Abstract
Importance:
Anti-vascular endothelial growth factor (anti-VEGF) medicines have revolutionized diabetic macular edema (DME) treatment. A recent randomized clinical trial comparing anti-VEGF agents for patients with decreased vision from DME found that at one year aflibercept (2.0-mg) achieved better visual outcomes than repackaged (compounded) bevacizumab (1.25-mg) or ranibizumab (0.3-mg); the worse the starting vision, the greater the treatment benefit with aflibercept. However, aflibercept and ranibizumab, respectively, are approximately 31 and 20 times more expensive than bevacizumab.
Objective:
To determine the incremental cost-effectiveness ratios (ICERs) of each of these agents for DME.
Design, Setting, Participants:
Post-hoc analysis of efficacy, safety, and resource utilization data at 1 one year follow-up from a Diabetic Retinopathy Clinical Research Network randomized clinical trial.
Main Outcomes and Measures:
ICERs for all trial participants and subgroups with baseline vision of approximate Snellen equivalent 20/32 to 20/40 (“better vision”) and baseline vision of approximate Snellen equivalent 20/50 or worse (“worse vision”). One-year trial data were used to calculate cost-effectiveness over one year for the 3 anti-VEGF agents; mathematical modeling then was used to project 10-year cost-effectiveness results.
Results:
For all participants, over one year, the ICERs of aflibercept and ranibizumab compared with bevacizumab were $1,110,000 per quality-adjusted life-year (QALY) and $1,730,000/QALY. Over 10 years, they were $349,000/QALY and $603,000/QALY, respectively. Compared with ranibizumab, aflibercept’s ICER was $648,000/QALY at one year and $203,000/QALY at ten years. For the subgroup with worse baseline vision, the 10-year ICERs of aflibercept and ranibizumab compared with bevacizumab were $287,000/QALY and $817,000/QALY, respectively. In eyes with decreased vision from DME, treatment costs of aflibercept and ranibizumab would need to decline by 69% and 80%, respectively, to reach a cost-effectiveness threshold of $100,000/QALY compared with bevacizumab over a 10-year horizon; for the subgroup with worse baseline vision, the costs would need to decline by 62% and 84%, respectively.
Conclusions and Relevance:
Aflibercept 2.0-mg and ranibizumab 0.3-mg are not cost-effective relative to bevacizumab for treatment of DME unless their prices decline substantially. These results highlight the challenges that clinicians, patients, and policy-makers face when safety and efficacy results are at odds with cost-effectiveness results.
A recent Diabetic Retinopathy Clinical Research Network (DRCR.net) comparative effectiveness trial showed for patients with diabetic macular edema (DME) and approximate Snellen equivalent baseline visual acuity (VA) 20/50 or worse, aflibercept produced greater average VA gains at one year than bevacizumab or ranibizumab. In contrast, no difference in average VA improvement was identified for patients with baseline VA of 20/32 to 20/40.1
These agents also vary substantially in cost. Based on 2015 wholesale acquisition costs, aflibercept (2.0-mg) costs $1,850,2 ranibizumab (0.3-mg) costs $1,170,2 and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25-mg of bevacizumab costs approximately $60 per dose.3 Considering that these medicines may be given 9 to 11 times in the first year of treatment1 and, on average, 17 times over 5 years,4 total costs can be substantial. In 2010, when these intravitreous agents were being used predominantly for age-related macular degeneration, ophthalmologic use of anti-vascular endothelial growth factor (anti-VEGF) therapy cost approximately $2 billion, or one-sixth of the entire Medicare Part B drug budget.3 In 2013, Medicare Part B expenditures for aflibercept and ranibizumab alone totaled $2.5 billion.5 Given these costs, the DRCR Network investigators believed it was important to analyze the relative cost-effectiveness of treating DME using each agent.
METHODS
Overview
In a post-hoc analysis, data from a randomized clinical trial was used to calculate clinical benefit, costs, and cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for DME.1 Using published cost and quality-of-life data, resource utilization and VA results from the trial were converted into estimates of overall medical costs and quality-adjusted life-years (QALYs) accrued during the first year of the trial.6 A mathematical model projected longer-term costs and health outcomes with each therapy (see Supplemental Material for details). Each therapy’s incremental cost-effectiveness ratio (ICER) was calculated, defined as the ratio of its incremental cost (in 2015 USD) to its incremental benefit (in QALYs) compared to the next-best therapy. Future outcomes were discounted at an annual rate of 3% to reflect their present value.
Because treatment efficacy in the DRCR.net trial differed significantly by baseline VA, the cost-effectiveness of these therapies also was assessed for “better” (approximate Snellen equivalent 20/32 to 20/40 [ETDRS letter score 78–69]) and “worse” (20/50 or worse [letter score <69]) VA subgroups.
Participants
The DRCR.net Protocol T trial included 660 patients randomly assigned to aflibercept, bevacizumab, or ranibizumab for DME. Patients were enrolled between August 22, 2012 and August 28, 2013; this analysis was performed between August 21, 2014 and November 7, 2015. The study adhered to the Declaration of Helsinki and was approved by local and centralized institutional review boards. Detailed procedures, protocol, and statistical methods have been reported previously.1
Participants were ≥18 years old and had one study eye with VA (Snellen equivalent) 20/32 to 20/320 due to DME. Among the 660 participants, 329 (50%) eyes were in the worse VA subgroup, and 331 (50%) in the better VA subgroup. Patients were followed every 4 weeks (termed “monthly” hereafter) for one year, with anti-VEGF injections provided on a monthly basis for the first six months in most cases. Thereafter, treatment was deferred if the eye was stable; laser treatment was added, if indicated, based on study-defined criteria. After deferring injections, if VA or macular thickness worsened due to DME, injections resumed until stability again occurred. If a participant’s non-study eye also required anti-VEGF treatment during the trial, it was given the same agent as the study eye. Participants were excluded for loss to follow-up (not including deaths) before the 1-year visit (n=29) or receiving an anti-VEGF agent other than randomized to receive (n=7), leaving a total of 624 participants receiving aflibercept (n=209), bevacizumab (n=207), or ranibizumab (n=208).
Quality-of-life
Participant VA levels at each visit were converted to QALYs using data from Brown and colleagues who linked VA in a patient’s better-seeing eye with health-related quality-of-life. Visual acuities were obtained from the trial, converted to Snellen acuities, and assigned a utility based on conversion tables.6 Quality-of-life levels at monthly visits during the first year were summed, providing an aggregate QALY value for the entire year for each participant.
Calculated quality-of-life was reduced for participants experiencing adverse events possibly caused by the study agent, including myocardial infarction (MI), cerebrovascular accident (CVA), endophthalmitis, retinal detachment, and vitreous hemorrhage. MI and CVA were assumed to reduce quality-of-life for the remainder of one’s life; other adverse events resulted in one-time quality-of-life decrements (eTable1). Over the one-year trial horizon, adverse events were identified and incorporated into the analysis based on patient-level trial data, differing nominally between treatment arms. Because a difference in adverse event rates and mortality between the treatment arms was not identified at 1 year in the trial or previous meta-analyses,1,7 the same pooled rates were used for all anti-VEGF agents in modeling projections beyond one year.
To assess cost-effectiveness beyond 1 year, a mathematical model based on prior cost-effectiveness analyses for DME was developed (eFigure 1).8,9 A prior trial using ranibizumab for DME showed relatively stable average VA from 1–5 years after treatment initiation;4 accordingly, the model assumes a patient’s VA at one year remains constant throughout the remainder of the patient’s life with ongoing monitoring and anti-VEGF therapy as needed. This assumption was varied widely in sensitivity analyses.
Costs
Overall costs were calculated by applying standardized unit costs to treatment and adverse event data from the trial, specifically including adverse and treatments with the potential to vary between arms (eTables 2 and 3). Injection costs were based on the average wholesale prices of each anti-VEGF agent and Medicare physician fees for administration in an office-based setting. Because trial protocol dictated that participants requiring treatment in the non-study eye receive the same agent as the study eye, and this could potentially affect quality-of-life outcomes, we included costs for both study-eye and non-study eye treatments. Adverse event costs were based upon studies of long-term costs of MI, CVA, and legal blindness.10,11 Costs not expected to vary among the treatment arms were not captured, including office visit costs, unrelated medical costs, and indirect costs such as caregiver burden. Thus, this analysis provides an accurate estimate of incremental costs between treatment strategies but not of overall medical costs associated with DME. Five-year data from a prior trial using ranibizumab was used to estimate the declining rates of injection and laser treatment over the longer term (eTable 1).4
Statistical analyses
Two-tailed t-tests assessed the significance of cost and quality-of-life differences in one-year data from the trial. Calculated P values reflect subject-level variance from trial data, but do not account for uncertainty in unit cost or quality-of-life data from outside sources.
Sensitivity analyses
To assess robustness of these results and explore how different assumptions might affect cost-effectiveness of the therapies, several sensitivity analyses were performed. In univariate and bivariate sensitivity analyses, effects of varying one or two key parameters at a time were assessed. In the base case analysis, quality-of-life was mapped to visual acuity in the patient’s better-seeing eye; a sensitivity analysis used data from the UK-based RESTORE trial of anti-VEGF therapy for DME to map quality-of-life to VA in the patient’s treated eye (whether it was the better- or worse-seeing eye).12 The effects of varying the time horizon of the modeling projections (1–30 years), changes in VA achieved from using the 3 agents over 10 years (±20 letters), adverse event rates (0–100% of base case), and others were explored. Costs of aflibercept and ranibizumab were varied to determine at what prices they would have an ICER below $100,000/QALY, a threshold commonly considered meaningful for determining cost-effectiveness in the United States.13–17
To assess uncertainty in model inputs, a probabilistic sensitivity analysis was performed. First, synthetic trial treatment groups were created by randomly drawing 200 participants from each trial arm, with replacement; values for model parameters including costs, quality-of-life, and adverse event rates then were drawn at random from distributions reflecting their uncertainty. This process was repeated 10,000 times with cost-effectiveness results calculated for each iteration to obtain a distribution of probabilities for each treatment strategy to be cost-effective at different societal “willingness-to-pay” values per QALY.
RESULTS
Quality-of-life
The mean (95% confidence interval (CI)) QALYs (Table 1) accrued during the first year of the trial in the aflibercept, bevacizumab, and ranibizumab arms, respectively, were 0.869 (0.857–0.880), 0.849 (0.835–0.862), and 0.857 (0.843–0.872). For participants with worse baseline vision, mean (95% CI) QALYs were 0.835 (0.817–0.854), 0.823 (0.807–0.840), and 0.829 (0.813–0.846); for those with better baseline vision, mean QALYs were 0.901 (0.891–0.911), 0.875 (0.855–0.895), and 0.884 (0.861–0.907), respectively (Table 1). Differences in mean QALYs between treatment arms were largest for aflibercept vs. bevacizumab among all participants (P = 0.03, eTable 4) and aflibercept vs. bevacizumab among those with better baseline vision (P = 0.02, eTable 4). All other comparisons had P>0.15 (eTable 4).
Table 1:
Cost-effectiveness outcomes
| 1-year horizon | 10-year horizon (projections) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (2015 USD) |
Utility (QALYs) |
ICER vs. bevacizumab ($/QALY)* | ICER vs. ranibizumab ($/QALY) | Cost (2015 USD) |
Utility (QALYs) |
ICER vs. bevacizumab ($/QALY) | ICER vs. ranibizumab ($/QALY)* | ||
| All patients | |||||||||
| Bevacizumab | 4,100 | 0.849 | - | 39,800 | 6.80 | - | |||
| Ranibizumab | 18,600 | 0.857 | 1,730,000 | 79,400 | 6.87 | 603,000 | |||
| Aflibercept | 26,100 | 0.869 | 1,110,000 | 648,000 | 102,500 | 6.98 | 349,000 | 203,000 | |
| Baseline visual acuity 20/50 or worse† | |||||||||
| Bevacizumab | 5,000 | 0.823 | - | 40,700 | 6.60 | - | |||
| Ranibizumab | 20,400 | 0.829 | 2,450,000 | 81,200 | 6.65 | 817,000 | |||
| Aflibercept | 28,100 | 0.835 | 1,870,000 | 1,270,000 | 104,500 | 6.82 | 287,000 | 135,000 | |
| Baseline visual acuity 20/32 to 20/40‡ | |||||||||
| Bevacizumab | 3,200 | 0.875 | - | 38,900 | 7.01 | - | |||
| Ranibizumab | 16,900 | 0.884 | 1,500,000 | 77,700 | 7.09 | 506,000 | |||
| Aflibercept | 24,100 | 0.901 | 798,000 | 422,000 | 100,600 | 7.14 | 474,000 | 428,000 | |
USD, United States Dollars; QALYs, quality-adjusted life-years; ICER, incremental cost-effectiveness ratio
Incremental cost-effectiveness ratios for ranibizumab and aflibercept are presented in comparison to bevacizumab. When comparing all three agents, ranibizumab would be dominated by aflibercept (lower utility, but higher incremental cost-effectiveness ratio than aflibercept). ICERs are calculated from unrounded cost and utility values, and thus may differ from values calculated based on the rounded costs and utilities in the table.
ETDRS letter score <69 (approximately 20/50 or worse).
ETDRS letter score 78 to 69 (approximately 20/32 to 20/40).
Of note, these outcomes may appear at odds with original trial results showing the greatest VA benefit of aflibercept vs. bevacizumab among the worse baseline vision group. This difference reflects the non-linear relationship between VA and quality-of-life, as well the fact that QALYs were summed over each month in this analysis, whereas VA was compared only at one year in the original trial (see Supplemental Material for further details).
Resources and costs
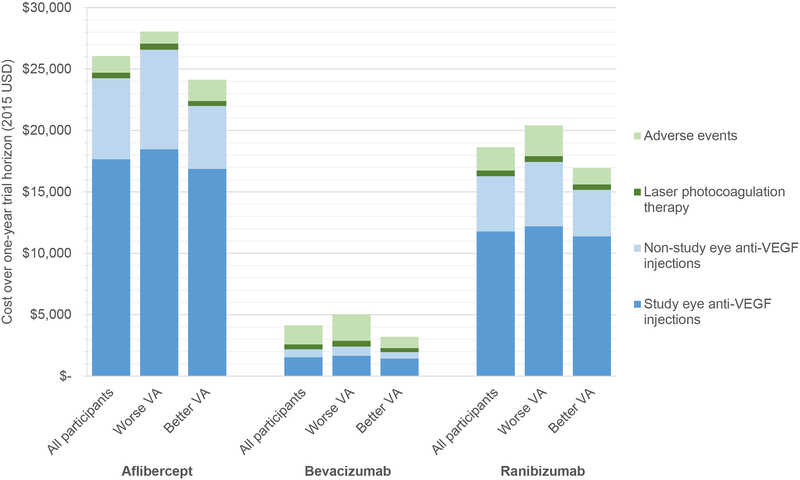
Total mean (95% CI) costs per participant over one year (including study eye and non-study eye anti-VEGF injections, laser photocoagulation therapy, and adverse events) in the aflibercept, bevacizumab, and ranibizumab groups, respectively, were $26,100 ($24,400–27,700), $4,100 ($3,000–5,200), and $18,600 ($17,100–20,200) (Figure 1, all differences P< 0.001). Overall mean costs were higher for those with worse baseline vision ($28,100, $5,000, and $20,400, respectively) and lower for those with better baseline vision ($24,100, $3,200, and $16,900, respectively).
Figure 1. Components of total cost over one-year trial horizon.
Costs in United States Dollars (USD) over one-year are shown divided into study and non-study eye anti-VEGF injections separately, laser, and adverse events. Costs are presented for all participants and the worse and better vision subgroups
The largest component of total cost was study-eye anti-VEGF injections, comprising 68%, 37%, and 63% of total cost in the aflibercept, bevacizumab, and ranibizumab groups, respectively (Figure 1). Regardless of baseline vision, study-eye anti-VEGF injection costs were higher with aflibercept compared to bevacizumab or ranibizumab, and with ranibizumab compared to bevacizumab (all differences P<0.001). A difference in cost for adverse events or laser photocoagulation therapy was not identified between treatment groups for any of the baseline vision subgroups.
One-year cost-effectiveness
For all participants, ICERs of aflibercept and ranibizumab compared with bevacizumab were $1,110,000/QALY and $1,730,000/QALY, respectively (Table 1). For the subgroup with worse baseline vision, ICERs were $1,870,000/QALY and $2,450,000/QALY; with better baseline vision, they were $798,000/QALY and $1,500,000/QALY. Compared with ranibizumab, aflibercept’s ICER for all participants was $648,000/QALY, for the worse baseline vision subgroup was $1,270,000/QALY, and for the better baseline vision subgroup was $422,000/QALY.
Longer-term projections
The mathematical model of longer-term results produced similar 3-year adverse event rates, 5-year survival, and life expectancy to other published studies of similar populations, supporting the validity of these projections (eTable 5).10–12 Projected over a 10-year horizon, among all participants, the worse baseline vision subgroup, and the better baseline vision subgroup, respectively, the difference in QALYs with aflibercept vs. bevacizumab was 0.18, 0.22, and 0.13 (Table 1). Among all participants, the worse baseline vision subgroup, and the better baseline vision subgroup, respectively, the ICERs of aflibercept and ranibizumab versus bevacizumab were $349,000/QALY and $603,000/QALY, $287,000/QALY and $817,000/QALY, and $474,000/QALY and $506,000/QALY (Table 1). Among all participants, the worse baseline vision subgroup, and the better baseline vision subgroup, respectively, the ICER of aflibercept vs. ranibizumab was $203,000/QALY, $135,000/QALY, and $428,000/QALY.
Drug cost thresholds
Table 2 shows how much the drug costs of aflibercept and ranibizumab would need to be reduced for them to become cost-effective ($100,000/QALY) relative to bevacizumab. Across varying time horizons and baseline vision subgroups, the per-dose cost of aflibercept would need to fall by 60–90%. Considering all patients, those with worse baseline vision, and those with better baseline vision, respectively, the cost per dose of aflibercept would need to fall below $240, $250, or $230 (vs. a current cost of $1,850) to become cost-effective relative to bevacizumab over one year and below $570, $700, or $410 for the same three groups over a 10-year horizon. Ranibizumab would require even larger cost declines to become cost-effective relative to bevacizumab (75%−95%, depending on the subgroup and the time horizon). eTables 6 and 7 show the costs required to reach alternative cost-effectiveness thresholds of $50,000/QALY and $150,000/QALY. When compared to ranibizumab, aflibercept’s cost would need to be reduced by 18% considering all patients, 9% for those with worse baseline vision, and 28% for those with better baseline vision to reach a cost-effectiveness threshold of $100,000/QALY at 10 years (eTable 8). eTables 9A–C show 10-year incremental cost effectiveness ratios for a wide range of costs for aflibercept or ranibizumab.
Table 2:
Cost thresholds for ranibizumab and aflibercept
| 1-year horizon | 10-year horizon (projections) | ||||||
|---|---|---|---|---|---|---|---|
| Current drug cost per dose (2015 USD)* | Maximum cost per dose to achieve ICER of $100,000/QALY relative to bevacizumab (2015 USD)* | Relative reduction from current cost | Maximum cost per dose to achieve ICER of $100,000/QALY relative to bevacizumab (2015 USD)* | Relative reduction from current cost | |||
| All patients | |||||||
| Ranibizumab | 1,170 | 100 | 91% | 230 | 80% | ||
| Aflibercept | 1,850 | 240 | 87% | 570 | 69% | ||
| Baseline visual acuity 20/50 or worse† | |||||||
| Ranibizumab | 1,170 | 94 | 92% | 190 | 84% | ||
| Aflibercept | 1,850 | 250 | 87% | 700 | 62% | ||
| Baseline visual acuity 20/32 to 20/40‡ | |||||||
| Ranibizumab | 1,170 | 94 | 92% | 260 | 77% | ||
| Aflibercept | 1,850 | 230 | 88% | 410 | 78% | ||
USD, United States Dollars; QALYs, quality-adjusted life-years; ICER, incremental cost-effectiveness ratio
Cost per dose includes drug cost and compounding cost for bevacizumab.
ETDRS letter score <69.
ETDRS letter score 78 to 69
Sensitivity analyses
In univariate sensitivity analyses, varying the time horizon (eFigure 2), adverse event rates with the three drugs (eFigure 3), longer-term anti-VEGF injection frequency (eFigure 4), and the methods used to convert vision into quality-of-life (eTable 10), aflibercept and ranibizumab never reached an ICER below $100,000/QALY in comparison to bevacizumab.
Because quality-of-life benefits of treatment were linked to vision in the better-seeing eye in the base case analysis, a subgroup of only those with better vision in the study eye versus the non-study eye was examined. In this subgroup, ICERs of aflibercept and ranibizumab, respectively, compared to bevacizumab were $467,000/QALY and $603,000/QALY at one year and $210,000/QALY and $231,000/QALY at ten years.
When simulating variable long-term VA outcomes, aflibercept reached an ICER below $100,000/QALY relative to bevacizumab only if aflibercept had unrealistic long-term gains in VA and bevacizumab had declines (Figure 2). For instance, if aflibercept produced a 12-letter average gain in VA during years 2–10 of treatment while bevacizumab produced a 13-letter average decline, then aflibercept would have an ICER below $100,000/QALY relative to bevacizumab. For aflibercept to reach an ICER below $100,000/QALY compared to ranibizumab at 10 years, it would require additional average VA gains of at least 5 letters relative to ranibizumab over years 2–10 (eFigure 5).
Figure 2. 10-year cost-effectiveness, visual acuity outcomes sensitivity analysis.
The aflibercept-bevacizumab ICER is shown varying assumptions for VA changes over 10 years (aflibercept, horizontal axis; bevacizumab, vertical axis). The changing color indicates the 10-year ICER based on VA change with each drug. ICER, incremental cost-effectiveness ratio.
A probabilistic sensitivity analysis assessed overall uncertainty in patient outcomes and parameter assumptions. eFigures 6A–D show the resulting estimates of the likelihood that each treatment will be optimal (i.e., cost-effective), defined as producing the greatest QALYs while maintaining an ICER below a set willingness-to-pay per QALY. Over a one-year horizon, there was a >95% likelihood that bevacizumab would be the optimal therapy, irrespective of baseline vision, as long as willingness-to-pay is less than $530,000/QALY. Over a ten-year horizon, bevacizumab would have a >90% likelihood of being optimal at a willingness-to-pay of $100,000/QALY, irrespective of baseline VA. For the subgroup of patients with worse baseline vision, over a ten-year horizon aflibercept is more likely to be optimal than bevacizumab or ranibizumab at willingness-to-pay values ≥$230,000/QALY.
DISCUSSION
In the first year results of a DRCR.net trial in eyes with VA 20/50 or worse from DME, aflibercept produced greater average VA gains compared with bevacizumab or ranibizumab. The current analysis suggests that VA benefits of aflibercept translate into modest quality-of-life improvements, but at a high cost relative to bevacizumab, with ICERs substantially higher than thresholds of $50,000-$150,000/QALY frequently cited in cost-effectiveness literature and U.S. guidelines. They remain above these threshold values even under broad alternative assumptions. It is unlikely that any realistic differences in VA achieved with the 3 agents over years 2–10 (in the range of changes seen in prior studies) would alter their relative cost-effectiveness.1,4,18–20
With rapidly rising U.S. healthcare costs, and given the widely varying costs of intravitreous anti-VEGF agents, it seems important that payers, policymakers, and providers consider both the costs and benefits of these agents. This analysis demonstrates that, from the payer or policymaker perspective, using bevacizumab rather than the more expensive agents would be cost-effective. Similarly, in contexts where bevacizumab is not available, 0.3-mg ranibizumab would be more cost-effective than aflibercept.
From the perspective of patients or providers, however, the decision seems less clear-cut. For some patients with DME, the expected additional visual benefits conferred by aflibercept over bevacizumab at one year, or perceived concerns over repackaging risks or lack of an FDA indication with bevacizumab may outweigh the added health system cost ($22,000 at one year), and may outweigh any added personal expenses, such as co-payments. This tension highlights the challenge of balancing varying perspectives of patients, providers, payers, and policymakers when efficacy results and cost-effectiveness are at odds, or when inconsistent comparative safety results across these agents are reported in the literature.
Study limitations include using trial data only through one year of follow-up; longer-term results relied on outside data sources and mathematical modeling. However, sensitivity analyses indicate that results beyond one year would have to be strikingly different from prior data on long-term anti-VEGF outcomes1,4,18–20 to alter the study findings. Next, the bevacizumab in this trial was repackaged or compounded into sterile vials, which might cost more than typical costs of repackaging. However, even if the price per bevacizumab dose was raised to $710 such that a whole 4 mL container is used for every injection with the excess discarded,2 thus forgoing the need for repackaging, bevacizumab remains the most cost-effective option (eTable 9). Additionally, quality-of-life outcomes were based on VA outcomes and prior data relating VA to quality-of-life; while VA has been shown to be a reliable predictor of quality-of-life, direct measurement of quality-of-life still would be preferable.6 Patient-specific factors, such as VA in the untreated eye, also could alter cost-effectiveness results for an individual patient. It is important to exercise caution in applying these results to other countries; while the data show that aflibercept and ranibizumab are not cost-effective in the U.S., differing cost structures or lower negotiated prices may alter their cost-effectiveness in other health systems.
Conclusions
Aflibercept 2.0-mg and ranibizumab 0.3-mg are not cost-effective relative to bevacizumab for treatment of DME unless their prices decline substantially. Likewise, in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab. From a societal perspective, bevacizumab as first-line therapy for DME would confer the greatest value, along with substantial cost-savings versus the other agents. These results highlight the challenges that clinicians, patients, and policy-makers face when safety and efficacy results are at odds with cost-effectiveness results.
Supplementary Material
Acknowledgements
Funding/Support:
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY23207, EY18817. The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication. Regeneron provided the aflibercept for protocol T. Genentech provided the ranibizumab for Protocol T and funds to DRCR.net to defray the study’s clinical site costs. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol.
Eric Ross and David Hutton had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
A complete list of all DRCR.net financial disclosures can be found at www.drcr.net
Footnotes
A list of Network contributors can be found in the supplemental material.
Diabetic Retinopathy Clinical Research Network clinical sites that participated on this protocol: see Supplemental Material accompanying The Diabetic Retinopathy Clinical Research Network*, Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. New Engl J Med 2015; 372(13):1193–1203.
REFERENCES
- 1.Diabetic Retinopathy Clinical Research N, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Red Book Online [database online]. Greenwood Village, CO: Truven Health Analytics, Inc.; 2016. [Google Scholar]
- 3.Department of Health and Human Services. Office of Inspector General. Medicare payments for drugs used to treat wet age-related macular degeneration. 2012;OEI-03-10-00360.
- 4.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal Ranibizumab for Diabetic Macular Edema with Prompt versus Deferred Laser Treatment: 5-Year Randomized Trial Results. Ophthalmology. 2015;122(2):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Government Accountability Office. Medicare part B: expenditures for new drugs concentrated among a few drugs, and most were costly for beneficiaries. 2015;GAO-16–12.
- 6.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48(2):204–223. [DOI] [PubMed] [Google Scholar]
- 7.Thulliez M, Angoulvant D, Le Lez ML, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014;132(11):1317–1326. [DOI] [PubMed] [Google Scholar]
- 8.Pershing S, Enns EA, Matesic B, Owens DK, Goldhaber-Fiebert JD. Cost-effectiveness of treatment of diabetic macular edema. Ann Intern Med. 2014;160(1):18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein JD, Newman-Casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013;120(9):1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonafede MM, Johnson BH, Richhariya A, Gandra SR. Medical costs associated with cardiovascular events among high-risk patients with hyperlipidemia. ClinicoEconomics and outcomes research : CEOR. 2015;7:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125(4):544–550. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P, Annemans L, Gallagher M, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol. 2012;96(5):688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert review of pharmacoeconomics & outcomes research. 2008;8(2):165–178. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance MeasuresA Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(21):2304–2322. [DOI] [PubMed] [Google Scholar]
- 15.Hutton D, Newman-Casey PA, Tavag M, Zacks D, Stein J. Switching to less expensive blindness drug could save medicare part B $18 billion over a ten-year period. Health affairs. 2014;33(6):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 17.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641. [DOI] [PubMed] [Google Scholar]
- 18.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. [DOI] [PubMed] [Google Scholar]
- 20.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.