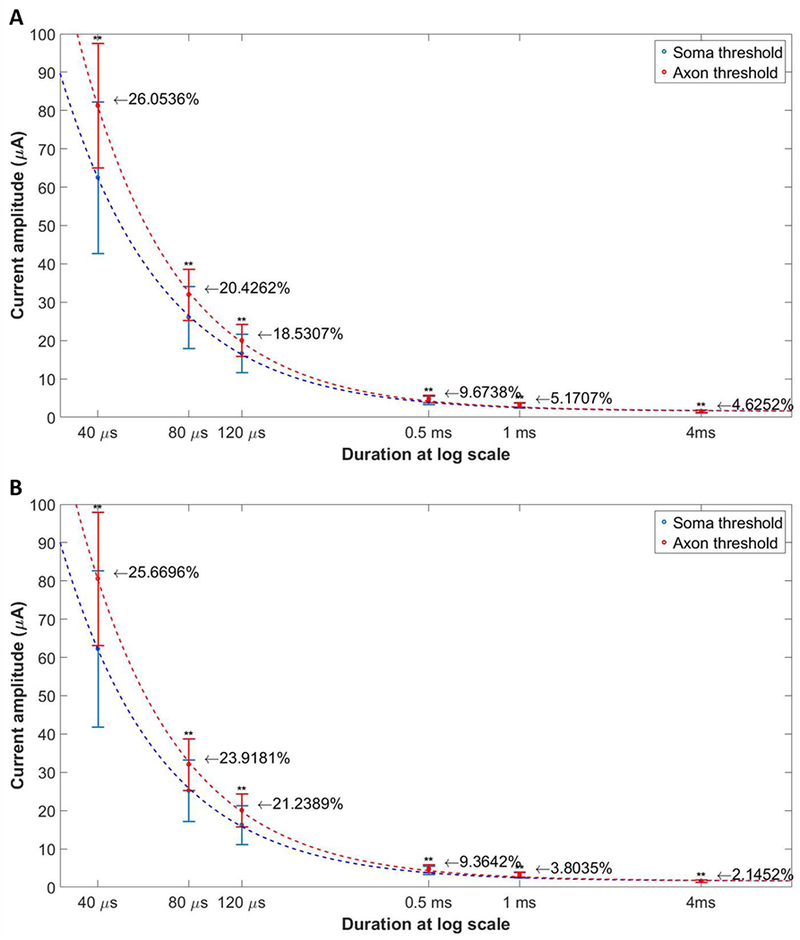
The strength duration curve plotted in terms of current amplitude for somatic and axonal activations respectively, from 20 regions of 5 WT mice (A) and 15 regions of 5 RD mice (B). The number listed near each error bar shows the percentage difference between mean soma and axon thresholds. Results indicated that stimulation with short duration pulse has greatest threshold difference, implying largest stimulation strength manipulation ranges for somatic versus axonal activations. ** means P < 0.01.
3.4. Phase Manipulation
Responses of RGCs generated by short (40 μs) and long duration (0.5 ms) symmetric cathodic-first pulses versus anodic-first (reverse) pulses with identical current amplitude are shown in Figure 9 (representative data). The spatial patterns of threshold maps of both WT and RD retinas demonstrate that symmetric anodic-first pulses produce more focal RGC responses compared with cathodic-first or asymmetric pulses, especially with 40 μs pulses, though the required current amplitudes are higher. While less pronounced, a similar phenomenon can also be observed for pulses with 0.5-ms duration. Comparing soma vs. axon activation across all ROIs at the mean threshold of all neurons for that ROI shows that short, anodic-first (reverse) pulses more preferentially evoke RGC somas and effectively avoid axonal stimulation, but at the cost of higher thresholds (Figure 10).
Figure 9:
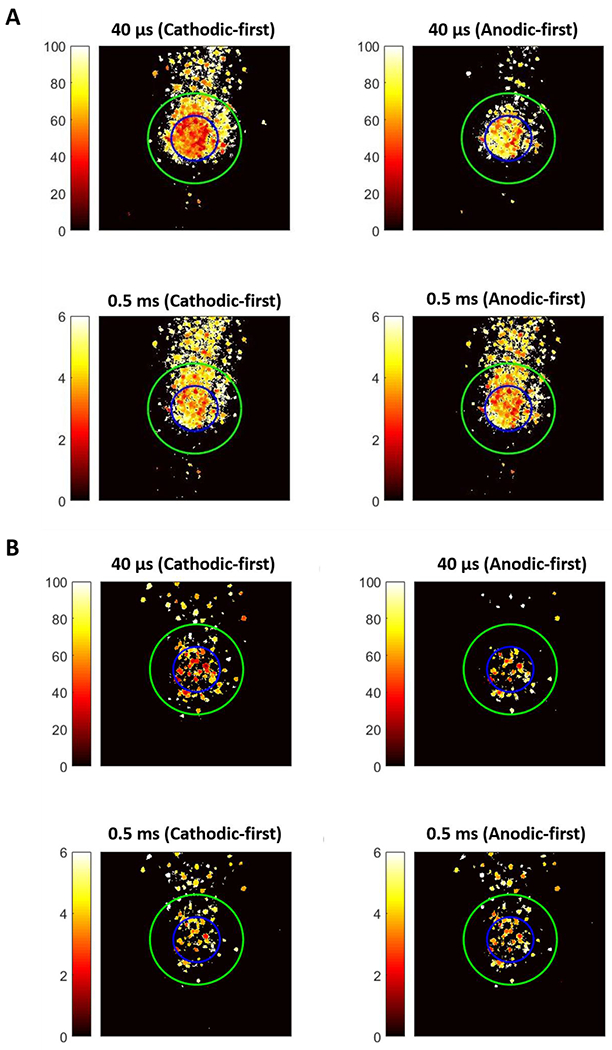
2-D spatial threshold maps for symmetric cathodic-first and anodic-first pulses with representative short duration (40 μs) and long duration (4 ms) in WT (Top) and RD (Bottom) retina. Identical setting as Figure 7.
Figure 10:
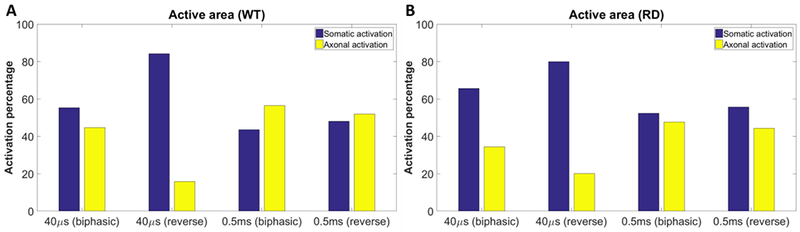
The active area statistical analysis for symmetric cathodic-first and anodic-first pulses with representative short duration (40 μs) and long duration (4 ms) from WT and RD mice (23 regions of 6 WT animals and 15 regions of 5 RD animals). (A) Results for WT group. (B) Results for RD group. The strength of stimulation is selected using the mean soma and axon threshold for stimulation respectively. The blue and yellow bars demonstrate the percentage of somatic and axonal activated regions, which clearly show that the symmetric anodic-first pulse with short duration can significantly confine the response area near to the electrode, thus activating RGC somatically and potentially forming more ideal visual percept.
3.5. Waveform Manipulation
Figure 11 compares the threshold maps of WT and RD retina for asymmetric anodic-first pulses of different ratios, symmetric cathodic-first, and anodic-first (reverse) pulses. The threshold of RGCs can be reduced by asymmetric waveforms, especially with a 20- or 10-times ratio. The leading anodic phase slightly hyperpolarizes the membrane potential of RGCs and conditions them to be more sensitive to stimulation, thus amplifying the depolarizing effect of the following cathodic pulse. However, when the ratio is reduced, as expected, the response patterns revert to the symmetric anodic-first pulse, which produces more localized maps but has higher threshold. In addition, the threshold maps suggest that a focal response can be elicited if proper stimulation current amplitude is used (10-20 μA for both representative WT and RD retinas). Response patterns elicited by asymmetric anodic-first pulsed and symmetric cathodic-first pulses were compared with 2D correlation analysis. The comparison was between response patterns from the same retina, but with different applied pulse shapes at varying amplitude. Response patterns evoked by symmetric cathodic-first pulses at a single amplitude that forming focal response served as baseline data and correlated with response patterns evoked by asymmetric anodic-first pulses with varying amplitudes (Figure 3) Average correlation was maximum when anodic first, asymmetric pulses used 50% (WT) and 66.6% (RD) of charge compared to symmetric cathodic-first pulses (Figure 12), suggesting improved efficiency for RGC stimulation.
Figure 11:
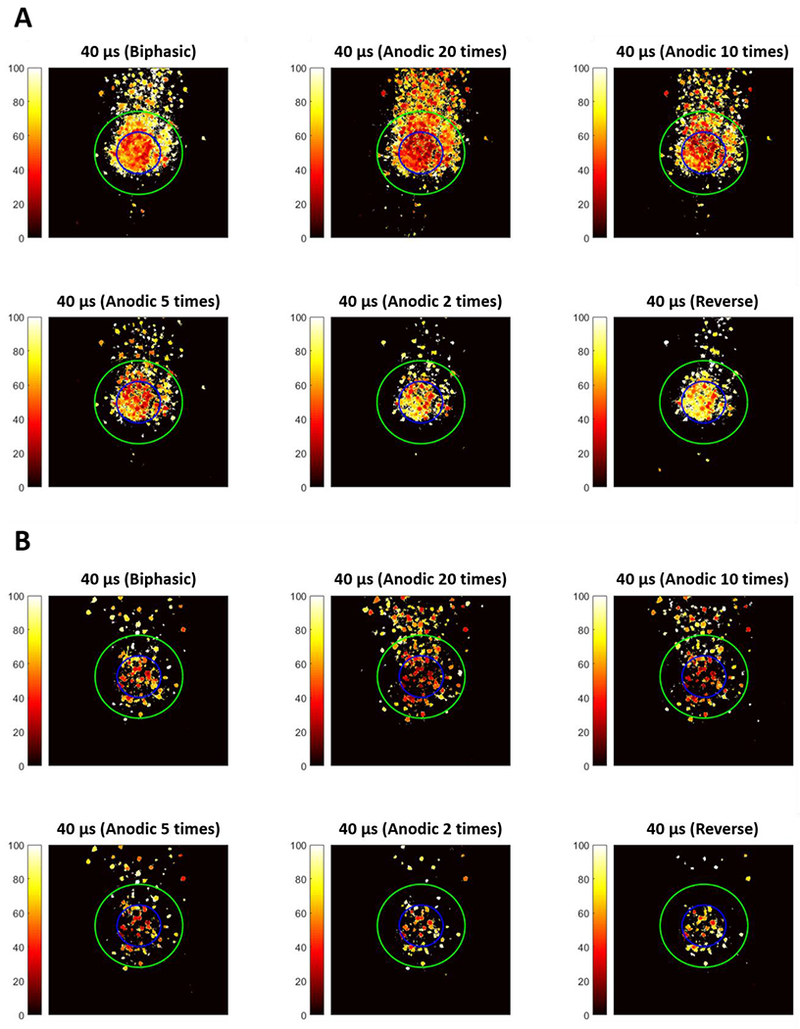
Spatial threshold maps for asymmetric anodic-first pulse (consistent with cathodic phase duration 40 μs) with different ratios and two controls (symmetric cathodic-first and anodic-first pulses), for representative WT (A) and RD (B) retina respectively. Data suggest that the thresholds for RGCs can be significantly reduced through asymmetric anodic-first pulses with phase ratio greater than 10 times. If the current amplitude is precisely controlled, a focal response near an electrode can still be achieved with the specially designed waveform. In contrast, when using phase ratio lower than 5 times, the responses become more localized but the thresholds of RGCs are universally increase. This finding is consistent with symmetric anodic-first (reverse) pulse since the short duration and high amplitude anodic phase hyperpolarize the membrane sharply, instead of making it hypersensitive.
Figure 12:
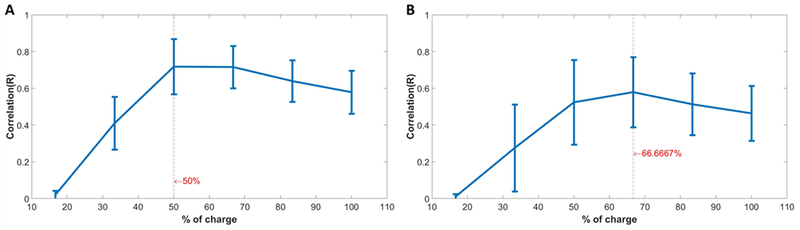
Correlations of response patterns between 20-times ratio asymmetric anodic-first pulse with different delivered charges and control symmetric cathodic-first pulses with fixed delivered charge (60 μA) that generally leads to focal response ((A): Result of WT; (B): Results of RD). The averaged correlation plot across multiple regions identifies the required amount of charge for 20-times ratio asymmetric anodic-first pulse to produce similar response as control symmetric cathodic-first pulse (23 regions of 6 WT animals and 15 regions of 5 RD animals).
4. Discussion
Using a transparent MEA platform combined with calcium imaging, we have developed a technique that provides the unique ability to visualize spatial patterns of RGC activation to electrical stimulation in real time. Using this technique, we have explored several aspects of the spatial response to epiretinal stimulation. Our findings support the possibility that preferential activation of RGCs somas (versus passing axons) can be achieved through varying stimulus durations, phases, and waveforms and without using inefficient pulsing strategies such as long duration pulses. The proposed waveforms have great potential to improve the retinal prosthetic implants through forming more ideal shape perception.
4.1. Short Duration Pulse for Direct RGC Activation
Various stimulation strategies have been used to target specific cell types in the retina, and selective activation of RGCs and BPs is possible by manipulation of stimulus duration. Studies have shown that more focal (to the stimulating electrodes) responses of RGCs can be achieved using indirect stimulation with long duration pulses (14). However, clinical testing demonstrated that thresholds gradually rose during the test session and some patients reported fading of brightness of percepts when using millisecond-scale pulses, implying that the retina was becoming desensitized (4). More evidence from animal studies reveals that indirect stimulation of RGCs originated from BP stimulation attenuate quickly (15, 25, 26). The mechanisms behind this phenomenon still remain unknown, however electrophysiology studies suggest multiple factors, including amacrine cell inhibition (15), depletion of synaptic vesicles (16), and BP calcium channel inactivation (27).
Short-duration pulses overcome this complicated issue by primarily targeting RGCs directly, as RGCs are capable of following high rates of electrical stimulation (28). The location on RGCs with the greatest potential in response to the rapid direct stimulation is the initial axon segment with its high density of voltage-gated channels, though method with higher temporal resolution is required to validate the actual depolarization site (29, 30). Our results further show that the RGC somas can be selectively activated with extremely short duration pulses (≤120 μs) of proper current amplitude, consistent with the findings about direct RGC stimulation by Sekirnjak et al. (31) and Jepson et al. (32). Axonal activation is problematic in that it underlies the elongated non-ideal percept reported by patients. However, the range of stimuli for selective activation of somas vs. axons is small (~25% of threshold).
4.2. Reverse Phases for Selective Activation
Symmetric biphasic cathodic-first pulses are a commonly used stimulation waveform for neural implants. The cathodic and anodic phase delivered in the specific order are designed to depolarize the neuron and to ensure net charge balance, respectively. In this project, we found that the symmetric anodic-first pulse can create a more focal response near an active electrode, suggesting a strategy for preferential somatic activation. As a trade-off, the overall RGCs thresholds for symmetric anodic-first are increased, as shown in recent research using MEA recording on RGC responses in retinal degeneration (rd1) mice (33). However, this stimulation pattern is still worth further exploration in clinical study if the increased thresholds of RGCs still remain within charge safety limit. Based on our findings, this phase alternation method has the greatest potential to form localized RGC activation, which implies high spatial resolution of retinal prosthesis.
4.3. Asymmetric Anodic-first Pulse for RGCs Thresholds Manipulation
Anode break excitation was first reported in the middle of last century to excite frog leg and squid axon models with hyperpolarization current (34, 35). When a small hyperpolarizing current is applied, the potential across the neuron membrane first increases (becomes more negative), which is followed by a conditioned reduction of threshold required for action potential. With the termination of the hyperpolarizing current, the membrane potential rapidly depolarizes. This findings agree with the Hodgkin-Huxley (HH) computational model (36). Hyperpolarization reduces the extent of inactivation among sodium channels, increasing the total pool of Na channels capable of chance opening flickers even at normal resting potential. Removal of hyperpolarization allows the chance openings of a sufficient number of Na channels to inject positive charge sufficient to offset the efflux of positive charge from resting-state potassium channels, and leads ultimately to the positive regenerative feedback that characterizes an action potential.
The design of the asymmetric anodic-first pulse was inspired by the concept of anode break excitation. Instead of using applying the anodic phase merely to achieve charge balance following an activating cathodic phase depolarization, the anodic phase of extended duration and small amplitude is applied first, and it serves to hyperpolarize and condition the membrane (by removing inactivation) to be easily excitable (relative to equilibrium). The following cathodic phase requires less energy for action potential initiation, and also serves to maintain overall charge balance. We also find that the RGC responses are localized to the region near the active electrode. Selective activation of RGC somas is possible with optimization of stimulation profile design. With proper adjustment and modification of the proposed pulse waveform, the same stimulation strategy also may offer great potential for threshold manipulation for other neural stimulation application, such as spinal cord (37) or peripheral nerves (38).
Others have investigated asymmetric pulses for retinal stimulation. Hadjinicolaou et al. found that short cathodic first pulses followed by a longer anodic pulse of lower amplitude (but equal charge) reduced the stimulus threshold relative to a symmetric pulse (39). This finding is likely due to the anodic following pulse, since it is lower current amplitude, having less of an attenuating effect on depolarization caused by the leading cathodic pulse. Loizos et al. used modeling to investigate long anodic pulses as a means of reducing spontaneous activity in the retina (40). Their model predicts that RGC responses can be elicited more reliably when long anodic pulses are applied in between short cathodic pulses, since the cathodic pulses will produce spikes from RGC and the anodic pulses will inhibit retinal activity. While both of these studies explored benefits of asymmetric pulsing, our study is different because we use asymmetric pulses to make the RGC more sensitive to stimulation, building on the principle of anode break excitation, followed by the cathodic pulse for depolarization. We found a significant decrease in threshold (33-50%) vs. the 10% difference noted by Hadjinicolaou. Calcium imaging does not allow us to study spontaneous activity, but standard electrophysiology should be able to test the model predictions from the Loizos paper.
4.4. Comparison to related work on spatiotemporal retinal stimulation
A number of other studies have sought to improve the resolution of retinal stimulation. Chichilnisky’s group have investigated using small electrodes (9-15 μm) in high-density arrays (31), and spatially patterned current injection to improve the spatial resolution (41). Palanker et al. have studied perforated membranes to promote cell proximity to implants (42), and photovoltaic subretinal arrays with high pixel density which allows for eye scanning (43). Freeman et al. have researched sinusoidal waveforms for selective activation of target cells (13), and other groups have explored selective activation of RGC types with high-frequency stimulation to improve visual perception (44, 45). Our approach was to use electrode sizes that are similar to clinical devices, specifically the Argus II. This will allow direct comparison of our response patterns to patient perceptions. We expect that stimulation with large electrodes will appear artificial, since multiple subgroups of retinal ganglion cells are being driven indiscriminately without considering individual information channels in the retina that encode the onset/offset of light and direction of motion. However, the concepts of the proposed simulation strategies might still be clinically deliverable for precepts with better spatial resolution and power efficiency, due to the similarity between the presented experimental model and existing epiretinal neural interfaces.
4.5. Limitations of Calcium Imaging
Viral vectors are excellent for calcium indicator expression in retina, owing to the ease of delivery and production (8). However, in practice, GCaMP-induced cytomorbidity, suggested to emerge from overexpression and interaction of the sensor CaM and/or M13 motifs with endogenous proteins (46), limits the timespan of calcium imaging experiments that can be performed. With the validation of a custom in vivo fundus imaging system (21), we found that the optimal expression window for proposed virus-transduced calcium indicator is around 3~4 weeks post injection, during which the cells do not exhibit abnormal cellular physiology, in agreement with the other studies (47, 48).
The other main drawback to calcium imaging in studies of WT retina is that the excitation light causes bleaching of photoreceptors, limiting the application to studies not involving light stimulation. To minimize photobleaching at photoreceptors while imaging calcium dynamics at inner retinal neurons, some groups have used two-photon excitation with infrared wavelength to selectively excite fluorescence in inner retinal neurons (49). For our studies, however, which rely on extracellular electrical stimulation (the method of stimulation with epiretinal prosthetic implants, such as the Argus II) of retinal neurons, even strong excitation light does not inhibit RGC or BC responsiveness.
5. Conclusion
In summary, we demonstrate an advanced imaging technique that can detect RGC responses across a microscope field at cellular-level resolution. The activation pattern in our in vitro preparation is a good reference for the retinal prostheses, since RGC response directly corresponds to the visual perceptions reported by patients. Experimental results showed a focal response can be achieved with relative short duration (≤120 μs) pulses, and can be improved by reversing the standard phase order. The RGCs threshold can further be significantly reduced through the special design of asymmetric anodic-first pulse. Our findings provide strong evidence to support development of high-resolution and low-power consumption retinal prostheses.
6. Reference
- 1.Stanga PE, Jalil A, Tsamis E, Papayannis A, Dorn JD, Greenberg RJ, et al. , editors. Argus II® electronic epiretinal prosthesis in advanced dry AMD: safety and feasibility study and preliminary functional results. The Annual Meeting of the Association for Research in Vision and Ophthalmology; 2016; Seattle WA. [Google Scholar]
- 2.Humayun MS, de Juan E Jr., Weiland JD, Dagnelie G, Katona S, Greenberg R, et al. Pattern electrical stimulation of the human retina. Vision research. 1999;39(15):2569–76. [DOI] [PubMed] [Google Scholar]
- 3.Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel JA, Stanga PE, et al. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology. 2012;119(4):779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP, et al. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci. 2011;278(1711):1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yue L, Weiland JD, Roska B, Humayun MS. Retinal stimulation strategies to restore vision: Fundamentals and systems. Prog Retin Eye Res. 2016;53:21–47. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja AK, Dorn JD, Caspi A, McMahon MJ, Dagnelie G, Dacruz L, et al. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. The British journal of ophthalmology. 2011;95(4):539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanduri D, Fine I, Horsager A, Boynton GM, Humayun MS, Greenberg RJ, et al. Frequency and amplitude modulation have different effects on the percepts elicited by retinal stimulation. Investigative ophthalmology & visual science. 2012;53(1):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitz AC, Behrend MR, Lee NS, Klein RL, Chiodo VA, Hauswirth WW, et al. Imaging the response of the retina to electrical stimulation with genetically encoded calcium indicators. Journal of neurophysiology. 2013;109(7):1979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg RJ, Velte TJ, Humayun MS, Scarlatis GN, de Juan E Jr. A computational model of electrical stimulation of the retinal ganglion cell. IEEE transactions on bio-medical engineering. 1999;46(5):505–14. [DOI] [PubMed] [Google Scholar]
- 10.Shah HA, Montezuma SR, Rizzo JF, 3rd. In vivo electrical stimulation of rabbit retina: effect of stimulus duration and electrical field orientation. Exp Eye Res. 2006;83(2):247–54. [DOI] [PubMed] [Google Scholar]
- 11.Fried SI, Hsueh HA, Werblin FS. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. Journal of neurophysiology. 2006;95(2):970–8. [DOI] [PubMed] [Google Scholar]
- 12.Sekirnjak C, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Electrical stimulation of mammalian retinal ganglion cells with multielectrode arrays. Journal of neurophysiology. 2006;95(6):3311–27. [DOI] [PubMed] [Google Scholar]
- 13.Freeman DK, Eddington DK, Rizzo JF, 3rd, Fried SI. Selective activation of neuronal targets with sinusoidal electric stimulation. Journal of neurophysiology. 2010;104(5):2778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weitz AC, Nanduri D, Behrend MR, Gonzalez-Calle A, Greenberg RJ, Humayun MS, et al. Improving the spatial resolution of epiretinal implants by increasing stimulus pulse duration. Sci Transl Med. 2015;7(318):318ra203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman DK, Fried SI. Multiple components of ganglion cell desensitization in response to prosthetic stimulation. Journal of neural engineering. 2011;8(1):016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen RJ, Rizzo JF, 3rd. Responses of ganglion cells to repetitive electrical stimulation of the retina. Journal of neural engineering. 2007;4(1):S1–6. [DOI] [PubMed] [Google Scholar]
- 17.Stronks HC, Dagnelie G. The functional performance of the Argus II retinal prosthesis. Expert Rev Med Devices. 2014;11(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahuja AK, Behrend MR, Kuroda M, Humayun MS, Weiland JD. An in vitro model of a retinal prosthesis. IEEE transactions on bio-medical engineering. 2008;55(6):1744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YC, Weiland JD, editors. Stimulation strategies for selective activation of retinal ganglion cells. 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER); 2017 25–28 May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. The Journal of comparative neurology. 2007;500(2):222–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YC, Walston ST, Chow RH, Weiland JD. GCaMP expression in retinal ganglion cells characterized using a low-cost fundus imaging system. Journal of neural engineering. 2017;14(5):056018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan LL, Lee EJ, Humayun MS, Weiland JD. Both electrical stimulation thresholds and SMI-32-immunoreactive retinal ganglion cell density correlate with age in S334ter line 3 rat retina. Journal of neurophysiology. 2011;105(6):2687–97. [DOI] [PubMed] [Google Scholar]
- 23.Lapicque L Recherches quantitatives sur l’excitation electrique des nerfs traitee comme une polarization. J Physiol Pathol Gen. 1907;9:620–35. [Google Scholar]
- 24.Geddes LA, Bourland JD. The strength-duration curve. IEEE transactions on bio-medical engineering. 1985;32(6):458–9. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Humayun MS, Weiland JD, Chen SJ, Margalit E, Piyathaisere DV, et al. Comparison of electrical stimulation thresholds in normal and retinal degenerated mouse retina. Jpn J Ophthalmol. 2004;48(4):345–9. [DOI] [PubMed] [Google Scholar]
- 26.Jensen RJ, Ziv OR, Rizzo JF, 3rd, Scribner D, Johnson L. Spatiotemporal aspects of pulsed electrical stimuli on the responses of rabbit retinal ganglion cells. Exp Eye Res. 2009;89(6):972–9. [DOI] [PubMed] [Google Scholar]
- 27.Hu C, Bi A, Pan ZH. Differential expression of three T-type calcium channels in retinal bipolar cells in rats. Vis Neurosci. 2009;26(2):177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai C, Ren Q, Desai NJ, Rizzo JF, 3rd, Fried SI. Response variability to high rates of electric stimulation in retinal ganglion cells. Journal of neurophysiology. 2011;106(1):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried SI, Lasker AC, Desai NJ, Eddington DK, Rizzo JF, 3rd. Axonal sodium-channel bands shape the response to electric stimulation in retinal ganglion cells. Journal of neurophysiology. 2009;101(4):1972–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeng J, Tang S, Molnar A, Desai NJ, Fried SI. The sodium channel band shapes the response to electric stimulation in retinal ganglion cells. Journal of neural engineering. 2011;8(3):036022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekirnjak C, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. High-resolution electrical stimulation of primate retina for epiretinal implant design. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(17):4446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jepson LH, Hottowy P, Weiner GA, Dabrowski W, Litke AM, Chichilnisky EJ. High-fidelity reproduction of spatiotemporal visual signals for retinal prosthesis. Neuron. 2014;83(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn KN, Ahn JY, Kim JH, Cho K, Koo KI, Senok SS, et al. Effect of Stimulus Waveform of Biphasic Current Pulse on Retinal Ganglion Cell Responses in Retinal Degeneration (rd1) mice. Korean J Physiol Pharmacol. 2015;19(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankenhaeuser B, Widen L. Anode break excitation in desheathed frog nerve. The Journal of physiology. 1956;131(1):243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guttman R, Hachmeister L. Anode break excitation in space-clamped squid axons. Biophys J. 1972;12(5):552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of physiology. 1952;117(4):500–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of Spinal Cord Stimulation and Their Role in Electrical Charge Delivery: A Review. Neuromodulation : journal of the International Neuromodulation Society. 2016;19(4):373–84. [DOI] [PubMed] [Google Scholar]
- 38.Araujo T, Candeias R, Nunes N, Gamboa H. Evaluation of Motor Neuron Excitability by CMAP Scanning with Electric Modulated Current. Neurosci J. 2015;2015:360648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadjinicolaou AE, Savage CO, Apollo NV, Garrett DJ, Cloherty SL, Ibbotson MR, et al. Optimizing the electrical stimulation of retinal ganglion cells. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2015;23(2):169–78. [DOI] [PubMed] [Google Scholar]
- 40.Loizos K, Marc R, Humayun M, Anderson JR, Jones BW, Lazzi G. Increasing Electrical Stimulation Efficacy in Degenerated Retina: Stimulus Waveform Design in a Multiscale Computational Model. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2018;26(6):1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jepson LH, Hottowy P, Mathieson K, Gunning DE, Dabrowski W, Litke AM, et al. Spatially patterned electrical stimulation to enhance resolution of retinal prostheses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(14):4871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palanker D, Huie P, Vankov A, Aramant R, Seiler M, Fishman H, et al. Migration of retinal cells through a perforated membrane: implications for a high-resolution prosthesis. Investigative ophthalmology & visual science. 2004;45(9):3266–70. [DOI] [PubMed] [Google Scholar]
- 43.Mathieson K, Loudin J, Goetz G, Huie P, Wang L, Kamins TI, et al. Photovoltaic Retinal Prosthesis with High Pixel Density. Nature photonics. 2012;6(6):391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo T, Lovell NH, Tsai D, Twyford P, Fried S, Morley JW, et al. , editors. Optimizing retinal ganglion cell responses to high-frequency electrical stimulation strategies for preferential neuronal excitation. 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER); 2015 22–24 April 2015. [Google Scholar]
- 45.Guo T, Yang CY, Tsai D, Muralidharan M, Suaning GJ, Morley JW, et al. Closed-Loop Efficient Searching of Optimal Electrical Stimulation Parameters for Preferential Excitation of Retinal Ganglion Cells. Frontiers in neuroscience. 2018;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasan MT, Friedrich RW, Euler T, Larkum ME, Giese G, Both M, et al. Functional fluorescent Ca2+ indicator proteins in transgenic mice under TET control. PLoS Biol. 2004;2(6):e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borghuis BG, Tian L, Xu Y, Nikonov SS, Vardi N, Zemelman BV, et al. Imaging light responses of targeted neuron populations in the rodent retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(8):2855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100(12):7319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


