Abstract
In this work, the study of photocatalytic hydrogen production by RGO/ZnIn2S4 with simultaneous degradation of organic amines was carried out in the presence of organic amines in wastewater as the sacrificial agents. The effects of several factors, such as organic amine types, pH value, catalyst concentration, organic amine concentration, and sunlight source, on the photocatalytic activity of RGO/ZnIn2S4 for H2 production were investigated. At the same time, its performance of degrading organic amines during H2 production was also examined. The results showed that the order of H2 production activity of RGO/ZnIn2S4 in six organic amine solutions was N(CH2CH3)3 > N(HOCH2CH2)3 > N(CH3)3 > HO(CH2)2NH2 > C6H5–N2> CO(NH2)2, and the highest H2 production was in N(CH2CH3)3 (triethylamine) solution, being 1597 μmol·g–1·h–1, which is 2.6 times as high as that using the aqueous solution mixture of Na2S and Na2SO3 as the sacrificial agent. In addition, when the pH was 13, the catalyst concentration was 1.0 g·L–1, and the triethylamine concentration was 1.0 mol·L–1, the photocatalytic activity was the highest. Furthermore, the relationship between triethylamine concentration and H2 production was analyzed according to the theory of dynamics.
1. Introduction
With the development of social economy, the problems of environmental pollution and energy shortage have become increasingly pressing.1−3 As a promising technology to generate new energy and solve environmental pollution, photocatalytic water-splitting for H2 production has received extensive attention.4−15 Currently, organic matters are usually used as sacrificial agents in the photocatalytic H2 evolution technology because it can significantly enhance the H2 production efficiency.16−20 Furthermore, using organic pollutants in wastewater as the sacrificial agent can serve two purposes at the same time: increasing H2 production and simultaneously removing the pollutant via oxidative decomposition.
Organic amines are common pollutants in the pharmaceutical, textile, and dyeing industries wastewater. The organic-amine-contaminated wastewater is high-nitrogen, poisonous, and difficult to biodegrade, thus threatening to the environment and human health. However, it is worth noting that the organic amines can be used as the effective sacrificial agents for photocatalytic H2 production. In 2004, Wu et al.21 studied the mechanism of oxygen-free photocatalytic H2 production by TiO2 in ethanolamine solution; the results showed that ethanolamine used as electron donor could improve H2 production by TiO2. In 2007, Yin et al.22 studied photocatalytic H2 production by TiO2 in three different ethanolamine solutions; it was found that H2 production with these three types of ethanolamine as the electron donors was much higher than that without electron donors, and the order of H2 production in these solutions was triethanolamine > diethanolamine > monoethanolamine. In 2012, Li et al.23 used ZnIn2S4 as the catalyst for photocatalytic H2 production in triethylamine solution; it was found that using triethylamine as an electron donor significantly increased the H2 production rate. In 2014, Peng et al.24 studied photocatalytic H2 production by ZnIn2S4 in the presence of three kinds of methylamine solutions; the results showed that the H2 production activity of the catalyst was improved by using three types of methylamine as the electron donors; the activities of ZnIn2S4 in the three solutions was trimethylamine > dimethylamine > monomethylamine. The current reports mainly focus on the study of using organic amines as sacrificial agents to enhance photocatalytic H2 production. Using organic amines in the wastewater as sacrificial agents to promote photocatalytic hydrogen evolution not only can achieve efficient conversion of pollutants and recovery of resource in water, but also can effectively prevent the organic-amine-contaminated wastewater to pollute the environment. However, the hydrogen production and simultaneous degradation of organic amines is rarely reported. Therefore, it is of great significance to study the simultaneous H2 production and organic amine degradation with organic amines as sacrificial agents.
The previous study25 of our research group showed that RGO/ZnIn2S4 has excellent photocatalytic H2 production activity and stability under visible light. Based on the previous research on RGO/ZnIn2S4, in this work, the effects of several factors, such as organic amine species, pH value, catalyst concentration, organic amine concentration, and solar light source, on the photocatalytic hydrogen production activity of RGO/ZnIn2S4 were investigated, and then investigation was carried out about the degradation of organic amines in wastewater in the process of photocatalytic H2 production. Furthermore, the relationship between triethylamine concentration and H2 production was analyzed according to the theory of dynamics.
2. Results and Discussion
2.1. Effects of Organic Amine Types on Photocatalytic H2 Production
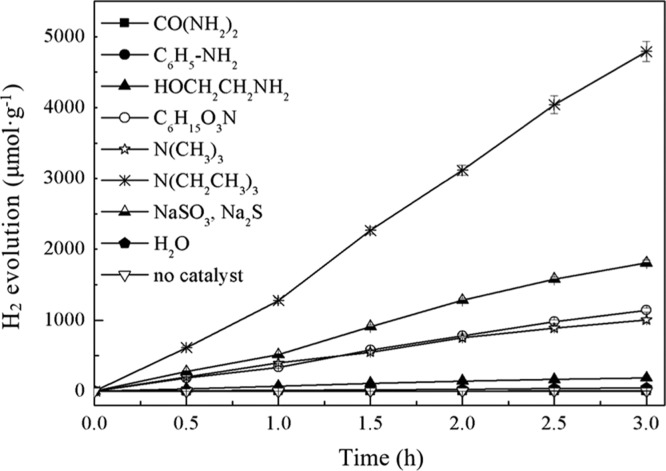
Figure 1 shows the photocatalytic H2 production efficiency with different types of organic amines (urea, aniline, ethanolamine, triethanolamine, trimethylamine, and triethylamine) as sacrificial agents. For comparison, the H2 production efficiency on the conditions of using Na2S–Na2SO3 as the sacrificial agent, without the sacrificial agent (pure water), and using triethylamine without catalyst were also performed and are shown in Figure 1.
Figure 1.
Photocatalytic H2 production in different types of organic amine solutions.
As can be seen from Figure 1, in the absence of RGO/ZnIn2S4, the H2 production rate in the presence of 1.0 mol·L–1 triethylamine was 16 μmol·g–1·h–1, which could be ignored in comparison with that obtained using RGO/ZnIn2S4. In the solution containing only water molecules, the H2 production rate by RGO/ZnIn2S4 was almost zero; however, by adding triethylamine at a concentration of 1.0 mol·L–1, the H2 production was greatly improved. This is mainly because in the solution containing only water molecules, although the RGO could transfer electrons well, the holes (h+) on the surface were not well transferred or consumed, thus making the holes and electrons easy to recombine, leading to the almost zero H2 production; however, after adding triethylamine in the solution, the holes (h+) were consumed through the oxidation reaction with the triethylamine adsorbed on the surface of RGO/ZnIn2S4, thus reducing the hole and electron recombination, making the H2 production greatly improved. At the same time, triethylamine was eventually decomposed into carbon dioxide, ammonia, and water.22 In addition, when triethylamine was used as the sacrificial agent, the H2 production reached 1597 μmol·g–1·h–1, which was 2.6 times as high as that obtained using the aqueous solution mixture of Na2S and Na2SO3 as the sacrificial agent.
It can also be seen from Figure 1 that when the organic amine concentration was 1.0 mol·L–1, the catalyst concentration was 1.0 g·L–1, and the solution pH was 13, the order of H2 production rate in the six organic amines was triethylamine (N(CH2CH3)3) > triethanolamine ((HOCH2CH2)3N) > trimethylamine (N(CH3)3) > ethanolamine (HO(CH2)2NH2) > aniline (C6H5–N2) > urea (CO(NH2)2). Among them, the activity order of N(CH2CH3)3 > (HOCH2CH2)3N > HO(CH2)2NH2 was consistent with the results reported by Li et al.23 According to Li, because of the strong electronegativity of the N atom, the electron density cloud of the adjacent −CH2– is reduced, making it vulnerable to be attacked by hydroxyl radical; in addition, the N atom and the photocatalyst can form a stable coordination bond, so that N(CH2CH3)3 can be easily adsorbed on the surface of the photocatalyst and can be easily degraded by photocatalysis.23 The combined effect makes the h+ easier to consume, the recombination rate of the photogenerated electron–hole pairs much lower, and the H2 production greatly improved. As for (HOCH2CH2)3N in the present study because the electronegativity of the O atom (3.44) in it was stronger than that of the N atom (3.04), the electrons at the N atom and the −CH2– were shifted toward the O atom, which increased the electron density cloud of −CH2– near the O atom. As a result, the ability of hydroxyl radical to attack −CH2– weakened, which made the photocatalytic H2 production in (HOCH2CH2)3N solution lower than that in N(CH2CH3)3. For N(CH3)3, the stability of its −CH3 was stronger than that of its −CH2–, so its ability of being oxidized by hydroxyl radicals was weakened, which caused the photocatalytic H2 production lower than that in (HOCH2CH2)3N solution. As for CO(NH2)2 and C6H5–N2, the former contained the p−π conjugation formed by the carbonyl group and two −NH2, whereas the latter contained the p−π conjugation formed by the benzene ring and N atoms; the conjugated structure made the internal energy in the molecules reduced, so that the molecules tended to be stable. Therefore, they were difficult to be oxidized by hydroxyl radicals, so that their recombination of the photon hole–electron pair greatly increased, resulting in the H2 production being almost zero.
2.2. Effects of Reaction Conditions on H2 Production When Using Triethylamine as Sacrificial Agent
2.2.1. Effects of the pH Values of the Solutions on H2 Production
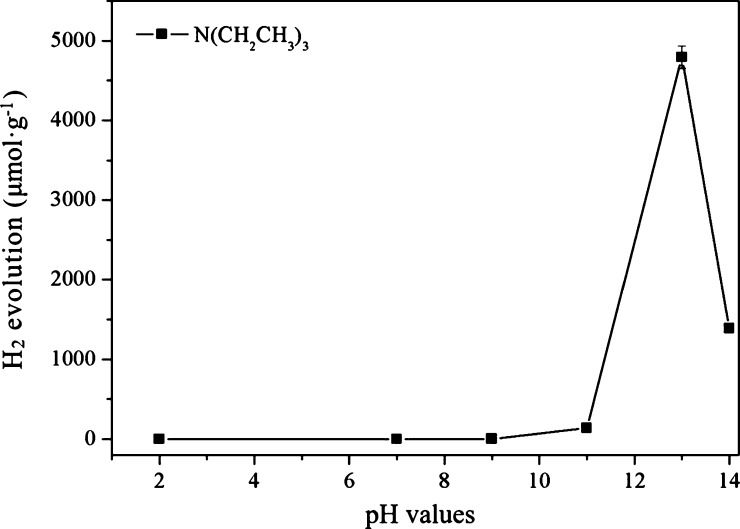
Figure 2 shows the photocatalytic H2 production efficiency with N(CH2CH3)3 as the sacrificial agent at the solutions with different pH values (2, 7, 9, 11, 13, 14). It can be seen that the H2 production was zero under either neutral or acidic conditions; when the pH was 9, H2 production increased; when the pH was 13, the H2 production efficiency was the highest; as the pH continued to increase, H2 production dropped sharply. These results are mainly because, at different pH values, N(CH2CH3)3 was adsorbed on the catalyst surface in different forms. When the pH of the solution was acidic or neutral (where the solution pH was adjusted with HCl), N(CH2CH3)3 was more likely to combine with hydrochloric acid to form triethylamine hydrochloride, which reduced the amount of triethylamine adsorbed on the catalyst surface, thus increasing the recombination rate of the photogenerated electron–hole pairs, hence reducing H2 production to a very low level or even zero. However, when the pH of the N(CH2CH3)3 solution was 13, most of the N(CH2CH3)3 existed in molecular form, which could be well adsorbed on the surface of the catalyst, so as to accelerate the separation of photogenerated electron–hole pairs, thus greatly improving H2 production. When the pH of N(CH2CH3)3 was higher than 13, the reduction potential of H+/H2 became very low and was not conducive to the generation of H2, resulting in a sharp decrease in H2 production.
Figure 2.
Photocatalytic H2 production with triethylamine as sacrificial agent at different pH values.
2.2.2. Effects of the Concentration of RGO/ZnIn2S4 on H2 Production
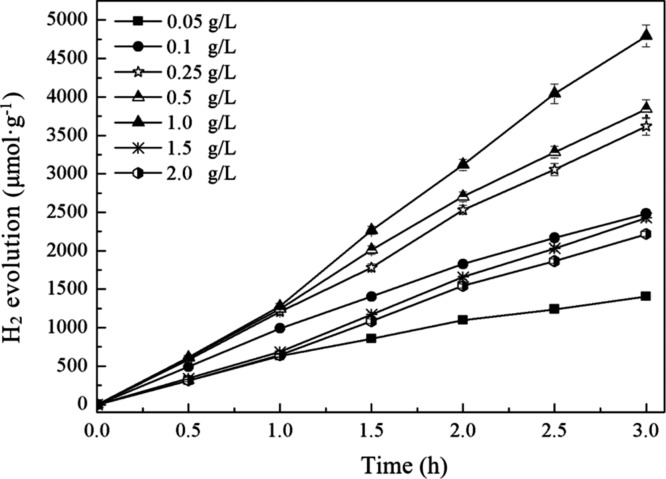
Figure 3 shows the photocatalytic H2 production efficiency with N(CH2CH3)3 as the sacrificial agent at different RGO/ZnIn2S4 dosages (0.05–2.0 g·L–1). It can be seen that, with the increase of catalyst concentration, the corresponding H2 production showed a trend of increasing first and then decreasing; in particular, when the concentration was 1.0 g·L–1, H2 production was the highest. This result is mainly because when the RGO/ZnIn2S4 concentration (0.05 g·L–1) was low, the reaction active sites of the catalyst were less, so it could not fully and effectively use the light energy, leading to low H2 production; when the RGO/ZnIn2S4 concentration gradually increased to 1.0 g·L–1, the reaction active sites of the catalyst increased as well, the catalyst could fully and effectively use the light energy to contact and react with N(CH2CH3)3, which increased the H2 production; when the concentration of RGO/ZnIn2S4 continued to increase, the over high concentration caused shading, which reduced the efficiency of the catalyst to accept photons, leading to a decrease in the H2 production.
Figure 3.
Photocatalytic H2 production with triethylamine as the sacrificial agent at different RGO/ZnIn2S4 dosages.
2.2.3. Effects of Triethylamine Concentrations on H2 Production
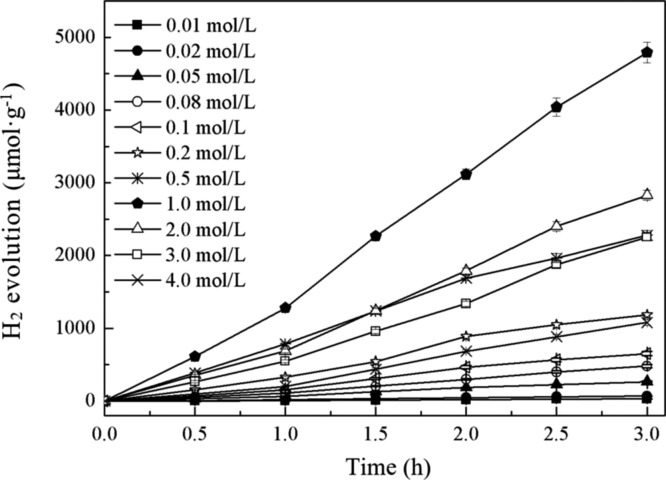
Figure 4 shows the photocatalytic H2 production efficiency with N(CH2CH3)3 of different concentrations (0.01–4.0 mol·L–1) as sacrificial agents. As can be seen from Figure 4, with the increase of N(CH2CH3)3 concentration, the corresponding H2 production showed a tendency to increase first and then decrease, with the highest appearing at the concentration of 1.0 mol·L–1. This result is mainly because when the N(CH2CH3)3 concentration was lower than 1.0 mol·L–1, 1.0 g·L–1 of the catalyst was not fully used; as the concentration increased from 0.1 to 1.0 mol·L–1, the utilization of the catalyst in the unit time was improved, so that the corresponding H2 production gradually increased; when the N(CH2CH3)3 concentration was higher than 1.0 mol·L–1, the surface active sites of RGO/ZnIn2S4 were limited, so H2 production was gradually reduced.
Figure 4.
Photocatalytic H2 production with triethylamine of different concentrations as sacrificial agents.
2.3. Photocatalytic Test under Sunlight
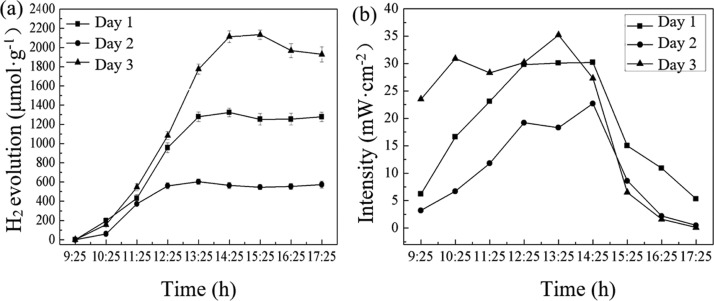
Figure 5a shows the photocatalytic H2 production with sunlight as a light source, the corresponding light intensities of which are shown in Figure 5b. The test conditions were as follows: 1.0 mol·L–1 of N(CH2CH3)3, 1.0 g·L–1 of catalyst, and the solution pH was 13. The solar-light-driven experiments were carried out for 3 days, between 9:25 and 17:25 each day on August 16 to August 18, 2015, at the E building roof of the Harbin Institute of Technology (Shenzhen), which is located in Shenzhen, China (N 22.59°, E 113.97°). As can be seen from Figure 5a, for the first 5 h of the reaction, all H2 production by RGO/ZnIn2S4 in the 3 days showed a tendency to increase gradually; at 14:25, all H2 production rates of the 3 days reached the maximum, that is 265, 121, and 423 μmol·g–1·h–1, respectively. When the reaction time exceeded 5 h, H2 production basically remained unchanged in a range of error. This is mainly because after 14:25, the intensity of the sunlight weakened, so that the photocatalytic reaction tended to stop. In addition, as can be seen from Figure 5a, H2 production of the 3 days differed significantly, with the production order being day 3 > day 1 > day 2. This is because within the first 5 h of the photocatalytic hydrogen production process, the sunlight intensity on day 3 was higher than that on either day 1 or day 2 and remained on a high level, so the H2 production on day 3 was the highest; and the sunlight intensity on day 1 increased gradually and was correspondingly higher than that on day 2, so that the H2 production rate on day 1 was higher than on day 2. It can be seen that the light intensity had a significant effect on H2 production by the catalyst. It is worth noting that, although this experiment was carried out in the winter, in the case of strong sunlight, the H2 production activity of the catalyst could be up to 26% of laboratory (350 W Xe lamp) results.
Figure 5.
Photocatalytic H2 production with sunlight as a light source and the corresponding light intensities. (a) H2 production; (b) light intensity.
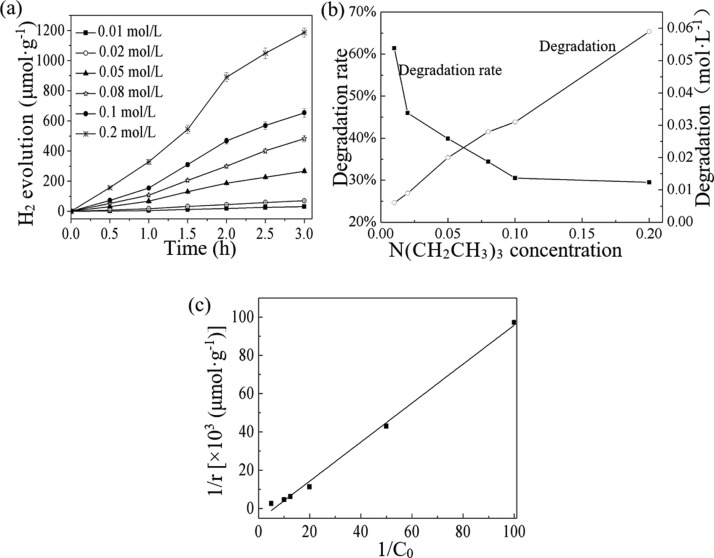
2.4. Kinetic Study of H2 Production Using Low-Concentration Triethylamine as Sacrificial Agent
Taking into account that the actual concentration of N(CH2CH3)3 in wastewater is not high, this study selected low-concentration (0.01–0.2 mol·L–1) N(CH2CH3)3 wastewater as the reaction solution to look at its degradation in the process of photocatalytic H2 production. Figure 6a,b show the simultaneous H2 production and the N(CH2CH3)3 degradation when using the low-concentration N(CH2CH3)3 as the sacrificial agent. As shown in Figure 6a, when the N(CH2CH3)3 concentration was low, with the increase of N(CH2CH3)3 concentration, the corresponding H2 production showed a gradually rising trend. As can be seen from Figure 6b, with the increase of N(CH2CH3)3 concentration, although the degradation rate of N(CH2CH3)3 gradually decreased, the total degradation amount gradually increased instead. It can be seen that when N(CH2CH3)3 in the wastewater was used as the sacrificial agent, hydrogen production and degradation of N(CH2CH3)3 can occur simultaneously.
Figure 6.
Simultaneous H2 production and organic degradation in low trimethylamine-concentration solutions and the corresponding kinetics analysis. (a) H2 production in low trimethylamine-concentration solution; (b) degradation of trimethylamine in different trimethylamine-concentration solutions; (c) kinetic analysis.
Figure 6c shows the kinetic analysis of the effect of N(CH2CH3)3 concentration on H2 production. As can be seen from Figure 6c, after linear transformation, H2 production was linear with the change of C6H15N concentration. The line met the characteristics of the Langmuir equation
where r, C, k, K are the hydrogen production rate, triethylamine concentration, surface reaction rate constant, and apparent adsorption equilibrium constant, respectively. By calculation, the apparent reaction constant k is 1.64 × 10–4 mol·h–1, the apparent adsorption equilibrium constant K is 5.97 × 10–3 L·mol–1, and R2 is 0.995.
2.5. Photocatalytic Oxidation Mechanism of Triethylamine
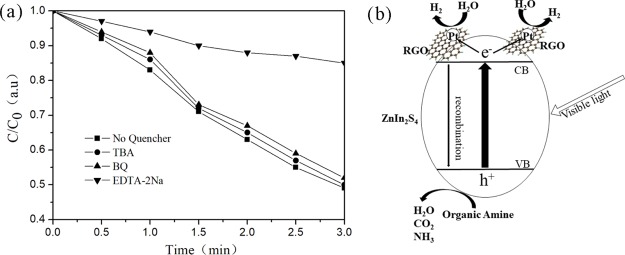
The detection of main oxides in a photocatalytic process is of great significance to reveal the photocatalytic mechanism. In the presence of tert-butyl alcohol (TBA, hydroxyl radical scavenger), 1,4-benzoquinone (BQ, superoxide radical scavenger), or disodium acetate (EDTA–2Na, hole scavenger), the main oxidation products in the photocatalytic degradation of triethylamine were detected by free radical and hole trapping experiments. As shown in Figure 7a, under visible light irradiation, the photodegradation rate of triethylamine decreased slightly with the addition of TBA and BQ, indicating that the hydroxyl radical and superoxide radical were not the main active substances for the degradation of triethylamine in these photocatalytic systems. However, the photodegradation rate of triethylamine decreased significantly with the addition of EDTA–2Na. The above results show that the holes play a major role in the degradation of triethylamine in RGO/ZnIn2S4 photocatalytic systems under visible light irradiation.
Figure 7.
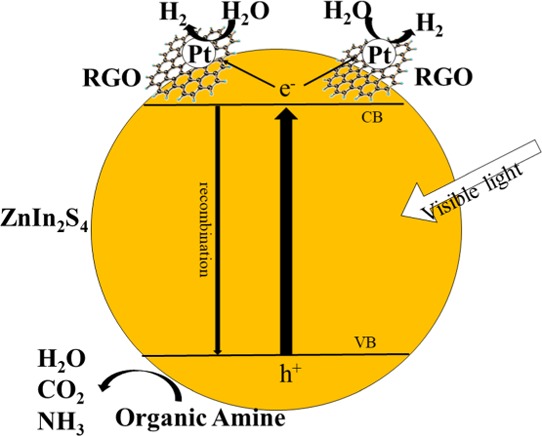
(a) Trapping experiments of the active species during the photocatalytic degradation of triethylamine over RGO/ZnIn2S4 sample under visible light irradiation. (b) Proposed photocatalytic mechanism of RGO/ZnIn2S4.
Based on the above experimental results, the degradation mechanism of triethylamine in RGO/ZnIn2S4 photocatalysis was proposed and is shown in Figure 7b. When ZnIn2S4 is irradiated with visible light, electrons will be excited to the conduction bands (CB), whereas holes will remain in the valence bands (VB). The electrons in the CB rapidly transported to the surface of Pt particles through RGO, and reacted with H2O to produce H2. Holes in the VB reacted with triethylamine and degraded them.
3. Conclusions
The presence of organic amine can promote hydrogen production from decomposition of water. The order of H2 generation activity of RGO/ZnIn2S4 in six organic amine solutions was N(CH2CH3)3 > N(HOCH2CH2)3 > N(CH3)3 > HO(CH2)2NH2 > C6H5–N2> CO(NH2)2, and the highest H2 production was in N(CH2CH3)3 solution, being 1597 μmol·g–1·h–1, 2.6 times as high as that by using the aqueous solution mixture of Na2S and Na2SO3 as sacrificial agent. In addition, when the pH was 13, the catalyst concentration was 1.0 g·L–1, and the N(CH2CH3)3 concentration was 1.0 mol·L–1, the photocatalytic activity was the highest. When N(CH2CH3)3 in wastewater is used as sacrificial agent, hydrogen production and degradation of N(CH2CH3)3 can occur simultaneously. The holes play a major role in the degradation of N(CH2CH3)3 in RGO/ZnIn2S4 photocatalytic systems under visible light irradiation; the relationship between the concentration of N(CH2CH3)3 and H2 production was in accordance with the Langmuir equation.
4. Experimental Section
4.1. Materials
All chemicals were of analytical grade and used as received without further purification. In(NO3)3·4H2O, ZnSO4·7H2O, H2O2 (30%), thioacetamide (TAA), potassium permanganate (K2MnO4), H2SO4 (purity ≥98%), NaNO3, absolute ethanol, glycerol, and organic amines(including triethylamine, triethanolamine, trimethylamine, ethanolamine, aniline, and urea) were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Na2S and Na2SO3 (purity ≥99.99%) were purchased from Sinopharm Chemical Reagent Co., Ltd., China. H2PtCl6·6H2O (purity ≥99.99%) was purchased from Shanghai July Chemical Co., Ltd., China. Graphite was purchased from Aladdin Reagent (Shanghai) Co., Ltd., China.
4.2. Preparation of RGO/ZnIn2S4
The RGO/ZnIn2S4 sample was prepared by an alcohothermal method. The preparation steps are consistent with the optimal procedure for the preparation of RGO/ZnIn2S4 in our previous work25 and are only briefly described herein.
For the synthesis of GO, graphite powder (5 g) and KMnO4 (20 g) were added into a flask (500 mL); then the mixture was vigorously stirred at 10 °C for 30 min and at 35 °C for 5 h, until a thick paste formed. Then, the mixture was diluted by deionized water (300 mL), treated with ultrasonic for 3 h, and diluted with distilled water (200 mL). The residual KMnO4 was destroyed through reduction by H2O2 (20 mL, 30%). Subsequently, the mixture was sufficiently washed with dilute HCl solution (5%) and deionized water. After drying in an air oven at 65 °C overnight, GO powder was obtained. The GO powder was then dispersed in absolute ethanol and washed 3 times with absolute ethanol, then a GO suspension was obtained. The as-prepared GO suspension was used directly in the next synthesis procedure.
For the synthesis of ZnIn2S4, ZnSO4·7H2O (0.735 g), In(NO3)3·4H2O (1.903 g), and TAA were dissolved in the solution mixed by absolute ethanol (40 mL) and glycerol (10 mL). The mixed solution (solution-1) was stirred for 30 min and then hydrothermally treated at 180 °C for 12 h, then cooled to room temperature naturally. A yellow precipitate was obtained, then filtered and washed with absolute ethanol and deionized water three times. After drying in vacuum at 80 °C, ZnIn2S4 was obtained.
For the synthesis of 0.1 wt % RGO/ZnIn2S4, a desired amount of GO was dropped into the solution-1 before heating.
4.3. Photocatalytic Reaction
Photocatalytic tests were carried out in an 868 mL gastight stainless steel reactor. The specific reactor characteristics were introduced in detail in our previous work.20,25 The light source was a 350 W Xe lamp (Shenzhen Stone-lighting Opto Device Co., Ltd., China), and a cut-off filter was used to remove the UV part of the light (λ > 420 nm). The light intensity was measured using a spectroradiometer (FZ-A, Photoelectric Instrument Factory of Beijing Normal University).
In all experiments, 200 mL deionized water containing a desired amount of catalyst (0.05, 0.1, 0.25, 0.5, 1.0, 1.5, or 2.0 g·L–1) and organic amine(0.01, 0.02, 0.05, 0.08, 0.1, 0.2, 0.5, 1.0, 2.0, 3.0, or 4.0 mol·L–1) was added into the reactor. Before light irradiation, argon gas was bubbled through the reaction mixture for 30 min to remove air from the reaction solution. Pt as a cocatalyst for the promotion of hydrogen evolution was photodeposited in situ on the photocatalyst from the precursor of H2PtCl6·6H2O, when there was special instruction. Hydrogen evolution was analyzed with a gas chromatograph (Shanghai Precision & Scientific Instrument Co., Ltd., GC-112A). Samples were taken at intervals of 60 min at 500 μL. Degradation of organic amines was analyzed in a high performance liquid chromatograph (LC2000, Shanghai Techcomp Instrument Ltd, China) using a Symmetry C18 column and a mobile phase consisting of 70% methanol (chromatographic pure) and 30% water. Before light irradiation, the suspension was stirred for 60 min in the dark to attain adsorption–desorption equilibrium of the organic amines on the surface of the photocatalyst. During the reaction, 2 mL suspension was withdrawn from the reaction vessel every 60 min. The photocatalyst was removed from the suspension by centrifugation.
Acknowledgments
All authors gratefully acknowledge support from the Special Fund for the Development of Strategic and New Industry in Shenzhen (no. JCYJ20150731104949789) and the Fund for Knowledge Innovation in Shenzhen (no. JCYJ20180507183621817).
The authors declare no competing financial interest.
References
- Zhou P.; Yu J.; Jaroniec M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. 10.1002/adma.201400288. [DOI] [PubMed] [Google Scholar]
- Ma X.; Jiang Q.; Guo W.; Zheng M.; Xu W.; Ma F.; Hou B. Fabrication of g-C3N4/Au/CdZnS Z-scheme photocatalyst to enhance photocatalysis performance. RSC Adv. 2016, 6, 28263–28269. 10.1039/c5ra27429j. [DOI] [Google Scholar]
- Hao L.; Kang L.; Huang H.; Ye L.; Han K.; Yang S.; Yu H.; Batmunkh M.; Zhang Y.; Ma T. Surface-Halogenation-Induced Atomic-Site Activation and Local Charge Separation for Superb CO2 Photoreduction. Adv. Mater. 2019, 1900546. 10.1002/adma.201900546. [DOI] [PubMed] [Google Scholar]
- Yu J.; Ran J. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364. 10.1039/c0ee00729c. [DOI] [Google Scholar]
- Xing M.; Qiu B.; Du M.; Zhu Q.; Wang L.; Zhang J. Spatially Separated CdS Shells Exposed with Reduction Surfaces for Enhancing Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2017, 27, 1702624. 10.1002/adfm.201702624. [DOI] [Google Scholar]
- Zheng D.; Pang C.; Wang X. The function-led design of Z-scheme photocatalytic systems based on hollow carbon nitride semiconductors. Chem. Commun. 2015, 51, 17467–17470. 10.1039/c5cc07867a. [DOI] [PubMed] [Google Scholar]
- Zhang L. J.; Li S.; Liu B. K.; Wang D. J.; Xie T. F. Highly Efficient CdS/WO3 Photocatalysts: Z-Scheme Photocatalytic Mechanism for Their Enhanced Photocatalytic H2 Evolution under Visible Light. ACS Catal. 2014, 4, 3724–3729. 10.1021/cs500794j. [DOI] [Google Scholar]
- Yu J.; Wang S.; Low J.; Xiao W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. 10.1039/c3cp53131g. [DOI] [PubMed] [Google Scholar]
- Li S.; Zhao Q.; Wang D.; Xie T. Work function engineering derived all-solid-state Z-scheme semiconductor-metal-semiconductor system towards high-efficiency photocatalytic H2 evolution. RSC Adv. 2016, 6, 66783–66787. 10.1039/c6ra14680e. [DOI] [Google Scholar]
- Nagaraju G.; Manjunath K.; Sarkar S.; Gunter E.; Teixeira S. R.; Dupont J. TiO2–RGO hybrid nanomaterials for enhanced water splitting reaction. Int. J. Hydrogen Energy 2015, 40, 12209–12216. 10.1016/j.ijhydene.2015.07.094. [DOI] [Google Scholar]
- Saadetnejad D.; Yıldırım R. Photocatalytic hydrogen production by water splitting over Au/Al-SrTiO3. Int. J. Hydrogen Energy 2018, 43, 1116–1122. 10.1016/j.ijhydene.2017.10.154. [DOI] [Google Scholar]
- Chen P.; Dong F.; Ran M.; Li J. Synergistic photo-thermal catalytic NO purification of MnO x /g-C3N4 : Enhanced performance and reaction mechanism. Chin. J. Catal. 2018, 39, 619–629. 10.1016/s1872-2067(18)63029-3. [DOI] [Google Scholar]
- Li J.; Zhang Z.; Cui W.; Wang H.; Cen W.; Johnson G.; Jiang G.; Zhang S.; Dong F. The Spatially Oriented Charge Flow and Photocatalysis Mechanism on Internal van der Waals Heterostructures Enhanced g-C3N4. ACS Catal. 2018, 8, 8376–8385. 10.1021/acscatal.8b02459. [DOI] [Google Scholar]
- Cui W.; Chen L.; Li J.; Zhou Y.; Sun Y.; Jiang G.; Lee S. C.; Dong F. Ba-vacancy induces semiconductor-like photocatalysis on insulator BaSO4. Appl. Catal., B 2019, 253, 293–299. 10.1016/j.apcatb.2019.04.070. [DOI] [Google Scholar]
- Chen F.; Huang H.; Guo L.; Zhang Y.; Ma T. The Role of Polarization in Photocatalysis. Angew Chem Int Ed Engl 2019, 58, 2–15. 10.1002/anie.201901361. [DOI] [PubMed] [Google Scholar]
- Salvador P.; Garcia Gonzalez M. L. G. Catalytic Role of Lattice Defects in the Photoasslsted Oxidation of Water at (001)n-TiO2 Rutile. J. Phys. Chem. 1992, 96, 10349–10353. 10.1021/j100204a046. [DOI] [Google Scholar]
- Munoz P.; Malekshoar G.; Ray M. B.; Zhu J.; Ray A. K. Sacrificial Hydrogen Generation from Formaldehyde with Pt/TiO2 Photocatalyst in Solar Radiation. Ind. Eng. Chem. Res. 2013, 52, 5023–5029. 10.1021/ie3029976. [DOI] [Google Scholar]
- Speltini A.; Sturini M.; Maraschi F.; Dondi D.; Fisogni G.; Annovazzi E.; Profumo A.; Buttafava A. Evaluation of UV-A and solar light photocatalytic hydrogen gas evolution from olive mill wastewater. Int. J. Hydrogen Energy 2015, 40, 4303–4310. 10.1016/j.ijhydene.2015.01.182. [DOI] [Google Scholar]
- Gao H.; Zhang J.; Wang R.; Wang M. Highly efficient hydrogen production and formaldehyde degradation by Cu2O microcrystals. Appl. Catal., B 2015, 172–173, 1–6. 10.1016/j.apcatb.2015.02.004. [DOI] [Google Scholar]
- Zhu R.; Tian F.; Yang R.; He J.; Zhong J.; Chen B. Z scheme system ZnIn2S4/RGO/BiVO4 for hydrogen generation from water splitting and simultaneous degradation of organic pollutants under visible light. Renewable Energy 2019, 139, 22–27. 10.1016/j.renene.2019.02.049. [DOI] [Google Scholar]
- Wu Y.; Lv G.; Li B. Hydrogen Production by Pt/TiO2 Anaerobic Photocatalytic Reforming Degradation of Aqueous Monoethanolamine. Acta Phys.—Chim. Sin. 2004, 20, 755–758. 10.3866/PKU.WHXB20040718. [DOI] [Google Scholar]
- Yin Z.; Li Y.; Peng S.; Lu G.; Li S. Photocatalytic Hydrogen Generation in the Presence of Ethanolamines over Pt/TiO2. J. Mol. Catal. 2007, 21, 155–161. [Google Scholar]
- Li Y.; Zhang K.; Peng S.; Lu G.; Li S. Photocatalytic hydrogen generation in the presence of ethanolamines over Pt/ZnIn2S4 under visible light irradiation. J. Mol. Catal. A: Chem. 2012, 363–364, 354–361. 10.1016/j.molcata.2012.07.011. [DOI] [Google Scholar]
- Peng S.; Ding M.; Yi T.; Li Y. Photocatalytic Hydrogen Evolution in the Presence of Pollutant Methylamines over Pt/ZnIn2S4 under Visible Light Irradiation. J. Mol. Catal. 2014, 5, 466–473. [Google Scholar]
- Tian F.; Zhu R.; Zhong J.; Wang P.; Ouyang F.; Cao G. An efficient preparation method of RGO/ZnIn2S4 for photocatalytic hydrogen generation under visible light. Int. J. Hydrogen Energy 2016, 41, 20156–20171. 10.1016/j.ijhydene.2016.08.063. [DOI] [Google Scholar]