Abstract
Functional three-dimensional (3D) microstructures incorporating accessible interiors have emerged as a versatile platform for biosystem applications. By configuring their 3D geometric features, these biosystem microdevices can accurately evaluate and control targeted bioenvironments. However, classical fabrication techniques based on photolithography-etching processes cannot precisely and programmably control the geometric of the entire hollow 3D microstructures. Here, we proposed the use of a two-photon polymerization (TPP)-based technique for the precise, straightforward, and customizable preparation of hollow 3D microstructure devices with small opening(s). Factors governing the formation of hollow 3D biosystem microdevices, including material composition, laser input, and (post-) development treatment, have been systematically investigated and a set of optimized conditions are presented as a starting point for the development of novel hollow biosystem microdevices. To evaluate the broad applicability of this approach, a series of tailored hollow 3D microdevices with small opening(s), including a micropore, microneedle, microelectrode, microvalve, and micromachine, were successfully prepared using our direct laser writing-TPP technique. To further validate the feasibility of these biosystem microdevices in practical implementations, we demonstrated the use of hollow 3D micropore devices for the robust resistive-pulse analysis of nanoparticles.
Introduction
Biosystem microdevices are microstructures used for the analysis or handling of biological compounds, and their development has both enhanced performance and increased throughput in modern biological research and applications.1−4 With recent advances in nano-/microfabrication technology having led to the implementation of multiscale, hollow, three-dimensional (3D) microstructure devices with accessible interiors, these benefits are stronger than ever.5,6 Hollow 3D microstructures engineered for both electrical and chemical functions have shown great potential for creating well-controlled microdevices that tackle the issues encountered in intricate biosystems, including biochemical sensing,7,8 pharmaceutical delivery,9 neural interfacing,10 biofluid manipulation,11 and biomedical therapy.11
To date, fabrication strategies utilized for hollow 3D microstructures have been dominated by photolithography–etching processes.12,13 However, because of the harsh processing conditions involved, candidate materials suitable for these methods are mostly limited to silicon-based inorganic substrates. Furthermore, these fabrication strategies can only generate limited geometries for hollow microstructures, resulting in a strong research focus on shapes like micropores and microneedles.5 Given the geometric constraints in chemical etching processes, it is currently not possible to control the geometric features of hollow structures in all three dimensions.14,15 Further geometric control of hollow 3D microstructures can be achieved using enhanced lithographic techniques, such as electron-beam lithographies for micropores and nanosphere lithographies for scaffolds.16,17 Nonetheless, these modified lithographic methods are still not able to precisely control the full 3D geometry of hollow microstructures, yielding pseudo-3D structures with a limited range of geometries and functions.12 Thus, there is a real desire to develop fabrication technologies that can accurately produce complex hollow 3D microstructures of any desired geometry.
Direct laser writing (DLW) via two-photon polymerization (TPP) is emerging as a powerful alternative to photolithographic methods for the rapid 3D fabrication of microstructures.18−20 TPP reactions can be spatiotemporally controlled, a capability that provides the possibility of reliably fabricating tailored hollow 3D microstructures with nanoscale resolution.21,22 During the TPP process, the photoresist polymerizes to generate insoluble polymeric networks, and by programming the laser focal point to move in 3D space, 3D microstructures can be formed.23,24 It is important to note that for TPP to create hollow 3D microstructures, the unpolymerized soluble photoresist trapped inside the structure must be thoroughly removed through opening holes leading into the inner hollow space.25 To our knowledge, no existing work has specifically explored the use of the DLW-TPP technique for the fabrication of tailored hollow 3D biosystem microdevices with small opening(s). Therefore, factors that ultimately determine the viability of TPP for the fabrication of hollow 3D biosystem microdevice structures, such as the broad range of processing settings, including both material issues (resist type, mechanical property and biocompatibility, etc.) and fabrication aspects (slicer parameters, laser power, development condition, etc.), remain unassessed.
Here, we propose the use of the DLW-TPP technique with optimized fabrication parameters for the precise preparation of tailored hollow 3D microstructure devices with accessible interiors. In this work, factors that govern the ultimate structural quality, including slicer parameters, laser input, post-development treatment, and material choice, were comprehensively investigated and are thoroughly discussed. To demonstrate the ubiquitous applicability of this DLW-TPP-based fabrication technique, we created a series of model hollow 3D microstructure devices that are representative of a broad variety of biosystem applications, such as micropores for nanoparticle sensing, microneedles for pharmaceutical delivery, microelectrodes for neural recording/stimulation, microvalves for biofluid controlling, and micromachines for biomedical therapy. Furthermore, to validate that this technique can create practical hollow 3D microstructures for biosystem utilizations, we fabricated hollow micropore devices with geometries tailored for resistive-pulse sensing (RPS) of nanoparticles. Because of the high resolution of this fabrication process, these micropore devices demonstrate robust analytical performance, providing calibration-free nanoparticle size measurements. Generally, these investigations suggest that the DLW-TPP technique serves as a promising fabrication platform for tailored hollow 3D biosystem microdevices with small opening(s).
Results and Discussion
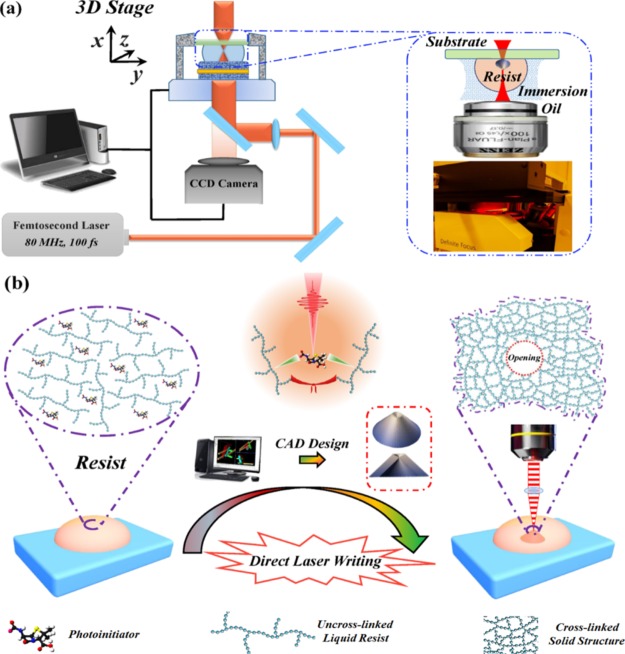
The DLW-TPP process for fabricating hollow 3D microdevices is illustrated in Figure 1. Here, the commercial photonics professional system (Nanoscribe GmbH, Germany) was used to perform the 3D DLW-TPP fabrication process. The underlying TPP mechanism and polymerization schemes are detailed in the Supporting Information. As shown in Figure 1a, the liquid photoresist was used as the immersion fluid between the microscope lens (25×, 0.8 NA, Zeiss) and the loading substrate. During DLW, a femtosecond laser pulse (80 MHz, 100 fs) was redirected and focused inside a droplet of the photoresist to trigger the TPP reactions that cross-link the polymeric resist material (see Figure 1b). Significantly, the reactive resist volume is strictly restricted to a nano-sized unit (voxel),26 allowing 3D fabrication of structures with nanoscale resolution (see Figure 1a).
Figure 1.
(a) Schematic illustration of a DLW-TPP system (Nanoscribe), oil immersion mode. Femtosecond laser pulse source: 780 nm, 80 MHz, 100 fs. Peak power: ∼25 kW. Highest fluence level: ∼1 J/cm2. Inset picture: XYZ-3D movable sample stage. (b) Schematic graph of the DLW process. Hollow 3D microstructures were sketched by a CAD program. Under femtosecond laser pulse irradiation, photoresist polymers are cross-linked to form solidified hollow 3D microstructure devices. In this work, the size of opening ranges from 500 nm to 6 μm.
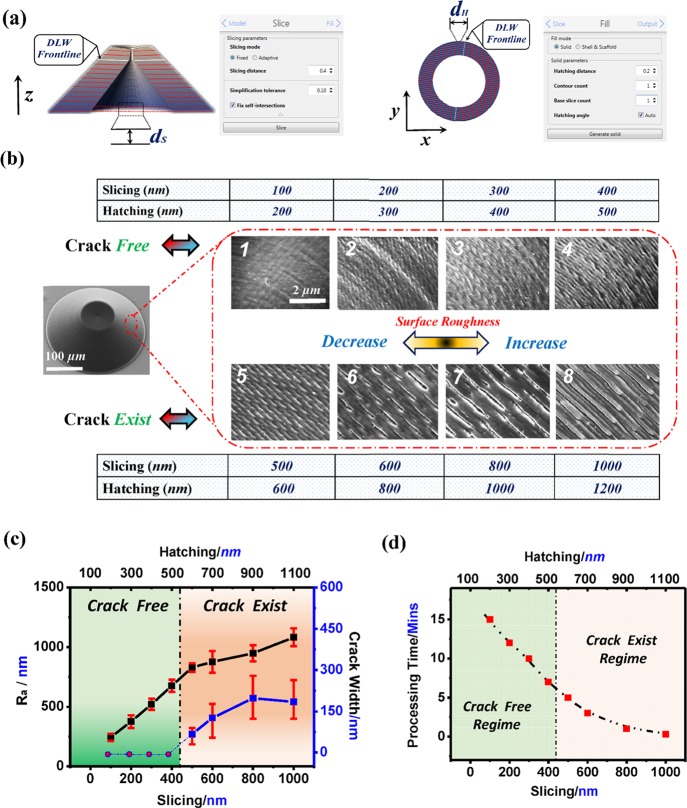
Control of microdevice surface properties in turn controls the functionality and performance of hollow 3D microdevices and is necessary to allow their uses in many biosystem applications.27,28 To evaluate the ability of DLW to precisely control surface properties, a series of conic hollow 3D micropore structures were fabricated under different processing conditions. In preparation, two structural processing settings—slicing distance and hatching distance (see Figure 2a)—were combined to regulate the exposure area of the material used in the DLW fabrication process. Figure 2b illustrates the surface morphologic profiles of the conic hollow 3D micropores prepared using different slicing–hatching parameters, ranging from nanoscale (slicing dxy: 100 nm, hatching lz: 200 nm) to micronscale (slicing dxy: 1000 nm, hatching lz: 1200 nm). For conical hollow micropores prepared by nanoscale (dxy: 100 nm, lz: 200 nm) slicing parameters, the structures displayed a flawless, smooth surface profile (Ra = 81 ± 10 nm). As the slicing and hatching distances were gradually increased, the hollow 3D microstructures showed a tendency toward a more rugged surface in appearance (see Figure 2c, blue line).
Figure 2.
Effects of structural processing settings on the surface morphologic profiles of prepared hollow conic 3D micropore structures. (a) Schematic slicing (left) and hatching (right) patterns for the DLW-TPP process of hollow 3D microstructures. The slicing and hatching parameter settings are realized by the DeScribe software. (b) Surface morphologic properties of the hollow 3D microstructures prepared using different-sized constituent units. Scale bar: 2 μm, apply to all SEM images. (c) Surface roughness of the hollow 3D microstructures vs structural processing conditions (left axis, black curve). The width of the cracks formed on the surface of hollow 3D microstructures vs structural processing conditions (right axis, blue curve). (d) Minimum required processing time for the fabrication of hollow 3D microstructures vs structural processing conditions.
As we further increase the slicing and hatching distances in TPP fabrication, cracks of varying sizes could be formed on the surface of the prepared hollow 3D microstructures (Figure 2c, black curve). These cracks can be controlled and may provide useful functions for biosystem microdevices, such as in pharmaceutical delivery systems requiring the controlled release of drug/vaccine products.29,30 Meanwhile, these parameters also affected the overall processing time of the DLW process (Figure 2d). Hollow 3D microstructures prepared using finer slicing–hatching parameters to give a crack-free surface required longer processing times. Taken together, hollow 3D biosystem microdevices with accurately controlled surface profiles can be realized via the DLW-TPP process.
The opening hole of a hollow 3D microdevice is essential for the overall performance quality in many biosystem implementations, that is, for the access of (bio)analytes into the interior functional structure. Therefore, to create high-performing hollow 3D biosystem microdevices, it is essential to optimize the processing parameters that govern the formation process of the opening hole (e.g., laser power and development conditions, etc.). As shown in Figure 3a, the quality of the opening hole could be dramatically influenced by the input laser power (fluence) levels. To quantitatively evaluate these laser-induced effects on the quality of formed circular opening holes, we herein introduce the ratio rcircularity (0 ≤ rcircularity ≤ 1), with which a higher rcircularity value corresponds to a higher quality hole. The results indicated that input laser pulses with low power levels (<40%) were not able to yield opening(s) with accurately defined geometric features as the applied laser was not strong enough to polymerize the resist material [Figure 3a, scanning electron microscopy (SEM) image 1–3].
Figure 3.
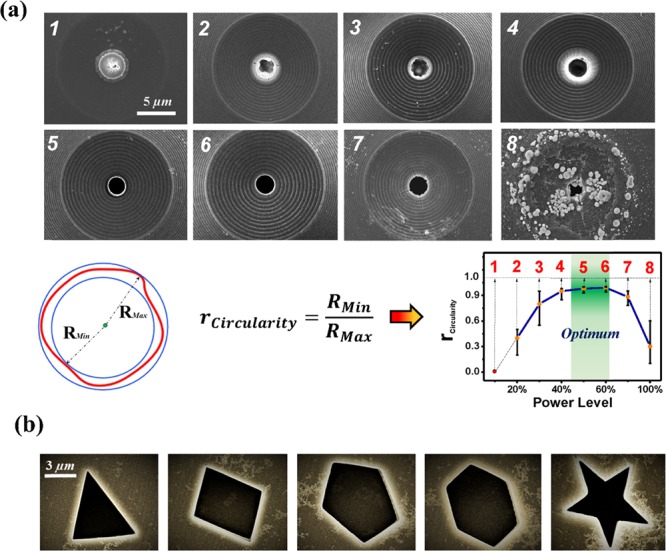
(a) Effect of the laser power level (fluence) on the quality of incorporated opening hole structures. Upper: SEM images of the built-in circular opening parts (Ø = 3 μm) prepared under different power levels. Down: Circularity ratio of the built-in circular opening parts prepared under different power levels. Full power level (100%): ∼1 J/cm2. Threshold power level: ∼40%, dynamic power range: 50–60%, damage power range: >70%. Scale bar: 5 μm, apply to all eight SEM images. (b) Opening holes with distinct geometric shapes incorporated within fabricated hollow 3D microstructure devices, left to right: triangle, rectangle, pentagon, hexagon, and pentagram. Scale bar: 3 μm, apply to all five SEM images.
As the laser fluence level approached the threshold (∼40% for IP-S resist), the opening holes showed clean and sharp circular shapes, exactly as CAD-designed. Within the working fluence range (50–60% for IP-S), the reactive resist volume was strictly confined to a single building unit (slicing dxy: 300 nm, hatching lz: 400 nm), creating accurately controlled opening hole structures with nanoscale resolution. Significantly, accurate formation of opening hole structures facilitates the wash-away process of the trapped resist during the development process, which is necessary to produce clean and accurate hollow 3D microdevices (Figure S2). Further increments in laser fluence above the working range would lead to opening structures with degraded quality (Figure 3a, SEM image 7 and 8). At higher fluence levels, the overexposed photoresist undergoes optical damage, which causes imperfections (e.g., cracks, surface debris, etc.) in the formed opening holes.31
Promisingly, this technique allows opening holes to be created based on arbitrary shapes. As shown in Figure 3b, opening hole structures with distinct geometric shapes (triangle, rectangular, pentagon, hexagon, and pentagram) were realized in hollow 3D microdevices. Our results suggest that the optimum laser power level for accurate opening hole formation is solely determined by the material properties of the utilized resist and is independent of the actual hole geometry (Table S1). The white ring visible at the entrance of the openings is likely an artifact of the platinum sputtering process used to coat the structures for SEM imaging, as the sharp edge of the opening cannot be easily coated with a uniform Pt layer using conventional sputtering processes. Therefore, DLW-TPP may serve as a universal platform for creating precise hollow 3D biosystem microdevices with tailored opening hole(s).
The suitable working temperature of biosystems varies broadly. To verify the extensive applicability of these hollow 3D micro-devices, it is essential to scrutinize their geometric stability under different temperatures. Figure S3 demonstrates the effects of increasing temperatures on the geometric properties of prepared hollow 3D microdevices. To quantify these temperature effects, we investigated the shrinkage rate of their opening holes. The temperature-induced geometric change was still below 5% even when the temperature was increased from room temperature to a high value (70 °C) and then rapidly cooled (−20 °C) to freeze the device in a shrunken state, indicating that these DLW-TPP-prepared hollow 3D microstructures possess good geometric stability over a broad temperature spectrum.
Hollow 3D microdevices with accessible interiors have been extensively investigated to resolve the thorny problems in biosystems.2,3,32,33 Importantly, biocompatibility of these microdevices could be modified either through surface modification/functionalization or by using biocompatible photoresists as the bulk material34−36 to seamlessly integrate with the biosystems being analyzed (Table S2). It is worthwhile to note that the design of the hollow interior can impact or even govern the fundamental functionality of the prepared hollow 3D microdevices. Promisingly, the biosystem microdevices fabricated in this paper using the DLW-TPP technique possess tailored geometric features both interior and exterior and offered customizable functionality and performance. As shown in Figure 4a,b, hollow microneedles suitable for enhanced pharmaceutical delivery were prepared. Through the fine-tuning of geometric features (i.e., surface roughness, surface cracks and interior hollow, etc.), drug/vaccine products loaded inside the hollow space or anchored on the microdevice surface could be released in a spatiotemporally controlled manner.27,29 Additionally, these solidified hollow 3D microneedle structures exhibited sufficient mechanical stability and rigidity to penetrate the skin without inducing any structural fracture (EYoung ≈ 2–3 GPa for IP-S resist, see Figure S4). Furthermore, highly controllable hollow microneedle array patches can be readily manufactured for various bioapplication purposes, as shown in Figure S5.
Figure 4.
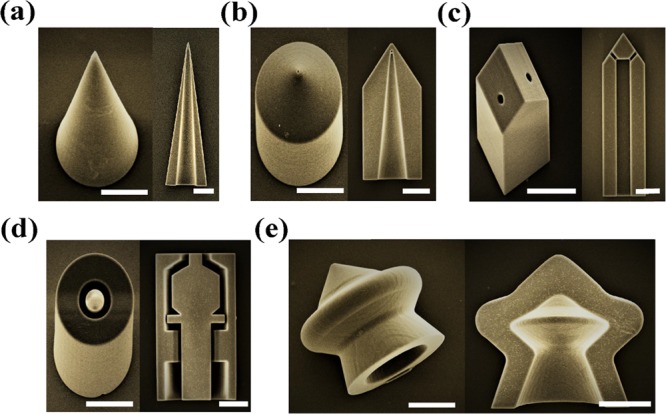
Series of CAD-designed hollow 3D microdevices were fabricated using the TPP-enabled DLW technique. Scale bar: 50 μm. (a,b) Hollow microneedles for enhanced pharmaceutical delivery. Left: Tilt angle-view. Right: Cross-section view. (c) Hollow microelectrode for neural recording and stimulation. Left: Tilt angle-view. Right: Cross-section view. (d) Hollow microvalve for biofluidic controlling. Left: Tilt angle-view. Right: Cross-section view. (e) Hollow microrobot for medical therapy. Left: Tilt angle-view. Right: Cross-section view.
Figure 4c shows a microelectrode that could simultaneously stimulate and record neural signals by integrating the functions of chemical stimulus delivery and electrical signal recording. The conductivity of microelectrodes fabricated by DLW-TPP can be tuned to improve their electrical signal recording capability, via coating of conducting metal thin films on the surface (Table S3) or by using intrinsically conductive photoresist materials.37,38 Importantly, the demonstrated hollow 3D microelectrode has a tip size smaller than 10 μm and features micropores allowing chemical delivery, sampling, or perfusion through the electrode. This would enable precise manipulations and interactions at the single-cell level.6,10Figure 4d demonstrates the possibility of a hollow microvalve structure containing sophisticated internal movable components. Such microvalves could be used to accurately control and sense biofluidic flow conditions in various biosystems with the advantage of being manufactured out of biocompatible materials. Figure 4e shows an example of a micromachine-inspired structure produced with the DLW-TPP technique. Similar structures have been fabricated using traditional chemical and photolithographic techniques in the literature, driven by self-propelling, chemical fuels, electric fields, or surface tension.39−41 The internal and external geometric features at the core of these devices could easily and accurately be produced via DLW-TPP based on simple CAD designs, greatly simplifying their production process. For use cases requiring individual, detached hollow 3D microdevices, ultrasonic treatments (∼10–30 s) have proven to be a robust tool that facilitates the release of these microdevices from the loading substrate (see Figure S6). Therefore, with its ability to precisely specify geometry and operate on a wide spectrum of photoresist materials, DLW-prepared hollow 3D microdevices would shed light on an extensive range of unexplored use cases.
To validate the practical applicability of these hollow 3D microdevices, we performed robust resistive-pulse analysis of nanoparticles using the DLW-TPP-prepared conic hollow 3D micropores. RPS has become a prominent technique for the high-throughput particle-by-particle characterization of colloid suspensions of individual (bio)analytes in biosystems.7,42,43 One crucial part of this characterization system is the sensitive nano-/microhollow hole, the preparation of which has been the subject of enormous interest in recent years. However, traditional preparation techniques suffer from several severe deficiencies: solid-state holes typically rely on etching, puncturing, or heating-and-pulling methods which do not give tight control over the internal pore geometry, whilst biological pores are limited to very small size regimes and are not suitable for all chemicals or analytes.
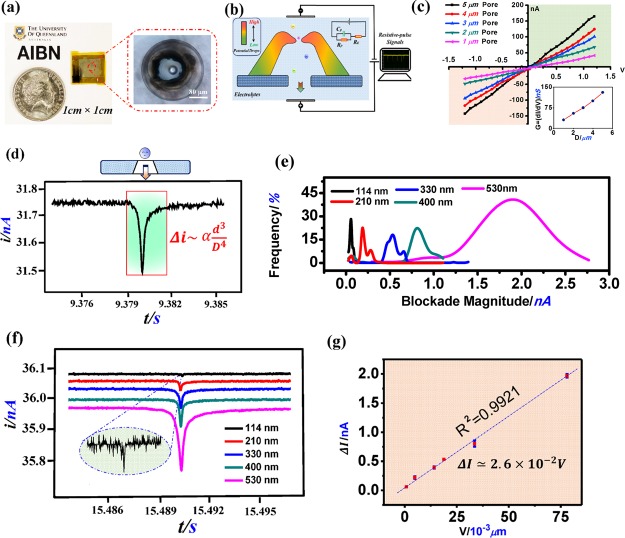
The DLW-TPP technique presented in this paper provides a feasible resolution for these critical issues encountered in RPS pore preparation. To explore this, we successfully prepared optimized hollow 3D micropores assembled on a plastic substrate (∼1 cm × 1 cm polyethylene terephthalate (PET), see Figures 5a and S7), for the high-performance resistive-pulse analysis of nanoparticles. The substrate microhole structure (≈Ø 100 μm) beneath the DLW-prepared conic micropore device was created via a hybrid additive–subtractive fabrication process employing both the method of this paper and our newly developed maskless 3D laser ablation technique44 (Figure S8). Figure 5b schematically shows the two-counter electrode configuration of the resistive-pulse characterization system, in which the prepared micropore structure works as an effective sensitive component for the RPS system (see Figure S9a).
Figure 5.
(a) DLW-TPP-prepared hollow 3D Microcone assembled on the PET substrate (∼1 cm × 1 cm). (b) Schematic graph of the resistive-pulse analysis system using the DLW-TPP-prepared hollow 3D conic microhole structure. A working voltage (Vw) is applied on two sides of the 3D microdevice. (c) i–v curves of hollow 3D microstructures integrated with different-sized circular openings, ranging from 1 to 5 μm. Working voltage range: −1.2 to 1.2 V, voltage step: 0.06 V. The down-right inset figure: The conductivity of the resistive-pulse characterization system vs opening size of the hollow 3D microhole devices. (d) Single current blockade induced by 300 nm PS nanoparticles passing through the hollow 3D microdevice with a 3 μm-sized circular opening hole. Vw: 0.3 V. (e) Current blockade magnitude (Δi) distribution of different types of PS nanoparticles (i.e., 114, 210, 300, 400, and 530 nm) characterized using the hollow 3D Microcone structure with a 3 μm-sized circular opening hole design. Vw: 0.3 V. (f) Combined single current blockade of different types of PS nanoparticles (114–530 nm) passing through a hollow 3D Microcone structure with a 3 μm-sized circular opening design. Vw: 0.3 V. Each individual signal was normalized to the same (current/time) scale. (g) Relationship of current blockade magnitude vs nanoparticle volume size in resistive-pulse analysis using the hollow 3D Microcone structure with a 3 μm-sized circular opening hole. Vw: 0.3 V.
In RPS characterization, the applied external working voltage induced the transient ionic current flowing through the hollow 3D micropore structure. We prepared hollow 3D micropore structures with variable-sized (Ø: 1–5 μm) circular opening hole. Static i–v curves of these hollow micropore structures were recorded in Figure 5c. The results showed that, at any particular working voltage, the magnitude of the transient ionic current (conductivity) linearly (R2 = 0.9927) increased with the circular opening hole size (see Figure S9c), a phenomenon that is modeled by Vogel et al.45
| 1 |
in which V is the applied voltage, DS is the small-side hole size, Dl is the large-side hole size, LC is the conical hole length, and ρ is the electrolyte resistivity (see Figure S9b). This study on the transient current behavior suggests that our DLW-TPP-prepared micropore devices could be used for practical RPS experiments.
To confirm this, we performed RPS-based nanoparticle size analysis to verify the robustness of these hollow 3D micropore devices. In testing, hollow 3D micropore structures with precisely defined circular opening holes (Ø = 3 μm) were prepared (see Figure S7). As shown in Figure 5d, a decrease in ionic current (“current blockade”) occurs as each individual nanoparticle translocates through the hollow micropore structure. The overall current plot and other analysis results for the RPS experiment are shown in Figure S10. Figure 5e shows the combined magnitude distributions of current blockades corresponding to each type of the analyzed nanoparticle solution (114–530 nm, see Figure S11). The magnitude of the mode current blockade increased remarkably as the analyzed nanoparticle size was increased from 114 to 530 nm (as modeled below by eq 2). We then calculated the volume of each type of nanoparticle based on its nominal size and recorded the corresponding current blockade magnitude (mode value) for each measurement (Table S5). These were plotted against each other, and the resulting graph (see Figure 5g) displays a highly linear relationship (R2 = 0.9921) between the nanoparticle volume and the magnitude of corresponding current blockade, as predicted by Kozak et al.46,47
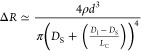 |
2 |
| 3 |
| 4 |
in which ΔR is the resistance change, d is the nanoparticle diameter, Δi is the current blockade, and V is nanoparticle volume size. As a result, these DLW-TPP-prepared hollow 3D micropore devices create new opportunities for the accurate, straightforward, and low-cost differentiation of nanoparticles/analytes in biosystems, via the use of the RPS technique (Figure 5f).
Conclusions
In conclusion, we have successfully developed a precise, controllable, and straightforward DLW-TPP-based additive fabrication strategy, for the preparation of tailored hollow 3D biosystem microdevices. Unlike conventional fabrication techniques, the DLW-TPP fabrication scheme provides superior control over the full geometric properties (both interior and exterior) of the fabricated device. With a well-established fabrication process, we now can systematically and accurately modulate the processing conditions, including slicer parameters, laser input, and development treatment, to tune the functionality of these customized hollow 3D biosystem microdevices. We have demonstrated that the tailored hollow 3D microstructure devices produced by our technique hold great potential for biosystem applications, including micropores for biochemical sensing, microneedles for pharmaceutical delivery, microelectrodes for neural recording, microvalves for biofluidic measurement and manipulation, and micromachines for biomedical therapy. To further confirm the suitability of the DLW-TPP-fabricated hollow 3D microdevices in practical biosystem implementations, we prepared hollow 3D micropores with accurately defined opening holes for the calibration-free resistive-pulse analysis of nanoparticles.
Experimental Section
Materials
Substrates for the loading of hollow 3D microstructure devices, including an indium tin oxide (ITO)-coated PET film (protective film on both sides, Shenzhen Jemstone Technology Co., Ltd.) and ITO-coated glass (Sigma-Aldrich Co.), were used as received. Milli-Q (Merck Millihole) deionized (DI) H2O was used for the phosphate-buffered saline (PBS) solution preparation, nanoparticle dilution, and ultrasonic treatment medium. A PBS tablet (Sigma-Aldrich Co.) was used for the preparation of PBS solution, dissolved with DI water. Resist materials for hollow 3D microstructure fabrication, including the IP-series resist (IP-L, IP-S, and IP-Dip, Nanoscribe GmbH), ORMOCER (Micro Resist Technology GmbH) and SU-8 (MicroChem Corp.), were used as received. Different types of polystyrene (PS) nanoparticles functionalized with carboxyl group, with a diameter size range from 114 to 530 nm, were purchased from Bangs Laboratories, Inc. (see Figure S11). Raw PS nanoparticle samples were diluted with 1× PBS solution, with a dilution ratio of 1:1000 to 1:10 000, to prepare suitable nanoparticle solutions for resistive-pulse analysis.
Direct Laser Writing
Externally accessible hollow 3D microstructures with built-in opening holes, including micropores, microneedles, microelectrodes, microvalves, and micromachines, were designed in an Autodesk AutoCAD program. These AutoCAD-generated standard triangle language (.STL) files were imported into the DeScribe software (Nanoscribe GmbH, Germany) for structural processing (slicing and hatching). This processed general writing language (.GWL) file was then transferred to the Nanoscribe Photonic Professional GT system (Nanoscribe GmbH, Germany. Figure S1) for the DLW of hollow 3D microstructures. This TPP-enabled DLW fabrication system was equipped with a FemtoFiber Pro NIR laser source (Toptica Photonics AG) operating with a pulse duration (τ) ≈ 100 fs and a repetition rate (f) of 80 MHz at 780 nm (λ). Transmittance (T) of laser beam through the IP-S resist at 780 nm was ∼0.75. The laser pulse was focused by a Zeiss Plan-Apochromat 25× 0.8 NA oil DIC M27 objective, with circular polarization at the entrance of the objective plane. With the aid of an integrated charge-coupled device camera (Figure 1a), the DLW-TPP process of hollow 3D microstructures can be visualized in real time (Video S1).
In the experiment, substrates coated with resist materials were mounted onto the Nanoscribe-controlled 3D XYZ piezo stage for the DLW-TPP process. For the hollow 3D microstructures fabricated with the IP-series resists (IP-L, IP-S, and IP-Dip) and ORMOCER, the liquid resist materials (∼100 μL) were directly dropped onto the loading substrate to initiate the DLW-TPP process. For the microfabrication using SU-8, prebaking (60 °C, 60 s) was required before laser irradiation. After DLW process, the SU-8-based structure was then processed with the post-exposure baking (60 °C, 2 min), followed by a third photoresist baking step (90 °C, 60 s) to harden the structure. Finally, these DLW-fabricated 3D microstructures were transferred into a corresponding development system (mixed organic solvents, Table S2) for a specific period to remove the un-cross-linked resist materials existing both inside and outside the hollow microstructures entirely.
Characterization
A JEOL IT-300 scanning electron microscope ( JEOL Ltd.) was used for the morphological characterization of these prepared hollow 3D microstructures. The surfaces of these microstructures analyzed by SEM were precoated with a 20 nm thick platinum film using a JEOL coating system. A Nikon Eclipse Ni–U optical system equipped with a Nikon Plan Fluor lens was employed for the optical imaging. Deposition of platinum films with different thicknesses (∼10–60 nm) on the surface of the resistive material was performed using the metal coating system, by controlling the deposition time and input current. Surface conductivity of the platinum-coated resist film was measured using a Fluke Multimeter. The surface roughness of the prepared hollow 3D micropore structures was measured and analyzed with the Dektak 150 stylus profiler (Bruker Corp.). The mechanical properties (Young’s modulus) of IP-S-based hollow microneedle structures were measured by the Asylum Research MFP-3D-Bio inverted optical atomic force microscope (Oxford Instruments), in an amplitude modulation–frequency modulation viscoelastic mapping mode. Confocal characterization imaging of the hollow 3D micropore structure was carried out by the confocal laser scanning microscopy (LSM) suite-Leica SP8 confocal laser scanning microscope (Leica AG). Ultrasonic treatment (∼10–30 s, Grant Instruments) was performed to release the hollow 3D microstructures from the loading substrate.
Resistive-Pulse Analysis
The prepared hollow 3D micropore structure used for resistive-pulse analysis was loaded on the PET substrate (∼1 cm × 1 cm). As shown in Figure S5, the substrate microhole (∼Ø 100 μm) fully covered by the upper DLW-TPP-prepared micropore structure was created by our previously developed laser ablation technique (Figure S5). The laser ablation process details were described above. A qNano system (Izon Science, New Zealand) was utilized for the resistive-pulse nanoparticle analysis. The micropore sample was incorporated into the measurement chamber unit of qNano (see Figure S6). PBS (1×, Sigma-Aldrich Co.) was used as the electrolyte for resistive-pulse analysis. For the study of the transient i–v curves of the hollow 3D micropore structures with a different-sized circular opening hole (1–5 μm), corresponding ionic currents were recorded at each applied voltage (−1.2 to 1.2 V, 0.6 V increment for each step). For the resistive-pulse analysis of PS nanoparticles, different types of nanoparticle (114–530 nm) solution samples (∼40 μL) were added onto the upper chamber of the measurement unit once the characterization system reached the stabilized states. In the experiment, the current blockades were collectively recorded and analyzed with the built-in qNano analysis.
Acknowledgments
The authors would like to acknowledge the financial support (DP160102836) from Australian Research Council for this research project. C.L. and F.A. would like to acknowledge the financial support from the Australian Government Research Training program (RTP) scholarship. The authors also acknowledge the expertise training and facilities support from the Australian National Fabrication Facility-Queensland Node (ANFF-Q) in The University of Queensland.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b03164.
TPP mechanism and resistive-pulse analysis; development process for 3D hollow microstructures; laser ablation of the substrate microhole for the 3D hollow micropore; nanoscribe system setup; development process for the DLW-TPP-prepared hollow 3D microstructures; heating effects on prepared hollow 3D microstructures; AFM characterization of the hollow microneedle surface coated with a Pt film; mechanical property of the microneedle-type hollow 3D microstructure; microneedle array; ultrosonic treatment to detach the prepared microstructure from the loading substrate; geometric characterization; SEM images of the laser-ablated substrate hole and different types of nanoparticles; resisitive-pulse system analysis and setup; development time for distinct-shaped opening structures; hollow 3D microstructures prepared using different types of resist materials; tunable surface conductivity of prepared hollow 3D microstructures; processing conditions of demonstrated 3D hollow biosystem microdevices; and volume size and ionic current magnitude for different types of nanoparticles (PDF)
Real-time DLW-TPP fabrication of hollow 3D microstructures (AVI)
Current trace curve of nanoparticles translocating through hollow micropores (AVI)
Confocal rotational images of 3D hollow microstructures (AVI)
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Ni J.; Wang Z.; Li Z.; Lao Z.; Hu Y.; Ji S.; Xu B.; Zhang C.; Li J.; Wu D.; Chu J. Multifurcate Assembly of Slanted Micropillars Fabricated by Superposition of Optical Vortices and Application in High-Efficiency Trapping Microparticles. Adv. Funct. Mater. 2017, 27, 1701939. 10.1002/adfm.201701939. [DOI] [Google Scholar]
- Lao Z.-X.; Hu Y.-L.; Pan D.; Wang R.-Y.; Zhang C.-C.; Ni J.-C.; Xu B.; Li J.-W.; Wu D.; Chu J.-R. Self-Sealed Bionic Long Microchannels with Thin Walls and Designable Nanoholes Prepared by Line-Contact Capillary-Force Assembly. Small 2017, 13, 1603957. 10.1002/smll.201603957. [DOI] [PubMed] [Google Scholar]
- Malinauskas M.; Žukauskas A.; Hasegawa S.; Hayasaki Y.; Mizeikis V.; Buividas R.; Juodkazis S. Ultrafast laser processing of materials: from science to industry. Light: Sci. Appl. 2016, 5, e16133 10.1038/lsa.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.; Sugioka K.; Midorikawa K. Microfabrication of 3D hollow structures embedded in glass by femtosecond laser for Lab-on-a-chip applications. Appl. Surf. Sci. 2005, 248, 172–176. 10.1016/j.apsusc.2005.03.078. [DOI] [Google Scholar]
- Kim Y.-C.; Park J.-H.; Prausnitz M. R. Microneedles for drug and vaccine delivery. Adv. Drug Delivery Rev. 2012, 64, 1547–1568. 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete Z. Technology of ultralong deep brain fluidic microelectrodes combined with etching-before-grinding. Microsyst. Technol. 2013, 21, 341–344. 10.1007/s00542-013-1985-7. [DOI] [Google Scholar]
- Kozak D.; Anderson W.; Vogel R.; Trau M. Advances in Resistive Pulse Sensors: Devices bridging the void between molecular and microscopic detection. Nano Today 2011, 6, 531–545. 10.1016/j.nantod.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell E. L. C. J.; Mayne L. J.; Billinge E. R.; Platt M. Emergence of tunable resistive pulse sensing as a biosensor. Anal. Methods 2015, 7, 7055–7066. 10.1039/c4ay03023k. [DOI] [Google Scholar]
- Ma G.; Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Controlled Release 2017, 251, 11–23. 10.1016/j.jconrel.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Pongrácz A.; Fekete Z.; Márton G.; Bérces Z.; Ulbert I.; Fürjes P. Deep-brain silicon multielectrodes for simultaneous in vivo neural recording and drug delivery. Sens. Actuators, B 2013, 189, 97–105. 10.1016/j.snb.2013.01.032. [DOI] [Google Scholar]
- Schizas C.; Melissinaki V.; Gaidukeviciute A.; Reinhardt C.; Ohrt C.; Dedoussis V.; Chichkov B. N.; Fotakis C.; Farsari M.; Karalekas D. On the design and fabrication by two-photon polymerization of a readily assembled micro-valve. Int. J. Adv. Manuf. Technol. 2009, 48, 435–441. 10.1007/s00170-009-2320-4. [DOI] [Google Scholar]
- Reznikova E. F.; Mohr J.; Hein H. Deep photo-lithography characterization of SU-8 resist layers. Microsyst. Technol. 2005, 11, 282–291. 10.1007/s00542-004-0432-1. [DOI] [Google Scholar]
- Ottow S.; Lehmann V.; Föll H. Processing of Three-Dimensional Microstructures Using Macroporous n-Type Silicon. J. Electrochem. Soc. 1996, 143, 385–390. 10.1149/1.1836442. [DOI] [Google Scholar]
- Mata A.; Fleischman A. J.; Roy S. Fabrication of multi-layer SU-8 microstructures. J. Micromech. Microeng. 2006, 16, 276–284. 10.1088/0960-1317/16/2/012. [DOI] [Google Scholar]
- Agirregabiria M.; Blanco F. J.; Berganzo J.; Arroyo M. T.; Fullaondo A.; Mayora K.; Ruano-López J. M. Fabrication of SU-8 multilayer microstructures based on successive CMOS compatible adhesive bonding and releasing steps. Lab Chip 2005, 5, 545–552. 10.1039/b500519a. [DOI] [PubMed] [Google Scholar]
- Tseng A. A.; Chen K.; Chen C. D.; Ma K. J. Electron beam lithography in nanoscale fabrication: recent development. IEEE Trans. Electron. Packag. Manuf. 2003, 26, 141–149. 10.1109/tepm.2003.817714. [DOI] [Google Scholar]
- Ströer F.; Hering J.; Eifler M.; Raid I.; von Freymann G.; Seewig J. Ultrafast 3D high precision print of micro structures for optical instrument calibration procedures. Addit. Manuf. 2017, 18, 22–30. 10.1016/j.addma.2017.09.001. [DOI] [Google Scholar]
- Harnisch E.; Russew M.; Klein J.; König N.; Crailsheim H.; Schmitt R. Optimization of hybrid polymer materials for 2PP and fabrication of individually designed hybrid microoptical elements thereof. Opt. Mater. Express 2015, 5, 456–461. 10.1364/ome.5.000456. [DOI] [Google Scholar]
- Sun H.-B.; Kawakami T.; Xu Y.; Ye J.-Y.; Matuso S.; Misawa H.; Miwa M.; Kaneko R. Real three-dimensional microstructures fabricated by photopolymerization of resins through two-photon absorption. Opt. Lett. 2000, 25, 1110–1112. 10.1364/ol.25.001110. [DOI] [PubMed] [Google Scholar]
- Kawata S.; Sun H.-B. Two-photon photopolymerization as a tool for making micro-devices. Appl. Surf. Sci. 2003, 208–209, 153–158. 10.1016/s0169-4332(02)01358-2. [DOI] [Google Scholar]
- Watanabe T.; Akiyama M.; Totani K.; Kuebler S. M.; Stellacci F.; Wenseleers W.; Braun K.; Marder S. R.; Perry J. W. Photoresponsive Hydrogel Microstructure Fabricated by Two-Photon Initiated Polymerization. Adv. Funct. Mater. 2002, 12, 611–614. . [DOI] [Google Scholar]
- Montemayor L. C.; Meza L. R.; Greer J. R. Design and Fabrication of Hollow Rigid Nanolattices via Two-Photon Lithography. Adv. Eng. Mater. 2013, 16, 184–189. 10.1002/adem.201300254. [DOI] [Google Scholar]
- Sun H.-B.; Maeda M.; Takada K.; Chon J. W. M.; Gu M.; Kawata S. Experimental investigation of single voxels for laser nanofabrication via two-photon photopolymerization. Appl. Phys. Lett. 2003, 83, 819–821. 10.1063/1.1598293. [DOI] [Google Scholar]
- Lao Z.; Hu Y.; Zhang C.; Yang L.; Li J.; Chu J.; Wu D. Capillary Force Driven Self-Assembly of Anisotropic Hierarchical Structures Prepared by Femtosecond Laser 3D Printing and Their Applications in Crystallizing Microparticles. ACS Nano 2015, 9, 12060–12069. 10.1021/acsnano.5b04914. [DOI] [PubMed] [Google Scholar]
- Li G.; Lu Y.; Wu P.; Zhang Z.; Li J.; Zhu W.; Hu Y.; Wu D.; Chu J. Fish scale inspired design of underwater superoleophobic microcone arrays by sucrose solution assisted femtosecond laser irradiation for multifunctional liquid manipulation. J. Mater. Chem. A 2015, 3, 18675–18683. 10.1039/c5ta05265c. [DOI] [Google Scholar]
- Sun H.-B.; Takada K.; Kim M.-S.; Lee K.-S.; Kawata S. Scaling laws of voxels in two-photon photopolymerization nanofabrication. Appl. Phys. Lett. 2003, 83, 1104–1106. 10.1063/1.1599968. [DOI] [Google Scholar]
- van der Maaden K.; Jiskoot W.; Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Controlled Release 2012, 161, 645–655. 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Xing J.-F.; Zheng M.-L.; Duan X.-M. Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. 10.1039/c5cs00278h. [DOI] [PubMed] [Google Scholar]
- Prausnitz M. R.; Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indermun S.; Luttge R.; Choonara Y. E.; Kumar P.; du Toit L. C.; Modi G.; Pillay V. Current advances in the fabrication of microneedles for transdermal delivery. J. Controlled Release 2014, 185, 130–138. 10.1016/j.jconrel.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Schaffer C. B.; Brodeur A.; Mazur E. Laser-induced breakdown and damage in bulk transparent materials induced by tightly focused femtosecond laser pulses. Meas. Sci. Technol. 2001, 12, 1784–1794. 10.1088/0957-0233/12/11/305. [DOI] [Google Scholar]
- Richter B.; Hahn V.; Bertels S.; Claus T. K.; Wegener M.; Delaittre G.; Barner-Kowollik C.; Bastmeyer M. Guiding Cell Attachment in 3D Microscaffolds Selectively Functionalized with Two Distinct Adhesion Proteins. Adv. Mater. 2016, 29, 1604342. 10.1002/adma.201604342. [DOI] [PubMed] [Google Scholar]
- Sakellari I.; Yin X.; Nesterov M. L.; Terzaki K.; Xomalis A.; Farsari M. 3D Chiral Plasmonic Metamaterials Fabricated by Direct Laser Writing: The Twisted Omega Particle. Adv. Opt. Mater. 2017, 5, 1700200. 10.1002/adom.201700200. [DOI] [Google Scholar]
- Cumpston B. H.; Ananthavel S. P.; Barlow S.; Dyer D. L.; Ehrlich J. E.; Erskine L. L.; Heikal A. A.; Kuebler S. M.; Lee I.-Y. S.; McCord-Maughon D.; Qin J.; Röckel H.; Rumi M.; Wu X.-L.; Marder S. R.; Perry J. W. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. 10.1038/17989. [DOI] [Google Scholar]
- Campbell M.; Sharp D. N.; Harrison M. T.; Denning R. G.; Turberfield A. J. Fabrication of photonic crystals for the visible spectrum by holographic lithography. Nature 2000, 404, 53–56. 10.1038/35003523. [DOI] [PubMed] [Google Scholar]
- Pitts J. D.; Campagnola P. J.; Epling G. A.; Goodman S. L. Submicron Multiphoton Free-Form Fabrication of Proteins and Polymers: Studies of Reaction Efficiencies and Applications in Sustained Release. Macromolecules 2000, 33, 1514–1523. 10.1021/ma9910437. [DOI] [Google Scholar]
- Blasco E.; Müller J.; Müller P.; Trouillet V.; Schön M.; Scherer T.; Barner-Kowollik C.; Wegener M. Fabrication of Conductive 3D Gold-Containing Microstructures via Direct Laser Writing. Adv. Mater. 2016, 28, 3592–3595. 10.1002/adma.201506126. [DOI] [PubMed] [Google Scholar]
- Vyatskikh A.; Delalande S.; Kudo A.; Zhang X.; Portela C. M.; Greer J. R. Additive manufacturing of 3D nano-architected metals. Nat. Commun. 2018, 9, 593. 10.1038/s41467-018-03071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y.; Peng F.; Wilson D. A. Motion Manipulation of Micro- and Nanomotors. Adv. Mater. 2017, 29, 1701970. 10.1002/adma.201701970. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Fu F.; Shang L.; Cheng Y.; Gu Z.; Zhao Y. Bioinspired Helical Microfibers from Microfluidics. Adv. Mater. 2017, 29, 1605765. 10.1002/adma.201605765. [DOI] [PubMed] [Google Scholar]
- Magdanz V.; Sanchez S.; Schmidt O. G. Development of a Sperm-Flagella Driven Micro-Bio-Robot. Adv. Mater. 2013, 25, 6581–6588. 10.1002/adma.201302544. [DOI] [PubMed] [Google Scholar]
- Seth Roberts G.; Kozak D.; Anderson W.; Broom M. F.; Vogel R.; Trau M. Tunable nano/micropores for particle detection and discrimination: scanning ion occlusion spectroscopy. Small 2010, 6, 2653–2658. 10.1002/smll.201001129. [DOI] [PubMed] [Google Scholar]
- Blundell E. L. C. J.; Healey M. J.; Holton E.; Sivakumaran M.; Manstana S.; Platt M. Characterisation of the protein corona using tunable resistive pulse sensing: determining the change and distribution of a particle’s surface charge. Anal. Bioanal. Chem. 2016, 408, 5757–5768. 10.1007/s00216-016-9678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.; Anderson W.; Antaw F.; Trau M. Maskless 3D Ablation of Precise Microhole Structures in Plastics Using Femtosecond Laser Pulses. ACS Appl. Mater. Interfaces 2018, 10, 4315–4323. 10.1021/acsami.7b18029. [DOI] [PubMed] [Google Scholar]
- Vogel R.; Willmott G.; Kozak D.; Roberts G. S.; Anderson W.; Groenewegen L.; Glossop B.; Barnett A.; Turner A.; Trau M. Quantitative sizing of nano/microparticles with a tunable elastomeric pore sensor. Anal. Chem. 2011, 83, 3499–3506. 10.1021/ac200195n. [DOI] [PubMed] [Google Scholar]
- Anderson W.; Kozak D.; Coleman V. A.; Jämting Å. K.; Trau M. A Comparative Study of Submicron Particle Sizing Platforms: Accuracy, Precision and Resolution Analysis of Polydisperse Particle Size distributions. J. Colloid Interface Sci. 2013, 405, 322–330. 10.1016/j.jcis.2013.02.030. [DOI] [PubMed] [Google Scholar]
- Anderson W.; Lane R.; Korbie D.; Trau M. Observations of Tunable Resistive Pulse Sensing for Exosome Analysis: Improving System Sensitivity and Stability. Langmuir 2015, 31, 6577–6587. 10.1021/acs.langmuir.5b01402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.