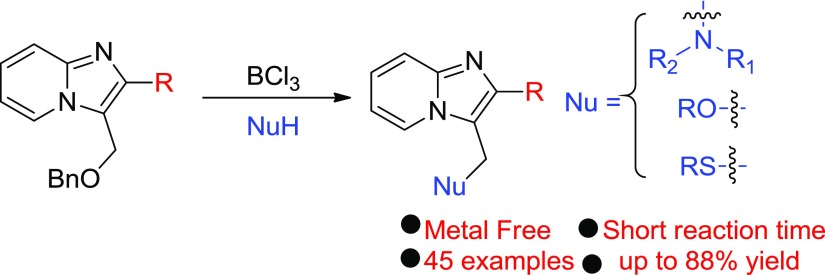
Abstract
An efficient BCl3-mediated reaction of imidazo[1,2-a]pyridines has been developed for the C–N, C–S, and C–O bond formation. The salient features of this method correspond to the substitution of different nucleophiles via in situ unconventional debenzylation. The developed process is applicable for the synthesis of a wide variety of ((3-amino/thio/alkoxy)-methyl)-imidazo[1,2-a]pyridines.
Introduction
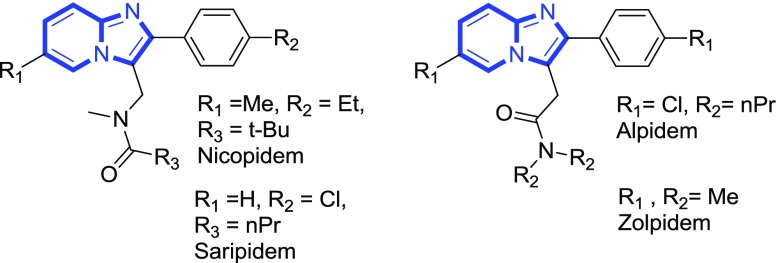
Substituted imidazopyridines represent an important class of fused heterocyclic compounds owing various pharmacological properties.1 Their analogues have been successfully used for pharmaceutical chemistry as well as in materials science.2 Marketed drugs like alpidem,3 zolpidem,4 nicopidem and saripidem,5 minodronic acid,6 and optically active GSK812397 drug7 are derived from the imidazo[1,2-a]pyridine scaffold (Figure 1).8
Figure 1.
Imidazo[1,2-a]pyridine-derived drugs.
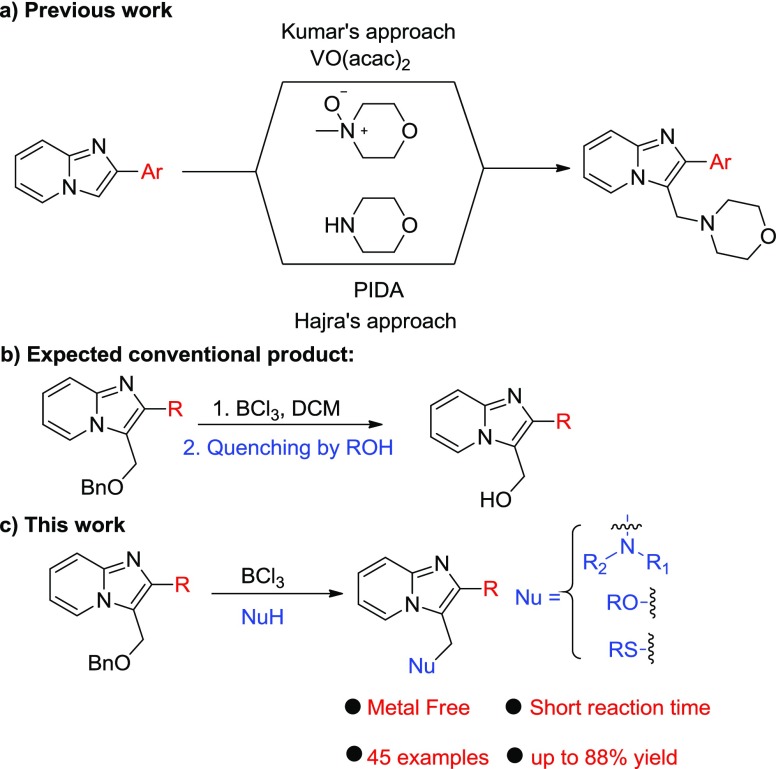
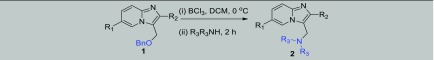
The construction of new C–N,9 C–S,10 and C–O11 bonds into organic molecules is highly appreciable in modern chemistry. Although in recent times a variety of substitutions on imidazopyridines have been made, there are no reports pertaining to a general approach for the synthesis of different C–X bonds at the C-3 methylene position of imidazo[1,2-a]pyridines. Recently, Hajra and group have presented the aminomethylation of imidazo-heterocycles with morpholine using (diacetoxyiodo)benzene, and Kumar et al. approached the synthesis by using N-methyl-morpholine oxide, which acts as a coupling partner as well as the oxidant.12 Both of these strategies, however, suffer from limitations in the context of (a) use of morpholine as only amine, (b) not a general C–X bond approach, (c) requirement of halo-substituted imidazopyridines as starting materials, (d) harsh reaction conditions, and (e) low atom economy. In this context, we developed a BCl3-mediated general C–X bond formation method around imidazopyridines that involve nucleophilic substitution reactions with a diverse array of amines, thiols, and alcohols at the C-3 methylene position (Scheme 1).
Scheme 1. Summary of Work.
Benzyl protection of a hydroxyl group is one of the most frequently used procedures in synthesis because of the mild conditions involved in its removal.13 Lewis acid has long been widely used as an ether-cleaving reagent because of its tolerance of several other functional groups in the target molecule.14
However, in the case of imidazopyridines, we presumed an aza-directed coupling with different nucleophiles through a resonance-stabilized intermediate A. In this perspective, we present BCl3-mediated different reactions of imidazopyridines 1 (synthesized as per our earlier approach)15 against different N/S/O nucleophiles that helped to generate different valuable carbon heterobonds.
Results and Discussion
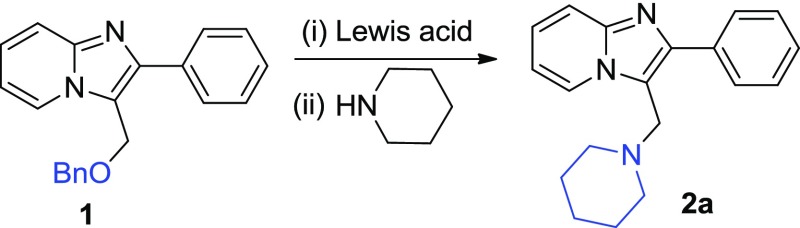
We commenced our study with 3-((benzyloxy) methyl)-2-phenylimidazo[1,2-a]pyridine 1 (1.0 equiv) as the model substrate to find out the optimized reaction conditions as summarized in Table 1. Initially, the reaction was performed using BCl3 (1 equiv) and piperidine (1 equiv) in dry dichloromethane (DCM) at −78 °C under a nitrogen atmosphere for 2 h. Under these conditions, 2a was isolated in 12% yield (Table 1, entry 1). Considering the gradual decrease in molarity of BCl3 solution over time due to high volatility of BCl3, 1.4 equiv of BCl3 was used, an improvement in yield (32%) of the desired product 2a (entry 2) was noticed.16
Table 1. Optimization of the Reaction Conditionsa.
| entry | Lewis acid (equiv) | amine (equiv) | T [°C] | yieldsb of 2a [%] |
|---|---|---|---|---|
| 1 | BCl3 (1) | 1 | –78 | 12 |
| 2 | BCl3 (1.4) | 1 | –78 | 32 |
| 3 | BCl3 (1.4) | 2 | –78 | 54 |
| 4 | BCl3 (1.4) | 3 | –78 | 77 |
| 5 | BCl3(1.4) | 3 | 0 | 80 |
| 6 | BCl3 (1.4) | 3 | 20 | 43 |
| 7 | 3 | 0 | 0 | |
| 8 | BF3·O(C2H5)2 (1.4) | 3 | –78 | 28 |
| 9 | BF3·O(C2H5)2 (1.4) | 3 | 0 | 40 |
| 10 | BBr3 (1.4) | 3 | –78 | 0 |
| 11 | BBr3 (1.4) | 3 | 0 | 18 |
| 12 | AlCl3 (1.4) | 3 | 0 | 0 |
General conditions: 1 (0.3 mmol), Lewis acid (0.3 mmol), and piperidine (0.3 mmol), in DCM at −78 °C under N2 for 2 h.
Isolated yield.
Following improvised yield of 2a, the reaction conditions particularly the change in the concentration of amine and variation in temperature were explored (entries 3–12). Primarily, screening of our reaction at different concentrations of amine against 1 was performed (entry 3 and 4), and the best result was observed with 3 equiv of amine (entry 4). In continuation, various reactions were conducted at different temperatures (entries 5–6). The reaction executed at 0 °C produced better yield as compared to reaction at 20 °C, indicating that 0 °C is an optimal temperature for this transformation. Further, in the absence of Lewis acid, no reaction was observed (entry 7). Subsequently, boron catalysts BF3O(C2H5)2 and BBr3 were also investigated in the presence of 3 equiv of piperidine (entries 8–10) at −78 °C and 0 °C, but BCl3 was found most appropriate in terms of yield (entry 5). Also, no product was observed in the case of AlCl3 (entry 12). Finally, the use of BCl3 (1.4 equiv) and amine (3 equiv) at 0 °C to room temperature (rt) under nitrogen was found to be the optimized reaction condition (entry 3).
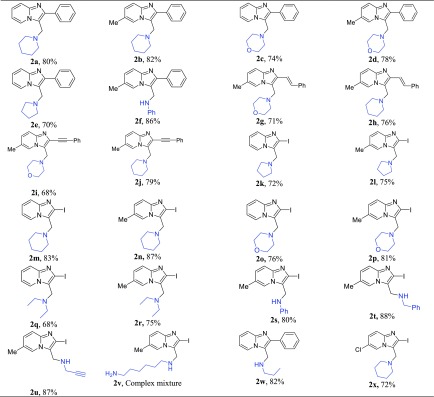
With these optimized reaction conditions in hand, the scope of the C3-methylene amination was demonstrated by testing a wide variety of cyclic, acyclic, and aromatic amines. We were pleased to find that in all tested reactions, the desired C3-aminomethylated-imidazo[1,2-a]pyridines (Table 2, entries 2a–2x) were produced in good yields (68–88%). 3-((Benzyloxy)methyl)-2-phenylimidazo[1,2-a]pyridine 1 reacted efficiently with piperidine and morpholine, giving the corresponding product 2a and 2c in 80 and 74% yields, respectively, and pyrrolidine yielded product 2e with comparable yield (70%).The presence of the electron-donating methyl group on imidazo[1,2-a]pyridines improved the yield to 82% (entries 2b and 2d). Reaction with aniline furnished product 2f in excellent yield (86%). Besides C2-phenyl substitution, the scope and generality of the reaction was also investigated on C2 alkenylated and alkynated imidazo[1,2-a]pyridines17 for amination, and these groups were well tolerated and afforded the products (entries 2g–2j) in good yield (68–79%). In addition, different experiments were performed between 2-iodo-imidazo[1,2-a]pyridines and various amines to afford the desired products (entries 2h–2x). In this case, yields were also good for all the reactions conducted irrespective of the nature of amine (Table 2). Reaction with piperidine (entry 2m, 83% yield) and morpholine (entry 2o, 76%) proceeded very smoothly and comparatively more reactive than pyrrolidine (entry 2l, 75% yield). The presence of the methyl group on imidazo[1,2-a]pyridines further enhanced the yield up to 87% (entry 2n) and chloro-substitution slightly decreased the yield (entry 2x, 72%). Aniline and benzylamine were also checked and afforded products 2s and 2t in excellent yields (80 and 88%, respectively). The use of alkyne as a triazole precursor18 encouraged us to perform reaction with propargyl amine and furnished the desired product 2u in 87% yield without any difficulties. The reaction was also compatible with the secondary amine diethylamine (entries 2q and 2r) as well as the primary amine n-propyl amine (entry 2w, 82% yield), while in the case of hexamethylene diamine (2v), an inseparable mixture was obtained. In general, we observed that irrespective the nature of imidazopyridines and amines, all of the tested reactions were efficiently transformed to desired products within 2 h.
Table 2. C3-Methylene Amination of Imidazo[1,2-a]pyridinesa,b.
Reaction conditions: 1 (0.3 mmol), BCl3 (0.42 mmol), and R3R3NH (0.9 mmol), in DCM at 0 °C to rt under N2 for 2 h.
Isolated yield.
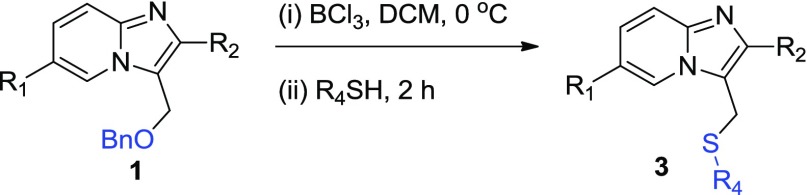
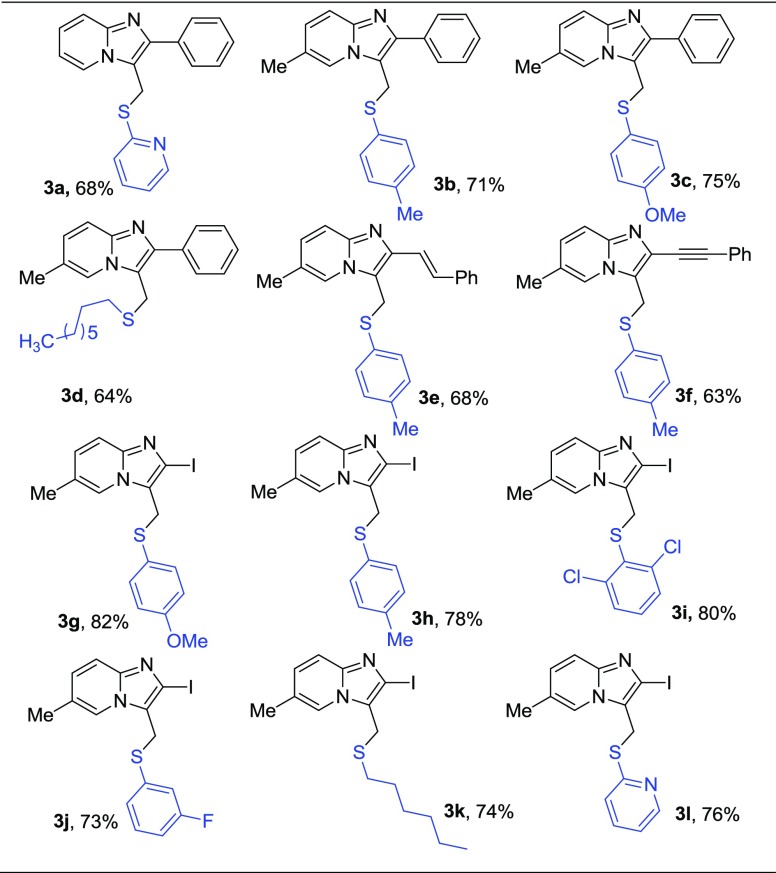
To investigate the practicability of this method, another set of experiments were performed between imidazopyridine 1 and thiols under the optimized reaction conditions and yields were generally good for all tested reactions (entries 3a–3l) (Table 3). Various substituted aromatic and aliphatic thiols proceeded well in this process. 2-Mercaptopyridine smoothly underwent reaction with 1, affording the desired product 3a in 68% yield. Additionally, the electron-donating group on the aromatic ring of thiophenol enhances the yield of the desired product (entries 3b–3c). Reaction with n-octyl mercaptan (entry 3d) afforded the desired compound in good yield (64%). Moreover, 2-alkenyl (entry 3e) and alkynyl (entry 3f) substitution on 1 dropped the yield to 68 and 63%, respectively. Importantly, mono- and dihalogen-substituted thiophenols (entry 3i and 3j) worked well, thus providing an opportunity for additional modifications of products. Notably, 2-iodo-substituted imidazo[1,2-a]pyridine 1 was also a good substrate for this reaction system (entries 3g–3l).
Table 3. C–S Bond Formation of Imidazo[1,2-a]pyridinesa,b.
Reaction conditions: 1 (0.3 mmol), BCl3 (0.42 mmol), and R4SH (0.9 mmol) in DCM at 0 °C under N2 for 2 h.
Isolated yields.
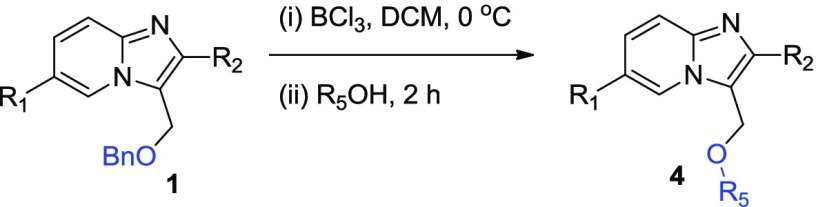
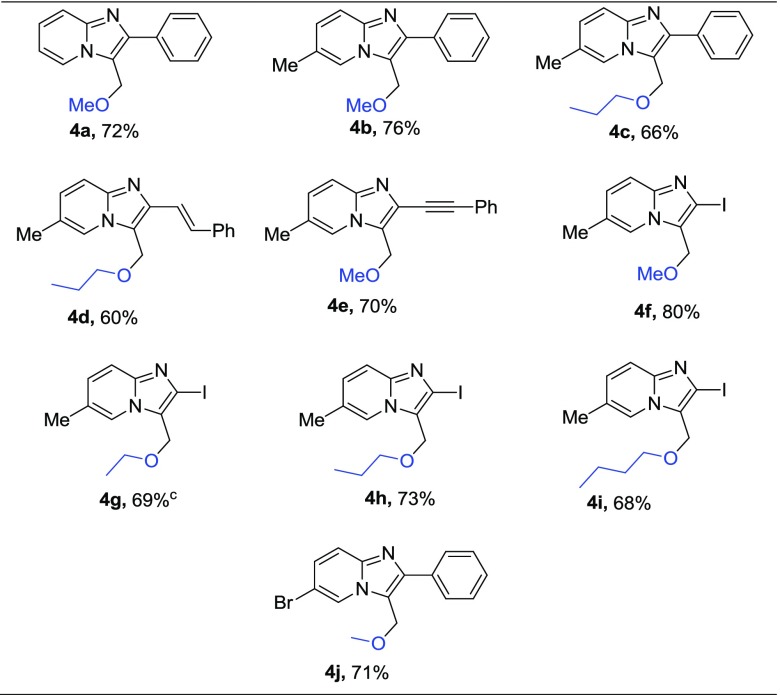
Next, we extended the method for the generation of different C–O bonds by treating different imidazo[1,2-a]pyridine 1 with different aliphatic alcohols as per the earlier optimal conditions and successfully isolated different products (entries 4a–4j) in good yield (60–80%, Table 4). As evident, despite change in the substitutions of 1, we isolated the desired products in moderate to good yield (60–80%). However, reactions with 2-iodo substitutions (entries 4e–4i) produced slightly better yields (68–80%). Furthermore, in these set of reactions, the change in the aliphatic alcohols as the coupling partner did not show a predominant effect in the reaction competence. We successfully isolated the desired products with most of the alcohols tested. However, we observed the best yields when reactions were conducted with methanol (entries 4a, 4b, 4e, 4f, and 4j) and also found that increase in alkyl chain length of alcohol (entries 4g, 4h, and 4i) and C-5 bromo substitution slightly decreased the yield.
Table 4. C–O Bond Formation of Imidazo[1,2-a]pyridinesa,b.
Reaction conditions: 1 (0.3 mmol), BCl3 (0.42 mmol), and R5OH (0.9 mmol), in DCM at 0 °C under N2 for 2h.
Isolated yield.
0–70 °C.
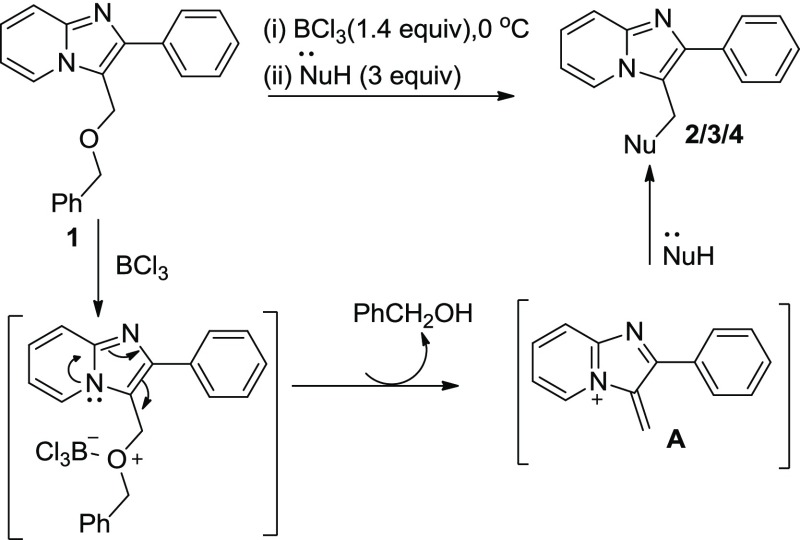
On the basis of our observation and previous reports,18 a plausible mechanism is proposed in Scheme 2. Probably, at the first step, 3-((benzyloxy)methyl)-2-phenylimidazo[1,2-a]pyridine 1 forms a complex with BCl3. This complex converts into intermediate A (supported by mass spectra) through aza-directed electronic delocalization. Subsequently, the intermediate A is readily coupled with nucleophiles to afford the desired compounds 2/3/4.
Scheme 2. Plausible Reaction Mechanism.
Conclusions
In summary, BCl3-mediated methodologies around imidazo[1,2-a]pyridines for the generation of different C–N, C–S, and C–O bonds through an in situ debenzylation are presented. The reaction demonstrated a broad substrate scope and excellent functional group tolerance at mild reaction conditions. These methods furnished a wide variety of 3-amino/thio/alkoxy 2-iodo-imidazo[1,2-a]pyridines in good yields and are perhaps ascertained because of aza-directed unusual coupling with different nucleophiles.
Experimental Section
General Information
All reactions and purity of the synthesized compounds were monitored by Merck thin-layer chromatography (TLC) using silica gel 60 F254 aluminum plates (20 × 20 cm). Visualization was accomplished by UV spectrometry at 254 nm light and exposure to iodine vapors. Buchi rotavapor was used for concentration of organic solvents. Column chromatographic purifications were performed on silica gel (100–200 mesh) for compound purification. 1H and 13C NMR spectra in CDCl3 were recorded with 400 and 500 MHz NMR instruments, respectively, using (CH3)4Si as an internal standard. Chemical shifts (δ) are expressed in parts per million (ppm, scale) referenced to the residual solvent (i.e., 1H 7.24 ppm and 13C 77.1 ppm for CDCl3). MestReNova software was used to process NMR spectra. Signal multiplicity is expressed as follows: s (singlet), br s (broad singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). J values are given in hertz (Hz). Mass spectra were obtained by using a Q-TOF-LC/MS spectrometer using electron spray ionization. Unless otherwise indicated, materials and solvents were purchased and used without further purification. All starting compounds 1 (3-((benzyloxy)methyl)-2-iodoimidazo[1,2-a]pyridine, 3-((benzyloxy) methyl)-2-arylimidazo [1,2-a]pyridine, 3-((benzyloxy)methyl)-2-(phenylethynyl)imidazo[1,2-a]pyridine, and 3-((benzyloxy)methyl)-2-styrylimidazo[1,2-a]pyridine) were synthesized compared with the spectroscopic data by following the literature reports.15
General Procedure for C3-Methylene C–N Bond Formation of Imidazo[1,2-a]pyridine (2)
In a round-bottom flask, 3-((benzyloxy)methyl)-2-phenylimidazo[1,2-a]-pyridine 1 (0.3 mmol) was dissolved in dry DCM (2 mL) at 0 °C under a nitrogen atmosphere. A BCl3 solution (1.0 M in methylene chloride) (0.42 mmol) was slowly added, and complex formation was confirmed by TLC. After that, temperature of reaction was increased up to rt and an amine (0.9 mmol) was added and stirred for 2 h (reaction progress was monitored by TLC). After completion, the reaction was quenched by water to stop the reaction. The aqueous solution was extracted with DCM (3 × 10 mL). The combined organic layer was washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuum. The residue was purified by a column (silica gel, 80/20 n-hexane/ethyl acetate) to give the desired product.
2-Phenyl-3-(piperidin-1-ylmethyl)imidazo[1,2-a]pyridine (2a)
Yield: (70 mg, 80%); 1H NMR (400 MHz, CDCl3): δ 8.56 (d, J = 6.8 Hz, 1H), 7.83 (d, J = 7.2 Hz, 2H), 7.67 (d, J = 9.0 Hz, 1H), 7.50–7.47 (m, 2H), 7.41–7.38 (m, 1H), 7.28–7.23 (m, 1H), 6.87–6.84 (m, 1H), 4.02 (s, 2H), 2.48 (br s, 4H), 1.59 (dt, J = 10.7, 5.4 Hz, 4H), 1.47 (d, J = 4.6 Hz, 2H); 13C NMR (126 MHz, CDCl3): δ 145.08 (C), 134.51 (C), 128.93 (CH), 128.48 (CH), 127.75 (CH), 125.71 (CH), 124.72 (CH), 117.12 (CH), 111.91 (CH), 54.07 (CH2), 52.17 (CH2), 25.74 (CH2), 24.14 (CH2); ESI-HRMS (m/z): calcd for C19H22N3 [M + H]+, 292.1814; found, 292.1811.
6-Methyl-2-phenyl-3-(piperidin-1-ylmethyl)imidazo[1,2-a]pyridine (2b)
Yield: (75 mg, 82%); 1H NMR (400 MHz, CDCl3): δ 8.24 (s, 1H), 7.81 (d, J = 7.3 Hz, 2H), 7.53 (d, J = 9.1 Hz, 1H), 7.46–7.42 (m, 2H), 7.36–7.33 (m, 1H), 7.06 (dd, J = 9.2, 1.4 Hz, 1H), 3.91 (s, 2H), 2.43 (s, 4H), 2.36 (s, 3H), 1.57–1.52 (m, 4H), 1.44 (d, J = 4.8 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 144.75 (C), 144.08 (C), 134.89 (C), 128.90 (CH), 128.34 (CH), 127.56 (CH), 127.48 (CH), 123.19 (CH), 121.22 (C), 116.46 (CH), 54.20 (CH2), 52.44 (CH2), 26.04 (CH2), 24.39 (CH2), 18.50 (CH3). ESI-HRMS (m/z): calcd for C20H24N3 [M + H]+, 306.1970; found, 306.1964.
4-((2-Phenylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (2c)12
Yield: (65 mg, 74%); 1H NMR (400 MHz, CDCl3): δ 8.44 (d, J = 6.9 Hz, 1H), 7.80 (d, J = 7.3 Hz, 2H), 7.65 (d, J = 9.0 Hz, 1H), 7.46 (d, J = 7.5 Hz, 2H), 7.39–7.35 (m, 1H), 7.25–7.20 (m, 1H), 6.85–6.81 (m, 1H), 3.98 (s, 2H), 3.69–3.65 (t, J = 4.8 Hz, 4H), 2.51–2.45 (t, J = 4.8 Hz, 4H); 13C NMR (101 MHz, CDCl3): δ 145.35 (C), 145.12 (C), 134.44 (C), 128.92 (CH), 128.49 (CH), 127.81 (CH), 125.34 (CH), 124.62 (CH), 117.32 (CH), 115.92 (C), 111.93 (CH), 67.01 (CH2), 53.23 (CH2), 52.15 (CH2). ESI-HRMS (m/z): calcd for C18H20N3O [M + H]+, 294.1606; found, 294.1605.
4-((6-Methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)morp-holine (2d)12
Yield: (72 mg, 78%); 1H NMR (400 MHz, CDCl3): δ 8.17 (s, 1H), 7.80 (d, J = 7.3 Hz, 2H), 7.55 (d, J = 9.1 Hz, 1H), 7.47–7.43 (m, 2H), 7.38–7.34 (m, 1H), 7.08 (dd, J = 9.1, 1.0 Hz, 1H), 3.95 (s, 2H), 3.70–3.66 (m, 4H), 2.51–2.47 (m, 4H), 2.38 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 145.07 (C), 144.12 (C), 134.51, 128.86 (CH), 128.45 (CH), 127.85 (CH), 127.71 (CH), 122.82 (CH), 121.58, 116.60 (CH), 115.67 (CH), 67.02 (CH2), 53.22 (CH2), 52.06 (CH2), 18.54 (CH3). ESI-HRMS (m/z): calcd C19H22N3O [M + H]+, 308.1763; found, 308.1761.
2-Phenyl-3-(pyrrolidin-1-ylmethyl)imidazo[1,2-a]pyridine (2e)
Yield: (58 mg, 70%); 1H NMR (400 MHz, CDCl3): δ 8.50 (d, J = 6.9 Hz, 1H), 7.79 (dd, J = 5.2, 3.2 Hz, 2H), 7.64 (d, J = 9.1 Hz, 1H), 7.46 (dd, J = 10.3, 4.7 Hz, 2H), 7.37 (d, J = 6.1 Hz, 1H), 7.24–7.19 (m, 1H), 6.82 (dd, J = 6.7, 5.9 Hz, 1H), 4.14 (s, 2H), 2.55 (br s, 4H), 1.77–1.74 (m, 4H); 13C NMR (101 MHz, CDCl3): δ 144.91 (C), 134.71 (C), 129.02 (CH), 128.92 (C), 128.48 (CH), 128.44 (C), 127.80 (C), 127.67 (CH), 125.44 (CH), 124.53 (CH), 117.20 (CH), 111.87 (CH), 53.68 (CH2), 48.61 (CH2), 23.60 (CH2). ESI-HRMS (m/z): calcd for C18H20N3 [M + H]+, 278.1657; found, 278.1654.
N-((2-Phenylimidazo[1,2-a]pyridin-3-yl)methyl)aniline (2f)
Yield: (76 mg, 86%); 1H NMR (400 MHz, CDCl3): δ 8.13 (d, J = 6.8 Hz, 1H), 7.78 (d, J = 7.1 Hz, 2H), 7.70 (d, J = 9.1 Hz, 2H), 7.46–7.43 (m, 2H), 7.38 (d, J = 7.3 Hz, 2H), 7.27–7.23 (m, 3H (merged with CDCl3)), 6.86–6.81 (m, 2H), 6.76 (d, J = 7.7 Hz, 2H), 4.70 (s, 4H); 13C NMR (101 MHz, CDCl3): δ 147.60 (C), 145.08 (C), 144.47 (C), 133.68 (C), 129.50 (CH), 128.80 (CH), 128.45 (CH), 128.16 (CH), 125.18 (CH), 124.18 (CH), 118.52 (CH), 117.51 (CH), 116.56 (C), 113.24 (CH), 112.70 (CH), 38.32 (CH2). ESI-HRMS (m/z): calcd for C20H18N3 [M + H]+, 300.1501; found, 300.1494.
4-((6-Methyl-2-styrylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (2g)
Yield: (71 mg, 71%); 1H NMR (400 MHz, CDCl3): δ 7.99 (s, 1H), 7.64 (d, J = 15.9 Hz, 1H), 7.57 (d, J = 7.4 Hz, 2H), 7.50 (d, J = 9.2 Hz, 1H), 7.38–7.34 (m, 2H), 7.28–7.24 (m, 1H), 7.18 (d, J = 15.9 Hz, 1H), 7.06 (dd, J = 9.2, 1.6 Hz, 1H), 3.85 (s, 2H), 3.70–3.66 (m, 4H), 2.52–2.47 (m, 4H), 2.35 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 144.70 (C), 142.37 (C), 137.47 (C), 130.68 (CH), 128.67 (CH), 128.48 (CH), 127.67 (CH), 126.63 (CH), 122.47 (CH), 121.37 (C), 118.02 (CH), 117.01 (C), 116.16 (CH), 66.93 (CH2), 53.50 (CH2), 51.37 (CH2), 18.57 (CH3). ESI-HRMS (m/z): calcd for C21H24N3O [M + H]+, 334.1919; found, 334.1914.
6-Methyl-3-(piperidin-1-ylmethyl)-2-styrylimidazo[1,2-a]pyridine (2h)
Yield: (76 mg, 76%); 1H NMR (400 MHz, CDCl3): δ 8.04 (s, 1H), 7.63 (d, J = 15.9 Hz, 1H), 7.57 (d, J = 7.5 Hz, 2H), 7.48 (d, J = 9.2 Hz, 1H), 7.37–7.34 (m, 2H), 7.22 (dd, J = 17.4, 11.7 Hz, 2H), 7.04 (dd, J = 9.2, 1.3 Hz, 1H), 3.80 (s, 2H), 2.43 (br s, 4H), 2.34 (s, 3H), 1.58–1.52 (m, 4H), 1.45 (br s, 2H); 13C NMR (101 MHz, CDCl3): δ 144.63 (C), 142.05 (C), 137.69 (C), 130.21 (CH), 128.61 (CH), 128.17 (CH), 127.48 (CH), 126.59 (CH), 122.78 (CH), 121.05 (C), 118.52 (CH), 118.19 (C), 116.07 (CH), 54.49 (CH2), 51.74 (CH2), 25.95 (CH2), 24.41 (CH2), 18.41 (CH3). ESI-HRMS (m/z): calcd for C22H26N3 [M + H]+, 332.2127; found, 332.2125.
4-((6-Methyl-2-(phenylethynyl)imidazo[1,2-a]pyridin-3-yl)methyl)-morpholine (2i)
Yield: (68 mg, 68%); 1H NMR (400 MHz, CDCl3): δ 8.02 (s, 1H), 7.58 (dd, J = 6.5, 3.1 Hz, 2H), 7.47 (d, J = 9.3 Hz, 2H), 7.36 (dd, J = 5.1, 1.8 Hz, 2H), 7.09 (dd, J = 9.2, 1.1 Hz, 1H), 3.92 (s, 2H), 3.71–3.68 (m, 4H), 2.54–2.52 (m, 4H), 2.37 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 144.39 (C), 132.17 (C), 132.07 (C), 131.90 (C), 131.63 (CH), 128.53 (CH), 128.44 (CH), 128.37 (CH), 123.03 (C), 122.28 (CH), 116.83 (CH), 92.77 (C), 82.66 (C), 66.92 (CH2), 53.50 (CH2), 52.00 (CH2), 18.43 (CH2). ESI-HRMS (m/z): calcd for C21H22N3O [M + H]+, 332.1763; found, 332.1755.
6-Methyl-2-(phenylethynyl)-3-(piperidin-1-ylmethyl)-imidazo-[1,2-a]pyridine (2j)
Yield: (78 mg, 79%); 1H NMR (400 MHz, CDCl3): δ 8.10 (s, 1H), 7.60 (dd, J = 7.4, 2.0 Hz, 2H), 7.47 (d, J = 9.2 Hz, 1H), 7.39–7.35 (m, 3H), 7.08 (d, J = 9.2 Hz, 1H), 3.89 (s, 2H), 2.48 (br s, 4H), 2.37 (s, 3H), 1.60–1.55 (m, 4H), 1.47 (br s, 2H); 13C NMR (101 MHz, CDCl3): δ 144.23 (C), 131.60 (CH), 128.39 (CH), 128.32 (CH), 128.05 (C), 123.75 (C), 123.18 (C), 122.64 (CH), 121.99 (C), 116.60 (CH), 92.45(C), 82.99 (C), 54.42 (CH2), 52.29 (CH2), 25.88 (CH2), 24.32 (CH2), 18.44 (CH3); ESI-HRMS (m/z): calcd for C22H24N3 [M + H]+, 330.1970; found, 330.1964.
2-Iodo-3-(pyrrolidin-1-ylmethyl)imidazo[1,2-a]pyridine (2k)
Yield: (71 mg, 72%); 1H NMR (400 MHz, CDCl3): δ 8.34 (d, J = 6.9 Hz, 1H), 7.52 (d, J = 9.1 Hz, 1H), 7.19–7.14 (m, 1H), 6.79 (dd, J = 6.8, 0.9 Hz, 1H), 3.93 (s, 2H), 2.54 (t, J = 6.5 Hz, 4H), 1.78–1.74 (m, 4H); 13C NMR (101 MHz, CDCl3): δ 147.18 (C), 124.74 (CH), 124.50 (CH), 123.26 (C), 116.65 (CH), 112.18 (CH), 94.58 (C), 53.81 (CH2), 49.90 (CH2), 23.65 (CH2). ESI-HRMS (m/z): calcd for C12H15IN3 [M + H]+, 328.0311; found, 328.0338.
2-Iodo-6-methyl-3-(pyrrolidin-1-ylmethyl)imidazo[1,2-a]pyridine (2l)
Yield: (77 mg, 75%); 1H NMR (400 MHz, CDCl3): δ 8.08 (s, 1H), 7.46 (d, J = 9.1 Hz, 1H), 7.04 (d, J = 9.2 Hz, 1H), 3.90 (s, 2H), 2.57 (br s, 4H), 2.37 (s, 3H), 1.79 (br s, 4H); 13C NMR (101 MHz, CDCl3): δ 146.27 (C), 127.81 (CH), 123.02 (C), 122.00 (CH), 121.85 (C), 115.99 (CH), 94.14 (C), 53.90 (CH2), 49.92 (CH2), 23.65 (CH2), 18.33 (CH3). ESI-HRMS (m/z): calcd for C13H17IN3 [M + H]+, 342.0467; found, 342.0459.
2-Iodo-3-(piperidin-1-ylmethyl)imidazo[1,2-a]pyridine (2m)
Yield: (85 mg, 83%); 1H NMR (400 MHz, CDCl3): δ 8.35 (d, J = 6.9 Hz, 1H), 7.51 (d, J = 9.1 Hz, 1H), 7.15 (dd, J = 9.0, 6.8, 1.2 Hz, 1H), 6.77 (dd, J = 6.8, 1.1 Hz, 1H), 3.73 (s, 2H), 2.39 (br s, 4H), 1.53–1.48 (m, 4H), 1.42 (br s, 2H); 13C NMR (101 MHz, CDCl3): δ 147.32 (C), 124.77 (CH), 124.70 (CH), 122.44 (C), 116.52 (CH), 112.03 (CH), 95.61 (C), 54.20 (CH2), 53.49 (CH2), 25.90 (CH2), 24.32 (CH2). ESI-HRMS (m/z): calcd for C13H17IN3 [M + H]+, 342.0467; found, 342.0449.
2-Iodo-6-methyl-3-(piperidin-1-ylmethyl)imidazo[1,2-a]pyridine (2n)
Yield: (93 mg, 87%); 1H NMR (400 MHz, CDCl3): δ 8.11 (s, 1H), 7.45 (d, J = 9.2 Hz, 1H), 7.04 (d, J = 10.8 Hz, 1H), 3.73 (s, 2H), 2.43 (br s, 4H), 2.36 (s, 3H), 1.58–1.52 (m, 4H), 1.46 (br s, 2H); 13C NMR (101 MHz, CDCl3): δ 146.46 (C), 127.84 (CH), 122.34 (CH), 121.96 (C), 121.75 (C), 115.95 (CH), 95.23 (C), 54.26 (CH2), 53.45 (CH2), 25.90 (CH2), 24.36 (CH2), 18.36 (CH3). ESI-HRMS (m/z): calcd for C14H18IN3 [M + H]+, 356.0624; found, 356.0624.
4-((2-Iodoimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (2o)
Yield: (78 mg, 76%); 1H NMR (400 MHz, CDCl3): δ 8.29 (d, J = 6.9 Hz, 1H), 7.54 (d, J = 9.1 Hz, 1H), 7.22–7.14 (m, 1H), 6.81 (t, J = 6.9, 1.0 Hz, 1H), 3.80 (s, 2H), 3.69–3.61 (m, 4H), 2.51–2.43 (m, 4H); 13C NMR (101 MHz, CDCl3): δ 147.47 (C), 124.97 (CH), 124.43 (CH), 121.42 (C), 116.81 (CH), 112.33 (CH), 96.04 (C), 66.88 (CH2), 53.21 (CH2), 53.09 (CH2). ESI-HRMS (m/z): calcd for C12H15IN3O [M + H]+, 344.0260; found, 339.0277.
4-((2-Iodo-6-methylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (2p)
Yield: (87 mg, 81%); 1H NMR (400 MHz, CDCl3): δ 8.06 (s, 1H), 7.49 (d, J = 9.2 Hz, 1H), 7.08 (dd, J = 9.2, 1.1 Hz, 1H), 3.79 (s, 2H), 3.70–3.68 (m, 4H), 2.52–2.50 (m, 4H), 2.39 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 146.59 (C), 128.10 (CH), 122.10 (CH), 121.99 (C), 121.04 (C), 116.12 (CH), 95.56 (C), 66.89 (CH2), 53.26 (CH2), 53.05 (CH2), 18.35 (CH3). ESI-HRMS (m/z): calcd for C13H17IN3O [M + H]+, 358.0416; found, 358.0403.
N-Ethyl-N-((2-iodoimidazo[1,2-a]pyridin-3-yl)methyl)ethanamine (2q)
Yield: (67 mg, 68%); 1H NMR (400 MHz, CDCl3): δ 8.44 (d, J = 6.2 Hz, 1H), 7.53 (d, J = 9.1 Hz, 1H), 7.20–7.14 (m, 1H), 6.78 (t, J = 6.8 Hz, 1H), 3.89 (s, 2H), 2.55 (q, J = 6.9 Hz, 4H), 1.05 (t, J = 7.1 Hz, 6H); 13C NMR (126 MHz, CDCl3): δ 147.29 (C), 128.40 (C), 124.75 (CH), 116.54 (CH), 112.01 (CH), 95.29 (C), 48.35 (CH2), 46.34 (CH2), 11.50 (CH3). ESI-HRMS (m/z): calcd for C12H17IN3 [M + H]+, 330.0467; found, 330.0459.
N-Ethyl-N-((2-iodo-6-methylimidazo[1,2-a]pyridin-3-yl)methyl)ethanamine (2r)
Yield: (77 mg, 75%); 1H NMR (400 MHz, CDCl3): δ 8.20 (s, 1H), 7.44 (d, J = 9.2 Hz, 1H), 7.04 (d, J = 9.2 Hz, 1H), 3.87 (s, 2H), 2.61–2.56 (m, 4H), 2.35 (s, 3H), 1.08 (t, J = 7.1 Hz, 6H); 13C NMR (101 MHz, CDCl3): δ 146.51 (C), 127.90 (C), 127.81 (C), 122.44 (CH), 115.95 (CH), 48.24 (CH2), 46.37 (CH2), 18.36 (CH3), 11.38 (CH3). ESI-HRMS (m/z): calcd for C13H19IN3 [M + H]+, 344.0624; found, 344.0616.
N-((2-Iodo-6-methylimidazo[1,2-a]pyridin-3-yl)methyl)aniline (2s)
Yield: (87 mg, 80%); 1H NMR (400 MHz, CDCl3): δ 7.91 (s, 1H), 7.48–7.45 (m, 1H), 7.28–7.25 (m, 2H), 7.04 (d, J = 9.1 Hz, 1H), 6.83 (t, J = 7.4 Hz, 1H), 6.79 (d, J = 7.7 Hz, 2H), 4.55 (s, 2H), 3.72 (br s, 1H), 2.31 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 147.72 (C), 146.60 (C), 129.45 (2× CH), 128.25 (C), 122.67 (C), 122.24 (C), 121.67 (CH), 118.74 (CH), 116.33 (CH), 113.61 (2× CH), 94.57 (C), 39.50 (CH2), 18.25 (CH3). ESI-HRMS (m/z): calcd for C15H15IN3 [M + H]+, 364.0311; found, 364.0304.
N-Benzyl-1-(2-iodo-6-methylimidazo[1,2-a]pyridin-3-yl)-methanamine (2t)
Yield: (100 mg, 88%); 1H NMR (400 MHz, CDCl3): δ 7.95 (s, 1H), 7.43 (d, J = 9.2 Hz, 1H), 7.37–7.23 (m, 5H merged with CDCl3), 7.01 (dd, J = 9.2, 1.5 Hz, 1H), 4.06 (s, 2H), 3.83 (s, 2H), 2.32 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 146.42 (C), 139.82 (C), 128.45 (CH), 128.25 (CH), 127.81 (CH), 127.20 (CH), 123.46 (C), 121.98 (CH), 116.13 (CH), 94.11 (C), 53.09 (CH2), 43.13 (CH2), 18.23 (CH3). ESI-HRMS (m/z): calcd for C16H16IN3 [M + H]+, 378.0467; found, 378.0462.
N-((2-Iodo-6-methylimidazo[1,2-a]pyridin-3-yl)methyl)prop-2-yn-1-amine (2u)
Yield: (85 mg, 87%); 1H NMR (400 MHz, CDCl3+ CD3OD): δ 8.04 (s, 1H), 7.38 (d, J = 9.2 Hz, 1H), 7.03 (d, J = 9.2 Hz, 1H), 4.13 (s, 2H), 3.38 (d, J = 2.3 Hz, 1H), 2.98 (s, 2H), 2.30 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 146.47 (C), 128.57 (CH), 122.68 (C), 121.92 (CH), 115.67 (CH), 93.81 (C), 81.23 (C), 72.40 (CH), 42.06 (CH2), 37.08 (CH2), 18.13 (CH3). ESI-HRMS (m/z): calcd for C12H13IN3 [M + H]+, 326.0154; found, 326.0156.
N-((2-Phenylimidazo[1,2-a]pyridin-3-yl)methyl)propan-1-amine (2w)
Yield: (65 mg, 82%); 1H NMR (400 MHz, CDCl3): δ 8.43 (d, J = 6.8 Hz, 1H), 7.76 (d, J = 7.4 Hz, 2H), 7.65 (d, J = 9.0 Hz, 1H), 7.50–7.47 (m, 2H), 7.41 (d, J = 7.2 Hz, 1H), 7.26–7.22 (m, 1H), 6.88–6.85 (m, 1H), 4.30 (s, 2H), 2.69 (t, J = 7.1 Hz, 2H), 1.58–1.53 (m, 2H), 0.93 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 144.38 (C), 134.40 (C), 128.80 (CH), 128.62 (CH2), 128.55(C), 127.87(C), 124.98 (CH), 124.81 (CH), 117.29 (CH), 112.15 (CH), 111.80 (CH), 51.03 (CH2), 42.63 (CH2), 22.60 (CH2), 11.68 (CH3). ESI-HRMS (m/z): calcd for C17H20N3 [M + H]+, 266.1657; found, 266.1643.
6-Chloro-2-iodo-3-(piperidin-1-ylmethyl)imidazo[1,2-a]pyridine (2x)
Yield: (81 mg, 72%); 1H NMR (400 MHz, CDCl3): δ 8.37 (s, 1H), 7.78 (d, J = 7.8 Hz, 2H), 7.59 (d, J = 9.5 Hz, 1H), 7.53–7.49 (m, 2H), 7.45 (d, J = 6.8 Hz, 1H), 7.34 (d, J = 9.5 Hz, 1H), 4.88 (s, 2H), 3.47 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 145.66 (C), 126.13 (CH), 123.08 (C), 122.61 (CH), 120.47 (C), 116.91 (CH), 96.06 (C), 54.25 (CH2), 53.52 (CH2), 25.88 (CH2), 24.29 (CH2). ESI-HRMS (m/z): calcd for C13H16ClIN3 [M + H]+, 376.0077; found, 376.0079.
General Procedure for C3-Methylene C–S Bond Formation of Imidazo[1,2-a]pyridine (3)
A solution of 3-((benzyloxy) methyl)-2-phenylimidazo[1,2-a]pyridine (0.3 mmol) in dry DCM (2 mL) was stirred at 0 °C under a nitrogen atmosphere. BCl3 solution (1.0 M in methylene chloride) (0.42 mmol) was slowly added, and complex formation was confirmed by TLC. Then, reaction was transferred to rt and a solution of an organosulfur in DCM (0.9 mmol) was added, and reaction was continuously stirred for 2 h (reaction progress was monitored by TLC). After completion, the reaction was quenched by water to stop the reaction. The aqueous solution was extracted with DCM (3 × 10 mL). The combined organic layer was washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuum. The residue was purified by a column (silica gel, 80/20 n-hexane/ethyl acetate) to give the desired product 3.
2-Phenyl-3-((pyridin-2-ylthio)methyl)imidazo[1,2-a]pyridine (3a)
Yield: (65 mg, 68%); 1H NMR (400 MHz, CDCl3): δ 8.49 (d, J = 4.4 Hz, 1H), 8.28 (d, J = 6.8 Hz, 1H), 7.88 (d, J = 7.3 Hz, 2H), 7.70 (d, J = 9.0 Hz, 1H), 7.57–7.49 (m, 3H), 7.43–7.39 (m, 1H), 7.27 (dd, J = 16.0, 5.2 Hz, 1H), 7.08 (dd, J = 6.7, 5.1 Hz, 1H), 6.89–6.87 (m, 2H), 5.07 (s, 2H); 13C NMR (101 MHz, CDCl3): δ 158.03 (C), 149.52 (CH), 145.21 (C), 144.70 (C), 136.26 (CH), 134.14 (C), 128.67 (CH), 128.53 (CH), 127.92 (CH), 124.74 (CH), 124.31 (CH), 122.67 (CH), 120.03 (CH), 117.54 (CH), 115.05 (C), 112.38 (CH), 24.64 (CH2). ESI-HRMS (m/z): calcd for C19H16N3S [M + H]+, 318.1065; found, 318.1027.
6-Methyl-2-phenyl-3-((p-tolylthio)methyl)imidazo[1,2-a]-pyridine (3b)
Yield: (73 mg, 71%); 1H NMR (400 MHz, CDCl3): δ 7.80 (s, 1H), 7.68–7.64 (m, 2H), 7.56 (d, J = 9.2 Hz, 1H), 7.42–7.39 (m, 2H), 7.35–7.32 (m, 1H), 7.21 (d, J = 8.1 Hz, 2H), 7.08 (dd, J = 9.2, 1.4 Hz, 1H), 7.04 (d, J = 7.9 Hz, 2H), 4.50 (s, 2H), 2.35 (s, 3H), 2.31 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 144.65 (C), 144.23 (C), 137.82 (C), 134.20 (C), 132.41 (CH), 130.92 (C), 129.84 (CH), 128.46 (CH), 128.42 (CH), 127.78 (CH), 127.70 (CH), 121.93 (C), 121.68 (CH), 116.89 (CH), 114.93 (C), 30.25 (CH2), 21.05 (CH3), 18.40 (CH3). ESI-HRMS (m/z): calcd for C22H21N2S [M + H]+, 345.1425; found, 345.1419.
3-(((4-Methoxyphenyl)thio)methyl)-6-methyl-2-phenylimidazo-[1,2-a]pyridine (3c)
Yield: (81 mg, 75%); 1H NMR (400 MHz, CDCl3): δ 7.83 (s, 1H), 7.73–7.70 (m, 2H), 7.59 (d, J = 9.2 Hz, 1H), 7.43 (dd, J = 10.2, 4.6 Hz, 2H), 7.36 (d, J = 7.3 Hz, 1H), 7.19–7.15 (m, 1H), 7.11 (dd, J = 9.1, 1.4 Hz, 1H), 6.92 (d, J = 7.7 Hz, 1H), 6.84–6.82 (m, 1H), 6.78 (dd, J = 8.3, 2.0 Hz, 1H), 4.57 (s, 2H), 3.70 (s, 3H), 2.36 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 159.95 (C), 144.12 (C), 135.98 (C), 129.90 (CH), 128.62 (CH), 128.42 (CH), 128.15 (C), 127.90 (CH), 123.52 (CH), 122.30 (C), 121.70 (CH), 122.26 (C), 116.82 (CH), 116.51 (CH), 114.52 (C), 113.48 (CH), 55.28 (OCH3), 29.47 (CH2), 18.44 (CH3). ESI-HRMS (m/z): calcd for C22H21N2OS [M + H]+, 361.1375; found, 361.1375.
6-Methyl-3-((octylthio)methyl)-2-phenylimidazo[1,2-a]pyridine (3d)
Yield: (70 mg, 64%); 1H NMR (400 MHz, CDCl3): δ 7.94 (s, 1H), 7.81 (d, J = 7.2 Hz, 2H), 7.57 (d, J = 9.1 Hz, 1H), 7.49–7.45 (m, 2H), 7.39–7.35 (m, 1H), 7.09 (dd, J = 9.2, 1.4 Hz, 1H), 4.22 (s, 2H), 2.40 (s, 3H), 2.38 (d, J = 7.6 Hz, 2H), 1.48–1.40 (m, 2H), 1.31–1.18 (m, 12H), 0.88 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 144.34 (C), 136.23 (C), 134.43 (C), 128.62 (CH), 128.56 (CH), 127.78 (CH), 121.90 (CH), 116.87 (CH), 39.29 (CH2), 31.81 (CH2), 29.26 (CH2), 29.20 (CH2), 29.17 (CH2), 28.55 (CH2), 25.36 (CH2), 22.64 (CH2), 18.48 (CH3), 14.06 (CH3). ESI-HRMS (m/z): calcd for C23H31N2S [M + H]+, 367.2208; found, 367.1207.
6-Methyl-2-styryl-3-((p-tolylthio)methyl)imidazo[1,2-a]pyridine (3e)
Yield: (76 mg, 68%); 1H NMR (400 MHz, CDCl3): δ 7.74 (s, 1H), 7.49 (d, J = 9.1 Hz, 1H), 7.44–7.37 (m, 3H), 7.34–7.30 (m, 2H), 7.24 (d, J = 7.2 Hz, 1H), 7.17 (d, J = 8.0 Hz, 2H), 7.08 (dd, J = 9.2, 1.4 Hz, 1H), 7.01 (d, J = 7.9 Hz, 2H), 6.55 (d, J = 15.8 Hz, 1H), 4.34 (s, 2H), 2.36 (s, 3H), 2.13 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 144.61 (C), 142.27 (C), 138.71 (C), 137.43 (C), 134.07 (CH), 130.35 (CH), 130.30 (C), 129.88 (CH), 128.48 (CH), 128.26 (CH), 127.45 (CH), 126.55 (CH), 121.67 (C), 121.34 (CH), 117.82 (CH), 116.95 (C), 116.50 (CH), 29.80 (CH2), 20.90 (CH3), 18.37 (CH3). ESI-HRMS (m/z): calcd for C24H23N2S [M + H]+, 371.1582; found, 371.1583.
6-Methyl-2-(phenylethynyl)-3-((p-tolylthio)methyl)imidazo-[1,2-a]pyridine (3f)
Yield: (70 mg, 63%); 1H NMR (400 MHz, CDCl3): δ 7.80 (s, 1H), 7.48–7.45 (m, 3H), 7.35–7.33 (m, 3H), 7.21 (d, J = 8.1 Hz, 2H), 7.09 (dd, J = 9.2, 1.3 Hz, 1H), 6.99 (d, J = 7.9 Hz, 2H), 4.43 (s, 2H), 2.36 (s, 3H), 2.20 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 145.54 (C), 138.02 (C), 132.97 (CH), 132.17 (C), 131.62 (CH), 130.15 (C), 129.81 (CH), 128.39 (C), 128.33 (CH), 128.24 (CH), 125.58 (C), 123.02 (C), 122.51(CH), 121.61 (CH), 117.04 (CH), 95.42 (C), 92.53 (C), 29.53 (CH2), 20.99 (CH3), 18.40 (CH3). ESI-HRMS (m/z): calcd for C24H21N2S [M + H]+, 369.1425; found, 369.1424.
2-Iodo-3-(((4-methoxyphenyl)thio)methyl)-6-methylimidazo[1,2-a]pyridine (3g)
Yield: (101 mg, 82%); 1H NMR (400 MHz, CDCl3): δ 7.77 (s, 1H), 7.45 (d, J = 9.2 Hz, 1H), 7.12 (d, J = 8.8 Hz, 2H), 7.04 (dd, J = 9.2, 1.5 Hz, 1H), 6.73 (d, J = 8.8 Hz, 2H), 4.20 (s, 2H), 3.76 (s, 3H), 2.35 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 160.34 (C), 146.23 (C), 136.43 (CH), 127.81 (CH), 123.31 (C), 122.34 (C), 121.5 (C), 121.41 (CH), 116.32 (CH), 114.64 (CH), 95.51 (C), 55.35 (OCH3), 31.48 (CH2), 18.32 (CH3). ESI-HRMS (m/z): calcd for C16H16IN2OS [M + H]+, 411.0028; found, 411.0022.
2-Iodo-6-methyl-3-((p-tolylthio)methyl)imidazo[1,2-a]pyridine (3h)
Yield: (92 mg, 78%); 1H NMR (400 MHz, CDCl3): δ 7.78 (s, 1H), 7.44 (d, J = 9.2 Hz, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.03 (d, J = 7.9 Hz, 3H), 4.27 (s, 2H), 2.33 (s, 3H), 2.30 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 146.30, 138.39, 133.71, 129.83, 129.68, 127.82, 122.35, 121.52, 121.39, 116.34, 95.50 (C), 30.81 (CH2), 21.15 (CH3), 18.31 (CH3) ESI-HRMS (m/z): calcd for C16H16IN2S [M + H]+, 395.0079; found, 395.0011.
3-(((2,6-Dichlorophenyl)thio)methyl)-2-iodo-6-methyl-imidazo-[1,2-a]pyridine (3i)
Yield: (107 mg, 80%); 1H NMR (400 MHz, CDCl3): δ 7.94 (s, 1H), 7.46 (d, J = 9.2 Hz, 1H), 7.31 (d, J = 7.8 Hz, 2H), 7.21 (dd, J = 8.7, 7.3 Hz, 1H), 7.07 (dd, J = 9.2, 1.5 Hz, 1H), 4.33 (s, 2H), 2.40 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 146.49 (C), 142.67 (C), 130.98 (CH), 128.68 (CH), 128.04 (CH), 122.52 (C), 121.61 (CH), 120.73 (C), 116.31 (CH), 95.06 (C), 29.25 (CH2), 18.38 (CH3). ESI-HRMS (m/z): calcd for C15H12Cl2IN2S [M + H]+, 448.9143; found, 448.9142.
3-(((3-Fluorophenyl)thio)methyl)-2-iodo-6-methylimidazo[1,2-a]pyridine (3j)
Yield: (87 mg, 73%); 1H NMR (400 MHz, CDCl3): δ 7.79 (s, 1H), 7.45 (d, J = 9.2 Hz, 1H), 7.18 (dd, J = 7.9, 5.5 Hz, 2H), 7.05 (d, J = 9.2 Hz, 1H), 6.90 (t, J = 8.4 Hz, 2H), 4.24 (s, 2H), 2.36 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 164.43 (C), 146.35 (C), 136.45(CH), 136.37 (CH), 127.96 (CH), 122.55 (C), 121.33 (CH), 121.03 (C), 116.44(CH) 116.29 (CH), 116.07 (CH), 95.73 (C), 31.10 (CH2), 18.32 (CH3). ESI-HRMS (m/z): calcd for C15H13FIN2S [M + H]+, 398.9828; found, 398.9932.
3-((Hexylthio)methyl)-2-iodo-6-methylimidazo[1,2-a]pyridine (3k)
Yield: (86 mg, 74%); 1H NMR (400 MHz, CDCl3): δ 7.88 (s, 1H), 7.46 (d, J = 9.2 Hz, 1H), 7.04 (dd, J = 9.2, 1.4 Hz, 1H), 4.00 (s, 2H), 2.40–2.35 (m, 5H), 1.58–1.50 (m, 2H), 1.34–1.20 (m, 6H), 0.84 (t, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 146.50 (C), 127.90 (CH), 122.32 (C), 121.67 (CH), 116.36 (CH), 94.83 (C), 31.39 (CH2), 31.29 (CH2), 29.68 (CH2), 28.52 (CH2), 25.66 (CH2), 22.51 (CH2), 18.35 (CH3), 14.00 (CH3). ESI-HRMS (m/z): calcd for C15H22IN2S [M + H]+, 389.0548; found, 389.0539.
2-Iodo-6-methyl-3-((pyridin-2-ylthio)methyl)imidazo[1,2-a]pyridine (3l)
Yield: (87 mg, 76%); 1H NMR (400 MHz, CDCl3): δ 8.52 (d, J = 4.9 Hz, 1H), 8.05 (s, 1H), 7.51–7.49 (m, 1H), 7.42 (d, J = 9.2 Hz, 1H), 7.17 (d, J = 7.9 Hz, 1H), 7.04 (dd, J = 7.2, 5.1 Hz, 1H), 6.99 (d, J = 9.2 Hz, 1H), 4.82 (s, 2H), 2.28 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 157.67(C), 149.32 (CH), 146.33 (C), 136.31 (CH), 127.82 (CH), 122.85 (CH), 122.32 (C), 122.12 (CH), 121.69 (C), 120.14 (CH), 116.24 (CH), 95.16 (C), 24.73 (CH2), 18.32 (CH3); ESI-HRMS (m/z): calcd for C14H13IN3S [M + H]+, 381.9875; found, 381.9863.
General Procedure for C3-Methylene C–O Bond Formation of Imidazo[1,2-a]pyridine (4)
A solution of 3-((benzyloxy) methyl)-2-phenylimidazo[1,2-a]pyridine (0.3 mmol) in dry DCM (2 mL) was stirred at 0 °C under a nitrogen atmosphere. BCl3 solution (1.0 M in methylene chloride) (0.42 mmol) was slowly added, and complex formation was confirmed by TLC. After that, reaction was allowed to raise temperature up to rt. Alcohol (500 μL) was added, and reaction was further stirred for another 2 h (reaction progress was monitored by TLC). After completion, the reaction was quenched by water to stop the reaction. The aqueous solution was extracted with DCM (3 × 10 mL). The combined organic layer was washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuum. The residue was purified by a column (silica gel, 80/20 n-hexane/ethyl acetate) to give the desired product 4.
3-(Methoxymethyl)-2-phenylimidazo[1,2-a]pyridine (4a)12
Yield: (51 mg, 72%); 1H NMR (400 MHz, CDCl3): δ 8.22 (d, J = 6.9 Hz, 1H), 7.79 (dd, J = 5.2, 3.3 Hz, 2H), 7.70 (d, J = 9.1 Hz, 1H), 7.51–7.47 (m, 2H), 7.41 (d, J = 7.3 Hz, 1H), 7.30–7.27 (m, 1H), 6.89 (dd, J = 7.3, 6.3 Hz, 1H), 4.88 (s, 2H), 3.43 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 145.64 (C), 145.23 (C), 134.00 (C), 128.78 (CH), 128.62 (CH), 128.07 (CH), 125.22 (CH), 124.37 (CH), 117.50 (CH), 116.79 (C), 112.54 (CH), 63.62 (CH2), 57.81 (CH3). ESI-HRMS (m/z): calcd for C15H15N2O [M + H]+, 239.1184; found, 239.1175.
3-(Methoxymethyl)-6-methyl-2-phenylimidazo[1,2-a]pyridine (4b)
Yield: (57 mg, 76%); 1H NMR (400 MHz, CDCl3): δ 7.98 (s, 1H), 7.77 (d, J = 7.2 Hz, 2H), 7.60 (d, J = 9.1 Hz, 1H), 7.49–7.45 (m, 2H), 7.40 (d, J = 7.3 Hz, 1H), 7.12 (dd, J = 9.2, 1.3 Hz, 1H), 4.85 (s, 2H), 3.43 (s, 3H), 2.38 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 144.19 (C), 134.02 (C), 128.73 (CH), 128.59 (CH), 128.49 (CH), 127.99 (CH), 122.36 (C), 122.05 (CH), 116.77 (CH), 116.52 (C), 63.64 (CH2), 57.74 (CH3), 18.36 (CH3). ESI-HRMS (m/z): calcd for C16H17N2O [M + H]+, 253.1341; found, 253.1337.
6-Methyl-2-phenyl-3-(propoxymethyl)imidazo[1,2-a]pyridine (4c)
Yield: (55 mg, 66%); 1H NMR (400 MHz, CDCl3): δ 7.99 (s, 1H), 7.79–7.77 (m, 2H), 7.56 (d, J = 9.2 Hz, 1H), 7.48–7.44 (m, 2H), 7.39–7.35 (m, 1H), 7.09 (dd, J = 9.2, 1.4 Hz, 1H), 4.88 (s, 2H), 3.50 (t, J = 6.6 Hz, 2H), 2.37 (s, 3H), 1.68–1.62 (m, 2H), 0.95 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 145.34 (C), 144.31 (C), 134.40 (C), 128.69 (CH), 128.53 (CH), 128.12 (CH), 127.82 (CH), 122.05 (CH), 122.03 (C), 116.83 (CH), 71.89 (CH2), 62.04 (CH2), 22.89 (CH2), 18.38 (CH3), 10.70 (CH3). ESI-HRMS (m/z): calcd for C18H21N2O [M + H]+, 281.1654; found, 281.1649.
6-Methyl-3-(propoxymethyl)-2-styrylimidazo[1,2-a]pyridine (4d)
Yield: (55 mg, 60%); 1H NMR (400 MHz, CDCl3): δ 7.94 (s, 1H), 7.67 (d, J = 15.9 Hz, 1H), 7.58 (d, J = 7.4 Hz, 2H), 7.54 (d, J = 9.2 Hz, 1H), 7.38–7.34 (m, 2H), 7.28–7.25 (m, 1H), 7.17 (d, J = 15.9 Hz, 1H), 7.10 (dd, J = 9.2, 1.5 Hz, 1H), 4.88 (s, 2H), 3.43 (t, J = 6.6 Hz, 2H), 2.35 (s, 3H), 1.64–1.59 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 144.61 (C), 141.95 (C), 137.32 (C), 131.46 (CH), 129.08 (CH), 128.65 (CH), 127.79 (CH), 126.71 (CH), 122.17 (CH), 122.07 (C), 118.01 (C), 117.59 (CH), 116.16 (CH), 71.54 (CH2), 60.80 (CH2), 22.85 (CH2), 18.28 (CH3), 10.60 (CH3). ESI-HRMS (m/z): calcd for C20H23N2O [M + H]+, 307.1810; found, 307.1808.
3-(Methoxymethyl)-6-methyl-2-(phenylethynyl)imidazo[1,2-a]pyridine (4e)
Yield: (58 mg, 70%); 1H NMR (400 MHz, CDCl3): δ 7.92 (s, 1H), 7.58 (dd, J = 6.5, 3.1 Hz, 2H), 7.48 (d, J = 9.2 Hz, 1H), 7.36–7.34 (m, 3H), 7.11 (dd, J = 9.2, 1.5 Hz, 1H), 4.89 (s, 2H), 3.36 (s, 3H), 2.35 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 144.60 (C), 131.69 (CH), 128.97 (CH), 128.82 (C), 128.52 (CH), 128.36 (CH), 122.91 (C), 122.82 (C), 121.97 (CH), 116.93 (CH), 92.72 (C), 82.33 (C), 62.98 (CH2), 57.43 (CH2), 18.28 (CH3). ESI-HRMS (m/z): calcd for C18H17N2O [M + H]+, 277.1341; found, 277.1326.
2-Iodo-3-(methoxymethyl)-6-methylimidazo[1,2-a]pyridine (4f)
Yield: (72 mg, 80%); 1H NMR (400 MHz, CDCl3): δ 7.92 (s, 1H), 7.46 (d, J = 9.2 Hz, 1H), 7.06 (dd, J = 9.2, 1.4 Hz, 1H), 4.73 (s, 2H), 3.31 (s, 3H), 2.34 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 146.75 (C), 128.51(CH), 122.70 (C), 121.86 (C), 121.72 (CH), 116.29 (CH), 95.87 (C), 64.14 (CH2), 57.35 (CH3), 18.22 (CH3). ESI-HRMS (m/z): calcd for C10H12IN2O [M + H]+, 302.9994; found, 302.9953.
3-(Ethoxymethyl)-2-iodo-6-methylimidazo[1,2-a]pyridine (4g)
Yield: (65 mg, 69%); 1H NMR (400 MHz, CDCl3): δ 7.96 (s, 1H), 7.46 (d, J = 9.2 Hz, 1H), 7.06 (dd, J = 9.2, 1.6 Hz, 1H), 4.78 (s, 2H), 3.53–3.47 (m, 2H), 2.35 (s, 3H), 1.20 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 146.55 (C), 128.40 (CH), 122.60 (C), 122.33 (C), 121.73 (CH), 116.28 (CH), 95.48 (C), 65.05 (CH2), 62.34 (CH2), 18.22 (CH3), 15.07 (CH3). ESI-HRMS (m/z): calcd for C11H14IN2O [M + H]+, 317.0151; found, 317.0150.
2-Iodo-6-methyl-3-(propoxymethyl)imidazo[1,2-a]pyridine (4h)
Yield: (72 mg, 73%); 1H NMR (400 MHz, CDCl3): δ 7.97 (s, 1H), 7.46 (d, J = 9.2 Hz, 1H), 7.06 (dd, J = 9.2, 1.6 Hz, 1H), 4.78 (s, 2H), 3.39 (t, J = 6.6 Hz, 2H), 2.35 (s, 3H), 1.64–1.55 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 146.71 (C), 128.38 (CH), 122.55 (C),122.38 (C), 121.78 (CH), 116.27 (CH), 95.44 (C), 71.33 (CH2), 62.55 (CH2), 22.77 (CH2), 18.21 (CH3), 10.55 (CH3). ESI-HRMS (m/z): calcd for C12H16IN2O [M + H]+, 331.0307; found, 331.0308.
3-(Butoxymethyl)-2-iodo-6-methylimidazo[1,2-a]pyridine (4i)
Yield: (70 mg, 68%); 1H NMR (400 MHz, CDCl3): δ 7.96 (s, 1H), 7.45 (d, J = 9.2 Hz, 1H), 7.05 (dd, J = 9.2, 1.4 Hz, 1H), 4.77 (s, 2H), 3.42 (t, J = 6.6 Hz, 2H), 2.34 (s, 3H), 1.57–1.51 (m, 2H), 1.37–1.30 (m, 2H), 0.87 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3): δ 146.67 (C), 128.38 (CH), 122.55 (CH), 122.38 (C), 121.76 (CH), 116.24 (CH), 95.43 (C), 69.42 (CH2), 62.55 (CH2), 31.59 (CH2), 19.29 (CH2), 18.20 (CH3), 13.78 (CH3). ESI-HRMS (m/z): calcd for C13H18IN2O [M + H]+, 345.0464; found, 345.0465.
6-Bromo-3-(methoxymethyl)-2-phenylimidazo[1,2-a]pyridine (4j)
Yield: (68 mg, 71%); 1H NMR (400 MHz, CDCl3): δ 8.37 (s, 1H), 7.78 (d, J = 7.8 Hz, 2H), 7.59 (d, J = 9.5 Hz, 1H), 7.51 (t, J = 7.5 Hz, 2H), 7.45 (d, J = 6.8 Hz, 1H), 7.34 (d, J = 9.5 Hz, 1H), 4.88 (s, 2H), 3.47 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 143.69 (C), 133.60 (C), 128.72 (CH), 128.57 (CH), 128.47 (C), 128.32 (CH), 124.60 (CH), 118.15 (CH), 117.25 (C), 107.21 (C), 63.53 (CH2), 58.04 (CH3). ESI-HRMS (m/z): calcd for C15H14BrN2O [M + H]+, 317.0290; found, 317.0287.
Acknowledgments
D.S., G.K., and D.D. thank DST and CSIR New Delhi, India, for research fellowship. This research work was financially supported by DST in the form of research grant DST-EMR/2016/002304, DST-EEQ/2016/000102, and IIIM communication no IIIM/2276/2019.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00035.
Copies of 1H and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Dymińska L. Imidazopyridines as a source of biological activity and their pharmacological potentials—Infrared and Raman spectroscopic evidence of their content in pharmaceuticals and plant materials. Bioorg. Med. Chem. 2015, 23, 6087–6099. 10.1016/j.bmc.2015.07.045. [DOI] [PubMed] [Google Scholar]; b Takada S.; Sasatani T.; Chomei N.; Adachi M.; Fujishita T.; Eigyo M.; Murata S.; Kawasaki K.; Matsushita A. Synthesis and Structure–Activity Relationships of Fused Imidazopyridines: A New Series of Benzodiazepine Receptor Ligands. J. Med. Chem. 1996, 39, 2844–2851. 10.1021/jm9600609. [DOI] [PubMed] [Google Scholar]; c Muniyan S.; Chou Y.-W.; Ingersoll M. A.; Devine A.; Morris M.; Odero-Marah V. A.; Khan S. A.; Chaney W. G.; Bu X. R.; Lin M.-F. Antiproliferative activity of novel imidazopyridine derivatives on castration-resistant human prostate cancer cells. Cancer Lett. 2014, 353, 59–67. 10.1016/j.canlet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kim O.; Jeong Y.; Lee H.; Hong S.-S.; Hong S. Design and synthesis of imidazopyridine analogues as inhibitors of phosphoinositide 3-kinase signaling and angiogenesis. J. Med. Chem. 2011, 54, 2455–2466. 10.1021/jm101582z. [DOI] [PubMed] [Google Scholar]; e Tobo A.; Tobo M.; Nakakura T.; Ebara M.; Tomura H.; Mogi C.; Im D.-S.; Murata N.; Kuwabara A.; Ito S.; Fukuda H.; Arisawa M.; Shuto S.; Nakaya M.; Kurose H.; Sato K.; Okajima F. Characterization of imidazopyridine compounds as negative allosteric modulators of proton-sensing GPR4 in extracellular acidification-induced responses. PLoS One 2015, 10, e0129334 10.1371/journal.pone.0129334. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Kaminski J. J.; Bristol J. A.; Puchalski C.; Lovey R. G.; Elliott A. J.; Guzik H.; Solomon D. M.; Conn D. J.; Domalski M. S. Antiulcer agents. 1. Gastric antisecretory and cytoprotective properties of substituted imidazo[1,2-a]pyridines. J. Med. Chem. 1985, 28, 876–892. 10.1021/jm00145a006. [DOI] [PubMed] [Google Scholar]
- a Genin M. J.; Gonzalez Valcarcel I. C.; Holloway W. G.; Lamar J.; Mosior M.; Hawkins E.; Estridge T.; Weidner J.; Seng T.; Yurek D.; Adams L. A.; Weller J.; Reynolds V. L.; Brozinick J. T. Imidazopyridine and Pyrazolopiperidine Derivatives as Novel Inhibitors of Serine Palmitoyl Transferase. J. Med. Chem. 2016, 59, 5904–5910. 10.1021/acs.jmedchem.5b01851. [DOI] [PubMed] [Google Scholar]; b Feng S.; Hong D.; Wang B.; Zheng X.; Miao K.; Wang L.; Yun H.; Gao L.; Zhao S.; Shen H. C. Discovery of Imidazopyridine Derivatives as Highly Potent Respiratory Syncytial Virus Fusion Inhibitors. ACS Med. Chem. Lett. 2015, 6, 359–362. 10.1021/acsmedchemlett.5b00008. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Chen G.; Liu Z.; Zhang Y.; Shan X.; Jiang L.; Zhao Y.; He W.; Feng Z.; Yang S.; Liang G. Synthesis and Anti-inflammatory Evaluation of Novel Benzimidazole and Imidazopyridine Derivatives. ACS Med. Chem. Lett. 2013, 4, 69–74. 10.1021/ml300282t. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lee H.; Kim S. J.; Jung K. H.; Son M. K.; Yan H. H.; Hong S.; Hong S.-S. A novel imidazopyridine PI3K inhibitor with anticancer activity in non-small cell lung cancer cells. Oncol. Rep. 2013, 30, 863–869. 10.3892/or.2013.2499. [DOI] [PubMed] [Google Scholar]; e Shao N.; Pang G.-X.; Yan C.-X.; Shi G.-F.; Cheng Y. Reaction of β-Lactam Carbenes with 2-Pyridyl Isonitriles: A One-Pot Synthesis of 2-Carbonyl-3-(pyridylamino)imidazo[1,2-a]pyridines Useful as Fluorescent Probes for Mercury Ion. J. Org. Chem. 2011, 76, 7458–7465. 10.1021/jo201273p. [DOI] [PubMed] [Google Scholar]; f Stasyuk A. J.; Banasiewicz M.; Cyrański M. K.; Gryko D. T. Imidazo[1,2-a]pyridines Susceptible to Excited State Intramolecular Proton Transfer: One-Pot Synthesis via an Ortoleva-King Reaction. J. Org. Chem. 2012, 77, 5552–5558. 10.1021/jo300643w. [DOI] [PubMed] [Google Scholar]; g Mutai T.; Tomoda H.; Ohkawa T.; Yabe Y.; Araki K. Switching of Polymorph-Dependent ESIPT Luminescence of an Imidazo[1,2-a]pyridine Derivative. Angew. Chem. 2008, 120, 9664–9666. 10.1002/ange.200803975. [DOI] [PubMed] [Google Scholar]
- Nair D. K.; Mobin S. M.; Namboothiri I. N. N. Synthesis of Imidazopyridines from the Morita-Baylis-Hillman Acetates of Nitroalkenes and Convenient Access to Alpidem and Zolpidem. Org. Lett. 2012, 14, 4580–4583. 10.1021/ol3020418. [DOI] [PubMed] [Google Scholar]
- Bagdi A. K.; Santra S.; Monir K.; Hajra A. Synthesis of imidazo[1,2-a]pyridines: a decade update. Chem. Commun. 2015, 51, 1555–1575. 10.1039/c4cc08495k. [DOI] [PubMed] [Google Scholar]
- Sahani R. L.; Liu R.-S. Development of Gold-catalyzed [4+1] and [2+2+1]/[4+2] Annulations between Propiolate Derivatives and Isoxazoles. Angew. Chem. 2017, 129, 1046–1050. 10.1002/ange.201610665. [DOI] [PubMed] [Google Scholar]
- a Kakimoto S.; Nagakura Y.; Tamura S.; Watabiki T.; Shibasaki K.; Tanaka S.; Mori M.; Sasamata M.; Okada M. Minodronic acid, a third-generation bisphosphonate, antagonizes purinergic P2X2/3 receptor function and exerts an analgesic effect in pain models. Eur. J. Pharmacol. 2008, 589, 98–101. 10.1016/j.ejphar.2008.05.011. [DOI] [PubMed] [Google Scholar]; b Tanishima S.; Morio Y. A review of minodronic acid hydrate for the treatment of osteoporosis. Clin. Interventions Aging 2013, 8, 185–189. 10.2147/cia.s23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Boggs S.; Elitzin V. I.; Gudmundsson K.; Martin M. T.; Sharp M. J. Kilogram-Scale Synthesis of the CXCR4 Antagonist GSK812397. Org. Process Res. Dev. 2009, 13, 781–785. 10.1021/op9000675. [DOI] [Google Scholar]; b Jenkinson S.; Thomson M.; McCoy D.; Edelstein M.; Danehower S.; Lawrence W.; Wheelan P.; Spaltenstein A.; Gudmundsson K. Blockade of X4-tropic HIV-1 cellular entry by GSK812397, a potent noncompetitive CXCR4 receptor antagonist. Antimicrob. Agents Chemother. 2010, 54, 817–824. 10.1128/aac.01293-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak N.; Gevorgyan V. General and Efficient Copper-Catalyzed Three-Component Coupling Reaction towards Imidazoheterocycles: One-Pot Synthesis of Alpidem and Zolpidem. Angew. Chem., Int. Ed. 2010, 49, 2743–2746. 10.1002/anie.200907291. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang H.; Wang Y.; Liang D.; Liu L.; Zhang J.; Zhu Q. Copper-Catalyzed Intramolecular Dehydrogenative Aminooxygenation: Direct Access to Formyl-Substituted Aromatic N-Heterocycles. Angew. Chem. 2011, 123, 5796–5799. 10.1002/ange.201100362. [DOI] [PubMed] [Google Scholar]
- a Bariwal J.; Van der Eycken E. C-N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 2013, 42, 9283–9303. 10.1039/c3cs60228a. [DOI] [PubMed] [Google Scholar]; b Shin K.; Kim H.; Chang S. Transition-Metal-Catalyzed C-N Bond Forming Reactions Using Organic Azides as the Nitrogen Source: A Journey for the Mild and Versatile C-H Amination. Acc. Chem. Res. 2015, 48, 1040–1052. 10.1021/acs.accounts.5b00020. [DOI] [PubMed] [Google Scholar]; c Raoufmoghaddam S. Recent advances in catalytic C-N bond formation: a comparison of cascade hydroaminomethylation and reductive amination reactions with the corresponding hydroamidomethylation and reductive amidation reactions. Org. Biomol. Chem. 2014, 12, 7179–7193. 10.1039/c4ob00620h. [DOI] [PubMed] [Google Scholar]; d Lam P. Y. S.; Clark C. G.; Saubern S.; Adams J.; Winters M. P.; Chan D. M. T.; Combs A. New aryl/heteroaryl C–N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 1998, 39, 2941–2944. 10.1016/s0040-4039(98)00504-8. [DOI] [Google Scholar]; e Bhunia S.; Pawar G. G.; Kumar S. V.; Jiang Y.; Ma D. Selected Copper-Based Reactions for C–N, C–O, C–S, and C–C Bond Formation. Angew. Chem., Int. Ed. 2017, 56, 16136–16179. 10.1002/anie.201701690. [DOI] [PubMed] [Google Scholar]; f Ley S. V.; Thomas A. W. Modern Synthetic Methods for Copper-Mediated C(aryl)–O, C(aryl)–N, and C(aryl)–S Bond Formation. Angew. Chem., Int. Ed. 2003, 42, 5400–5449. 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- a Postigo A. Synthetically useful carbon-carbon and carbon-sulphur bond construction mediated by carbon- and sulphur-centred radicals in water and aqueous media. RSC Adv. 2011, 1, 14–32. 10.1039/c1ra00372k. [DOI] [Google Scholar]; b Chauhan P.; Mahajan S.; Enders D. Organocatalytic Carbon-Sulfur Bond-Forming Reactions. Chem. Rev. 2014, 114, 8807–8864. 10.1021/cr500235v. [DOI] [PubMed] [Google Scholar]; c Bastug G.; Nolan S. P. Carbon-Sulfur Bond Formation Catalyzed by [Pd(IPr*OMe)(cin)Cl] (cin = cinnamyl). J. Org. Chem. 2013, 78, 9303–9308. 10.1021/jo401492n. [DOI] [PubMed] [Google Scholar]
- a Kuwabe S.-i.; Torraca K. E.; Buchwald S. L. Palladium-Catalyzed Intramolecular C–O Bond Formation. J. Am. Chem. Soc. 2001, 123, 12202–12206. 10.1021/ja012046d. [DOI] [PubMed] [Google Scholar]; b Muci A. R.; Buchwald S. L.. Practical palladium catalysts for CN and CO bond formation. In Cross-Coupling Reactions; Springer, 2002; p 131. [Google Scholar]; c Canty A. J.; Denney M. C.; Skelton B. W.; White A. H. Carbon–Oxygen Bond Formation at Organopalladium Centers: The Reactions of PdMeR(L2) (R = Me, 4-tolyl; L2= tmeda, bpy) with Diaroyl Peroxides and the Involvement of Organopalladium(IV) Species. Organometallics 2004, 23, 1122–1131. 10.1021/om030644q. [DOI] [Google Scholar]; d Rosen B. M.; Quasdorf K. W.; Wilson D. A.; Zhang N.; Resmerita A.-M.; Garg N. K.; Percec V. Nickel-catalyzed cross-couplings involving carbon– oxygen bonds. Chem. Rev. 2010, 111, 1346–1416. 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Mondal S.; Samanta S.; Singsardar M.; Hajra A. Aminomethylation of Imidazoheterocycles with Morpholine. Org. Lett. 2017, 19, 3751–3754. 10.1021/acs.orglett.7b01594. [DOI] [PubMed] [Google Scholar]; b Kaswan P.; Porter A.; Pericherla K.; Simone M.; Peters S.; Kumar A.; DeBoef B. Oxidative Cross-Coupling of sp3- and sp2-Hybridized C-H Bonds: Vanadium-Catalyzed Aminomethylation of Imidazo[1,2-a]pyridines. Org. Lett. 2015, 17, 5208–5211. 10.1021/acs.orglett.5b02539. [DOI] [PubMed] [Google Scholar]
- a Gaunt M. J.; Yu J.; Spencer J. B. Rational Design of Benzyl-Type Protecting Groups Allows Sequential Deprotection of Hydroxyl Groups by Catalytic Hydrogenolysis. J. Org. Chem. 1998, 63, 4172–4173. 10.1021/jo980823v. [DOI] [Google Scholar]; b Sartori G.; Ballini R.; Bigi F.; Bosica G.; Maggi R.; Righi P. Protection (and Deprotection) of Functional Groups in Organic Synthesis by Heterogeneous Catalysis. Chem. Rev. 2004, 104, 199–250. 10.1021/cr0200769. [DOI] [PubMed] [Google Scholar]; c Jarowicki K.; Kocienski P. Protecting groups. J. Chem. Soc., Perkin Trans. 1 2001, 18, 2109–2135. 10.1039/b103282h. [DOI] [Google Scholar]
- a Ryley J. F. The mode of action of proguanil and related antimalarial drugs. Br. J. Pharmacol. 1953, 8, 424–430. 10.1111/j.1476-5381.1953.tb01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b McOmie J. F. W.; Watts M. L.; West D. E. Demethylation of aryl methyl ethers by boron tribromide. Tetrahedron 1968, 24, 2289–2292. 10.1016/0040-4020(68)88130-x. [DOI] [Google Scholar]; c Hou Z.-W.; Mao Z.-Y.; Melcamu Y. Y.; Lu X.; Xu H.-C. Electrochemical Synthesis of Imidazo-Fused N-Heteroaromatic Compounds through a C–N Bond-Forming Radical Cascade. Angew. Chem. 2018, 130, 1652–1655. 10.1002/ange.201711876. [DOI] [PubMed] [Google Scholar]; d Warner A. J.; Churn A.; McGough J. S.; Ingleson M. J. BCl3 -Induced Annulative Oxo- and Thioboration for the Formation of C3-Borylated Benzofurans and Benzothiophenes. Angew. Chem., Int. Ed. 2017, 56, 354–358. 10.1002/anie.201610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dheer D.; Rawal R. K.; Singh V.; Sangwan P. L.; Das P.; Shankar R. β-CD/CuI catalyzed regioselective synthesis of iodo substituted 1,2,3-triazoles, imidazo[1,2- a ]-pyridines and benzoimidazo[2,1- b ]thiazoles in water and their functionalization. Tetrahedron 2017, 73, 4295–4306. 10.1016/j.tet.2017.05.081. [DOI] [Google Scholar]; b Dheer D.; Reddy K. R.; Rath S. K.; Sangwan P. L.; Das P.; Shankar R. Cu(I)-catalyzed double C-H amination: synthesis of 2-iodo-imidazo[1,2-a]pyridines. RSC Adv. 2016, 6, 38033–38036. 10.1039/c6ra02953a. [DOI] [Google Scholar]; c Nitha P. R.; Joseph M. M.; Gopalan G.; Maiti K. K.; Radhakrishnan K. V.; Das P. Chloroform as a carbon monoxide source in palladium-catalyzed synthesis of 2-amidoimidazo[1,2-a]pyridines. Org. Biomol. Chem. 2018, 16, 6430–6437. 10.1039/c8ob01486h. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Tracy J. S. Organic Synthesis. Use of Alkynes as a Key to Innovation in Designing Structure for Function. Isr. J. Chem. 2018, 58, 18–27. 10.1002/ijch.201700077. [DOI] [Google Scholar]
- Dheer D.; Singh V.; Shankar R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. 10.1016/j.bioorg.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Fuji K.; Ichikawa K.; Node M.; Fujita E. Hard acid and soft nucleophile system. New efficient method for removal of benzyl protecting group. J. Org. Chem. 1979, 44, 1661–1664. 10.1021/jo01324a017. [DOI] [Google Scholar]; b Akiyama T.; Shima H.; Ozaki S. Trimethylsilyl Chloride-Tin(II) Chloride-Anisole: A Novel Selectivep-Methoxybenzyl Ether Cleavage Reagent. Synlett 1992, 415–416. 10.1055/s-1992-21364. [DOI] [Google Scholar]; c Bouzide A.; Sauvé G. Lewis acid-catalyzed deprotection of p-methoxybenzyl ether. Synlett 1997, 1153–1154. 10.1055/s-1997-990. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.