Abstract
The neural basis of reading and writing has been a source of inquiry as well as controversy in the neuroscience literature. Reading has been associated with both left posterior ventral temporal zones (termed the “visual word form area”) as well as more dorsal zones, primarily in left parietal cortex. Writing has also been associated with left parietal cortex, as well as left sensorimotor cortex and prefrontal regions. Typically, the neural basis of reading and writing are examined in separate studies and/or rely on single case studies exhibiting specific deficits. Functional neuroimaging studies of reading and writing typically identify a large number of activated regions but do not necessarily identify the core, critical hubs. Last, due to constraints on the functional imaging environment, many previous studies have been limited to measuring the brain activity associated with single-word reading and writing, rather than sentence-level processing. In the current study, the brain correlates of reading and writing at both the single- and sentence-level were studied in a large sample of 111 individuals with a history of chronic stroke using voxel-based lesion symptom mapping (VLSM). VLSM provides a whole-brain, voxel-by-voxel statistical analysis of the role of distinct regions in a particular behavior by comparing performance of individuals with and without a lesion at every voxel. Rather than comparing individual cases or small groups with particular behavioral dissociations in reading and writing, VLSM allowed us to analyze data from a large, well-characterized sample of stroke patients exhibiting a wide range of reading and writing impairments. The VLSM analyses revealed that reading was associated with a critical left inferior temporo-occipital focus, while writing was primarily associated with the left supramarginal gyrus. Separate VLSM analyses of single-word versus sentence-level reading showed that sentence-level reading was uniquely associated with anterior to mid-portions of the middle and superior temporal gyri. Both single-word and sentence-level writing overlapped to a great extent in the left supramarginal gyrus, but sentence-level writing was associated with additional underlying white matter pathways such as the internal capsule. These findings suggest that critical aspects of reading and writing processes diverge, with reading relying critically on the ventral visual recognition stream and writing relying on a dorsal visuo-spatial-motor stream.
Keywords: Reading, Writing, Aphasia, Temporal lobe, Parietal lobe, Alexia, Agraphia, Supramarginal gyrus, Inferior parietal cortex
1. Introduction
Reading and writing are relatively recent human developments that originated several thousand years ago (Powell, 2009). As such, the human brain did not evolve to support these skills but rather these abilities had to be built upon pre-existing neural structures (Dehaene, 2009). The precise nature and location of these structures has been a source of debate in the neuroscience literature (Cohen et al., 2002; Price and Devlin, 2003). One particular issue in this debate is the extent to which reading and writing are mediated by overlapping cognitive and neural processes (Kemmerer, 2014; Tainturier and Rapp, 2003). Both are complex communicative skills involving the precise coordination of numerous componential processes. Intuitively, one would expect that some core aspects of these skills would overlap, given their heavily intertwined content, development, and usage (Lorch, 2013). Early case studies around the turn of the 20th century by Dejerine and others highlighted the importance of left posterior cortex in both reading and writing. Dejerine (1891) claimed that the left angular gyrus was a critical word center for both reading and writing based on his case, Mr. C, who initially had pure alexia (an impairment in reading without aphasia) followed five years later by a sudden onset of agraphia (an impairment in writing). At autopsy, however, Dejerine noted two distinct lesions: An older lesion in left inferior occipito-temporal cortex and a more recent lesion situated in the angular gyrus/inferior parietal cortex (Dehaene, 2009; Hanley and Kay, 2003; Marshall and Newcombe, 1973). Consistent with this case, lesions associated with pure alexia are commonly localized to left posterior inferior temporal cortex (Dehaene, 2009). In contrast, lesion sites associated with agraphia reported in the literature have been quite varied (Assal et al., 1970; see Lorch, 2013 for a review) and include left inferior parieto-occipital cortex (Gerstmann, 1930; Levine et al., 1988), left posterior parietal cortex (Auerbach and Alexander, 1981), left posterior parietotemporal cortex (Kinsbourne and Rosenfield, 1974; Roux et al., 2014), left posterior middle frontal cortex (Exner, 1881; Roux et al., 2009), as well as subcortical regions (Laine and Marttila, 1981; Tanridag and Kirshner, 1985).
Modern imaging has enabled a more detailed analysis of lesions associated with specific reading and writing impairments, as well as the networks active in healthy individuals during the execution of these skills (Dehaene, 2009; Price and Friston, 2002; Price et al., 2003; Price and Mechelli, 2005; Ripamonti et al., 2014; Turkeltaub et al., 2002). With respect to reading, a number of both lesion and functional neuroimaging studies have pointed to the critical importance of a portion of left ventral temporo-occipital cortex known as the visual word form area (Cohen et al., 2000, 2002; Dehaene, 2009; Mani et al., 2008). This area forms a core part of a larger reading network that has been suggested to comprise occipital cortex for processing printed/written features, anterior left temporal cortex for processing meaning, posterior parietal cortex for top-down attention and serial reading, and left inferior fronto-insular regions for oral reading (Dehaene, 2009; Jobard et al., 2003; Nakamura et al., 2012; Price, 2012). Other regions have also been implicated, including the left posterior superior temporal gyrus, pre-central gyrus (bilaterally), superior temporal sulcus (bilaterally), right superior frontal gyrus, and the cerebellum (Mechelli et al., 2003; Price et al., 2003).
In the modern era, much less attention has been dedicated to identifying the brain networks associated with writing (also referred to as spelling in the literature, depending on the task; Kemmerer, 2014; Lorch, 2013; Rapp and Dufor, 2011; Rapp et al., 2015; Starrfelt and Shallice, 2014). A recent meta-analysis of 11 functional imaging studies reported that the most consistent regions associated with writing were the left inferior temporal cortex, fusiform gyrus, inferior frontal cortex, and the posterior intraparietal sulcus (though not the angular gyrus, which has been frequently implicated in lesion studies; Purcell et al., 2011a). Another meta-analysis by Planton et al. (2013) also found that the left intraparietal sulcus was a consistent region associated with writing, but in addition found that the left middle frontal gyrus and right cerebellum were involved as well. As with the reading literature, it is unclear from functional neuroimaging studies which of these regions within the reading network are most critical to the process of writing and which are simply recruited as part of the overall activity.
Most relevant to the current investigation, only a few studies have attempted to identify the neural basis of both reading and writing in the same individuals. Tsapkini and Rapp (2010) studied an individual with a brain tumor who underwent surgical resection of the middle and anterior left fusiform gyrus and adjacent regions of the inferior temporal gyrus. He subsequently exhibited deficits in orthographic processing during reading (mainly reduced speed) and spelling of words (errors such as writing tipe for type). Philipose et al. (2007) used diffusion- and perfusion-weighted MRI in a large group of acute stroke patients to identify brain regions critical for (oral) reading and written spelling of words and pronounceable pseudowords (Philipose et al., 2007). They found that impairments in reading and writing were most strongly associated with hypoperfusion and/or infarcts in left posterior temporal cortex (Brodmann’s area (BA) 37), inferior parietal cortex (predominantly BA 40 but also BA 39 more weakly), and posterior superior temporal cortex. These regions were implicated in reading and writing both words and pseudowords.
Functional imaging studies in healthy individuals have also suggested some degree of overlap in the neural correlates of reading and writing. Rapp and Dufor (2011) and Rapp and Lipka (2011) reported that the left inferior frontal gyrus was activated in addition to the left mid-fusiform gyrus in healthy individuals during both reading and writing. Purcell et al. (2011b) found that both reading and writing relied on common lexical representations in left occipito-temporal cortex. In that study, writing words (by typing) uniquely activated the inferior frontal gyrus, middle/superior frontal gyrus, supramarginal gyrus, superior parietal lobe, and fusiform gyrus. A conjunction analysis of reading and writing revealed an overlap in the left inferior frontal gyrus and occipito-temporal cortex, with an area in occipito-temporal cortex lateral and superior to the visual word form area that was uniquely associated with writing.
Given the mixed findings with respect to the location and overlap between reading and writing processes in the brain, the main goal of the current study was to identify regions critical for these two learned skills in a single group of individuals. The second goal of this study was to additionally contrast the neural correlates of single-word versus sentence-level reading and writing, as previous literature has focused primarily on single words (Perfetti and Bolger, 2004). To address these goals, we conducted a retrospective, whole-brain analysis of lesion data from a group of 111 individuals with a history of left hemisphere stroke using voxel-based lesion symptom mapping (VLSM). VLSM identifies discrete brain regions that are most critical to a particular cognitive process on a voxel-by-voxel basis. Participants were tested and imaged in the chronic stage of recovery, when both lesion site and behaviors are relatively stable, and we used very stringent methods for evaluating the significance of regions in the VLSM analyses so that only the most critical regions would be highlighted.
Unlike previous case studies of alexia and agraphia involving single or small groups of cases, VLSM allowed for the analysis of lesion data from a large group of individuals who suffered from a range of reading and writing impairments that could be quantified as continuous performance scores rather than a binary cut-off (e.g., alexic versus not alexic, agraphic versus not agraphic; Ripamonti et al., 2014). In this way, VLSM could pinpoint the precise areas that, when damaged, lead to impaired reading and writing performance. Also, unlike functional imaging in healthy individuals which identifies all active regions, VLSM identifies those brain areas that are most critical to the behavior under study, including both grey and white matter regions. Furthermore, by studying individuals in the chronic phase of stroke, we were able to identify brain regions that are so critical to reading and writing, they are not compensated over time by plastic changes in neighboring or homologous brain regions.
To our knowledge, this study is the first to use a whole-brain, voxel-based lesion analysis to compare brain regions associated with reading and writing in a large cohort of chronic stroke patients. While we did not have complete lesion coverage of the ventral brain surface, our analysis did include coverage of ventral occipito-temporal regions previously identified by Dehaene, Cohen and colleagues as the visual word form area (Cohen et al., 2002, 2008). Based on the current literature, our first hypothesis was that both reading and writing would share common neural substrates in left posterior occipito-temporal cortex but that writing would show a unique reliance on regions in left sensorimotor and parietal cortex. Our second hypothesis was that, compared to single words, sentence-level reading and writing would be associated with additional left middle temporal regions, as well as left inferior frontal regions associated with working memory.
2. Methods
2.1. Participants
Language and brain imaging data were retrospectively analyzed from 111 individuals (28 female) in our database who met the following criteria: History of a single left hemisphere stroke, pre-morbidly right-handed, English as a first language, chronic phase of stroke (> 6 months), minimum 8th grade education, no other neurologic history, no severe psychiatric history (e.g., bipolar disorder, schizophrenia), concurrent brain imaging, no visual agnosia, normal/corrected-to-normal vision and hearing, and ability to comply with task instructions. The mean age of the sample was 60.6 years (SD = 11.5, range 31–86), mean education was 14.8 years (SD = 28.1, range 8–20), and mean months post-stroke was 49.2 months (SD = 50.1, range 11–271). These 111 participants were selected from the entire database of 601 patients because they met the above criteria and had been administered the reading and writing tasks. The primary reasons for exclusion of the other individuals were: 1) the presence of right hemisphere and/or multiple strokes, 2) no brain imaging data, and 3) no reading and writing data.
Based on performance on the Western Aphasia Battery (WAB; Kertesz, 1982), the participants included 37 individuals with anomic aphasia, 25 with Broca’s aphasia, 5 with conduction aphasia, 2 with global aphasia, 1 with transcortical sensory aphasia, 12 with Wernicke’s aphasia, and 29 who scored within normal limits (≥ 93.8 out of 100 points). This latter group includes individuals with very mild or no aphasic symptoms. Therefore, the sample of individuals included in this study exhibited a wide range of language impairments. There was no minimum aphasia score required for inclusion in the study, as long as participants met the inclusion/exclusion criteria described above. There was also a wide range of reading and writing deficits. Only a single patient in the sample had a pure alexia, that is, extremely impaired reading ability with relatively good overall language ability (in the mildly anomic range) and very little writing impairment. There were six individuals who exhibited a disproportionate impairment in writing, with minimal reading impairment and only a mild aphasia.
Although all individuals were pre-morbidly right-handed, some participants used their left hand for writing due to weakness/paralysis. However, writing performance was scored on accuracy only, and no points were deducted for poor penmanship or slowed writing. Furthermore, as can be seen in the figures below, motor regions were not the main focus of the VLSM results.
Informed consent was obtained from all study participants, and the study was carried out in accordance with the Helsinki Declaration and approved by the VA Northern California Institutional Review Board.
2.2. Materials and procedures
2.2.1. Behavioral tasks
All participants were tested on the WAB, which assesses a number of speech and language processes, including speech production, auditory comprehension, repetition, naming, reading, and writing. Standard administration and scoring was followed as described in the WAB manual (Kertesz, 1982). The only exception was simplified instructions, including gestures, to convey instructions to individuals with comprehension deficits. Individuals who still could not comply with task instructions were discontinued and thus were not included in this analysis.
For the current study, we analyzed participants’ scores from four WAB Reading subtests that involve single-word reading and sentence-level reading. (We excluded reading subtests that require additional speech and language processes that can confound reading performance, for example, oral reading and auditory word-matching). The three single-word reading subtests on the WAB require participants to 1) point to an actual object that matches a presented word, 2) point to the picture of an object that matches a presented word, and 3) point to a word that matches a picture. The sentence-level reading subtest requires participants to read a series of sentences that are missing a final word (e.g., Farmers often grow wheat, corn, and other grain. They can also produce____), and point to the word that best completes the sentence (in this example, coal, tractor, earth, or vegetables). Thus, both the sentence-level reading subtest and the single-word reading subtests all involve reading for comprehension.
The Writing section of the WAB comprises seven subtests. In order to most closely parallel the reading subtests described above, we analyzed data from two subtests that involved single-word writing (writing-to-dictation) and sentence-level writing (picture description). The single-word writing subtest required participants to write dictated words, consisting of common, high-frequency, concrete nouns similar to those in the single-word reading subtests described above. Scoring is based on accuracy (not penmanship), and partial credit is given for misspellings. The sentence-level writing task required participants to write a description of a black and white drawing of a picnic scene that stayed in view as they wrote. As per manual instructions, scoring is based on the number of sentences and words generated, with partial credit given for incomplete sentences and single words, and points deducted for spelling and paraphasic errors. Participants’ writing was not scored on accuracy (of content), organization, or relevance, and thus task scores primarily reflected their ability to generate sentences/words. As described above, participants with visual deficits and/or agnosia were not included in this study and thus visual perception impairment was minimized as a potential confound.
2.2.2. Brain imaging and lesion reconstructions
Participants’ lesions were reconstructed from 3D MRI T1 scans or 3D CT scans when MRI was contraindicated. Imaging was acquired in the chronic phase of stroke close to the time of testing, so that acute effects of the stroke had stabilized. For 49 individuals, high-resolution T1-weighted 3D MRI scans were obtained on a 1.5 T Phillips Eclipse scanner. T1-weighted images were acquired with a Spoiled Gradient Recall (SPGR) sequence (TR/TE = 15/4.47 ms, FOV = 240 mm, 256 × 256 imaging matrix, flip angle = 35°, 0.94 × 1.3 × 0.94 mm3 voxels, 212 coronal slices). Participants’ lesions were outlined directly on the patient’s T1 digital MRI image using MRIcro (Rorden and Brett, 2000) and then registered with the MNI template using the standard nonlinear spatial normalization procedure from SPM5 (Statistical Parametric Mapping, Wellcome Trust Centre for Neuroimaging). A cost function masking procedure was used to avoid distortions due to the presence of the lesion (Brett et al., 2001). T2 and FLAIR images were yoked to the T1 images in MRIcro to verify the extent of the lesion.
When 3D digital MRI images were not available, lesions were drawn from hard-copy CT (n = 34) or MRI films (n = 28). The CT scanner was a Siemens Somatom Emotion 16 CT scanner with 3 × 3 × 3 mm imaging, and MRI images were collected on the 1.5 T scanner described above. Lesions were outlined onto an 11-slice, standardized template (based on the atlas by DeArmond et al., 1989) by a board-certified neurologist who was blind to participants’ clinical presentation as well as the predictions of the study. Reliability with this technique has been previously demonstrated (Friedrich et al., 1998; Knight et al., 1988). The brain templates were then digitized and non-linearly transformed into MNI space (Collins et al., 1994) with SPM5. This transformation was achieved using 50 control point pairs to match anatomical features on the two templates. Slices were then aligned using a local weighted mean transformation implemented by the Matlab cpselect, cp2tform and imtransform image processing toolbox functions.
An overlay map of participants’ lesions is shown in Fig. 1. The overlay map only includes voxels affected in a minimum of five participants in each voxel, consistent with the constraints of the VLSM analyses (see below). As can be seen, the extent of coverage in the analyses included much of the left cerebral hemisphere and underlying white matter. The average lesion volume for the sample was 102.1 cc (SD = 85.6, range = 1–421 cc).
Fig. 1.
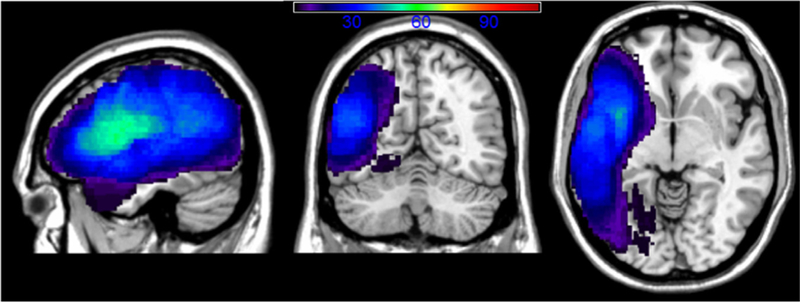
Lesion overlay map showing the degree of overlap across participants’ lesions, with a minimum of 5 participants’ lesions in each voxel. This 5-patient cut-off was used in order to reflect the VLSM analyses shown below, which also used this cut-off to minimize spurious results.
We also generated an estimated power map based on a medium effect size of 0.5 and alpha set at 0.01. As shown in Fig. 2, power ranged from 0.4 to 1.0 across most of the same brain regions identified by the lesion overlay map. Brain regions that were not represented by the overlay and power maps were not included in the predictions for this study.
Fig. 2.
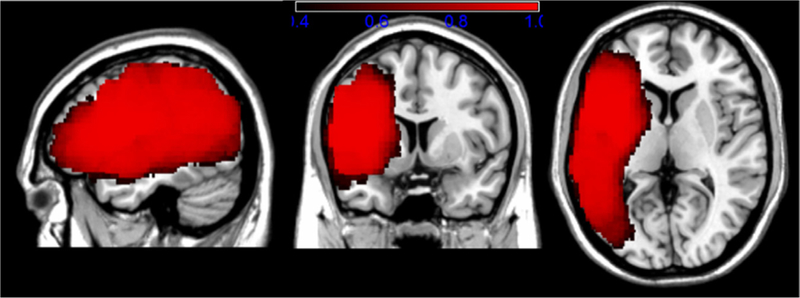
Estimated power map based on a medium effect size and alpha set at 0.01. Legend indicates power range from 0.4 (in black) to 1.0 (in bright red).
2.2.3. VLSM analyses
In order to identify the critical grey and white matter correlates of reading and writing, participants’ lesion reconstructions and behavioral data were analyzed using voxel-based lesion symptom mapping (VLSM; freely available at https://langneurosci.mc.vanderbilt.edu/resources.html). In this version of VLSM, linear regression functions are estimated at every voxel, comparing behavioral performance (here, reading and writing scores) in individuals with and without a lesion in each voxel. Analyses were limited to those voxels that had at least 5 individuals with a lesion in that voxel in order to minimize biased regression parameter estimates. Permutation testing was used to determine the cut-off for a significant cluster size, based on 1000 iterations and alpha for the voxelwise threshold set at 0.05 (see Kimberg et al., 2007). This is a relatively conservative method to account for the large number of comparisons made across the brain. In addition, all VLSM analyses included lesion volume as a covariate. The auditory comprehension score on the WAB was used as an additional covariate for all VLSM analyses of writing due to the nature of the instructions in the writing tasks, particularly writing to dictation. Auditory comprehension on the WAB is tested by yes/no questions, word recognition, and sequential commands. The figures shown below in the results highlight only those voxels surpassing the significance threshold. Identification of the brain regions associated with the significant voxels in each map was made with the Brodmann, AAL, and Johns Hopkins white matter atlas templates in MRIcron.
3. Results
3.1. Behavioral findings
Mean performance on the single-word reading subtests was 17.0 out of a possible 18 points (SD = 3.2, range = 1–18) and 28.2 out of a possible 40 points (SD = 12.5, range = 0–40) on the sentence-level reading subtest, reflecting the fact that participants were generally very good at reading and understanding single words, but considerably less successful and more variable when they had to read and comprehend sentences. On the Writing section, participants’ mean performance on single-word writing was 6.3 out of a possible 10 points (SD = 4.2, range = 0–10) and 15.8 out of a possible 34 points on sentence-level writing (SD = 13.9, range = 0–34).
3.2. VLSM findings: overall reading versus writing
To compare brain regions critical for reading versus writing, we ran a VLSM analysis of the overall Reading score (single-word and sentence-level reading combined) using the overall Writing score (single-word and sentence-level writing combined) as a covariate, and vice versa. These analyses allowed us to visualize critical regions associated with reading and writing, while minimizing the impact of general processes common to both. As described above, these analyses also included lesion volume and auditory comprehension (for Writing analysis) as covariates.
Fig. 3 shows the significant regions associated with Reading (in blue) when the overall Writing score was covaried, and the regions associated with Writing (in red) when the overall Reading score was covaried. The critical area for Reading was left occipito-temporal cortex, with a peak t-value of 7.42 located at the border of the lingual and fusiform gyri (− 30, − 58, − 2). The other remaining significant voxels for Reading included adjacent white matter (the optic radiations) and a few voxels in inferior parietal cortex. The critical areas remaining for Writing, after covarying the overall Reading score, were primarily located in the left inferior parietal cortex, namely, the supramarginal gyrus. There were also significant voxels in superior temporal cortex including Heschl’s gyrus, the angular gyrus, sensorimotor cortex, mid-frontal cortex, and underlying white matter. The peak t-value of 4.34 was located in the supramarginal gyrus (− 44, − 46, 42). The maximal additional variance explained by the Writing foci over and above that of reading and other covariates was 60% at the location of maximal statistical significance, and was 59% for Reading over and above Writing, both indicative of moderately-sized peak effects of predictive importance (Abdi, 2010).
Fig. 3.
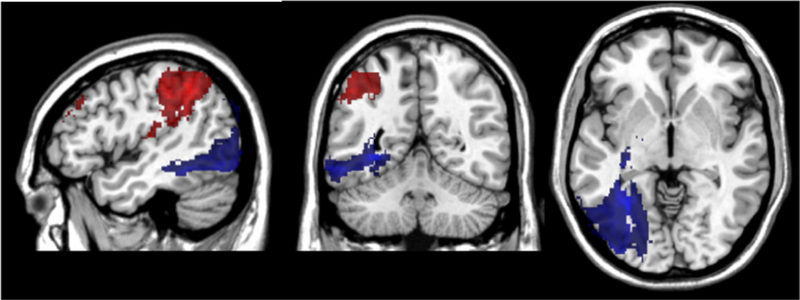
VLSM maps showing neural correlates of Reading (in blue) when overall Writing score is included as a covariate, and neural correlates of Writing (in red) when overall Reading score is used as a covariate.
3.3. VLSM findings: single-word reading versus sentence-level reading
Since much of the literature on reading and writing/spelling has focused on single words, it was of interest to map the neural correlates of single-word and sentence-level processing in both reading and writing. Fig. 4 shows separate VLSM maps for single-word reading and sentence-level reading with lesion volume included as a covariate in both maps. The significant voxels associated with single-word reading are shown in the red VLSM map, while voxels associated with sentence-level reading are shown in the green VLSM map. The overlap of the two VLSM maps is shown in yellow. As described above, only significant voxels are displayed that exceeded the permutation testing-based threshold for each respective map. For single-word reading, significant voxels were located primarily in the left temporo-occipital cortex and underlying white matter, with some extension into left posterior lateral temporal and inferior parietal cortex. The largest t-value (8.44) was centered at the border of the left lingual and fusiform gyri (− 30, − 58, — 2). Sentence-level reading was also associated with left temporo-occipital cortex and lateral temporal cortex, but had a more anterior extension that included significant voxels in the superior portion of the temporal pole, inferior and posterior insula, and the white matter adjacent to the insula. The peak t-value for sentence-level reading was 4.74, located in the white matter underlying the left middle temporal gyrus (− 36, − 54, − 2).
Fig. 4.
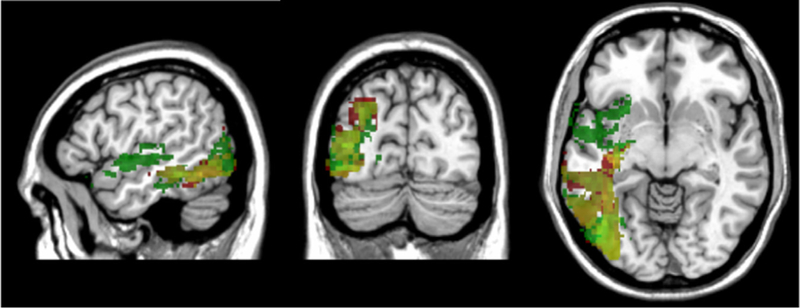
VLSM maps of single-word reading (red) and sentence-level reading (green), with yellow indicating regions of overlap. Lesion volume was used as a covariate in both maps
3.4. VLSM findings: single-word writing versus sentence-level writing
Next, we ran separate VLSM analyses of single-word writing and sentence-level writing on the WAB, with lesion volume and auditory comprehension included as covariates in both analyses. The significant voxels associated with single-word writing are shown in the red VLSM map, while voxels associated with sentence-level writing are shown in the green VLSM map. The overlap of the two VLSM maps is shown in yellow (see Fig. 5). As can be seen, the majority of significant voxels for both single-word and sentence-level writing were located primarily in the left supramarginal gyrus and the underlying white matter. For single-word writing, the majority of significant voxels were located in the left supramarginal gyrus with some extension into the angular gyrus, postcentral gyrus, and Heschl’s gyrus. The maximum t-value for single-word writing was 5.16, located at the border of the supramarginal gyrus and somatosensory cortex (− 50, − 30, 44).
Fig. 5.
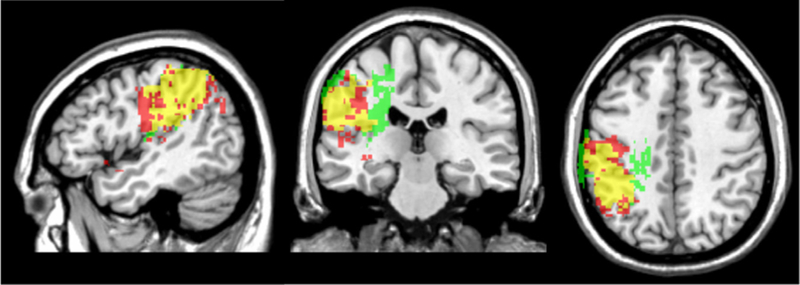
VLSM maps of single-word writing (red), sentence-level writing (green), and regions of overlap (yellow). Lesion volume was used as a covariate in both maps.
The areas associated with sentence-level writing were similar to single-word writing, including a large overlapping area primarily in the left supramarginal gyrus, as well as voxels in the postcentral gyrus and Heschl’s gyrus, but uniquely included underlying white matter regions corresponding to the corona radiata, external capsule and posterior limb of the internal capsule. A small percentage of significant voxels were also present in the white matter underlying parietal cortex, corresponding to the location of the posterior/dorsal portion of the superior longitudinal fasciculus. The maximum t-value for sentence-level writing was 4.47 in the internal capsule (− 28, − 18, 18).
4. Discussion
In the current study, we used a whole-brain, voxel-based, statistical approach to identify the brain areas critical for reading and writing at both the single-word and sentence-level. Voxel-based lesion symptom mapping (VLSM) allowed us to directly compare these abilities in a single, large group of well-characterized left hemisphere stroke patients suffering from a wide range of reading and writing impairments. Previous findings in smaller case studies and in healthy individuals have been mixed with respect to the degree to which reading and writing processes overlap in the brain. We found a robust dissociation between brain regions underlying these two abilities, with reading associated primarily with left posterior inferior/ventral temporal cortex (fusiform/lingual border) and writing associated primarily with left inferior parietal cortex, specifically the supramarginal gyrus.
The current findings are generally in keeping with a number of previous studies that have identified posterior ventral temporal cortex as a critical region for single-word reading. A portion of this region, sometimes called the visual word form area, has been implicated in a large number of different reading tasks, involving both words and non-words (Cohen et al., 2000, 2002; Dehaene, 2009; Mani et al., 2008). In the current study, the peak voxel associated with reading was at the fusiform/lingual gyrus border, just superior to the coordinates delineated by Cohen, Dehaene and colleagues for the visual word form area. Earlier studies of reading reported activation in both the left fusiform and lingual gyri (Fiez and Petersen, 1998), consistent with the current findings. While we did not have complete coverage of the ventral temporal surface of the brain, due to the nature of our patient sample and because we limited the VLSM analyses to those voxels with a minimum of 5 individuals with lesions in each voxel, we did have lesion coverage in the visual word form area based on coordinates identified by Cohen, Dehaene and colleagues (e.g., Cohen et al., 2002, 2008). Nonetheless, we repeated the primary analyses of overall reading and writing with a minimum of 3 (rather than 5) individuals in each voxel so that the ventral temporal surface was more completely sampled. The results did not change: The critical region for reading remained in the same area of the fusiform/lingual border.
Although this left posterior, ventral temporal area has been consistently associated with reading, there is some controversy as to the specificity of this region as an area devoted to word forms; it has been argued that this region also subserves other non-reading functions that involve visual-verbal integration, such as picture naming (Mani et al., 2008; Price and Devlin, 2003; Price et al., 2006; Vogel et al., 2013). For example, Binder et al. (2003) noted that left occipito-temporal regions are activated to a greater extent by object naming than by reading, suggesting that this region is not specific to orthographic processing but may “act as an interface in the retrieval of phonology from visual input.” While we did not directly address this issue of specificity in the current study, our previous VLSM study of naming identified the left mid-posterior middle temporal gyrus and underlying white matter as most critical to picture naming (Baldo et al., 2013). This region is distinct from the more ventral region associated with single-word reading in the current study. Further work to directly compare brain regions associated with these different modalities using matched stimuli is ongoing.
Unlike many previous studies of reading, all the tasks in the current study involved so-called “reading-for-comprehension.” That is, performance reflected participants’ ability to understand the words they were reading, rather than simply reading words aloud (which can be accomplished without any understanding). Our findings for single-word reading were generally consistent with previous studies demonstrating the critical nature of the ventral temporo-occipital cortex in reading. Our inclusion of higher-level sentence reading (for comprehension) showed additional involvement of more anterior and mid-temporal regions. These additional left temporal regions are likely associated with higher-level comprehension required for integrating semantic information across sentences (Price, 2012).
Our finding that writing is primarily dependent on left parietal cortex is generally consistent with a subset of findings in the literature that have reported this region as a critical component of writing and a cause of agraphia in case studies (Basso et al., 1978; Roeltgen and Heilman, 1984). There has been less agreement with respect to which portions of left parietal cortex are most critical, however (Sakurai et al., 2010). In the current study, the left supramarginal gyrus appeared to play the most critical role, consistent with a number of studies (e.g., Scarone et al., 2009). Similar to other studies that have assessed more motoric aspects of writing (Planton et al., 2013), we also found a small cluster of significant voxels in descending motor fibers, namely, the corona radiata and the posterior limb of the internal capsule (in sentence-level writing). The impact of motor dysfunction was minimized in our study because participants could use their unaffected left hand to write when necessary, and the writing subtests were scored only for content, not penmanship. Writing was also associated with some voxels in the postcentral gyrus that correspond to the hand area in the current study, but writing is typically associated with multi-modal brain regions in left parietal cortex where we found the majority of significant voxels.
Although writing performance was strongly associated with left parietal cortex in our analyses, there was some concern that we may have inadvertently factored out some ventral temporal regions by including auditory comprehension as a covariate (since some pictures are used as stimuli to test auditory comprehension). Therefore, we re-ran the VLSM analysis of the writing scores without auditory comprehension as a covariate, and it did not alter the left parietal focus for writing. In addition, we ran a VLSM analysis on the auditory comprehension score itself, and the significant voxels were primarily in posterior, lateral middle temporal cortex as we have reported elsewhere (Baldo and Dronkers, 2018), with no significant voxels in ventral temporal/visual word form regions.
The current findings are not consistent with some previous studies that have identified left frontal cortex as a critical region involved in writing (Katanoda et al., 2001; Sugihara et al., 2006; Roux et al., 2010). This may be due to the fact that, unlike some of these previous studies, the effects of motor impairment were minimized in the current writing tasks (as described above) and working memory demands were low. Also, some previous lesion and imaging studies have implicated left superior parietal cortex in writing (and sometimes reading; Auerbach and Alexander, 1981; Menon and Desmond, 2001), but there was no involvement of this brain region in the current analyses. Rather, we found that the supramarginal gyrus was most strongly associated with writing. The discrepancy may be due to the distinction between a more inferior (parietal) agraphia in which word/letter knowledge is disrupted versus a more superior (parietal) agraphia that is associated with an apraxic syndrome (i.e., impaired spatially-guided movements; Alexander et al., 1992). Last, previous studies have sometimes reported a role for the visual word form area in writing, but we did not find evidence of this in the current study.
A unique aspect of the current study was the analysis of both reading and writing performance in a single, large group of brain-injured individuals. As described above, we found evidence of a strong dissociation between these processes. A previous study in acute stroke patients (Philipose et al., 2007) reported common areas of hypoperfusion/infarct for reading and writing in a more posterior region of left temporo-parietal cortex. That study differed from the current one as it involved oral reading, included both words and pseudowords, and did not include sentence-level processing. Perhaps most importantly, our study differed in that it included only individuals who were many months and years post-stroke. These differences in study sample provide important, complementary information. Philipose et al. identified brain regions involved in reading and writing prior to any plasticity or reorganization. In contrast, the present study suggests that left posterior ventral temporal cortex and the supramarginal gyrus are apparently so critical for reading and writing, respectively, they cannot be compensated for, even after years of recovery.
Another unique aspect of the current study is that we included both single-word and sentence-level reading and writing. Previous studies in patients and healthy controls have focused primarily on single-word reading or writing, which allows for greater control and ability to manipulate experimental factors, but may not be representative of reading and writing at the sentence level. As mentioned above, we found a dissociation between regions associated with single-word reading and sentence-level reading, as the latter included additional regions in left anterior to mid-temporal cortex. Sentence-level reading likely relies on additional lexical-semantic representations and integration processes along the lateral temporal axis (Noppeney et al., 2005; Vandenberghe et al., 2002). With respect to writing, the regions critical for single-word and sentence-level tasks were largely overlapping and centered in inferior parietal cortex, although sentence-level writing was additionally associated with a number of underlying white matter pathways. More work needs to be done using better-matched word- and sentence-level stimuli and using sentence-level (or higher) stimuli that fully evaluate the brain networks involved in real-world reading and writing tasks.
As a complement to the VLSM analyses reported above, we also inspected our data for individuals who exhibited disproportionate impairments in reading or writing. There were six individuals who had relatively pure agraphia (i.e., greatly impaired writing performance with very mild aphasia and minimal reading impairment) and one individual with relatively pure alexia (i.e., greatly impaired reading performance with very mild aphasia and minimal writing impairment). When we overlaid the lesions from the six agraphic individuals, they were centered in left inferior parietal cortex, with the greatest area of overlap in the underlying white matter. None of the six patients’ lesions extended into the ventral temporal-occipital regions that we found to be associated with reading. Our single case of pure alexia had a large posterior cerebral artery stroke that encompassed left posterior ventral/inferior temporal-occipital cortex (including the lingual and fusiform gyri) and also extended dorsally into the angular gyrus. Significantly, the supramarginal gyrus was not affected in this individual, which likely accounts for her relatively preserved writing skills.
This study examined performance from a large group of individuals with a range of deficits in order to identify critical regions for reading and writing using VLSM. This type of large-scale, retrospective analysis allowed us to identify 111 stroke patients out of a much larger pool who met strict inclusion/exclusion criteria. Reading and writing performance was measured by the WAB, a commonly-used research and clinical tool, which lends itself to comparing results across studies as well as providing data relevant to the clinical realm. As much as possible, we selected WAB reading and writing conditions that had parallel task demands and used comparable, concrete nouns as stimuli. Also, unlike some previous studies of reading and writing, we minimized the potential confound of speech impairments by omitting oral reading and oral spelling subtests from the analysis. However, in order to amass data from over 100 patients, we had to rely on a retrospective analysis that did not include specialized testing with experimentally-matched stimuli.
Another limitation of the current study is that the use of a standardized language battery did not allow us to tease apart subcomponents of reading and writing. Writing has been proposed to involve the activation of a generic graphemic representation, conversion of the orthographic representation into a specific allographic pattern, and production of the corresponding sequence of graphomotor movements (Bonin et al., 2015; Planton et al., 2013; Rapp and Caramazza, 1997; Roux et al., 2009, 2014; Yuan and Brown, 2015). Results from the current study likely reflect the first two components, as participants were mostly at ceiling on separate subtests (not analyzed here) in which they had to write more automatic sequences like their names. Additional writing processes were also involved in the written picture description task as participants wrote sentences to describe a visual scene. However, since scoring was based on the number of complete sentences and single words in this task (not on accuracy of perception or completeness/organization), the VLSM results reflect primarily the ability to generate written sentences/words.
With respect to reading, the main cognitive subcomponents include visual processing of the printed or written features, generation and recognition of the orthographic form, accessing or converting the graphic representation into its phonological form, and the activation and integration of corresponding semantic representations (Carreiras et al., 2014; Dehaene and Cohen, 2011; Jobard et al., 2003). The WAB reading subtests used in the current study likely reflect all of these processes, with single-word reading more reliant on the first steps and sentence-level reading more reliant on the latter steps.
The use of VLSM is complementary to other techniques used to identify brain-behavior correlates. For example, VLSM can identify brain areas on a voxel-by-voxel basis that are critical for a given process, based on a continuous measure of impairment severity across a large sample, while case studies provide the opportunity to do more detailed testing and better characterize the nature of the processes subserved by these regions. The current study also complements smaller group studies that employ rigorously-matched experimental stimuli in order to tease apart the neural correlates of distinct subcomponents of reading or writing (Rapp et al., 2015). Also complementary, functional imaging studies in healthy individuals and acute stroke patients provide a snapshot of the larger network of regions recruited during a particular activity. In contrast, VLSM (in chronic patients) identifies primarily those regions most critical for a particular task that are not susceptible to plasticity or diaschitic changes. Another advantage of VLSM is that there is no need to identify regions of interest a priori and no need to define what is considered “impaired performance.”
The current study did not make predictions about brain regions where there was limited lesion coverage, such as the frontal pole, cerebellum, and other subcortical regions such as the basal ganglia. Some of these regions have been implicated in functional imaging and case studies, and may become involved when the core network is affected (Booth et al., 2007; Planton et al., 2013). For example, focal lesions to the putamen/internal capsule have been associated with impaired writing ability (Tanridag and Kirshner, 1985), and micrographia is a common symptom of basal ganglia-related diseases such as Parkinson’s (Marsden and Obeso, 1994). A number of focal cerebellar cases have been reported that present with an apraxic agraphia (Mariën et al., 2007; for a review, see De Smet et al., 2011). As described above, this form of agraphia is associated with spatial distortions and poor letter formation, and has also been associated with left superior parietal cortex. Apraxic agraphia has also been reported following damage to the left thalamus (Ohno et al., 2000). Interestingly, while the structural lesion in this case was focal in the left thalamus, functional imaging showed reduced activity in both the thalamus and the left dorsolateral premotor cortex (see also Osawa et al., 2013), illustrating the complex interactions of a broader network involving both subcortical and cortical regions.
Also, we only analyzed the role of the left hemisphere in reading and writing due to the availability of predominantly left hemisphere stroke patients in our participant database. The right hemisphere has been reported to come online during reading and writing in cases of injury/dysfunction in the left hemisphere (Cohen et al., 2004; Coltheart, 2000) and during higher-level discourse processing (similar to spoken language; St George et al., 1999). Because this was not a functional imaging study, we were not able to assess the degree to which this compensation took place in the current sample of chronic left hemisphere patients, but it may account for some of the observed variability. For example, one study showed that the right visual word form area homologue was activated during reading in a child who underwent resection of the left occipito-temporal region. Similarly, Tsapkini et al. (2011) showed compensation in bilateral anterior temporal cortex for reading and writing deficits following a left fusiform resection. Additional combined lesion/functional imaging studies such as these are needed to provide insight into such complex interactions of the reading and writing network during the recovery phase of acquired brain injury.
Another way to think about the dissociation between reading and writing observed in the current study is that of a functional distinction between dorsal versus ventral pathways. Such a model has been used to characterize distinct neural pathways underlying different components of speech and language, for example, a ventral stream for mapping sound to meaning and a dorsal stream for mapping sound onto motor (articulatory) representations (Hickok and Poeppel, 2000, 2004). In an analogous way, one can think of reading and writing as reliant on these two functional pathways: Reading is associated with the ventral stream that extends from posterior/ventral occipito-temporal cortex along the lateral temporal surface and is critical for visual recognition and semantic integration, while writing is most associated with the dorsal stream that extends from parietal to frontal cortex and is critical for visuospatial processing and guided movement (but see Carreiras et al., 2014; Cohen et al., 2008; Zhou et al., 2016 for discussion of ventral versus dorsal dissociation in reading alone). In the healthy individual, these two streams interact, for example, with dorsal stream processes exerting attentional control over the ventral, perceptual processes, so that a deficit in visual attention (in the dorsal stream) can lead to basic reading deficits (Boden and Giaschi, 2007; Lawton and Shelley-Tremblay, 2017; Vidyasagar and Pammer, 2010).
In summary, the current study used a whole-brain, voxel-based approach to identify critical brain regions associated with reading and writing in a large group of over 100 left hemisphere stroke patients. The focus of the current paper was not to establish the precise location of the visual word form area based on this sample of chronic lesion patients (much elegant work has gone into this previously), but rather to compare/contrast the regions critical for reading versus writing and for single-word versus sentence processing. We found that the critical brain regions associated with reading and writing (fusiform-lingual border and supramarginal gyrus, respectively) were strongly dissociated. This type of whole-brain, lesion analysis approach has certain advantages over smaller patient studies and functional imaging in healthy participants, but there were also limitations due to the retrospective nature of the approach. Findings from this VLSM study complement data from other research modalities and further our understanding of the neural correlates of reading and writing.
Acknowledgments
This material is based on work supported by the U.S. Department of Veterans Affairs, Office of Research and Development CSR&D Grant (CX000254–04). The contents reported/presented within do not represent the views of the Department of Veterans Affairs or the United States Government. We would like to thank our lab colleagues for their comments on earlier versions of the manuscript and most importantly, the research participants who took part in this study.
References
- Abdi H, 2010. Partial least squares regression and projection on latent structure regression (PLS regression). Wiley Interdiscip. Rev.: Comput. Stat 2 (1), 97–106. [Google Scholar]
- Alexander MP, Fischer RS, Friedman R, 1992. Lesion localization in apractic agraphia. Arch. Neurol 49 (3), 246–251. [DOI] [PubMed] [Google Scholar]
- Assal G, Chapuis G, Zander E, 1970. Isolated writing disorders in a patient with stenosis of the left internal carotid artery. Cortex 6 (2), 241–248. [DOI] [PubMed] [Google Scholar]
- Auerbach SH, Alexander MP, 1981. Pure agraphia and unilateral optic ataxia associated with a left superior parietal lobule lesion. J. Neurol. Neurosurg. Psychiatry 44 (5), 430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Arévalo A, Patterson JP, Dronkers NF, 2013. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston naming test. Cortex 49 (3), 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF, 2018. Lesion studies. In: de Groot A, Hagoort P (Eds.), Research Methods in Psycholinguistics and the Neurobiology of Language Wiley Blackwell, Hoboken, NJ. [Google Scholar]
- Basso A, Taborelli A, Vignolo LA, 1978. Dissociated disorders of speaking and writing in aphasia. J. Neurol. Neurosurg. Psychiatry 41 (6), 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L, 2003. Neural correlates of lexical access during visual word recognition. J. Cogn. Neurosci 15 (3), 372–393. [DOI] [PubMed] [Google Scholar]
- Boden C, Giaschi D, 2007. M-stream deficits and reading-related visual processes in developmental dyslexia. Psychol. Bull 133 (2), 346. [DOI] [PubMed] [Google Scholar]
- Bonin P, Méot A, Lagarrigue A, Roux S, 2015. Written object naming, spelling to dictation, and immediate copying: different tasks, different pathways? Q. J. Exp. Psychol 68 (7), 1268–1294. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T, 2007. The role of the basal ganglia and cerebellum in language processing. Brain Res 1133, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J, 2001. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14 (2), 486–500. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Armstrong BC, Perea M, Frost R, 2014. The what, when, where, and how of visual word recognition. Trends Cogn. Sci 18 (2), 90–98. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F, 2000. The visual word form area. Brain 123 (2), 291–307. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S, 2002. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain 125 (5), 1054–1069. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Henry C, Bourgeois M, Larroque C, Sainte-Rose C, Hertz-Pannier L, et al. , 2004. Learning to read without a left occipital lobe: right-hemispheric shift of visual word form area. Ann. Neurol 56 (6), 890–894. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Vinckier F, Jobert A, Montavont A, 2008. Reading normal and degraded words: contribution of the dorsal and ventral visual pathways. Neuroimage 40 (1), 353–366. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC, 1994. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr 18 (2), 192–205. [PubMed] [Google Scholar]
- Coltheart M, 2000. Deep dyslexia is right-hemisphere reading. Brain Lang 71 (2), 299–309. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Fusco MM, Dewey MM, 1989. Structure of the Human Brain: A Photographic Atlas Oxford University Press, New York, NY. [Google Scholar]
- Dehaene S, 2009. Reading in the Brain: The New Science of How We Read Penguin, New York, NY. [Google Scholar]
- Dehaene S, Cohen L, 2011. The unique role of the visual word form area in reading. Trends Cogn. Sci 15 (6), 254–262. [DOI] [PubMed] [Google Scholar]
- Dejerine J, 1891. Sur un cas eccite verbale avec agraphie, suivi d′autopsie. C. R. Soc. du Biol 43, 197–201. [Google Scholar]
- De Smet HJ, Engelborghs S, Paquier PF, De Deyn PP, Mariën P, 2011. Cerebellar-induced apraxic agraphia: a review and three new cases. Brain Cogn 76 (3), 424–434. [DOI] [PubMed] [Google Scholar]
- Exner S, 1881. Untersuchungenüber die Lokalisation der Functioneninder Grosshirnrindedes Menschen 104. Wien, Braumuller, pp. 21–49. [Google Scholar]
- Fiez JA, Petersen SE, 1998. Neuroimaging studies of word reading. Proc. Natl. Acad. Sci. USA 95 (3), 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich FJ, Egly R, Rafal RD, Beck D, 1998. Spatial attention deficits in humans: a comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology 12 (2), 193. [DOI] [PubMed] [Google Scholar]
- Gerstmann J, 1930. Zur Symptomatologie der Herderkrankimgen in der Übergangsregion der unteren Parietal-und mittleren Okzipitalhirnwindung. Dtsch. Z. für Nervenheilkd 116 (1–6), 46–49. [Google Scholar]
- Hanley JR, Kay J, 2003. Monsieur C: Dejerine’s case of alexia without agraphia. Class. Cases Neuropsychol 2, 57–73. [Google Scholar]
- Hickok G, Poeppel D, 2004. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 92 (1), 67–99. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D, 2000. Towards a functional neuroanatomy of speech perception. Trends Cogn. Sci 4 (4), 131–138. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N, 2003. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage 20 (2), 693–712. [DOI] [PubMed] [Google Scholar]
- Katanoda K, Yoshikawa K, Sugishita M, 2001. A functional MRI study on the neural substrates for writing. Hum. Brain Mapp 13 (1), 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer D, 2014. Cognitive Neuroscience of Language Psychology Press, New York. [Google Scholar]
- Kertesz A, 1982. Western Aphasia Battery Test Manual Psychological Corporation, San Antonio, TX. [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF, 2007. Power in voxel-based lesion-symptom mapping. J. Cogn. Neurosci 19 (7), 1067–1080. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M, Rosenfield DB, 1974. Agraphia selective for written spelling: an experimental case study. Brain Lang 1 (3), 215–225. [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth C, 1988. The effects of lesions of superior temporal gyrus and inferior parietal lobe on temporal and vertex components of the human AEP. Electroencephalogr. Clin. Neurophysiol 70 (6), 499–509. [DOI] [PubMed] [Google Scholar]
- Laine T, Marttila RJ, 1981. Pure agraphia: a case study. Neuropsychologia 19 (2), 311–316. [DOI] [PubMed] [Google Scholar]
- Lawton T, Shelley-Tremblay J, 2017. Training on movement figure-ground discrimination remediates low-level visual timing deficits in the dorsal stream, improving high-level cognitive functioning, including attention, reading fluency, and working memory. Front. Hum. Neurosci 11, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DN, Mani RB, Calvanio R, 1988. Pure agraphia and Gerstmann’s syndrome as a visuospatial-language dissociation: an experimental case study. Brain Lang 35 (1), 172–196. [DOI] [PubMed] [Google Scholar]
- Lorch M, 2013. Written language production disorders: historical and recent perspectives. Curr. Neurol. Neurosci. Rep 13 (8), 369. [DOI] [PubMed] [Google Scholar]
- Mani J, Diehl B, Piao Z, et al. , 2008. Evidence for a basal temporal visual language center cortical stimulation producing pure alexia. Neurology 71 (20), 1621–1627. [DOI] [PubMed] [Google Scholar]
- Mariën P, Verhoeven J, Brouns R, De Witte L, Dobbeleir A, De Deyn PP, 2007. Apraxic agraphia following a right cerebellar hemorrhage. Neurology 69 (9), 926–929. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA, 1994. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain 117 (4), 877–897. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Newcombe F, 1973. Patterns of paralexia: a psycholinguistic approach. J. Psycholinguist. Res 2 (3), 175–199. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kuo WJ, Pegado F, Cohen L, Tzeng OJ, Dehaene S, 2012. Universal brain systems for recognizing word shapes and handwriting gestures during reading. Proc. Natl. Acad. Sci. USA 109 (50), 20762–20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ, 2003. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J. Cogn. Neurosci 15 (2), 260–271. [DOI] [PubMed] [Google Scholar]
- Menon V, Desmond JE, 2001. Left superior parietal cortex involvement in writing: integrating fMRI with lesion evidence. Cogn. Brain Res 12 (2), 337–340. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Duncan JS, Koepp MJ, 2005. Reading skills after left anterior temporal lobe resection: an fMRI study. Brain 128 (6), 1377–1385. [DOI] [PubMed] [Google Scholar]
- Ohno T, Bando M, Nagura H, Ishii K, Yamanouchi H, 2000. Apraxic agraphia due to thalamic infarction. Neurology 54 (12), 2336–2339. [DOI] [PubMed] [Google Scholar]
- Osawa A, Maeshima S, Yamane F, Uemiya N, Ochiai I, Yoshihara T, Tanahashi N, et al. , 2013. Agraphia caused by left thalamic hemorrhage. Case Rep. Neurol 5 (1), 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA, Bolger DJ, 2004. The brain might read that way. Sci. Stud. Read 8 (3), 293–304. [Google Scholar]
- Philipose LE, Gottesman RF, Newhart M, et al. , 2007. Neural regions essential for reading and spelling of words and pseudowords. Ann. Neurol 62 (5), 481–492. [DOI] [PubMed] [Google Scholar]
- Planton S, Jucla M, Roux FE, Démonet JF, 2013. The “handwriting brain”: a meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 49 (10), 2772–2787. [DOI] [PubMed] [Google Scholar]
- Powell BB, 2009. Writing: Theory and History of the Technology of Civilization John Wiley & Sons, Malden, MA. [Google Scholar]
- Price CJ, 2012. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62 (2), 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, 2003. The myth of the visual word form area. Neuroimage 19 (3), 473–481. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ, 2002. Functional imaging studies of neuropsychological patients: applications and limitations. Neurocase 8 (5), 345–354. [DOI] [PubMed] [Google Scholar]
- Price CJ, Gorno-Tempini ML, Graham KS, Biggio N, Mechelli A, Patterson K, Noppeney U, 2003. Normal and pathological reading: converging data from lesion and imaging studies. Neuroimage 20, S30–S41. [DOI] [PubMed] [Google Scholar]
- Price CJ, McCrory E, Noppeney U, Mechelli A, Moore CJ, Biggio N, Devlin JT, 2006. How reading differs from object naming at the neuronal level. Neuroimage 29 (2), 643–648. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mechelli A, 2005. Reading and reading disturbance. Curr. Opin. Neurobiol 15 (2), 231–238. [DOI] [PubMed] [Google Scholar]
- Purcell J, Turkeltaub PE, Eden GF, Rapp B, 2011a. Examining the central and peripheral processes of written word production through meta-analysis. Front. Psychol 2, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Napoliello EM, Eden GF, 2011b. A combined fMRI study of typed spelling and reading. Neuroimage 55 (2), 750–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Caramazza A, 1997. From graphemes to abstract letter shapes: levels of representation in written spelling. J. Exp. Psychol.: Hum. Percept. Perform 23 (4), 1130. [DOI] [PubMed] [Google Scholar]
- Rapp B, Dufor O, 2011. The neurotopography of written word production: an fMRI investigation of the distribution of sensitivity to length and frequency. J. Cogn. Neurosci 23 (12), 4067–4081. [DOI] [PubMed] [Google Scholar]
- Rapp B, Fischer-Baum S, Miozzo M, 2015. Modality and morphology: what we write may not be what we say. Psychol. Sci 26 (6), 892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Lipka K, 2011. The literate brain: the relationship between spelling and reading. J. Cogn. Neurosci 23 (5), 1180–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripamonti E, Aggujaro S, Molteni F, Zonca G, Frustaci M, Luzzatti C, 2014. The anatomical foundations of acquired reading disorders: a neuropsychological verification of the dual-route model of reading. Brain Lang 134, 44–67. [DOI] [PubMed] [Google Scholar]
- Roeltgen D, Heilman K, 1984. Lexical agraphia Brain. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M, 2000. Stereotaxic display of brain lesions. Behav. Neurol 12 (4), 191–200. [DOI] [PubMed] [Google Scholar]
- Roux FE, Dufor O, Giussani C, Wamain Y, Draper L, Longcamp M, Démonet JF, 2009. The graphemic/motor frontal area Exner’s area revisited. Ann. Neurol 66 (4), 537–545. [DOI] [PubMed] [Google Scholar]
- Roux FE, Draper L, Köpke B, Démonet JF, 2010. Who actually read Exner? Returning to the source of the frontal “writing centre” hypothesis. Cortex 46 (9), 1204–1210. [DOI] [PubMed] [Google Scholar]
- Roux FE, Durand JB, Réhault E, Planton S, Draper L, Démonet JF, 2014. The neural basis for writing from dictation in the temporoparietal cortex. Cortex 50, 64–75. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Asami M, Mannen T, 2010. Alexia and agraphia with lesions of the angular and supramarginal gyri: evidence for the disruption of sequential processing. J. Neurol. Sci 288 (1), 25–33. [DOI] [PubMed] [Google Scholar]
- Scarone P, Gatignol P, Guillaume S, Denvil D, Capelle L, Duffau H, 2009. Agraphia after awake surgery for brain tumor: new insights into the anatomo-functional network of writing. Surg. Neurol 72 (3), 223–241. [DOI] [PubMed] [Google Scholar]
- St George M, Kutas M, Martinez A, Sereno MI, 1999. Semantic integration in reading: engagement of the right hemisphere during discourse processing. Brain 122 (7), 1317–1325. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Shallice T, 2014. What’s in a name? The characterization of pure alexia. Cogn. Neuropsychol [DOI] [PMC free article] [PubMed]
- Sugihara G, Kaminaga T, Sugishita M, 2006. Interindividual uniformity and variety of the “writing center”: a functional MRI study. Neuroimage 32 (4), 1837–1849. [DOI] [PubMed] [Google Scholar]
- Tainturier MJ, Rapp B, 2003. Is a single graphemic buffer used in reading and spelling? Aphasiology 17 (6–7), 537–562. [Google Scholar]
- Tanridag O, Kirshner HS, 1985. Aphasia and agraphia in lesions of the posterior internal capsule and putamen. Neurology 35 (12) (1797–1797). [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Rapp B, 2010. The orthography-specific functions of the left fusiform gyrus: evidence of modality and category specificity. Cortex 46 (2), 185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Vindiola M, Rapp B, 2011. Patterns of brain reorganization subsequent to left fusiform damage: fMRI evidence from visual processing of words and pseudowords, faces and objects. Neuroimage 55 (3), 1357–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA, 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16 (3), 765–780. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ, 2002. The response of left temporal cortex to sentences. J. Cogn. Neurosci 14 (4), 550–560. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Pammer K, 2010. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends Cogn. Sci 14 (2), 57–63. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Petersen SE, Schlaggar BL, 2013. Matching is not naming: a direct comparison of lexical manipulations in explicit and implicit reading tasks. Hum. Brain Mapp 34 (10), 2425–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Brown S, 2015. Drawing and writing: an ALE meta-analysis of sensorimotor activations. Brain Cogn 98, 15–26. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang X, Xia Z, Bi Y, Li P, Shu H, 2016. Neural mechanisms of dorsal and ventral visual regions during text reading. Front. Psychol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]


