Abstract
INTRODUCTION:
Freezing of gait (FOG) is a debilitating, late motor complication of Parkinson’s disease (PD) that occurs in 50–80% of patients. Gait freezing significantly worsens quality of life by decreasing mobility and increasing falls. Studies have shown that patients with episodic freezing episodes also have deficits in continuous gait. We evaluated whether there was an objective gait correlate to the increased stumbling reported by many patients with gait freezing.
METHODS:
PD subjects and healthy controls (HC) were enrolled after IRB approval. Subjects with more than 1 fall/day or a Montreal Cognitive Assessment score <10 were excluded. Subjects walked at their normal pace, 8 lengths of a 20×4 foot pressure-sensor mat. Data was collected and analyzed using PKMAS software (Protokinetics) and statistical analysis performed using SPSS 22 (IBM).
RESULTS:
72 age matched subjects (22 PD FOG, 27 PD no-FOG, and 23 HC) were enrolled. Disease duration and Hoehn & Yahr scores were not significantly different between the PD groups. Mean dimensions of foot strike were not significantly different between groups, but PD FOG subjects had increased step-to-step variability in foot strike as measured by the percent coefficient of variation (%CV) in foot strike length compared to PD no-FOG and HC, independent of stride velocity. In PD no-FOG subjects, fallers also had higher variability in foot strike length compared to non-fallers.
CONCLUSION:
PD subjects with FOG had increased variability in foot strike suggesting that in addition to stride length variability, foot strike variability could contribute to imbalance leading to falls.
Keywords: freezing of gait, falls, Parkinson’s disease (5 max)
INTRODUCTION
Freezing of gait (FOG) is an episodic phenomenon manifested by the feet “sticking to the ground” for several seconds during active movement [1, 2]. Up to 90% of patients with PD have been reported to experience gait freezing by later stages of disease [3, 4], but early development of FOG has been associated with earlier development of postural instability, dyskinesias, and depression [4]. Compared with PD subjects without freezing, freezers have been reported to differentially regulate gait initiation [5–7], steady-state continuous gait [8–13], and turning [11, 14]. Cognitive reserve may play a role in FOG as freezers had greater deficits in executive function [15], set-shifting [16], and conflict resolution [17]. Earlier development of FOG has also been correlated with an earlier age of onset of cognitive decline [4].
PD patients with infrequent freezing episodes or levodopa responsive FOG often report feeling unsteady when walking and “stumbling”, even at peak levodopa efficacy. Since PD gait disorder classically is noted as a shuffling of the feet, we hypothesized that the “stumbling” behaviors reported could be related to differential regulation of foot strike in freezers. To answer this question, we evaluated continuous gait patterns in PD patients with and without FOG as well as healthy age matched controls, using a pressure sensor impregnated gait mat to measure foot strike.
METHODS
Subjects with idiopathic PD, based upon the UK Brain Bank criteria [18], were recruited from the Movement Disorders Clinic at the University of Arkansas for Medical Sciences after obtaining approval from the institutional review board and in compliance with the Declaration of Helsinki guidelines for research involving human subjects. Family members were asked to participate as healthy controls. All subjects provided written informed consent in accordance with the Declaration of Helsinki prior to any study activities being performed. Subjects in the PD FOG group met previously defined criteria for probable or definite FOG [19] (assessed by movement disorders trained neurologist - T.V). Exclusion criteria included more than 1 fall/day, Montreal cognitive assessment (MoCA) score <10, and the use of anti-dopaminergic medications in the year prior to assessment.
Clinical assessments
All subjects received a complete Unified Parkinson’s Disease Scale (UPDRS) [20] assessment (by T.V), a Hoehn and Yahr staging score [21], the Giladi Freezing of Gait Questionnaire (FOG-Q) [22], and were administered previously validated cognitive screening tests including the Montreal Cognitive Assessment (MoCA) [23], the Frontal Assessment Battery (FAB) [24], and the Scales for Outcome in Parkinson’s Disease - Cognition (SCOPA-Cog) [25]. Each subjects daily equivalent levodopa dose at the time of the assessment was calculated based on 100% bioavailability for immediate release formulations (Sinemet IR and Stalevo), and 70% bioavailability for extended release formulations (Sinemet CR and Rytary) [26].
Gait protocol and analysis
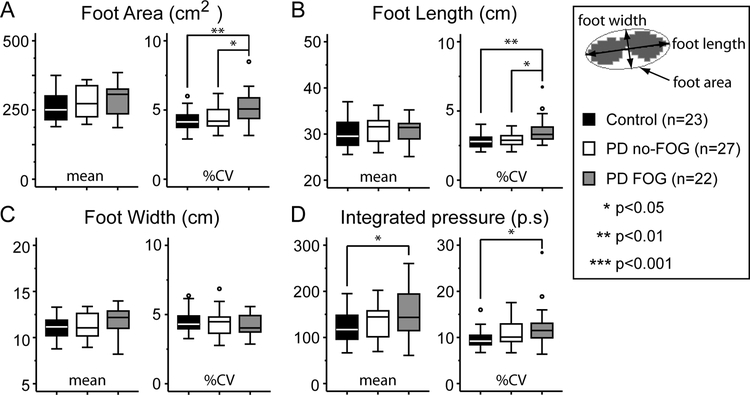
All PD subjects who were on levodopa were assessed in the levodopa ON medication state. All subjects were asked to walk at their comfortable pace, eight full lengths of a 20 foot long by 4 foot wide pressure sensor impregnated mat, Zeno Walkway (ProtoKinetics, Havertown, PA). Subjects were instructed to turn off the mat at both ends before making the return run. Data were collected and analyzed using Protokinetic Movement Analysis Software (PKMAS, ProtoKinetics). The software’s algorithm auto selects right and left feet. We independently analyzed each trial for selection accuracy and made corrections as appropriate. The first and last steps on the mat were excluded in order to avoid acceleration and deceleration effects associated with the edge of the mat. Trial periods in which patients stopped during the course of the walk, or had episodes consistent with freezing or hesitation were excluded from analysis. Gait kinematics (stride length, stride width, stride velocity, gait cycle time, stance and swing phase percentages, and single support and total double support percentages) were calculated by the software using previously defined algorithms. Foot area, foot length, and foot width were also analyzed using an inbuilt algorithm. Briefly, the software estimates an ellipse around each foot step and uses the major axis of the ellipse as the foot length, the minor axis as the foot width, and the area of the ellipse as the foot area (Fig 2A).
Statistical analysis
Analysis was performed using SPSS version 24 (IBM). Normality of data for each individual parameter was assessed using the Schapiro-Wilk test. Statistical significance between groups for normally distributed data was calculated using a one-way ANOVA with a post-hoc Bonferonni correction for multiple comparisons, otherwise the Kruskal-Wallace test with a post-hoc Bonferonni correction was used. The chi-square test was used to look for group differences in medical conditions. Pearson’s correlation coefficients were calculated for %CV in gait parameters compared to stride velocity. Univariate analysis using stride velocity as a covariate with post-hoc Bonferroni correction was performed on %CV variables. A step-wise multivariate model using FOG-Q scores as the dependent variable was also employed.
RESULTS
72 subjects were enrolled in this study including 23 healthy controls, 27 subjects with PD without freezing of gait (no-FOG), and 22 subjects with PD with freezing of gait (FOG). Mean age of subjects enrolled did not differ between groups (Table 1). There were more female participants in the control group as these were primarily spousal volunteers. Gastrointestinal and genitourinary conditions, and depression and anxiety were more common in the PD group compared to control (Supplementary Table 1) but there were no significant differences in the incidence of medical or neurologic conditions or surgical procedures between groups. Disease duration was similar between the groups (Table 1). FOG subjects had higher UPDRS motor and FOG-Q scores than no-FOG subjects, and were on a higher daily levodopa dose. The time from, and the dosage of, the last levodopa dose prior to assessments was not significantly different between PD groups (Table 1).
Table 1 -.
Demographics
| Data | Statistics | |||||
|---|---|---|---|---|---|---|
| Control (n=23) | PD no-FOG (n=27) | PD FOG (n=22) | Control vs. PD no-FOG | Control vs. PD FOG | PD no-FOG vs. PD FOG | |
| Age (years) | 61.4 ± 9.2 | 66.5 ± 6.2 | 65.7 ± 9.4 | n.s | n.s | n.s. |
| Gender (percent female) | 61% | 41% | 33% | - | - | - |
| Right handed | 87% | 81% | 87% | - | - | - |
| MoCA score | 27.7 ± 1.8 | 26.5 ± 2.7 | 24.7 ± 3.5 | n.s. | 0.003 | n.s. |
| FAB score | 16.7 ± 1.3 | 16.4 ± 2.3 | 13.7 ± 2.9 | n.s | 0.001 | 0.001 |
| SCOPA-Cog score | 29.7 ± 4.3 | 26.0 ± 4.5 | 21.7 ± 5.3 | 0.027 | <0.001 | 0.009 |
| Disease duration (years) | - | 7.3 ± 6.4 | 8.7 ± 5.5 | - | - | n.s. |
| Hoehn & Yahr score | 0.0 ± 0.0 | 1.7 ± 0.4 | 2.3 ± 0.7 | - | - | n.s. |
| FOG-Q score | 0.1 ± 0.4 | 2.5 ± 2.9 | 11.4 ± 3.5 | <0.001 | <0.001 | <0.001 |
| Subjects with falls | 0 | 19% (5/27) | 41% (9/22) | - | - | - |
| Fall frequency (/month) | 0.2 ± 0.1 (n=5) | 1.9 ± 3.8 (n=9) | - | - | n.s. | |
| UPDRS Part III Score (motor) | 0.8 ± 0.9 | 12.0 ± 5.7 | 18.8 ± 9.6 | <0.001 | <0.001 | 0.004 |
| Total UPDRS Score | 2.8 ± 2.3 | 23.1 ± 8.1 | 35.6 ± 11.0 | <0.001 | <0.001 | <0.001 |
| Total daily levodopa dose (mg) | - | 475.9 ± 358.5 | 720.0 ± 357.0 | - | - | 0.030 |
| Time to last levodopa dose (hrs) | - | 2.9 ± 3.5 | 2.0 ± 1.1 | - | - | n.s |
| Last levodopa dose (mg) | - | 145.7 ± 57.7 | 185.0 ± 74.5 | - | - | n.s. |
Gait dynamics:
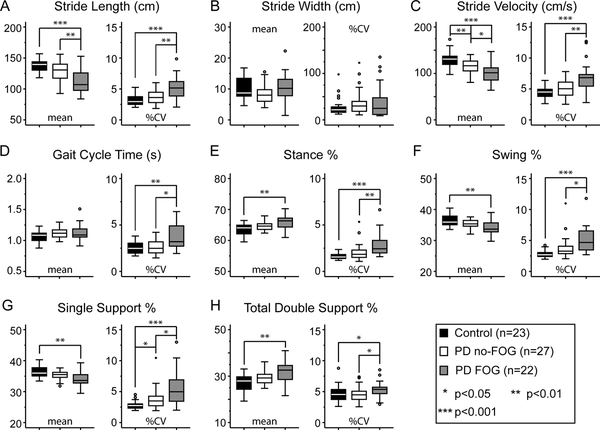
In our cohort, mean stride length was significantly shorter (Fig 1A) and mean stride velocity significantly slower (Fig 1C) in the FOG group compared to both the control and no-FOG groups. Mean stride velocity was also significantly slower in the no-FOG compared to the control group (Fig 1C). Mean percentage of time spent in the stance phase of the gait cycle (Fig 1E), and the mean total double support percentage (Fig 1H), were significantly higher in the FOG group compared to controls, with a corresponding decrease in the percent time spent in swing phase and single support (Fig 1F, G). There was a trend towards similar differences in gait cycle phases between PD groups that did not reach statistical significance. Mean stride width and mean gait cycle time were not significantly different between the groups.
Fig 1. Continuous gait measures.
Mean (left panel) and percent coefficient of variation (%CV; right panel) objective gait measures for controls (black bars), PD no-FOG (white bars) and PD FOG (gray bars). Statistics reported for ANOVA (parametric) or Mann-Whitney test (non-parametric).
Overall gait variability was higher in the FOG group as evidenced by an increased coefficient of variability (%CV) compared to the control and no-FOG groups. A higher %CV in single support percent was observed between the control and no-FOG groups (Fig 1G).
In order to measure foot strike, we analyzed total foot area, foot length, and foot width along with the integrated pressure applied during each step. FOG subjects exhibited higher variability in foot strike with a 30% increase in %CV in the foot strike area (Fig 2A right), compared to both controls and no-FOG subjects despite a similar mean foot strike area between groups (Fig 2A left). The variability in foot strike was primarily due to a 25% increase in %CV in the length of foot strike (Fig 2B right), as %CV in foot strike width (Fig 2C right) was similar. In order to exclude gross differences in foot size between the groups, we normalized the mean foot dimensions for each individual during continuous gait with the pressure trace of their feet when standing still. Once again, no differences between the mean normalized foot length, foot width, or foot area were observed. Subjects with FOG applied more pressure during each step and had increased variability in step-to-step pressure applied as measured by an increased %CV compared to controls but not the no-FOG group (Fig 2D). Mean integrated pressure values, when normalized to each individuals’ weight, were also significantly different between PD-FOG and controls (p=0.048; one-way ANOVA).
Fig 2. Measurement of foot strike.
Mean (left panel) and percent coefficient of variation (%CV; right panel) objective gait measures for controls (black bars), PD no-FOG (white bars) and PD FOG (gray bars). Statistics reported for ANOVA (parametric) or Mann-Whitney test (non-parametric).
Approximately 41% of FOG subjects reported falls in the 3 months prior to their gait assessments compared to 19% no-FOG subjects (Table 1). To determine whether foot strike variability plays a role in fall risk, we split subjects with PD into fallers and non-fallers. Within the no-FOG group, foot strike variability (measured as %CV in foot length) was significantly higher in those with falls (n=5) compared to those without falls (n=22), although the number of subjects in the falls group was small (p=0.031, one-way ANOVA) (Table 2). Foot strike variability was higher in both fallers and non-fallers with FOG compared to no-FOG non-fallers (p=0.009 and p=0.002 respectively, one-way ANOVA) using group wise comparisons. After Bonferroni correction, only the FOG non-fallers had a statistically higher foot strike variability compared to the no-FOG non-fallers, possibly due to the smaller numbers in the other groups (Table 2). Variability in the integrated pressure of the foot strike, stride length, and stride velocity were not different between groups (Table 2).
Table 2 –
Analysis of asynchronicity and falls
| PD no-FOG no-falls (n=22) |
PD no-FOG falls (n=5) |
PD FOG no-falls (n=13) |
PD FOG falls (n=9) |
|
|---|---|---|---|---|
| Foot length %CV | 2.8 ± 0.5 | 3.3 ± 0.3* | 3.7 ± 1.1** | 3.5 ± 0.9** |
| Integrated pressure %CV | 10.9 ± 3.0 | 12.1 ± 3.2 | 12.1 ± 5.5 | 12.7 ± 3.0 |
| Stride length %CV | 3.7 ± 1.2 | 3.9 ± 1.0 | 5.5 ± 2.8* | 5.7 ± 2.1** |
| Stride velocity %CV | 5.0 ± 1.5 | 5.4 ± 0.9 | 6.6 ± 2.3* | 7.5 ± 2.5** |
p<0.05,
p<0.01 vs. PD no-FOG no-falls
In order to test whether decreased stride velocity was the cause of increased variability (%CV) between the PD groups, we looked for correlations against mean stride velocity (Supplementary Table 2). Foot length, stride length, stride velocity, swing %, and single support % variability were inversely correlated with mean stride velocity in both groups. A univariate analysis using mean stride velocity as a covariate with post-hoc Bonferroni correction showed variability in foot length (p=0.03), stride length (p=0.02) and stride velocity (p=0.03) significantly differed between the PD groups.
We tested whether steady-state gait parameters in the FOG group were more predictive of freezing severity. Using the FOG-Q score as a dependent variable in a step-wise multivariate regression model with mean and %CV of the 12 gait parameters as independent variables (p=0.05 for inclusion), only the mean integrated pressure was included in the final model (R2=0.216; p=0.029). Using only questions 3–6 of the FOG-Q as a more direct assessment of FOG severity (frequency + maximal and average length of freezes) as the dependent variable in the same analysis, all parameters were excluded in the step-wise model.
DISCUSSION
In our cohort of PD subjects and age matched healthy controls, we found that during continuous gait, independent of freezing episodes, subjects with FOG had increased foot strike variability. This variability was primarily along the axis of motion as measured by the length of their foot strike (Fig 2). Foot strike variability was not due to differences in gait velocity as it remained significant between the FOG and no-FOG groups using stride velocity as a covariate (Supplementary Table 2). Additionally, this finding was present despite subjects being evaluated in the levodopa medicated state. Previous studies have shown that levodopa reduces stride (or step) time variability in both FOG and no-FOG subjects [9, 10]. Times when subjects shuffled or dragged their feet more along the ground would be visualized as increased foot length on the pressure trace, while an incomplete heel strike, for example, would be visualized as decreased foot length. Both of these phenomena could result in imbalance either by the foot catching on the ground with the shuffle or drag, or the decreased area of foot strike leading to less secure footing. This could be what patients describe as increased “stumbling” even during the levodopa ON medication state, as opposed to the actual freezing episodes described as the sensation of their “feet sticking to the ground”. Alternatively, increased episodes of longer foot strikes could potentially be reported as the feet sticking to the ground, since it is in contact for a longer period of time. In the future, real time analysis of gait may allow us to actively correlate these changes to patient perceptions of symptoms, which was not possible with our current evaluation technology.
We used an estimated ellipse around each foot step to calculate the foot length and width. While the measured foot length closely approximated the major axis of the surrounding ellipse (which exhibited the most variability in foot strike), foot width (estimated as the short axis of the ellipse) likely overestimates true foot width. However, as this estimation was the same for all subjects, this represents a systematic error across the study population and should not affect our overall results. Subjects were allowed to wear their own shoes, so differences in the material of the soles of shoes might influence foot strike. However, as shoe choice was random across all subject groups it should not bias towards any given group.
FOG subjects had an increased mean integrated pressure during a step and increased variability in step-step integrated pressure. By definition, integrated pressure provides us with a single number combining (i) the pressure applied, (ii) over the foot area in contact with the mat, (iii) for the time period the foot is in contact with the mat. As such, any of these three gait characteristics, or a combination thereof, could be individually changing in PD-FOG subjects. As discussed above, foot area and foot length were more variable but the mean dimensions were not changed in PD-FOG subjects. The time of foot contact with the ground, however, was longer in FOG patients compared with controls and no-FOG subjects, as shown by an increased percent time in the stance phase of the gait cycle (Fig 1), as well as an absolute increase in mean stance time (data not shown). As such, the increase in integrated pressure in PD-FOG could be a function of increased contact time, an increase in downward pressure, or both. This could be experienced as the heaviness that PD subjects sometimes report. Similarly, the increased variability in absolute stance time (or stance phase percent) and pressure applied, when resulting in a longer heavier step, could be a precursor to a freezing event or even a micro-freezing event itself that is not amenable to subjective visualization by the naked eye due to the rapid time scale. While our analysis excluded trials in which clear episodic freezing episodes were evident, future studies to correlate these variables and determine if they precede a freezing episode would help clarify this possibility.
While there was no difference in foot strike variability between fallers and non-fallers in the FOG group, interestingly, the few subjects in the no-FOG group who reported falls in the 3 months prior to the gait assessments did have increased foot strike variability compared with non-fallers in the group. While the number of fallers was small (n=5), increased foot strike variability in this group compared to non-fallers was on the order of that seen in the FOG freezer group, with no difference in stride length variability or stride velocity variability. Two possible explanations for this are considered. Foot strike variability may be an independent predictor of imbalance as both FOG and no-FOG subjects with falls had increased foot stride variability. Arguing against this would be the fact that FOG non-fallers also had increased foot strike variability. Alternatively, increasing foot strike variability could be an early marker of development of FOG. Longitudinal follow-up of the no-FOG group with increased foot-strike variability to determine if they convert to a freezing phenotype would help differentiate these two possibilities.
Only mean integrated pressure was inversely correlated with FOG-Q in a multivariate regression model. If we excluded questions 1–2, which ask about general gait disturbance, none of the gait factors correlated with the FOG-Q score. This suggests one of the following: (i) subjects with more severe gait freezing apply less pressure when stepping, which may in turn contribute to the increased imbalance, or (ii) the FOG-Q is a good predictor of subjective but not objective gait severity. We are unable to differentiate these based on our study.
A number of models have been proposed in regards to development of episodes of gait freezing (for review see Nieuboer and Giladi 2013 [27]); (i) the Threshold model [28], (ii) Interference model [29], (iii) Cognitive model [30], and (iv) Decoupling model [5]. Our results provide further evidence, as has been previously suggested [2, 27, 28], that these models are not independent of one another and likely integrate to explain FOG in different situations. The presence of increased foot strike variability adds support for the Threshold model, where continuous gait disturbances lower the threshold for development of a freezing episode, although the increased variability in integrated pressure seen in FOG subjects could be interpreted as micro-freezing episodes. Our data also reproduced previously described steady state gait changes used in support of this hypothesis. In support of the Cognitive model, as previously shown [15, 16], FOG subjects in our cohort had relative deficits on both the MoCA and SCOPA-Cog. In addition, similar to prior cohorts [15], our cohort showed specific declines in frontoparietal networks based on the lower FAB scores, compared to no-FOG subjects and controls. In freezers, MoCA scores, but not FAB or SCOPA-Cog scores, were also correlated with variability in integrated pressure and foot strike length, but not stride length or stride velocity (data not shown).
Our study is looking at a subgroup of subjects that presented to a tertiary care referral center, although there was no recruitment bias among the population offered enrollment. As UAMS has the only academic movement disorder clinic in the state, we also are not selecting for particular subpopulations of patients that present for provider sub-specialty interests. This is reflected in subjects enrolled with a wide range of motor disease duration (from 1–25 years), with an equally broad range of motor and functional disease severity as evidenced by the ON medication motor UPDRS ranging from 5–39/108, and the total ON medication UPDRS ranging from 8–58/176.
In summary, we describe novel features of foot strike variability in PD patients with FOG compared with their non-freezing or age matched healthy counterparts. These findings support an integrated model of episodic freezing and suggest gait characteristics that provide physiologic correlates to subjective symptomatology. Future longitudinal cohorts will allow us to determine how these features evolve over time, and whether they can be predictive of future development of FOG in PD patients treated with levodopa.
Supplementary Material
Acknowledgements
This work was supported in part by the University of Arkansas Clinician Scientist Program and by the NIGMS IDeA Program Center of Excellence award P30 GM110702. We also appreciate the mentorship and advice of Dr. Charlotte Hobbs.
Funding sources: The work was supported in part by funding from the University of Arkansas for Medical Sciences’ Clinician Scientist Program and by the NIGMS IDeA Program Center of Excellence award P30 GM110702.
Footnotes
Data statement:As this data was collected from human subjects with disease states, the IRB under which the data was collected does not allow for general sharing of raw data.
Ethics: This study was approved by the University of Arkansas’ institutional review board (IRB# 203234) and complies with the guidelines in the Declaration of Helsinki for research involving human subjects.
Other: All authors have agreed to conditions noted on the Authorship and Contributorship Form. This work has not been accepted for prior publication. The authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research. The corresponding author guarantees the accuracy of the references. The corresponding author had full access to all the data and has the right to publish any and all data.
Declaration of interest:
Jesal Shah is a medical student at the University of Arkansas for Medical sciences. She has no financial disclosures.
Lakshmi Pillai is a research technician at the University of Arkansas for Medical Sciences and received salary support from the Clinician Scientist Training program grant to Tuhin Virmani.
Shannon Doerhoff is an Advanced practice nurse employed by the University of Arkansas for Medical Sciences and has no other financial disclosures.
Dr. Williams is a Professor in Biostatics employed at the University of Arkansas for Medical Sciences. He has served on the advisory board for Core Metabolics, and received grant support from the NIGMS IDeA Program Center of Excellence award P30 GM110702.
Dr. Larson-Prior is a Professor of Psychiatry, Neurobiology & Developmental Sciences, & Neurology employed by the University of Arkansas. She received grant support from NSF EPScOR and NIH SBIR.
Dr. Garcia-Rill is Director of the Center for Translational Neuroscience and a Professor of Neurobiology & Developmental Sciences employed by the University of Arkansas for Medical sciences and he also received salary support from the University of Arkansas for Medical Sciences’ Clinician Scientist Program for mentoring Dr. Virmani. He has also received salary support as the PI of NIH award P30 GM110702, and has received reviewer fees from the NIH, as well as international grant agencies and has served as a consultant on the Las Vegas COBRE and the Chilean Millennium Project. He has also received Royalties from Elsevier.
Dr. Virmani is Director of the Movement Disorders Program and an Assistant Professor of Neurology employed by the University of Arkansas for Medical Sciences. He received salary and grant support from the University of Arkansas for Medical Sciences Clinician Scientist program as well as grant support from the NIGMS IDeA Program Center of Excellence award P30 GM110702.
REFERENCES
- [1].Fahn S The freezing phenomenon in parkinsonism. Adv Neurol. 1995;67:53–63. [PubMed] [Google Scholar]
- [2].Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destee A, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. 2014;71:884–90. [DOI] [PubMed] [Google Scholar]
- [4].Virmani T, Moskowitz CB, Vonsattel JP, Fahn S. Clinicopathological characteristics of freezing of gait in autopsy-confirmed Parkinson’s disease. Mov Disord. 2015;30:1874–84. [DOI] [PubMed] [Google Scholar]
- [5].Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Okada Y, Fukumoto T, Takatori K, Nagino K, Hiraoka K. Abnormalities of the first three steps of gait initiation in patients with Parkinson’s disease with freezing of gait. Parkinsons Dis. 2011;2011:202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, Coyne TJ, et al. A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain. 2011;134:2085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord. 2001;16:1066–75. [DOI] [PubMed] [Google Scholar]
- [9].Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149:187–94. [DOI] [PubMed] [Google Scholar]
- [10].Gilat M, Shine JM, Bolitho SJ, Matar E, Kamsma YP, Naismith SL, et al. Variability of Stepping during a Virtual Reality Paradigm in Parkinson’s Disease Patients with and without Freezing of Gait. PLoS One. 2013;8:e66718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Peterson DS, Plotnik M, Hausdorff JM, Earhart GM. Evidence for a relationship between bilateral coordination during complex gait tasks and freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:1022–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Ann Neurol. 2005;57:656–63. [DOI] [PubMed] [Google Scholar]
- [13].Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain. 2009;132:2151–60. [DOI] [PubMed] [Google Scholar]
- [14].Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning. Mov Disord. 2010;25:2563–70. [DOI] [PubMed] [Google Scholar]
- [15].Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord. 2008;23:395–400. [DOI] [PubMed] [Google Scholar]
- [16].Shine JM, Naismith SL, Palavra NC, Lewis SJ, Moore ST, Dilda V, et al. Attentional set-shifting deficits correlate with the severity of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:388–90. [DOI] [PubMed] [Google Scholar]
- [17].Vandenbossche J, Deroost N, Soetens E, Zeischka P, Spildooren J, Vercruysse S, et al. Conflict and freezing of gait in Parkinson’s disease: support for a response control deficit. Neuroscience. 2012;206:144–54. [DOI] [PubMed] [Google Scholar]
- [18].Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord. 2012;18:149–54. [DOI] [PubMed] [Google Scholar]
- [20].Fahn S, Elton R. The Unified Parkinson’s Disease Rating Scale In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. p. 153–63, [Google Scholar]
- [21].Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. [DOI] [PubMed] [Google Scholar]
- [22].Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord. 2009;24:655–61. [DOI] [PubMed] [Google Scholar]
- [23].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- [24].Slachevsky A, Villalpando JM, Sarazin M, Hahn-Barma V, Pillon B, Dubois B. Frontal assessment battery and differential diagnosis of frontotemporal dementia and Alzheimer disease. Arch Neurol. 2004;61:1104–7. [DOI] [PubMed] [Google Scholar]
- [25].Marinus J, Visser M, Verwey NA, Verhey FR, Middelkoop HA, Stiggelbout AM, et al. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61:1222–8. [DOI] [PubMed] [Google Scholar]
- [26].Mittur A, Gupta S, Modi NB. Pharmacokinetics of Rytary((R)), An Extended-Release Capsule Formulation of Carbidopa-Levodopa. Clin Pharmacokinet. 2017;56:999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nieuwboer A, Giladi N. Characterizing freezing of gait in Parkinson’s disease: models of an episodic phenomenon. Mov Disord. 2013;28:1509–19. [DOI] [PubMed] [Google Scholar]
- [28].Plotnik M, Giladi N, Hausdorff JM. Is freezing of gait in Parkinson’s disease a result of multiple gait impairments? Implications for treatment. Parkinsons Dis. 2012;2012:459321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lewis SJ, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:333–8. [DOI] [PubMed] [Google Scholar]
- [30].Vandenbossche J, Deroost N, Soetens E, Coomans D, Spildooren J, Vercruysse S, et al. Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Front Hum Neurosci. 2012;6:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.