Abstract
Green nanotechnology incorporates the principles of green chemistry and green engineering to fabricate innocuous and eco-friendly nanoassemblies to combat the problems affecting the human health or environment. Subsequently, amalgamation of green nanotechnology with drug delivery area has actually commenced a new realm of “green nanomedicine”. The burgeoning demand for green nanotechnology-driven drug delivery systems has led to the development of different types of delivery devices, like inorganic (metallic) nanoparticles, quantum dots, organic polymeric nanoparticles, mesoporous silica nanoparticles, dendrimers, nanostructured lipid carriers, solid lipid nanoparticles, etc. The present article deals with a brief account of delivery devices produced from green methods and describes site-specific drug delivery systems (including their pros and cons) and their relevance in the field of green nanomedicine.
1. Introduction
Drug delivery systems (DDS) are extensively studied and proliferated to ameliorate the efficacy and administration of active pharmaceutical compounds, such as drugs, vaccines, antibodies, enzymes, peptides, proteins, etc.1 The exigency to develop an advanced type of DDS lies in the limitations associated with conventional DDS (emulsions, suspensions, and solutions), e.g., first pass effect, instability, intolerance, fluctuations in plasma drug levels, no sustained effect, limited effectiveness, lack of selectivity, poor bioavailability, high dosage, etc.2,3 To achieve targeted delivery and to avoid rapid degradation of drugs or protection from clearance, a plethora of controlled DDS have been developed. During the years 1950–1980, the first generation of DDS was developed, dedicated toward transdermal and orally sustained release systems. However, between 1980 and 2010, the second generation (2G) of DDS came into being, which majorly focused on green nanotechnology.4 The third generation (from 2010) of DDS will have to be advanced much beyond 2G to overcome both physiochemical (poor water solubility, controlled drug release kinetics) and biological (specific target site delivery) barriers with the help of nontoxic excipients.
“Nanotechnology” is the term coined by Taniguchi in 1974, but its conceptual foundation was laid down by the Nobel laureate Richard Feynman in his well-known lecture “There’s Plenty of Room at the Bottom” on Dec 29, 1959. It involves manipulation, reduction, and fabrication of materials on a nanoscale with dimensions between 1 and 100 nm and characteristic properties, like improved stability, good strength, cost effectiveness, biocompatibility, definite and specific targeting, etc. It is predominantly the very small size and a large surface-to-volume ratio of nanoparticles (NPs) that accounts for significant differences in their chemical and physical properties. The importance of exploiting NPs as potent DDS (for the prevention and therapy of different diseases) resides in their improved bioavailability; controlled and sustained drug release; high drug loading capacity; prolonged circulation time; enhanced intracellular penetration and targeted delivery to specific sites or organs; protection of the active ingredient against physiological pH, enzymes, and moisture; usage of various ways of administration, including parenteral, oral, nasal, intraocular; and many more.5
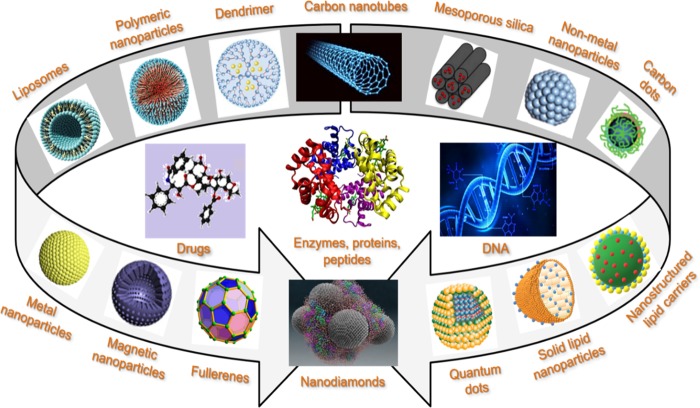
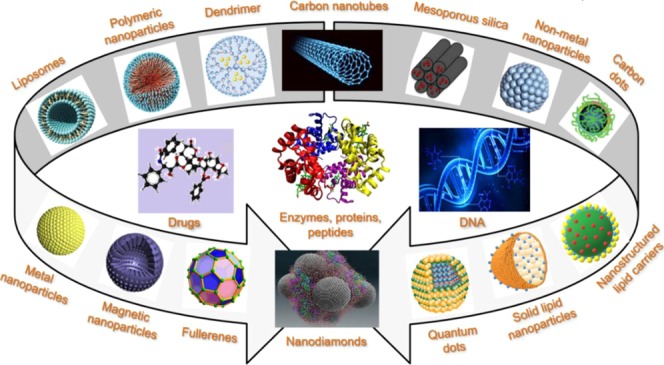
To date, a variety of nanocarriers, such as metal/metal oxide NPs, nonmetal NPs, quantum dots, polymeric NPs, silica NPs, carbon nanomaterials, liposomes, dendrimers, nanostructured lipid carriers, solid lipid nanoparticles, etc., have been designed to carry diverse molecules, such as drugs, peptides and proteins, DNA/RNA, antibodies, etc.5Figure 1 shows the structures of different nanovectors and types of pharmaceutical ingredients that can be efficiently targeted and delivered.
Figure 1.
Structures of different assemblies of colloidal systems designed to carry diverse molecules, such as drugs, enzymes, peptides, proteins, DNA, etc.
In the light of enhancing the quality of NPs for making them application specific with a sustained process of production, it became a prerequisite to synthesize the NPs using a green approach by taking into account the 12 principles of green chemistry.6 The green synthesis employs simple, cost-effective, eco-friendly, and readily available raw materials, with no harmful chemicals, no toxic by-products, and less steps in the procedure. The present article provides a succinct overview of different nanovectors (because of their unique properties, such as biocompatibility, biodegradability, amended cellular uptake, long shelf life with minimum drug loss, and minute toxicity) employed in drug delivery applications to address issues associated with traditional dosage forms in recent years. A brief account of NPs (namely, inorganic and organic NPs) has been outlined ahead, including their green synthesis and drug delivery applications.
2. Results and Discussion
2.1. Drifting from Classical to Greener Methods for Nanomaterial Synthesis
Over past decades, NPs have been synthesized using nongreen methods, the prominent ones being the chemical methods, which frequently employed hazardous chemicals and engendered serious concerns due to their toxicity. In traditional chemical methods, reducing and stabilizing agents were exploited for the reduction of metal ions and to prevent undesired agglomeration of produced NPs, respectively, but being bio-incompatible, they pose a threat to the environment. In the literature,7−10 classic cases have been documented that frequently report on the use of harmful reducing agents for the fabrication of silver (Ag), gold (Au), palladium (Pd), copper (Cu), and other NPs.7
Dhas and co-workers8 synthesized Cu NPs using thermal reduction method by reducing copper(II) hydrazine carboxylate in an aqueous solution. The fabrication of Ag NPs was carried out in micellar solutions using organic solvents and toxic surfactants. Chou et al.9 formulated spherical Ag NPs by chemical reduction method using formaldehyde as the reducing agent. Nickel and co-workers10 formulated Ag colloids of size 40–70 nm showcasing long-term stability in the presence of hydrazine hydrochloride (as a reducing agent).
Further, despite a great performance of quantum dots prepared using organometallic synthesis, the process requires the use of nongreen conditions, high-energy input, and toxic chemicals [dimethylcadmium (Cd(Me)2), hydrogen telluride (H2Te)], and harmful solvents [tri-n-octylphosphine oxide, hexadecyl amine, and trioctylphosphine as coordinating agents].11
Emulsion polymerization being one of the fastest methods has been often used for the preparation of polymeric NPs. Polyacrylamide NPs fabricated by emulsion polymerization method involved the use of toxic surfactants, monomers, initiators, and hazardous organic solvents (toluene, chloroform, and n-hexane),12,13 which need to be subsequently eliminated from the NPs.14
In recent years,15,16 it was realized by the scientific community that there is a need to develop simpler and greener methodologies in which exceptionally dangerous and unstable antecedents can be replaced by a nonpoisonous source. Moreover, to circumvent the negative drawbacks associated with conventional chemicals, integrating NPs by green nanotechnology seems to be a far better approach as it not only eliminates the use of toxic and expensive chemicals but also consumes lesser energy and generates environmentally benign products and by-products. For example, for synthesizing Ag and Au NPs, green reducing agents, like citrate, ascorbic acid, and many more, have been explored regularly. Even greener synthesis of Ag NPs on the surface of mesoporous silica nanoparticles have been reported17 using the simple and environment-friendly approach with tyrosine as the reducing agent. Palem et al.18 fabricated Rhubarb-based Ag NPs and chitosan cross-inked Ag nanocomposites using eco-friendly in situ synthesis in which active ingredients from the Rhubarb extract were attached to Ag NPs. The greener methodology in the QD arrangement includes the utilization of plant/microorganisms-interceded biosynthesis, a field that is able to produce highly fluorescent QDs under ambient conditions via a sustainable process.11 Moreover, the preparation of lipid NPs, such as solid lipid nanoparticles and nanostructured lipid carriers, has been carried out using greener methods, such as microemulsion, phase-inversion temperature, high-pressure homogenization, ultrasonication, and many other methods.19−22 These environment-friendly approaches do not require the use of organic solvents. Thus, synthesis of NPs utilizing natural constituents and greener methods has become a superior alternative with numerous advantages over classical chemical methods.
2.2. Inorganic NPs for Drug Delivery Applications
2.2.1. Metallic NPs and Their Oxides
Metallic NPs are widely exploited in the arena of biomedical science and engineering owing to their various advantages, such as the large surface area that allows the guest molecules (drug, probes, or protein) to bind, absorb, or entrap within the surface of the particles. Metallic NPs, such as Ag, Au, platinum (Pt), Pd, Cu, selenium (Se), iron (Fe), and their oxides, such as zinc oxide (ZnO), iron oxide (Fe2O3/Fe3O4), etc. have drawn significant attention as potential DDS because of their emerging applications in the field of clinical diagnostics and therapy.23 They are extensively used in the formulation of products ranging from pharmaceuticals and cosmetics to medical, e.g., Au NPs have been widely exploited in biomedical applications,24 disease diagnostics,25 and pharmaceuticals.26,27 Ag NPs have found widespread utilization in wound dressings, pharmaceutics, and medical implant coatings28,29 due to anti-inflammatory and antibacterial properties that pace up the wound healing process. Pt and Pd NPs have gained importance in biomedical30 and antibacterial applications,31 respectively. Cu NPs32 and Se NPs33 are largely utilized in antibacterial applications, cosmetic formulations, and medical treatments. ZnO NPs have never ceased to interest the researchers due to their good antimicrobial, antibacterial, and disinfectant properties, which led to the development of dermatological substances for curing inflammation and itching.34
Several methods, such as chemical methods, sol gel, and laser ablation, are reported in the literature,35,36 for the synthesis of metallic NPs, but these methods are not sustainable since the chemicals (formaldehyde, toluene, and many more) used are highly reactive and known to pose a potential threat to the environment. With the rising demand of metallic NPs in nanomedicine, it has become even more important to synthesize these NPs using benign, reliable, and environment-friendly green methods. There exist several routes for the synthesis of metal/metal oxide NPs from their corresponding metal salts. The approaches used for the synthesis of metallic NPs mainly involves the following: (a) top-down approach (NPs are formed by size reduction from a suitable starting material) and (b) bottom-up approach (NPs are synthesized from the smaller things, and the nanostructured building blocks of NPs are formed at the initial stage that further leads to the formation of final NPs).37
Metallic NPs have been studied as efficient pharmaceutical drug carriers for more than 30 years to improve the antitumor efficiency. Chen et al.38 fabricated methotrexate (MTX, a first-generation anticancer drug)-loaded Au NPs and evaluated the anticancer effect of the drug in Lewis lung carcinoma (LL2) and verified that the anticancer efficiency of MTX-Au NPs in LL2 cells was higher (more than 17-fold sensitive) than that of the free MTX drug solution.
Au NPs were also prepared by Bhumkar et al.26 for the transmucosal delivery of insulin drug and demonstrated a significant reduction of the blood glucose level via a transmucosal route in diabetic rats. In addition, the efficiency of functionalized 3-mercaptopropionic acid-capped Au NPs in drug delivery against K562/ADM cells was evaluated by Li and co-workers.39
Thirumurugan et al.40 reported the biological synthesis of noble-metal NPs (Pt and Ag NPs) using curry leaves and neem extracts. Doxorubicin (DOX), an anticancer drug, was encapsulated in synthesized NPs and its in vitro study was assessed against MCF-7 cell lines. On comparison of DOX-coated Ag NPs with the DOX-coated Pt NPs in terms of the percent inhibition against the MCF-7 cell line, a better inhibition value (IC50) was found for Ag NPs over Pt NPs. The concentration-dependent toxicity of both the drug-coated NPs in MCF-7 cell lines was observed.
Thambiraj et al.41 fabricated functionalized Au NPs by the chemical reduction method (using sodium citrate as the reducing and the capping agent) and obtained spherical shape NPs of the size of 21.75 nm. Docetaxel (DTX), an anticancer drug, was loaded in Au NPs, and cell culture studies were carried out using A549 cell line (lung cancer cell line) to evaluate the cytotoxicity effect of Au NPs and DTX at different intervals of time and at different concentrations. Excellent cell death by Au NPs was achieved as compared with DTX.
Mehnath et al.42 formulated poly(biscarboxyphenoxy)phosphazene-stabilized Au NPs for entrapment of Camptotheca (CPT, a quinolone alkaloid), and the release behavior of CPT as well as in vitro cytotoxicity effects were studied toward human breast cancer cells. The results show that the CPT@PCPP Au NPs were readily internalized by cancer cells owing to a charge reversal behavior as compared to the CPT drug alone. After internalization, the CPT released within the cytoplasm further enhanced the therapeutic effect through intercellular binding to Topoisomerase-1 and showed antiproliferative activities with respect to MDA-MB-231 cells.
Polyethylene-glycolylated Au NPs conjugated with Arg-Gly-Asp (RGD) peptide were fabricated by Wu et al.43 with the objective of targeting the cancer cells expressing RGD-binding integrins like α5 and αv integrins. These cells that were subjected to radiation treatment showed much lower invasion in the presence of Au NPs and suggested that the use of the integrin-tagged Au NPs could be a potential clinical route to arrest breast cancer development.
Banu et al.44 focused on Phoenix dactylifera pollen extract to synthesize Ag and Au NPs and screened their toxicity on MCF-7 breast cancer cell line and showed signs of apoptotic cell death. A significant level of dose-dependent cytotoxicity of metallic NPs as compared with pollen extract (no cytotoxicity) was shown due to the presence of anticancerous bioflavonoids on the surface of metallic NPs. Wang et al.45 synthesized cobalt ferrite (CoFe2O4) in the presence of l-cysteine (Lys) for encapsulation of DOX that exhibited a superb drug loading capacity and pH-sensitive drug release behavior. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay results showed an insignificant level of cytotoxicity of Lys-coated-CoFe2O4 NPs over 24 h, making them a suitable carrier for drug delivery applications.
Metallic NPs prepared using green methods are being used to act as a potential DDS owing to their chemical surface properties and different morphology in the field of pharmaceutics.
2.2.2. Quantum Dots (QDs)
QDs have proven themselves as an indispensable tool in the field of biological imaging, sensing, and detection, which has further encouraged scientists to develop entities for translational and clinical research. Being potent fluorescent probes, QDs are frequently employed in quantitative imaging and detection.46 QDs with therapeutic functionalities and assimilated targeting have become outstanding materials to study the drug delivery in cells and small animals.
To date, a broad range of QDs have been fabricated, such as carbon dots (C-QDs), silicon QDs (Si-QDs), graphene QDs (G-QDs), nitrogen-doped QDs (N-QDs), Ag2Se, Ag2S, ZnS, InP, etc. because of their good biocompatibility and excellent optical properties. Numerous drugs have been effectively incorporated within QDs to deliver them at the specific target site. Moreover, synthesis of QDs carried out by traditional methods requires the use of heavy metal precursor and organic solvents that pose a potential hazard to the atmosphere. In this field, the only way to make the process “greener” is to adapt benign synthetic approaches that include the use of environmentally renewable raw materials as coordinating solvents, such as oleic acid, castor oil, and olive oil.
Lee et al.47 fabricated the DOX-loaded green C-dots from cyanobacteria using an eco-friendly approach and reported a good loading efficiency (95%). Upon in vitro and in vivo delivery of DOX, the DOX-loaded C-QDs induced the death of MCF-7 and HepG2 cancer cells as well as tumor cells of mice, showcasing the improved anticancer efficiency of the drug-loaded QDs as compared to that of the free DOX injection. The first exploration of mitochondria as a drug delivery system in C-QDs for administration of the DOX showed that mitochondria as carriers are compatible with the C-QDs (prepared by the hydrothermal method) and preserve the properties of C-QDs.48 Moreover, the mitochondria delivery system not only increased the retention time but also improved the C-QDs’ biodistribution in organs. The mitochondria-based approach to deliver the DOX was found to be nontoxic, as mitochondria do not have the bacterial virulence that could be dangerous to cancer patients. The present pattern in the advancement of nanotheranostic platforms requires the perfect nanomaterials to ought to have great fluorescence, higher security, low poisonous quality, and be environmentally friendly. Excellent magnetic and fluorescent properties of gallium, nitrogen, sulfur, and gadolinium elements lead to the formation of magnetofluorescent C-QDs (GdNS@C-QDs) that can be functionalized with folic acid for targeting dual modal fluorescence/magnetic resonance imaging. DOX was incorporated within the functionalized C-QDs and high entrapment efficiency (>80%) with a pH-sensitive drug release was reported.49
Olerile and group50 fabricated paclitaxel (PTX)-loaded CdTe/CdS/ZnS QDs by emulsion evaporation method, which displayed a biphasic pattern of drug release with a 75% growth inhibition rate and then exploited them in target detection of the H22 tumor. Khodadadei et al.51 synthesized MTX-loaded N-QDs (of size 10 nm) by the hydrothermal method (using urea as a nitrogen source and pyrolysis of citric acid as a carbon source) and used as an effective anticancer drug delivery system. By using the hydrothermal technique, Fe3O4–Ag2O QD/cellulose nanofibers (of size 62.5 nm) for encapsulation of two types of medicines (etoposide and methotrexate) were fabricated by Fakhri et al.52 for anticancer application. Drugs were found equally distributed on the surface and showed a slow and steady release with the total amount of release of 78.94% for etoposide and 63.84% for methotrexate. The QDs synthesized during hydrothermal carbonization method exhibited attractive properties, such as low cost and nontoxicity.
The effectiveness of 5-fluorouracil (5-FU), an anticancer drug, was increased by using a suitable nanocarrier system that targeted the folate receptors of malignant tumor tissues. Yaghini et al.53 synthesized the Mn-ZnS QDs conjugated with folic acid (FA) and chitosan (CS) biopolymer for the encapsulation of 5-FU. The formed 5-FU@FACSMn/ZnS QDs showed the selective antitumor effect in 4TI breast cancer cell line and MDA-MB231 breast cancer line. Thakur et al.54 exploited the green chemistry protocol for the fabrication of aqueous soluble G-QDs (of size 5 nm) using one-pot microwave-assisted heating (cow milk as a precursor) method followed by encapsulation of anticancer drug-Berberine hydrochloride (BHC) for simultaneous imaging and drug delivery. Further, the theranostic G-QD@Cys-BHC complex showed a higher cytotoxic effect on different cancerous cell lines, such as 50% cell death obtained on MDA-MB-231 cell line and L929 cell line in 24 and 48 h, respectively. An eco-friendly and green route was adopted by Sarkar et al.55 to fabricate C-QDs using aloe vera gel via the pathway of carbonization, and its applicability in delivering vancomycin in presence or absence of β-CD, aiming to eliminate its complicacy of poor absorption in the gastrointestinal tract, was demonstrated. Fluorescent C-dots were synthesized by hydrothermal method via pasteurized milk (as a carbon source) that exhibited several advantages, such as good biocompatibility, environment friendliness, small size, excitation-dependent fluorescence property, and multifunctional groups (−NH2, −OH, C=O, and COOH). Mehta and co-workers56 reported that the MTT assay of bare CQs and Lis-loaded C-QD nanoassembly effectively targets the Hela cells whereas the bare C-QDs displayed a significant survival rate of Hela cells, suggesting the biocompatibility of the C-QDs.
As a potent imaging probe, QDs have played a significant role in in vitro drug release diagnostics and fundamental biology (due to their flexible drug linking, tunable and uniform size, doping mechanisms, and a large surface-to-volume ratio) that has supported new avenues of research.
2.3. Organic Nanoparticles for Drug Delivery Application
2.3.1. Polymeric Nanoparticles (PNPs)
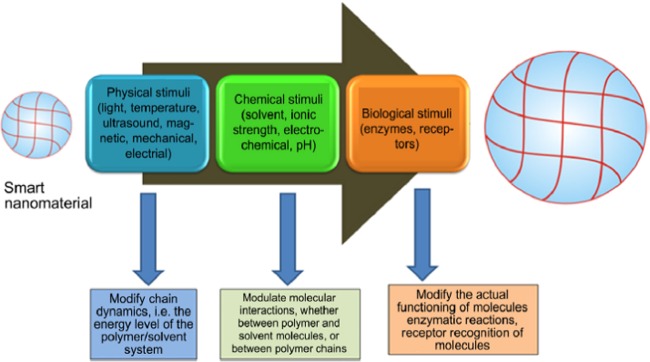
PNPs have gained significant attention owing to their unique optical, electrical, and optoelectrical qualities, as well as interesting applications in biomedical sciences, sensing, drug delivery, and many more. PNPs are the solid nanoparticulates in the size range 10–1000 nm synthesized from the biodegradable and biocompatible polymers.57 Preparation of PNPs mainly includes two steps: (1) preparation of the NPs either by gelation or polymerization of monomers and (2) preparation of emulsified system (emulsions, microemulsion, and nanoemulsion). Commonly used natural polymers exploited for the fabrication of the PNPs are gelatin, chitosan, sodium alginate, albumin, and synthetic polymers, such as polyactides, polyaniline, polyglycolides, poly(lactic-co-glycolides), polyorthoesters, polycaprolactone, poly(vinyl alchohol), polyglutamic acid, poly(vinyl alcohol), poly(methyl methacrylate), and many more.58 Polymers show a drastic chemical and physical change in response to the physical stimuli (pH, temperature), chemical stimuli (numerous signaling molecules), or biological stimuli (enzyme) that makes them potent drug delivery vehicles (Figure 2).
Figure 2.
Description of stimuli-responsive polymers and their responses. Reproduced with permission from ref (41).
Green synthesis of PNPs is an area of intense scientific and technological interest. PNPs prepared using natural ingredient as a stabilizing agent play an important role in preventing the agglomeration during storage. Several environmental-friendly reagents (poly(ethylene glycol) (PEG), poly(vinyl pyrrolidone) (PVP) etc.) have been used in preparation of PNPs to prevent aggregation in emulsion polymerization process. Saini and co-workers59 demonstrated that surface alteration of PNPs with hydrophilic polymers, like poly(ethylene glycol) (PEG), poly(ethylene oxide) (PEO), and Tween 80, increases the prolonged circular time of the drug and decreases the opsonization of drug in the body. Electroresponsive polymers have been studied as a smart nanomaterial for drug delivery as they can shrink, swell, or bend in response to the electric field.60 A novel acid responsive PNP, i.e., Bi (PEG-PLA) Pt(IV), was fabricated by using ring-opening polymerization (ROP with drug cisplatin) method by which cisplatin analogue prodrug was covalently linked to the hydrophobic segment of two PEG–PLA copolymer chains through the pH-sensitive hydrazone bond for effective drug delivery applications.61
In the field of cancer nanotechnology, a variety of polymeric micelle-based anticancer drugs have been well-known to attain high and selective accumulation at the tumor site. Du et al.62 fabricated a drug conjugated nanoparticulate system (pH sensitive) to deliver the anticancer drug DOX. The polymeric drug carrier responds to the tumor by intracellular and extracellular pH gradients by chemically defined mechanisms that simultaneously promote drug accumulation at the tumor site through the enhanced permeability and retention effect and enhance the drug delivery efficiency.
Nystatin (an antifungal drug), having a very short time at the target tissue, was incorporated in the bio adhesive PNPs by Roque et al.63 and obtained a high adhesion capacity to oral mucosa and a prolonged drug release profile in the in vitro and in vivo studies, respectively. A modified version of the solvent evaporation method for the preparation of d,l-lactide/glycolide NPs for delivery of water soluble and insoluble drugs (5-fluorouracil and Indomethacin) prospectively was developed by Niwa et al.64 The entrapment efficiency of formed submicron size PNPs for indomethacin was found to be up to 75% with an initial burst release profile. In the region of multidrug resistance in chemotherapy, triblock copolymer of poly(ethylene glycol)-block-poly(l-lysine)-block-polyaspartyl(N-(N′,N′-diisopropylaminoethyl)) (PEG-PLL-PAsp) was synthesized for the first time to enable the code delivery of BCL-2siRNA and DOX in hepatic carcinoma therapy. As obtained in in vitro and in vivo studies, the nanosized complex regulates the antiapoptotic gene (BCL-2) to the cells and finally, obtains a synergistic anticancer effect using PNPs.65
2.3.2. Mesoporous Silica Nanoparticles (MSNs)
MSNs are usually proposed as drug delivery matrices recognized by their morphological characterstics, such as pore volume, large surface area, and easily modifiable surface properties. The most common mesoporous silica material includes MCM-48, MCM-41, and SBA-15 with the pore size of 2–10 nm, which have two/three-dimensional cubic characteristic features.66 Initially, Mann et al. reported the fabrication of MSNs; however, after the work of Victor Lin, the term MSN became more popular.67 In 2001, for the first time, mesoporous silica material MCM-41 was reported as a drug delivery system. Many hydrophobic drugs have limited applications due to their poor water solubility, which makes the adsorption of drug in the gastrointestinal tract poor. Zhang and co-workers68 fabricated the MSNs and reported an improved dissolution rate of telmisartan (TEL) as compared to that of the crude TEL powder.
The channel in MSNs keeps the drug within the pores in an amorphous/noncrystalline state that facilitates the drug dissolution. Site-specific administration and long-term release are prerequisite to achieve sustainable drug delivery in the body. MSNs employed for sustained drug delivery have been divided into two groups:
-
(a)
unmodified silica materials (where interaction with the drug molecules and functional group delays drug dissolution and enables a sustained drug release) and
-
(b)
modified silica NPs (includes the regulated pore size).
Application of nanomaterials in the era of plant sciences has been extensively investigated to overcome the negative impact to environment caused by the toxic solvents used in the preparation of the silica nanoparticles. Qu et al.69 synthesized the MSNs using nontoxic surfactants (didodecyldimethylammonium bromide (DDAB) and cetyltrimethylammonium bromide (CTAB)) and proved that the drug release behavior is related to the size of particles, i.e., related to the pore channel length. Modification of MSNs with the functional groups, such as −Cl, −NH2, −CN, or −SH, or by polymers affects the rate of the drug release profile. Chitosan (biodegradable and environment-friendly polymer)-functionalized MSNs were developed by Sun and co-workers,70 and the ability of the chitosan to shrink (in an alkaline medium and swell in an acidic medium) was shown to regulate the drug release in a simulated gastrointestinal fluid. MSNs based on stimuli-responsive controlled drug delivery systems were developed by applying controls such as gatekeepers over the pore entrance due to which the drug cannot escape out from silica carriers unless the carriers are exposed to external stimuli (redox potential, temperature, and pH). The different types of gatekeepers developed by researchers are shown in Figure 3. Type I gatekeepers include solid NPs (ZnO, CdS, and Fe3O4) attached by the covalent bond to the pore opening, type III are attached to the pore openings by the covalent/noncovalent interactions, whereas the linear molecule (type II) and multilayer (type IV) gatekeepers are attached to the surface by covalent bonds or absorbed on it. Macrocyclic β-cyclodextrin (β-CD)-capped MSN-based drug delivery systems offer a typical pH-responsive sustained release.71
Figure 3.
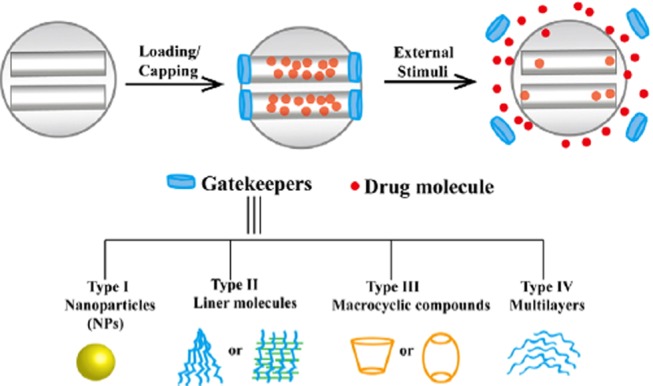
Different gatekeepers of MSNs on the pore outlets for stimuli-responsive controlled drug delivery systems. Reproduced with permission from ref (53).
Lidocaine, an anesthetic drug, has low bioavailability. To overcome this shortcoming, lidocaine was loaded into MCM-41 and amine-modified MCM-41 (the inclusion complex) using various components (CTAB, tetraethyl orthosilicate) by Nafisi et al.72 Higher drug release and skin permeation were obtained for the functionalized complex over pure lidocaine and lido/MSM41 that can be attributed to the electrostatic interaction of positively charged lido/MCM41-NH2 with the negatively charged skin membrane cells. In addition, novel MSNs having the hierarchical sievelike structure of NPs were synthesized by Gao and co-workers73 using the centrifugal method at room temperature. The developed approach lead to the formation of MSNs of size 40–100 nm and a higher drug loading efficiency with a sustained release of the DOX.
Methotrexate was loaded within the MSNs, a multifunctional nanotheranostic system based on MCM-41 and functionalized with peptides, by Farooq et al.74 Titana-coated MSNs for improving the bioavailability of sodium nitroprusside (SNP) were synthesized, and the effect of coating was observed on the biocompatibility and drug release profile. The MSNs were prepared by the surfactant template-directed method at a rate of dilution of 0.08% per min over 2.5 h for TiMSN–SNP conjugate (as compared with uncoated SNP).
Szewczyk et al.75 fabricated the amino-modified MSNs as a potential bifunctional drug delivery system for cefazolin (an antibiotic drug). The MSNs (SBA-15) were formed via the greener sol–gel method, and further, the surface functionalization was carried out by postgrafting synthesis and higher encapsulation with a slow and sustained release over 7 days was demonstrated.
2.3.3. Dendrimers
Dendrimers derived their name from the Greek word “dendron” meaning “reminiscent of a tree”. Dendrimers are unimolecular, micellar nanostructures of the size of around 10 nm, with a well-defined, highly branched, three-dimensional symmetrical structure with a pattern resembling a tree branching out from a central point. These are polymeric molecules (having a compact spherical shape) consisting of three distinct architectural regions: a focal core having two or more reactive groups, interior layers of branched repeating units (generations) covalently attached to the core, and a high density of terminal functional groups at the periphery.76 To serve as potential drug carriers in DDS, the above mentioned three domains can be tailored. It is their branching and dendritic nature that facilitates the drug encapsulation onto the outside surface of dendrimers despite their small sized polymeric structure.77
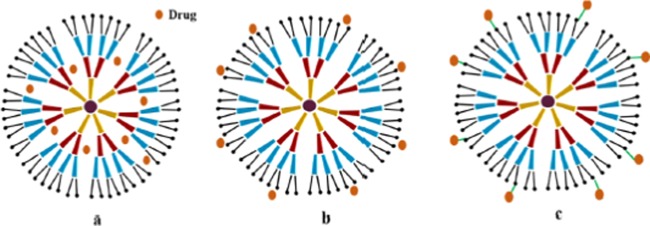
Frequently used dendrimers in the drug delivery studies typically incorporate one or more of the mentioned polymers: melamine, polyamidoamine (PAMAM), poly(l-glutamic acid) (PG), polypropyleneimine, polyethyleneimine, poly(ethylene glycol) (PEG), chitin, etc. The drug loading potential of the dendrimers can be increased by controlling the association of the guest (drug) molecule with the terminal groups of the dendrimers. Convergence and divergence are the two approaches using which dendrimers can be synthesized.78 A divergent approach is also termed as an ascending approach that involves construction of the dendrimers from the core and building of the core toward the periphery using two basic operations. Most often, the one-pot divergent approach is exploited to synthesize water soluble, photoluminescent, biocompatible, and biodegradable “green” bifunctional dendrimers like polyurea dendrimers synthesized in supercritical carbon dioxide (scCO2). In contrast to the divergent synthesis, the convergent method initiates at the periphery and proceeds toward the core through one-to-one coupling of polymers. The dendritic assembly formulated using the convergent approach is poly(aryl ether) framework, also known as the Frechet-type framework. The modes responsible for the interaction of the dendrimers with drugs are represented in Figure 4.
Figure 4.
Different ways of drug interaction: (a) physical entrapment of drugs, (b) adsorption of drug molecules on the surface by intermolecular interaction, and (c) conjugation of drug molecules to the surface groups of dendrimer. Reproduced with permission from ref (60).
Wang et al.79,80 reported that the electrostatic interaction occurs between the PAMAM dendrimers and the carboxyl group of the ibuprofen (drug) and assessed that at pH 10.5, ibuprofen molecules were attached with the PAMAM dendrimers, which results in the enhancement of solubility of the drug. The covalent bonding of the dendrimers to the terminal groups of the dendrimers can be done with the help of chemical inserts, such as PEG, p-nitrobenzoic acid, etc. Yang and co-workers81 reported that penicillin V(XII) conjugation with the dendrimers occurs through the ester and amide linkages via the PEG spacer. In addition, amide linkage results in the amide bond stability and the ester linkage between the drug and dendrimers provides the controlled release. Two types of mechanisms are documented in the literature82 with regard to dendrimer drug delivery. On the basis of the first mechanism, the in vivo cleavage of the covalent bond between the dendrimers and drug in the presence of enzymes leads to drug release whereas the second mechanism states that the drug release occurs due to the alteration of physical conditions, like temperature and pH.
Ma et al.83 described the fabrication of polyester dendrimers using the protocatechuic acid (as a repeating unit), which could serve as an efficient anticancer drug delivery vehicle. Similarly, Abedi-gaballu et al.84 prepared the PAMAM-based dendrimers, which exhibited higher potential for the targeted cancer therapy. To overcome the poor rates of transcutaneous delivery, PAMAM dendrimers were complexed with the drug (Ketoprofen, Diflunisal), which improved the drug penetration through the skin.85 Further, PAMAM dendrimers were fabricated to deliver dexamethasone (DEX) for the treatment of diabetic retinopathy (DR). Formulations were assessed in terms of cell permeability and cytotoxicity, and results showed that the anionic dendrimers have no significant toxicity for human corneal cells in comparison with the DEX solution alone at a concentration of 1 mg/mL.86 Du et al.87 synthesized the hyaluronic acid (HA) and poly(lactide) (PLA) co-modified half-generation PAMAM dendrimers by using the divergent method to enhance the delivery of docetaxel. In this study, PLA was coupled with the hydroxyl group within the core of the PAMAM and HA (ionic linear polysaccharides) and used to protect the positive charge of the PAMAM that reduced the toxicity and improved the bioavailability of the drug. Surface modification of PAMAM dendrimers is one of the important steps to reduce the hemolytic as well as cytotoxic nature of the PAMAM due to presence of the positively charged amino groups. Modification of the PAMAM by the natural or anionic functional groups prevents the interaction of the PAMAM dendrimers with the biological membrane and helps them to become nontoxic, biocompatible, and environment friendly.
2.4. Nanoparticles Based on Solid Lipids
2.4.1. Solid Lipid Nanoparticles (SLNs)
SLNs are at the forefront of nanocarriers having numerous potential applications in research and clinical medicine, drug delivery, etc.88 SLNs are termed as submicron colloidal carriers, which are made up of a solid lipid core matrix stabilized in an aqueous solution of emulsifier. The ability of the SLNs to encapsulate the drugs within nanocarriers offers a new prototype (having unique size-dependent properties) that can be used for secondary and tertiary levels of drug targeting.89
In 1990, Gasco and co-workers actively engaged in developing SLNs of the size range 10–1000 nm. The commonly exploited solid lipids for preparing SLNs are triacylglycerols, acylglycerols, triglycerides, fatty acids, and waxes. The lipophilic moiety of SLNs within the lipid matrix as well as in nanoparticulate matrix provides protection to chemically labile drugs.89 Owing to their large surface area and small size, SLNs can be functionalized with ligands, antibodies, moieties, and other functional groups to target the specific locations or cells within the body.
For the first time, the SLNs were employed to encapsulate the peptide drugs (thymopentin,([D-Trp-6] LHRH)) by using the W/O/W microemulsion-based method.90,91 Further, Ugazio and co-workers92 incorporated hydrophobic peptide using the w/o microemulsion technique. SLNs consisting of the excipients stearic acid, emulsifying wax, octadecyl alcohol, and cetyl palmitate were synthesized and employed for the delivery of the anticancer drugs. Baek et al.93 fabricated the surface-modified PTX-loaded SLNs with hydroxypropyl-β-cyclodextrin (to prevent the oxidation of the lipids and solubilize the drugs) by using the emulsification solvent evaporation method that showed an average size of 251 nm with 71% loading capacity. Further, Pooja et al.94 prepared SLNs coated with wheat germ agglutinin to improve the oral drug delivery of paclitaxel and studied the in vitro anticancer activity against A549 lung cancer cells. SLNs coated with the d-α-tocopherol poly(ethylene glycol) succinate or Tween 80 were synthesized by the solvent diffusion method to enhance the oral delivery of the DTX. In cardiovascular-related disorders, SLNs have been used to increase the drug plasma concentration and extend the circulation time in the body. Nimodipine-loaded SLNs were synthesized using high-pressure homogenization method using poloxamer 188 (surfactant), soya lecithin (co surfactant), and palmitic acid (lipid).95 The stability test displayed no significant changes in the shape and polydispersity of SLNs for 3 months, and the oral bioavailability of nimodipine (8 mg/kg) was found to be 2-fold greater as compared to that of free drug solution in rats. Lipid-based nanosystems stand out due to their nontoxic, biocompatible, and environment-friendly composition that includes stearic acid, cetyl palmitate, glyceryl mono stearate, soya lecithin, and many more.
Chitosan biopolymer has been widely used in the pharmaceutical field as it exhibits a number of interesting properties, such as greener nanotechnology, nontoxicity, and biodegradability. Chitosan-coated SLNs were prepared by Zariwala et al.96 to deliver iron supplements using double emulsion solvent evaporation process (consists of stearic acid), and particle sizes of a submicron range (300–500 nm) were obtained. Iron adsorption was evaluated in vitro by using Caco-2 cell line on which equal doses of iron formulations were added to determine intracellular ferritin protein concentration. Results showed that the iron adsorption from SLN-Fe (containing 589.98 ± 40.83 mg/mg cell protein) and SLN–Fe–chitosan (containing 642.77 ± 29.67 mg/mg cell protein) was found to be 13.42 and 24.9%, respectively, as compared with ferrous sulfate.
SLNs functionalized with chitosan and PEG polymers were prepared by Matthias et al.97 using nanoprecipitation method for the delivery of the PTX for lung tumor therapy. Coated SLNs significantly decreased the in vitro inhibitory concentration of PTX in M109-HiFR cells. Olmesartan medoxomil (a BCS class II drug)-loaded SLNs were prepared by a hot homogenization process to increase their oral bioavailability. The in vivo pharmakinetic studies revealed that the olmesartan medoxomil-loaded SLNs showed a higher Cmax of 1610 mg/mL and increased the relative bioavailability by almost 2.3-fold than the marketed formulation.98 Mehrad and co-workers99 reported SLNs of size <220 nm with spherical morphology using microemulsification method. They employed palmitic acid as the solid lipid and stabilized them with a protein isolate for increasing the physiochemical stability of β-carotene by encapsulating it in SLNs. Kanwar et al.100 formulated SLNs by solvent injection method and achieved a high entrapment efficiency and loading efficiency for antileprosy drugs, namely, rifampicin and dapsone. A lower toxicity and optimal size stability of the prepared SLNs with a sustained release was demonstrated.
2.4.2. Nanostructured Lipid Carriers (NLCs)
NLCs are the second-generation lipid nanocarriers, developed by Muller et al.89 in the year 1999. NLCs comprise a mixture of liquid lipid and solid lipid matrix (to create more imperfection in the matrix of NLCs) stabilized in an aqueous surfactant solution. NLCs were formulated to overcome the problems associated with the first-generation SLNs by increasing the loading capacity and preventing the drug expulsion (Figure 5). The lipidlike glyceryl dibehenate, glyceryl palmitostearate, tripalmitin, and stearic acid (as solid lipids); caprylic/capric triglycerides, vitamin E and derivatives, lauryl polyoxylglycerides, soya lecithin, and squalene (liquid lipids); and polysorbates, poloxamers (188, 407), macrogol-15-hydroxy stearate, and polyoxylcastor oil (as surfactants) are generally used in the preparation of the NLCs.101,102 The NLCs can be used in the form of particulate by the intestine and transferred to various organs of lymphatic systems. Drug can be encapsulated within the NLCs and administered through different routes, such as pulmonary (celecoxib, dexamethasone, and montelukast), oral (etoposide, lovastatin, and spironolactone), intravenous (artemether, bufadienolides, and β-elemene), and ocular (cyclosporine A, flurbiprofen, ibuprofen, and oxofloxacin).103
Figure 5.
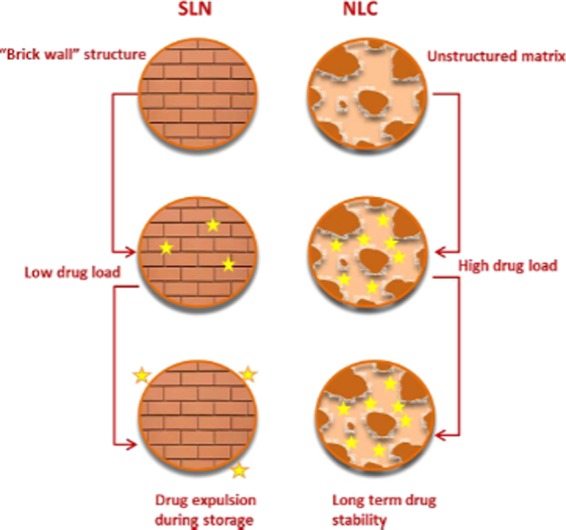
Pictorial differentiation of SLN and NLC structures, highlighting the advantages of NLCs over SLNs. Reproduced with permission from ref (82).
A number of physical methods (ultrasonication, high-pressure homogenization, supercritical fluid technique, and many more) with reasonable modification in their methodology have been carried out for the controlled synthesis of NPs owing to their energy requirements, less usage of hazardous chemicals, and higher yield.104Figure 6 displays that the absorption mechanisms of a drug from the NLCs can be elaborated stepwise starting from degradation of lipid by enzymes within the gut that further leads to the formation of micelles in bile salts.105 Beloqui et al.106 fabricated the saquinavir-loaded NLCs by a high-pressure homogenization technique [Precirol ATO-5, (solid lipid), Miglyol 812 (liquid lipid), Tween 80, and poloxamer 188 (as surfactants)] and showcased that dextran–protamine coating significantly enhanced the permeability of saquinavir across Caco-2 monolayers in comparison with the uncoated NPs.
Figure 6.
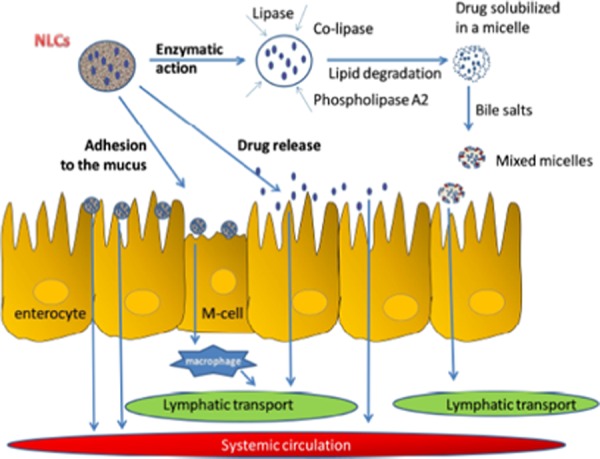
Absorption mechanisms of a drug from NLCs in the gastrointestinal tract. Reproduced with permission from ref (83).
In another study, Khan et al.107 targeted the tacrolimus-loaded NLCs by using capmul MCM C8 and compritol 888 ATO (lipid phase) for oral delivery and observed a slow and sustained release in the body. Kaithwas et al.108 loaded olmesartan medoxomil (OLM, BCS class II drug) within the NLCs and carried out its in vitro cellular uptake study and in vivo pharmokinetic study. Results demonstrated that the cellular uptake was 5.2-fold higher as compared to that of the free drug (when incubated with Caco-2) cells and AUCtotal and Cmax values obtained by the pharmacokinetic study of OLM-NLC were found to be significantly higher than those with free drug suspension.
Fathi et al.109 fabricated NLCs loaded with Simvastatin (SIM, an antiperlipidemic drug) by emulsification solvent evaporation method followed by ultrasonication, and in vivo pharmodynamics and in vitro pharmokinetic studies were carried out. Results showed a 4-fold increase in bioavailability as compared with the SIM suspension in in vivo studies. Further, for ocular drug delivery purposes, NLCs loaded with curcumin were synthesized by Lakhani and co-workers110 using the hot emulsification technique and NLCs of a particle size of 66 nm with a higher entrapment efficiency (>96%) were obtained and further, their ex vivo trans corneal permeability was examined. The trans corneal permeation and the flux of synthesized curcumin-loaded NLCs were found to be 2.5-fold higher as compared with the curcumin-based suspension.
Moreover, the potential of the NLC for antifungal delivery of voriconazole (VOR) to deeper regions of the nail plate was shown by Rocha and co-workers.111 Results obtained demonstrated the high loading capacity (74.52 ± 2.13%) of VOR-NLC, and the permeation data established that the VOR amount extracted was similar for all formulations, i.e., 2.42 ± 0.26 μg/cm2 (unloaded VOR), 2.52 ± 0.36 μg/cm2 (VOR-NLC), and 2.41 ± 0.60 μg/cm2 (VOR-NLC-Ur). Successive extractions showed that the amount of VOR retained in deeper regions was significantly higher for VOR-NLC, or VOR-NLC-Ur demonstrated the potential of NLCs as a topical delivery system. Further, Kelidari et al.112 prepared the fluconazole-loaded NLCs using the ultrasonication method and investigated the efficiency of the optimized formulation on Candida species. NLCs having spherical morphology of the size of 126.4 nm and an entrapment efficiency of 93.6% were reported. Oral bioavailability of an antipsychotic drug Olanzapine (BCS class II drug) was enhanced through NLCs prepared by Jawahar et al.113 NLCs were prepared by using the solvent diffusion method consisting of olanzapine, stearyl amine, soya lecithin, glyceryl tripalmitate, and castor oil in the organic phase and Pluronic F-68 in the aqueous phase. A 5-fold increase in the oral bioavailability of NLCs was reported compared with the olanzapine suspension, from which it can be inferred that NLCs provide a sustained release of the drug.
Overall, the biocompatible and biologically nontoxic nature of various lipids is responsible for the development of NLCs as a promising and sustained drug delivery vehicle.
3. Conclusions
Current research in the synthesis of NPs using plant extracts has opened a new era of development of nontoxic methods for preparation of NPs. The formation of NPs using natural substances has a major edge over methods in terms of its interaction and effect on the environment. Green chemistry has appeared as a new concept in the implementation of chemical processes to avoid the use of hazardous substances. Various reports showed the superiority of green synthesized NPs from natural and biological sources over chemical methods. The biological reagents and biocompatible processes do not require the use of harsh chemicals. Several reports demonstrated that the nanomaterials synthesized using green methods are found to be more stable as compared to those using classical methods.
In the present review, a brief description of inorganic NPs, metallic NPs and quantum dots, and organic NPs, polymeric NPs, mesoporous silica NPs, dendrimers, SLNs, etc., has been highlighted, comprising their preparation and applications. Most of these syntheses have been carried out in research laboratories at a small scale, but researchers are engaged in scaling up these processes so as to explore the potential and applications of nanomaterials in health science, environment, and many more to fulfill future demands. Although innumerable reports are available for the fabricated nanoassemblies that are employed in the drug delivery applications, the inbuilt potential of these exuberant nanocarriers/nanomedicine has not been exploited completely to treat a wide range of diseases in a safe and effective manner.
In today’s scenario, plant-mediated eco-friendly protocols and surfactants provide a green tool for the synthesis of nanomaterials. It is necessary to adopt safer alternative methods to synthesize different nanoassemblies. Recent trends of developing green-triggered technologies for the fabrication of the nanomaterial depict their enormous importance in science and nanotechnology.
Acknowledgments
R.K. gratefully acknowledges CSIR and DAAD for the fellowship. J.R. is grateful to UGC for the junior research fellowship, S.K.M. acknowledges DST-PURSE II. D.B.S. is grateful to DBT New Delhi for the award of the Ramalingaswami fellowship.
The authors declare no competing financial interest.
References
- Anselmo A. C.; Mitragotri S. An Overview of Clinical and Commercial Impact of Drug Delivery Systems. J. Controlled Release 2014, 190, 15–28. 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans M.; Lowman A. Biodegradable Nanoparticles for Drug Delivery and Targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. 10.1016/S1359-0286(02)00117-1. [DOI] [Google Scholar]
- Kumar B.; Jalodia K.; Kumar P.; Gautam H. K. Recent Advances in Nanoparticle-Mediated Drug Delivery. J. Drug Delivery Sci. Technol. 2017, 41, 260–268. 10.1016/j.jddst.2017.07.019. [DOI] [Google Scholar]
- Park K. Controlled Drug Delivery Systems: Past Forward and Future Back. J. Controlled Release 2014, 190, 3–8. 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosa A.; Reddi S.; Saha R. N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. 10.1016/j.biopha.2018.04.055. [DOI] [PubMed] [Google Scholar]
- Jahangirian H.; Lemraski E. G.; Webster T. J.; Rafiee-Moghaddam R.; Abdollahi4 Y. A Review of Drug Delivery Systems Based on Nanotechnology and Green Chemistry: Green Nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. 10.2147/IJN.S127683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverberi A. P.; Kuznetsov N. T.; Meshalkin V. P.; Salerno M.; Fabiano B. Systematical Analysis of Chemical Methods in Metal Nanoparticles Synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. 10.1134/s0040579516010127. [DOI] [Google Scholar]
- Dhas N. A.; Raj C. P.; Gedanken A. Synthesis, Characterization, and Properties of Metallic Copper Nanoparticles. Chem. Mater. 1998, 10, 1446–1452. 10.1021/cm9708269. [DOI] [Google Scholar]
- Chou K.; Ren C. Synthesis of Nanosized Silver Particles by Chemical Reduction Method. Mater. Chem. Phys. 2000, 64, 241–246. 10.1016/S0254-0584(00)00223-6. [DOI] [Google Scholar]
- Nickel U.; Castell A.; Po K.; Schneider S. A Silver Colloid Produced by Reduction with Hydrazine as Support for Highly Sensitive Surface-Enhanced Raman. Langmuir 2000, 16, 9087–9091. 10.1021/la000536y. [DOI] [Google Scholar]
- Martínez Bonilla C. A.; Kouznetsov V. V. “Green” Quantum Dots: Basics, Green Synthesis, and Nanotechnological Applications. Green Nanotechnol. 2016, 173–192. [Google Scholar]
- Peng Z. A.; Peng X. Formation of High-Quality CdTe, CdSe, and CdS Nanocrystals Using CdO as Precursor. J. Am. Chem. Soc. 2001, 123, 183–184. 10.1021/ja003633m. [DOI] [PubMed] [Google Scholar]
- Ge C.; Xu M.; Liu J.; Lei J.; Ju H. Facile Synthesis and Application of Highly Luminescent CdTe Quantum Dots with an Electrogenerated Precursor. Chem. Commun. 2008, 19, 450–452. 10.1039/B714990E. [DOI] [PubMed] [Google Scholar]
- Ekman B. O. Improved Stability of Proteins Immobilized in Microparticles Prepared by modified emulsion polymerisation technique. J. Pharma. Sci. 1976, 38, 1975–1978. [DOI] [PubMed] [Google Scholar]
- Lowe P. J.; Temple C. S. Calcitonin and Insulin in Isobutylcyanoacrylate Nanocapsules: Protection Against Proteases and Effect on Intestinal Absorption in Rats. J. Pharm. Pharmacol. 1994, 1251, 547–552. 10.1111/j.2042-7158.1994.tb03854.x. [DOI] [PubMed] [Google Scholar]
- Nagavarma B. V. N.; Yadav H. K. S.; Ayaz A.; Vasudha L. S.; Shivakumar H. G. Different techniques for preparation of polymeric nanoparticles—A Review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Ratirotjanakul W.; Sioloetwong T.; et al. Green Synthesis of AgNPs Coated Mesoporous Silica Nanoparticles Using Tyrosine as Reducing/Stabilising Agent. Mater. Sci. Forum 2018, 928, 89–93. 10.4028/www.scientific.net/MSF.928.89. [DOI] [Google Scholar]
- Palem R. R.; Ganesh S. D.; Kronekova Z.; Sláviková M.; Saha N.; Saha P. Green Synthesis of Silver Nanoparticles and Biopolymer Nanocomposites: A Comparative Study on Physico-Chemical, Antimicrobial and Anticancer Activity. Bull. Mater. Sci. 2018, 41, 1–11. 10.1007/s12034-018-1567-5. [DOI] [Google Scholar]
- Malik P.; Shankar R.; Malik V.; Sharma N.; Mukherjee T. K. Green Chemistry Based Benign Routes for Nanoparticle Synthesis. J. Nanopart. Res. 2014, 2014, 302429 10.1155/2014/302429. [DOI] [Google Scholar]
- Bevilacqua A.; Cibelli F.; Corbo M. R.; Sinigaglia M. Effects of High-Pressure Homogenization on the Survival of Alicyclobacillus acidoterrestris in a Laboratory Medium. Lett. Appl. Microbiol. 2007, 45, 382–386. 10.1111/j.1472-765X.2007.02219.x. [DOI] [PubMed] [Google Scholar]
- Rehman M.; Khan M. A.; Khan W. S.; Shafique M. Fabrication of Niclosamide Loaded Solid Lipid Nanoparticles: In Vitro Characterization and Comparative in Vivo Evaluation. Artif. Cells, Nanomed., Biotechnol. 2017, 46, 1–9. 10.1080/21691401.2017.1396996. [DOI] [PubMed] [Google Scholar]
- Gao S.; Mcclements D. J. Formation and Stability of Solid Lipid Nanoparticles Fabricated Using Phase Inversion Temperature Method. Colloids Surf., A 2016, 499, 79–87. 10.1016/j.colsurfa.2016.03.065. [DOI] [Google Scholar]
- Shah M.; Fawcett D.; Sharma S.; Tripathy S. K.; Poinern G. E. J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. A.; Rivera Gil P.; Zhang F.; Zanella M.; Parak W. J. Biological Applications of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- Torres-Chavolla E.; Ranasinghe R. J.; Alocilja E. C. Characterization and Functionalization of Biogenic Gold Nanoparticles for Biosensing Enhancement. IEEE Trans. Nanotechnol. 2010, 9, 533–538. 10.1109/TNANO.2010.2052926. [DOI] [Google Scholar]
- Bhumkar D. R.; Joshi H. M.; Sastry M.; Pokharkar V. B. Chitosan Reduced Gold Nanoparticles as Novel Carriers for Transmucosal Delivery of Insulin. Pharm. Res. 2007, 24, 1415–1426. 10.1007/s11095-007-9257-9. [DOI] [PubMed] [Google Scholar]
- Cai W.; Gao T.; Hong H.; Sun J. Applications of Gold Nanoparticles in Cancer Nanotechnology. Nanotechnol., Sci. Appl. 2008, 1, 17–32. 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzajani F.; Ghassempour A.; Aliahmadi A.; Esmaeili M. A. Antibacterial Effect of Silver Nanoparticles on Staphylococcus aureus. Res. Microbiol. 2011, 162, 542–549. 10.1016/j.resmic.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Pollini M.; Paladini F.; Catalano M.; Taurino A.; Licciulli A.; Maffezzoli A.; Sannino A. Antibacterial Coatings on Haemodialysis Catheters by Photochemical Deposition of Silver Nanoparticles. J. Mater. Sci.: Mater. Med. 2011, 22, 2005–2012. 10.1007/s10856-011-4380-x. [DOI] [PubMed] [Google Scholar]
- Hrapovic S.; Liu Y.; Male K. B.; Luong J. H. T. Electrochemical Biosensing Platforms Using Platinum Nanoparticles and Carbon Nanotubes. Anal. Chem. 2004, 76, 1083–1088. 10.1021/ac035143t. [DOI] [PubMed] [Google Scholar]
- West P. R.; Ishii S.; Naik G. V.; Emani N. K.; Shalaev V. M.; Boltasseva A. Searching for Better Plasmonic Materials Paul. Laser Photonics Rev. 2010, 4, 795–808. 10.1002/lpor.200900055. [DOI] [Google Scholar]
- Lee H.-J.; Lee G.; Jang N. R.; Yun J. H.; Song J. Y.; Kim B. S. Biological Synthesis of Copper Nanoparticles Using Plant Extract. Nanotechnology 2011, 1, 371–374. [Google Scholar]
- Prasad K. S.; Patel H.; Patel T.; Patel K.; Selvaraj K. Biosynthesis of Se Nanoparticles and Its Effect on UV-Induced DNA Damage. Colloids Surf., B 2013, 103, 261–266. 10.1016/j.colsurfb.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Basnet P.; Inakhunbi Chanu T.; Samanta D.; Chatterjee S. A Review on Bio-Synthesized Zinc Oxide Nanoparticles Using Plant Extracts as Reductants and Stabilizing Agents. J. Photochem. Photobiol., B 2018, 183, 201–221. 10.1016/j.jphotobiol.2018.04.036. [DOI] [PubMed] [Google Scholar]
- Kim M.; Osone S.; Kim T.; Higashi H.; Seto T. Synthesis of Nanoparticles by Laser Ablation: A Review. Materials 2016, 1–11. 10.14356/kona.2017009. [DOI] [Google Scholar]
- Ahlawat D. S.; Kumari R. Synthesis and Characterization of Sol–Gel Prepared Silver Nanoparticles. Int. J. Nanosci. 2014, 13, 1–8. 10.1142/S0219581X14500045. [DOI] [Google Scholar]
- Thakkar K. N.; Mhatre S. S.; Parikh R. Y. Biological Synthesis of Metallic Nanoparticles. Nanomedicine 2010, 6, 257–262. 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Chen Y. H.; Tsai C. Y.; Huang P. Y.; Chang M. Y.; Cheng P. C.; Chou C. H.; Chen D. H.; Wang C. R.; Shiau A. L.; Wu C. L. Methotrexate Conjugated to Gold Nanoparticles Inhibits Tumor Growth in a Syngeneic Lung Tumor Model. Mol. Pharm. 2007, 4, 713–722. 10.1021/mp060132k. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang X.; Wang C.; Chen B.; Dai Y.; Zhang R.; Song M.; Lv G.; Fu D. The Enhancement Effect of Gold Nanoparticles in Drug Delivery and as Biomarkers of Drug-Resistant Cancer Cells. ChemMedChem 2007, 2, 374–378. 10.1002/cmdc.200600264. [DOI] [PubMed] [Google Scholar]
- Thirumurugan A.; Blessy V.; Karthikeyan M.. Comparative Study on Doxorubicin Loaded Metallic Nanoparticles in Drug Delivery Against MCF-7 Cell Line, Applications of Nanomaterials; Elsevier Ltd, 2018; pp 303–313. [Google Scholar]
- Thambiraj S.; Hema S.; Shankaran D. R. Functionalized Gold Nanoparticles for Drug Delivery Applications. Mater. Today: Proc. 2018, 5, 16763–16773. 10.1016/j.matpr.2018.06.030. [DOI] [Google Scholar]
- Mehnath S.; Arjama M.; Rajan M.; Arokia M. Polyorganophosphazene Stabilized Gold Nanoparticles for Intracellular Drug Delivery in Breast Carcinoma Cells. Process Biochem. 2018, 72, 152–161. 10.1016/j.procbio.2018.06.006. [DOI] [Google Scholar]
- Wu P. H.; Onodera Y.; Ichikawa Y.; Rankin E. B.; Giaccia A. J.; Watanabe Y.; Qian W.; Hashimoto T.; Shirato H.; Nam J.-M.; et al. Targeting Integrins with RGD-Conjugated Gold Nanoparticles in Radiotherapy Decreases the Invasive Activity of Breast Cancer Cells. Int. J. Nanomed. 2017, 12, 5069–5085. 10.2147/IJN.S137833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu H.; Renuka N.; Faheem S. M.; Ismail R.; Singh V.; Saadatmand Z.; Khan S. S.; Narayanan K.; Raheem A.; Premkumar K.; et al. Gold and Silver Nanoparticles Biomimetically Synthesized Using Date Palm Pollen Extract-Induce Apoptosis and Regulate P53 and Bcl-2 Expression in Human Breast Adenocarcinoma Cells. Biol. Trace Elem. Res. 2018, 186, 122–134. 10.1007/s12011-018-1287-0. [DOI] [PubMed] [Google Scholar]
- Wang G.; Zhou F.; Li X.; Li J.; Ma Y.; Mu J.; Zhang Z.; Che H.; Zhang X. Controlled Synthesis of L-Cysteine Coated Cobalt Ferrite Nanoparticles for Drug Delivery. Ceram. Int. 2018, 44, 13588–13594. 10.1016/j.ceramint.2018.04.193. [DOI] [Google Scholar]
- Ghasemi Y.; Peymani P.; Afifi S. Quantum Dot: Magic Nanoparticle for Imaging, Detection and Targeting. Acta Bio Med. Atenei Parmensis 2009, 80, 156–165. [PubMed] [Google Scholar]
- Lee H. U.; Park S. Y.; Park E. S.; Son B.; Lee S. C.; Lee J. W.; Lee Y.; Kang K. S.; Kim M. Il; Park H. G.; et al. Photoluminescent Carbon Nanotags from Harmful Cyanobacteria for Drug Delivery and Imaging in Cancer Cells. Sci. Rep. 2014, 4, 4665 10.1038/srep04665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Q.; Wang Z.; Hao S.; Sun L.; Nisic M.; Cheng G.; Zhu C.; Wan Y.; Ha L.; Zheng S. Y. Mitochondria-Based Aircraft Carrier Enhances in Vivo Imaging of Carbon Quantum Dots and Delivery of Anticancer Drug. Nanoscale 2018, 10, 3744–3752. 10.1039/C7NR08816G. [DOI] [PubMed] [Google Scholar]
- Chiu S. H.; Gedda G.; Girma W. M.; Chen J. K.; Ling Y. C.; Ghule A. V.; Ou K. L.; Chang J. Y. Rapid Fabrication of Carbon Quantum Dots as Multifunctional Nanovehicles for Dual-Modal Targeted Imaging and Chemotherapy. Acta Biomater. 2016, 46, 151–164. 10.1016/j.actbio.2016.09.027. [DOI] [PubMed] [Google Scholar]
- Olerile L. D.; Liu Y.; Zhang B.; Wang T.; Mu S.; Zhang J.; Selotlegeng L.; Zhang N. Near-Infrared Mediated Quantum Dots and Paclitaxel Co-Loaded Nanostructured Lipid Carriers for Cancer Theragnostic. Colloids Surf., B 2017, 150, 121–130. 10.1016/j.colsurfb.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Khodadadei F.; Safarian S.; Ghanbari N. Methotrexate-Loaded Nitrogen-Doped Graphene Quantum Dots Nanocarriers as an Efficient Anticancer Drug Delivery System. Mater. Sci. Eng., C 2017, 79, 280–285. 10.1016/j.msec.2017.05.049. [DOI] [PubMed] [Google Scholar]
- Fakhri A.; Tahami S.; Nejad P. A. Preparation and Characterization of Fe3O4-Ag2O Quantum Dots Decorated Cellulose Nanofibers as a Carrier of Anticancer Drugs for Skin Cancer. J. Photochem. Photobiol., B 2017, 175, 83–88. 10.1016/j.jphotobiol.2017.08.032. [DOI] [PubMed] [Google Scholar]
- Yaghini E.; Turner H. D.; Le Marois A. M.; Suhling K.; Naasani I.; MacRobert A. J. In Vivo Biodistribution Studies and Ex Vivo Lymph Node Imaging Using Heavy Metal-Free Quantum Dots. Biomaterials 2016, 104, 182–191. 10.1016/j.biomaterials.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M.; Mewada A.; Pandey S.; Bhori M.; Singh K.; Sharon M.; Sharon M. Milk-Derived Multi-Fluorescent Graphene Quantum Dot-Based Cancer Theranostic System. Mater. Sci. Eng., C 2016, 67, 468–477. 10.1016/j.msec.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Sarkar N.; Sahoo G.; Das R.; Prusty G.; Swain S. K. Carbon Quantum Dot Tailored Calcium Alginate Hydrogel for PH Responsive Controlled Delivery of Vancomycin. Eur. J. Pharm. Sci. 2017, 109, 359–371. 10.1016/j.ejps.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Mehta V. N.; Chettiar S. S.; Bhamore J. R.; Kailasa S. K.; Patel R. M. Green Synthetic Approach for Synthesis of Fluorescent Carbon Dots for Lisinopril Drug Delivery System and Their Confirmations in the Cells. J. Fluoresc. 2017, 27, 111–124. 10.1007/s10895-016-1939-4. [DOI] [PubMed] [Google Scholar]
- Vauthier C.; Bouchemal K. Expert Review Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharma Res. 2009, 26, 1025–1058. 10.1007/s11095-008-9800-3. [DOI] [PubMed] [Google Scholar]
- Nagavarma B. V. N.; Yadav H. K. S.; Ayaz A.; Vasudha L. S.; Shivakumar H. G. Different Techniques for Preparation of Polymeric Nanoparticles - A Review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Saini R. K.; Bagri L. P.; Bajpai A. K.; Mishra A.. Responsive Polymer Nanoparticles for Drug Delivery Applications. Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Woodhead Publishing, 2018; Vol. 1, pp 289–320. [Google Scholar]
- Yoon S. G.; Kim I. Y.; Kim S. I.; Kim S. J. Swelling and Electroresponsive Characteristics of Interpenetrating Polymer Network Hydrogels. Polym. Int. 2005, 54, 1169–1174. 10.1002/pi.1825. [DOI] [Google Scholar]
- Aryal S.; Hu C. J.; Zhang L. Polymer-Cisplatin Conjugate Delivery. ACS Nano 2010, 4, 251–258. 10.1021/nn9014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J. Z.; Du X. J.; Mao C. Q.; Wang J. Tailor-Made Dual PH-Sensitive Polymer-Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- Roque L.; Castro P.; Molpeceres J.; Viana A. S.; Roberto A.; Reis C.; et al. Bioadhesive Polymeric Nanoparticles as Strategy to Improve the Treatment of Yeast Infections in Oral Cavity: In-Vitro and Ex-Vivo Studies. Eur. Polym. J. 2018, 104, 19–31. 10.1016/j.eurpolymj.2018.04.032. [DOI] [Google Scholar]
- Niwa T.; Takeuchi H.; et al. Preparations of Biodegradable Nanospheres of Water-Soluble and Insoluble Drugs with D,L-Lactide/Glycolide Copolymer by a Novel Spontaneous Emulsification Solvent Diffusion Method, and the Drug Release Behavior. J. Controlled Release 1993, 25, 89–98. 10.1016/0168-3659(93)90097-O. [DOI] [Google Scholar]
- Sun W.; Chen X.; Xie C.; Wang Y.; Lin L.; Zhu K.; Shuai X. Co-Delivery of Doxorubicin and Anti-BCL-2 SiRNA by PH-Responsive Polymeric Vector to Overcome Drug Resistance in In Vitro and In Vivo HepG2 Hepatoma Model. Biomacromolecules 2018, 19, 2248–2256. 10.1021/acs.biomac.8b00272. [DOI] [PubMed] [Google Scholar]
- Kao K. C.; Mou C. Y. Pore-Expanded Mesoporous Silica Nanoparticles with Alkanes/Ethanol as Pore Expanding Agent. Microporous Mesoporous Mater. 2013, 169, 7–15. 10.1016/j.micromeso.2012.09.030. [DOI] [Google Scholar]
- Mehmood A.; Ghafar H.; Yaqoob S.; Gohar U. F.; Ahmad B.. Mesoporous Silica Nanoparticles: A Review. J. Dev. Drugs 2017, 06. 10.4172/2329-6631.1000174. [DOI] [Google Scholar]
- Zhang Y.; Jiang T.; Zhang Q.; Wang S. Inclusion of Telmisartan in Mesocellular Foam Nanoparticles: Drug Loading and Release Property. Eur. J. Pharm. Biopharm. 2010, 76, 17–23. 10.1016/j.ejpb.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Qu F.; Zhu G.; Lin H.; Zhang W.; Sun J.; Li S.; Qiu S. A Controlled Release of Ibuprofen by Systematically Tailoring the Morphology of Mesoporous Silica Materials. J. Solid State Chem. 2006, 179, 2027–2035. 10.1016/j.jssc.2006.04.002. [DOI] [Google Scholar]
- Sun L.; Wang Y.; Jiang T.; Zheng X.; Zhang J.; Sun J.; Sun C.; Wang S. Novel Chitosan-Functionalized Spherical Nanosilica Matrix As an Oral Sustained Drug Delivery System for Poorly Water-Soluble Drug Carvedilol. ACS Appl. Mater. Interfaces 2013, 5, 103–113. 10.1021/am302246s. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhao Q.; Han N.; Bai L.; Li J.; Liu J.; Che E.; Hu L.; Zhang Q.; Jiang T.; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomedicine 2015, 11, 313–327. 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Nafisi S.; Samadi N.; Houshiar M.; Maibach H. I. Mesoporous Silica Nanoparticles for Enhanced Lidocaine Skin Delivery. Int. J. Pharm. 2018, 550, 325–332. 10.1016/j.ijpharm.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Gao Q.; Xie W.; Zhao L.; Wang Y.; Zhang W.; Cai Q. Synthesis of Hierarchical Sieve-like Mesoporous Silica Nanoparticle Aggregates via Centrifugal Method for Drug Delivery System. Chin. Chem. Lett. 2018, 29, 1804–1810. 10.1016/j.cclet.2018.09.006. [DOI] [Google Scholar]
- Dumas A.; Couvreur P. Palladium: A Future Key Player in the Nanomedical Field?. Chem. Sci. 2015, 6, 2153–2157. 10.1039/C5SC00070J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk A.; Prokopowicz M. Amino-Modified Mesoporous Silica SBA-15 as Bifunctional Drug Delivery System for Cefazolin: Release Profile and Mineralization Potential. Mater. Lett. 2018, 227, 136–140. 10.1016/j.matlet.2018.05.059. [DOI] [Google Scholar]
- Huang D.; Wu D. Biodegradable Dendrimers for Drug Delivery. Mater. Sci. Eng., C 2018, 90, 713–727. 10.1016/j.msec.2018.03.002. [DOI] [PubMed] [Google Scholar]
- De Jong W. H.; Borm P. J. A. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. 10.2147/IJN.S596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherje A. P.; Jadhav M.; Dravyakar B. R.; Kadam D. Dendrimers: A Versatile Nanocarrier for Drug Delivery and Targeting. Int. J. Pharm. 2018, 548, 707–720. 10.1016/j.ijpharm.2018.07.030. [DOI] [PubMed] [Google Scholar]
- Wang F.; Cai X.; Su Y.; Hu J.; Wu Q.; Zhang H.; Xiao J.; Cheng Y. Reducing Cytotoxicity While Improving Anti-Cancer Drug Loading Capacity of Polypropylenimine Dendrimers by Surface Acetylation. Acta Biomater. 2012, 8, 4304–4313. 10.1016/j.actbio.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Wang X.; Guerrand L.; Wu B.; Li X.; Boldon L.; Chen W. R.; Liu L. Characterizations of Polyamidoamine Dendrimers with Scattering Techniques. Polymers 2012, 4, 600–616. 10.3390/polym4010600. [DOI] [Google Scholar]
- Yang H.; Lopina S. T. Penicillin V-Conjugated PEG-PAMAM Star Polymers. J. Biomater. Sci., Polym. Ed.. 2003, 14, 1043–1056. 10.1163/156856203769231556. [DOI] [PubMed] [Google Scholar]
- Kesharwani P.; Cairul M.; Mohd I.; Giri N.; Jain A.. Dendrimers in Targeting and Delivery of Drugs; Elsevier Inc., 2017; pp 363–388. [Google Scholar]
- Ma X.; Tang J.; Shen Y.; Fan M.; Tang H.; Radosz M. Facile Synthesis of Polyester Dendrimers from Sequential Click Coupling of Asymmetrical Monomers. J. Am. Chem. Soc. 2009, 131, 14795–14803. 10.1021/ja9037406. [DOI] [PubMed] [Google Scholar]
- Abedi-gaballu F.; Dehghan G.; Ghaffari M.; Yekta R.; et al. PAMAM Dendrimers as Efficient Drug and Gene Delivery Nanosystems for Cancer Therapy. Appl. Mater. Today 2018, 12, 177–190. 10.1016/j.apmt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.; Xu Z.; Ma M.; Xu T. Dendrimers as Drug Carriers: Applications in Different Routes of Drug Administration. J. Pharm. Sci. 2008, 97, 123–143. 10.1002/jps.21079. [DOI] [PubMed] [Google Scholar]
- Yavuz B.; Pehlivan S. B.; Vural I.; Ünlü N. In Vitro/In Vivo Evaluation of Dexamethasone - PAMAM Dendrimer Complexes for Retinal Drug Delivery. J. Pharm. Sci. 2015, 104, 3814–3823. 10.1002/jps.24588. [DOI] [PubMed] [Google Scholar]
- Du X.; Yin S.; Wang Y.; Gu X.; Wang G.; Li J. Hyaluronic Acid-Functionalized Half-Generation of Sectorial Dendrimers for Anticancer Drug Delivery and Enhanced Biocompatibility. Carbohydr. Polym. 2018, 202, 513–522. 10.1016/j.carbpol.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Kanwar R.; Bhar R.; Kumar S. Designed Meso-Macroporous Silica Framework Impregnated with Copper Oxide Nanoparticles for Enhanced Catalytic Performance. ChemCatChem 2018, 10, 2087–2095. 10.1002/cctc.201701630. [DOI] [Google Scholar]
- Müller R. H.; Mäder K.; Gohla S. Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery - a Review of the State of the Art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Morel S.; Rosa Gasco M.; Cavalli R. Incorporation in Lipospheres of [d-Trp-6]LHRH. Int. J. Pharm. 1994, 105, R1–R3. 10.1016/0378-5173(94)90466-9. [DOI] [Google Scholar]
- Morel S.; Ugazio E.; Cavalli R.; Gasco M. R. Thymopentin in Solid Lipid Nanoparticles. Int. J. Pharm. 1996, 132, 259–261. 10.1016/0378-5173(95)04388-8. [DOI] [Google Scholar]
- Ugazio E.; Cavalli R.; Gasco M. R. Incorporation of Cyclosporin A in Solid Lipid Nanoparticles (SLN). Int. J. Pharm. 2002, 241, 341–344. 10.1016/S0378-5173(02)00268-5. [DOI] [PubMed] [Google Scholar]
- Baek J. S.; So J. W.; Shin S. C.; Cho C. W. Solid Lipid Nanoparticles of Paclitaxel Strengthened by Hydroxypropyl-β-Cyclodextrin as an Oral Delivery System. Int. J. Mol. Med. 2012, 30, 953–959. 10.3892/ijmm.2012.1086. [DOI] [PubMed] [Google Scholar]
- Pooja D.; Kulhari H.; Kuncha M.; Rachamalla S. S.; Adams D. J.; Bansal V.; Sistla R. Improving Efficacy, Oral Bioavailability, and Delivery of Paclitaxel Using Protein-Grafted Solid Lipid Nanoparticles. Mol. Pharm. 2016, 13, 3903–3912. 10.1021/acs.molpharmaceut.6b00691. [DOI] [PubMed] [Google Scholar]
- Chalikwar S. S.; Belgamwar V. S.; Talele V. R.; Surana S. J.; Patil M. U. Formulation and Evaluation of Nimodipine-Loaded Solid Lipid Nanoparticles Delivered via Lymphatic Transport System. Colloids Surf., B 2012, 97, 109–116. 10.1016/j.colsurfb.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Zariwala M. G.; Elsaid N.; Jackson T. L.; Corral F.; Farnaud S.; Somavarapu S.; Renshaw D. A Novel Approach to Oral Iron Delivery Using Ferrous Sulphate Loaded Solid Lipid Nanoparticles. Int. J. Pharm. 2013, 456, 400–407. 10.1016/j.ijpharm.2013.08.070. [DOI] [PubMed] [Google Scholar]
- Rosière R.; Van Woensel M.; Gelbcke M.; Mathieu V.; Hecq J.; Mathivet T.; Vermeersch M.; Van Antwerpen P.; Amighi K.; Wauthoz N. New Folate-Grafted Chitosan Derivative to Improve Delivery of Paclitaxel-Loaded Solid Lipid Nanoparticles for Lung Tumor Therapy by Inhalation. Mol. Pharm. 2018, 15, 899–910. 10.1021/acs.molpharmaceut.7b00846. [DOI] [PubMed] [Google Scholar]
- Pandya N. T.; Jani P.; Vanza J.; Tandel H. Solid Lipid Nanoparticles as an Efficient Drug Delivery System of Olmesartan Medoxomil for the Treatment of Hypertension. Colloids Surf., B 2018, 165, 37–44. 10.1016/j.colsurfb.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Mehrad B.; Ravanfar R.; Licker J.; Regenstein J. M.; Abbaspourrad A. Enhancing the Physicochemical Stability of β-Carotene Solid Lipid Nanoparticle (SLNP) Using Whey Protein Isolate. Food Res. Int. 2018, 105, 962–969. 10.1016/j.foodres.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Kanwar R.; Gradzielski M.; Mehta S. K. Biomimetic Solid Lipid Nanoparticles of Sophorolipids Designed for Antileprosy Drugs. J. Phys. Chem. B 2018, 122, 6837–6845. 10.1021/acs.jpcb.8b03081. [DOI] [PubMed] [Google Scholar]
- Kanwar R.; Gradzielski M.; Prevost S.; Kaur G.; Clemens D.; Appavou M.; Kumar S. Effect of Lipid Chain Length on Nanostructured Lipid Carriers: Comprehensive Structural Evaluation by Scattering Techniques. J. Colloid Interface Sci. 2019, 534, 95–104. 10.1016/j.jcis.2018.08.066. [DOI] [PubMed] [Google Scholar]
- Kanwar R.; Kaur G.; Mehta S. K. Revealing the Potential of Didodecyldimethylammonium Bromide as Efficient Scaffold for Fabrication of Nano Liquid Crystalline Structures. Chem. Phys. Lipids 2016, 196, 61–68. 10.1016/j.chemphyslip.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Khosa A.; Reddi S.; Saha R. N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. 10.1016/j.biopha.2018.04.055. [DOI] [PubMed] [Google Scholar]
- Kanwar R.; Rathee J.; Patil M. T.; Mehta S. K.. Microemulsions as Nanotemplates: A Soft and Versatile Approach. In Microemulsion—Chemical Nanoreactor, 2019. [Google Scholar]
- Beloqui A.; del Pozo-Rodríguez A.; Isla A.; Rodríguez-Gascón A.; Solinís M. Á. Nanostructured Lipid Carriers as Oral Delivery Systems for Poorly Soluble Drugs. J. Drug Delivery Sci. Technol.. 2017, 42, 144–154. 10.1016/j.jddst.2017.06.013. [DOI] [Google Scholar]
- Beloqui A.; Solinís M. Á.; Rieux A. D.; Préat V.; Rodríguez-Gascón A. Dextran-Protamine Coated Nanostructured Lipid Carriers as Mucus-Penetrating Nanoparticles for Lipophilic Drugs. Int. J. Pharm. 2014, 468, 105–111. 10.1016/j.ijpharm.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Khan S.; Shaharyar M.; Fazil M.; Hassan Q.; Baboota S.; Ali J. Tacrolimus-Loaded Nanostructured Lipid Carriers for Oral Delivery- in Vivo Bioavailability Enhancement. Eur. J. Pharm. Biopharm. 2016, 109, 149–157. 10.1016/j.ejpb.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Kaithwas V.; Dora C. P.; Kushwah V.; Jain S. Nanostructured Lipid Carriers of Olmesartan Medoxomil with Enhanced Oral Bioavailability. Colloids Surf., B 2017, 154, 10–20. 10.1016/j.colsurfb.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Fathi H. A.; Allam A.; Elsabahy M.; Fetih G.; El-Badry M. Nanostructured Lipid Carriers for Improved Oral Delivery and Prolonged Antihyperlipidemic Effect of Simvastatin. Colloids Surf., B 2018, 162, 236–245. 10.1016/j.colsurfb.2017.11.064. [DOI] [PubMed] [Google Scholar]
- Lakhani P.; Patil A.; Taskar P.; Ashour E.; Majumdar S. Curcumin-Loaded Nanostructured Lipid Carriers for Ocular Drug Delivery: Design Optimization and Characterization. J. Drug Delivery Sci. Technol. 2018, 47, 159–166. 10.1016/j.jddst.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha K. A. D.; Krawczyk-Santos A. P.; Andrade L. M.; Souza L. C.; Marreto R. N.; Gratieri T.; Taveira S. F. Voriconazole-Loaded Nanostructured Lipid Carriers (NLC) for Drug Delivery in Deeper Regions of the Nail Plate. Int. J. Pharm. 2017, 531, 292–298. 10.1016/j.ijpharm.2017.08.115. [DOI] [PubMed] [Google Scholar]
- Kelidari H. R.; Moazeni M.; Babaei R.; Saeedi M.; Akbari J.; Parkoohi P. I.; Nabili M.; Gohar A. A.; Morteza-Semnani K.; Nokhodchi A. Improved Yeast Delivery of Fluconazole with a Nanostructured Lipid Carrier System. Biomed. Pharmacother. 2017, 89, 83–88. 10.1016/j.biopha.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Jawahar N.; Kumar P.; Arun R.; Selvaraj J.; Anbarasan A.; Sathianarayanan S.; Nagaraju G. International Journal of Biological Macromolecules Enhanced Oral Bioavailability of an Antipsychotic Drug through Nanostructured Lipid Carriers. Int. J. Biol. Macromol. 2018, 110, 269–275. 10.1016/j.ijbiomac.2018.01.121. [DOI] [PubMed] [Google Scholar]