Abstract
The synthesis of 2,3-disubstituted benzo[b]thiophenes with selective placement of a chlorine moiety at the 3 position while maintaining diversity at the 2 position has only been accomplished by a handful of conditions in the past. The development of a greener, less expensive, and simpler method is paramount for the exploration of innovative compounds for application in medicinal and materials chemistry. Herein, the first reported copper-catalyzed electrophilic chlorocyclization method was developed and employed across diverse substrates to generate highly functionalized 2,3-disubstituted benzo[b]thiophenes and 2,3,5-trisubstituted thiophenes in very high yields. This method was optimized in both ethanol and acetonitrile in a comparative solvent study. The utility of this method was further expanded beyond chlorocyclization by changing the sodium halide to generate bromo- and iodocyclization products in excellent yields.
Introduction
The significant inclusion of chlorine moieties in heterocyclic compounds in the development of drug molecules since 1984 has seen more than 60 approved drugs including lornoxicam, alprazolam, loracarbef, cefaclor, and chlorphenamine.1 Chlorination of drug molecules is an established method to optimize molecular properties in absorption, distribution, metabolism, excretion, and toxicity; structure; and half-life.2 The intrinsic properties of halogens provide diverse reactivity, but regioselective synthesis of heterocyclic molecules containing halogens has proven to be challenging. Common methodologies for synthesizing halogenated heterocycles include electrophilic aromatic substitution with toxic and corrosive reagents,3 conversion of amine to halogen via explosive diazonium salts,4 and directed metalation using pyrophoric lithium and magnesium reagents.5 The development of a green, regioselective, and effective methodology for the synthesis of chlorinated heterocycles is an urgent need.
Heterocyclic core structures such as benzo[b]thiophene and thiophenes functionalized at the 2 and 3 positions have demonstrated diverse medicinal applications. Compounds containing benzo[b]thiophene derivatives are known for their use as antimicrobial,6 anticancer,7 antiviral,8 antidepressant,9 antifungal,10 anti-inflammatory,11 analgesic,12 and estrogen receptor-mediating agents.13 The introduction of chlorine at the 3 position enhances the anticancer and antiviral properties of compounds.7,8 Similar molecules with a bromine at the 3 position showed improved analgesic and anti-inflammatory activity.14
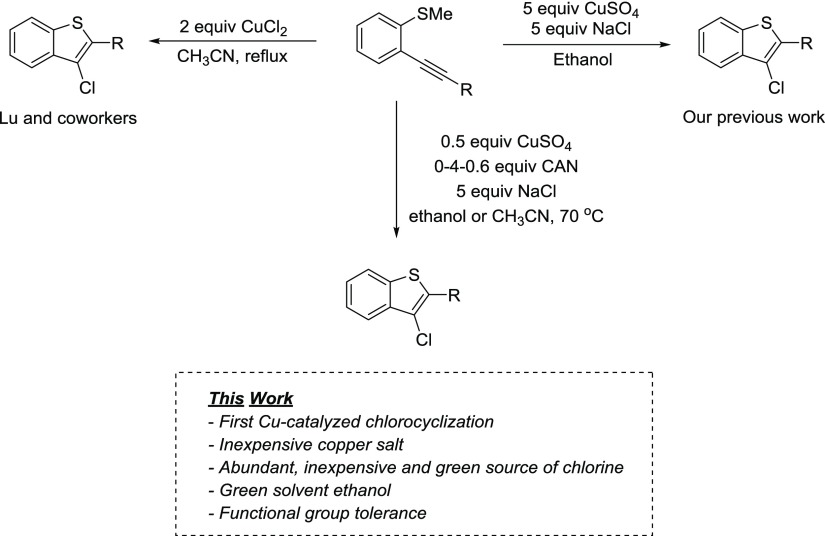
Until recently, generating 3-chlorosubstituted benzo[b]thiophenes while maintaining diverse functionality at the 2 position has proven difficult. Wu provided a methodology that generated good yields of chloro-/bromocyclized derivatives that required 2 equiv of CuCl2 or CuCl2/PdCl2.15 Palladium is a remarkable metal with innumerable applications, but the use of a large amount of expensive Pd and an excess of copper incurs a financial challenge. Wang used N-chlorosuccinimide to generate moderate yields of desired 3-chlorobenzo[b]thiophenes but required the use of an electron-rich ynamide starting substrate.16 Lu’s work was the first to report chlorocyclization with high yields but required 2 equiv of CuCl2.17 We recently reported a potent method using relatively inexpensive CuSO4 along with NaCl for generating 2,3-disubstituted benzo[b]thiophene derivatives with the desired chlorine moiety at the 3 position.18 The yields were higher and reaction conditions were milder compared to previous methodologies, but an excess of CuSO4 was required. The need for the development of a reduced amount of transition metal has prompted our research on the development of simple and high-yielding copper-catalyzed electrophilic chlorocyclization reactions for the synthesis of benzo[b]thiophenes and thiophenes. The development of a copper-catalyzed chlorocyclization reaction would be a boon in the advancement of synthesizing chlorinated heterocyclic compounds.
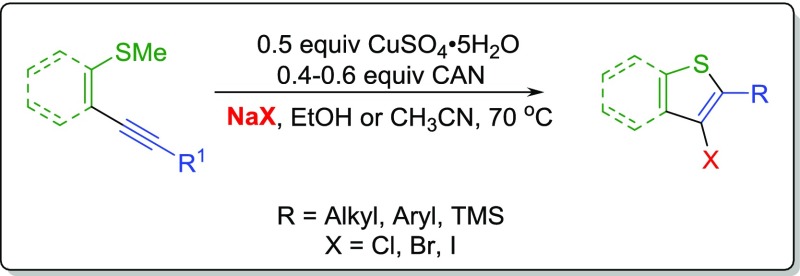
In this work, we report the first copper-catalyzed electrophilic chlorocyclization reaction using an external oxidant (Scheme 1). The introduction of an oxidant to generate a catalytic copper cycle in Wacker oxidation has been explored thoroughly by Stahl and co-workers.19 Herein, we explored a similar copper catalytic cycle to reduce the amount of CuSO4 required by employing an external oxidant. Interestingly, this has never been explored in electrophilic chlorocyclization reactions.
Scheme 1.
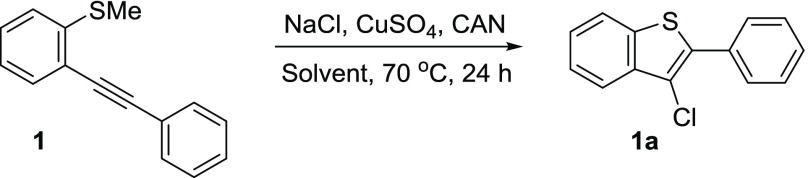
Thioanisole-containing compounds are known to generate sulfoxides or sulfones in the presence of an oxidant,20 and therefore, our first concern was to find an oxidant that would not form oxidation byproducts while converting copper(I) to copper(II). As our starting point, 1 equiv of starting alkynyl thioanisole 1, 0.5 equiv of CuSO4, 5 equiv of NaCl, and 3 mL of acetonitrile were allowed to react for an appropriate amount of time at 70 °C using a chosen oxidant along with co-oxidant oxygen (Table 1). A series of oxidants such as para-quinone, (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO), hydrogen peroxide, and sodium hypochlorite were investigated, which resulted in dismal yields (entries 2–5). Selectfluor generated moderate yields of the product, but it simultaneously produced byproducts, evident from thin-layer chromatography (TLC) of the reaction mixture, that were not isolated (entry 6). The cyclization attempt with 1 equiv of cerium ammonium nitrate (CAN) along with co-oxidant O2 resulted in no product (entry 7). However, addition of CAN 30 min after the start of the reaction resulted in a 90% yield of the desired product (entry 8). Further optimization was performed to lower the amount of CAN. We found that the sequential addition of a total of 0.4 equiv of CAN over the period of 6 h generated a 99% product in 24 h (entry 9). Our attempts to lower the amount of copper below 0.5 equiv failed, as reducing CuSO4 resulted in the lower yields of the product (entries 10 and 11). It should be noted that the reaction was also attempted at room temperature, but no desired product was obtained. A series of copper catalysts were selected to determine which catalyst worked the best under our reaction conditions. Copper (II) salts such as CuCl2 and copper(II) acetate generated the desired cyclized product 1a in 97 and 93% yields, respectively (entries 13 and 14). The copper(I) salt CuCl resulted in the formation of 1a in a 92% yield, suggesting that CAN was used for performing the desired oxidation of Cu(I) to Cu(II) in the catalytic cycle (entry 15). This inference was further supported when it was found that employing Cu(0) for the chlorocyclization reaction generated 1a in a 57% yield (entry 16). CuSO4 was selected as the copper salt as it is inexpensive, and the SO42– anion is a weakly coordinating anion in the reaction allowing for a diversity of halogens with the same copper salt by altering the sodium halide. In search for a better solvent system, we explored solvents such as methanol, tert-butanol, water, nitromethane, ethyl acetate, and toluene (entries 17–22). It was found that ethanol, methanol, and ethyl acetate gave promising yields, whereas tert-butanol, water, nitromethane, and toluene resulted in undesirably poor yields. Because of its environmentally benign properties, ethanol was selected as the solvent of choice for further optimization. Modifications were made to generate higher yields of the product by increasing the amount of CAN to 0.6 equiv, shortening the sequential addition time to 3 h, and allowing the reaction to run for 48 h (entries 23–25).
Table 1. Optimization of the Copper-Catalyzed Chlorocyclization Reactiona.
| entry | catalyst (equiv) | oxidant (equiv) | solvent | % yield |
|---|---|---|---|---|
| 1 | CuSO4 (0.5) | CH3CN | 28 | |
| 2 | CuSO4 (0.5) | p-quinone (1.0) | CH3CN | 26 |
| 3 | CuSO4 (0.5) | TEMPO (1.0) | CH3CN | 25 |
| 4 | CuSO4 (0.5) | H2O2 (1.0) | CH3CN | 36 |
| 5 | CuSO4 (0.5) | NaClO (1.0) | CH3CN | 12 |
| 6 | CuSO4 (0.5) | selectfluor (1.0) | CH3CN | 59 |
| 7 | CuSO4 (0.5) | CAN (1.0) | CH3CN | trace |
| 8 | CuSO4 (0.5) | CAN (0.4) | CH3CN | 90b |
| 9 | CuSO4(0.5) | CAN (0.4) | CH3CN | 99c |
| 10 | CuSO4 (0.4) | CAN (0.4) | CH3CN | 91c |
| 11 | CuSO4 (0.3) | CAN (0.4) | CH3CN | 88c |
| 12 | CAN (0.4) | CH3CN | trace | |
| 13 | CuCl2 (0.5) | CAN (0.4) | CH3CN | 97c |
| 14 | Cu(OAc)2 (0.5) | CAN (0.4) | CH3CN | 93c |
| 15 | CuCl (0.5) | CAN (0.4) | CH3CN | 92c |
| 16 | Cu(0) (0.5) | CAN (0.4) | CH3CN | 57c |
| 17 | CuSO4 (0.5) | CAN (0.4) | methanol | 71c |
| 18 | CuSO4 (0.5) | CAN (0.4) | t-butanol | 39c |
| 19 | CuSO4 (0.5) | CAN (0.4) | water | 9c |
| 20 | CuSO4 (0.5) | CAN (0.4) | CH3NO2 | 30c |
| 21 | CuSO4 (0.5) | CAN (0.4) | EtOAc | 68c |
| 22 | CuSO4 (0.5) | CAN (0.4) | toluene | 21c |
| 23 | CuSO4 (0.5) | CAN (0.4) | EtOH | 72d |
| 24 | CuSO4 (0.5) | CAN (0.5) | EtOH | 87d |
| 25 | CuSO4(0.5) | CAN (0.6) | EtOH | 98d |
All reactions were performed using 0.30 mmol of the alkyne, 5 equiv of NaCl, and 5 mL of solvent in the presence of O2 balloon at 70 °C for 24 h.
CAN was added 30 min after the reaction was started.
CAN was added in increments of 0.1 equiv every 2 h.
CAN was added in increments of 0.1 equiv every 30 min, and the reaction was carried out for 48 h.
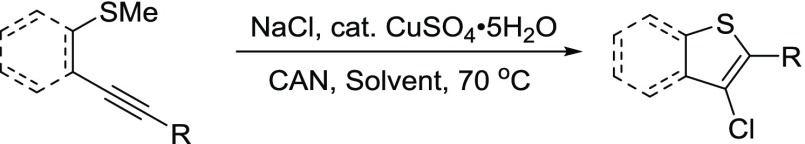
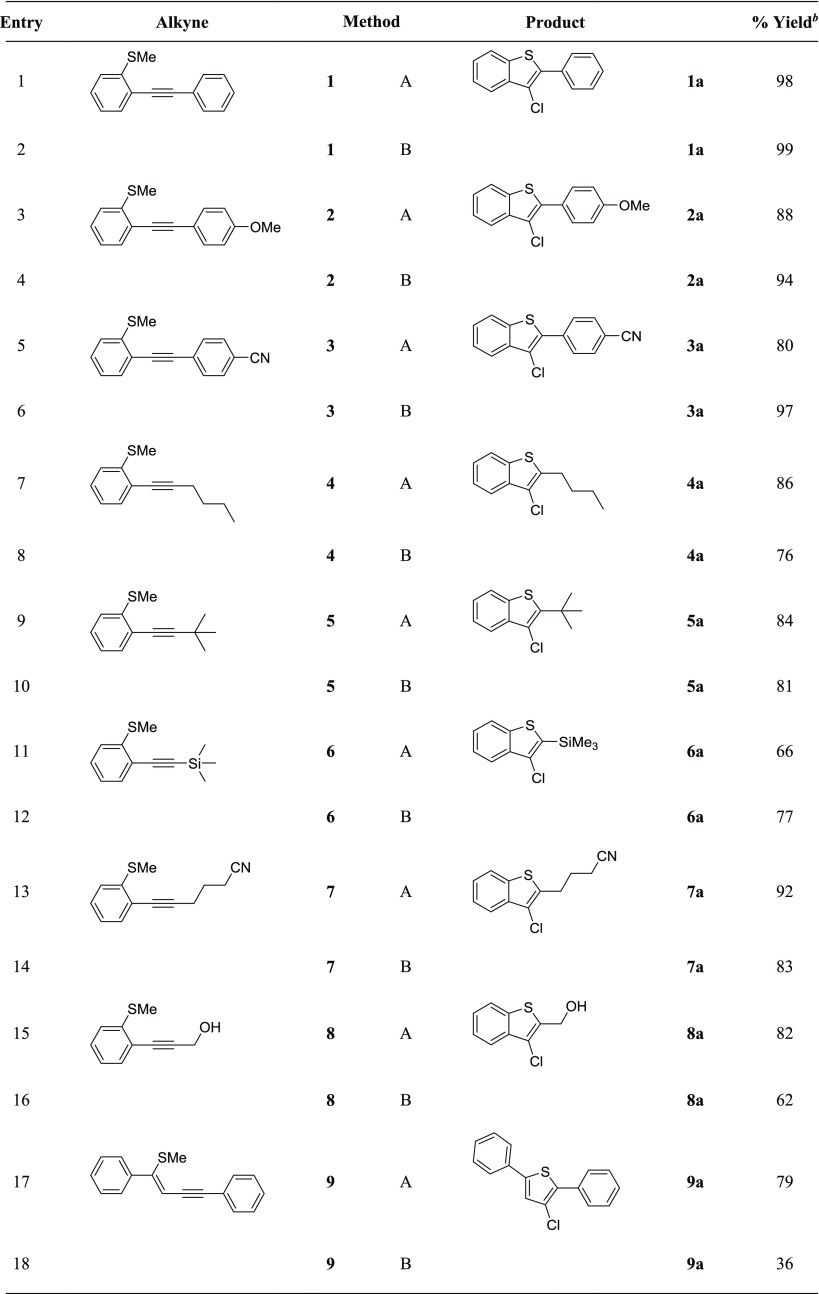
Once the optimal conditions were established, each reaction was performed using 0.3 mmol of starting 2-alkynylthioanisole, 1.5 mmol of the appropriate sodium halide, 0.15 mmol of CuSO4, and 5 mL of solvent in the presence of O2. The amount of CAN employed was a total of 0.12 and 0.18 mmol in the acetonitrile and ethanol reactions, respectively. Various substrates were tested to provide an overview of our reaction’s viability. Each substrate was tested in acetonitrile and ethanol for a direct comparison. First, we investigated the chlorocyclization reaction using a variety of thioanisole substrates 1–9 (Table 2). As mentioned earlier, the phenyl substrate 1 resulted in the formation of 3-chlorobenzo[b]thiophene 1a in a near-perfect yield of 98 and 99% in ethanol and acetonitrile, respectively. The electronically rich p-methoxyphenyl substrate proceeded highly favorably to generate 2a with comparable yields of 88 and 94%. When electron-deficient p-cyanophenyl-substituted alkynyl thioanisole 3 was subjected to our cyclization condition, the resulting cyclized product 3a was obtained in a high yield of 97% in acetonitrile and 80% in ethanol. The observed yields with electron-rich methoxy and electron-poor nitrile groups suggest that changing the electronics of the remote phenyl group does not have a significant effect on the reaction yield.
Table 2. Copper-Catalyzed Electrophilic Chlorocyclization Substrate Studya.
Method A. All reactions were performed using 0.30 mmol of the alkyne, 5 equiv of NaCl, 0.5 equiv of CuSO4, 0.6 equiv of CAN, and 5 mL of ethanol with an O2 balloon at 70 °C for 48 h. Method B. All reactions were performed using 0.30 mmol of the alkyne, 5 equiv of NaCl, 0.5 equiv of CuSO4, 0.4 equiv of CAN, and 5 mL of CH3CN with an O2 balloon at 70 °C for 24 h.
Isolated yield.
A linear alkyl chain substrate 4 resulted in 4a with an excellent yield of 86% when ethanol was employed as the solvent and 76% yield with acetonitrile using method B. The cyclization reaction was completed with ease with a sterically large tert-butyl group and resulted in comparable yields of 84 and 81% in both solvents. Ethanol was found to be superior with most substrates with the exception of trimethylsilyl (TMS) and p-methoxyphenyl alkynes. The TMS-substituted alkyne, when subjected to our reaction conditions, generated a side product resulting from the addition of Cl2 across the alkyne along with 6a as observed from gas chromatography–mass spectrometry (GC–MS). In acetonitrile, a higher yield of 6a was obtained, and less alkyne addition product was observed. Alcohol and nitrile groups were well tolerated as the reaction proceeded with success and resulted in the formation of desired cyclized products 7a and 8a in excellent yields of 92 and 82%, respectively, in ethanol and slightly lower yields of 83 and 62%, respectively, in acetonitrile. There was a dramatic difference of yield in generating the thiophene product. The reaction was carried out smoothly with substrates that contributed electronically. In summary, the chlorocyclization yields seemed to be better across the board when ethanol was employed as the solvent except with the TMS and p-methoxyphenyl groups.
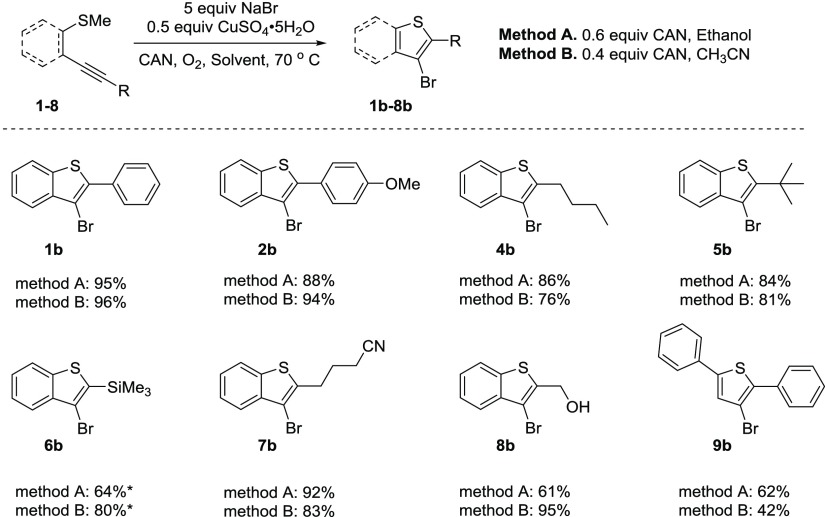
After these encouraging results from chlorocyclization, we employed bromocyclization reaction conditions with NaBr instead of NaCl while keeping the other conditions the same. The bromocyclization reaction resulted in higher or comparable yields of the desired bromocyclized product when compared with the existing literature procedures. A similar trend of higher yields in ethanol versus acetonitrile was observed with most of the substrates except alkynes substituted with TMS, p-methoxyphenyl, and hydroxymethyl. Cyclization of TMS substrate 6 in MeCN resulted in the desired benzo[b]thiophene 6b in an 80% yield, whereas in ethanol the yield was only 64%. In both reactions, a side product was obtained where a bromine molecule was added to the alkyne instead of performing the desired cyclization reaction, as determined by NMR (Scheme 2).
Scheme 2.
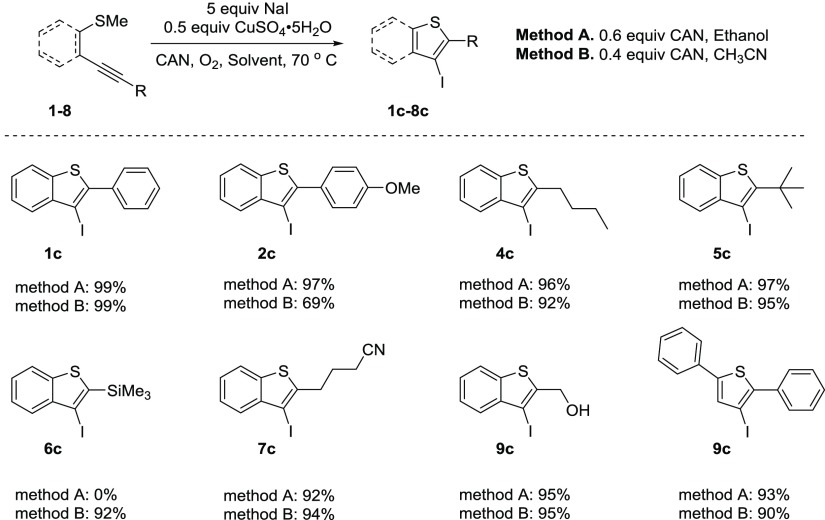
Additionally, sodium iodide was employed as the source of electrophile in our cyclization reaction to generate the desired 3-iodinated benzo[b]thiophene (Scheme 3). The sodium iodide trials generated high yields of the cyclized 3-iodobenzo[b]thiophenes. Once again, the iodocyclization reaction resulted in higher or comparable yields of the desired 3-iodobenzo[b]thiophenes compared with current literature procedures. The only exception occurred with TMS and electron-rich p-methoxyphenyl-substituted alkynes. The p-methoxyphenylalkynyl group resulted in significantly lower yields in acetonitrile compared to ethanol, whereas the TMS-substituted alkyne failed to generate any iodocyclized product in ethanol but generated a clean 92% yield in acetonitrile.
Scheme 3.
It is well established that CuI2 is unstable and decomposes to CuI and I2; therefore, the mechanism for iodocyclization seems to involve molecular I2 as the electrophile, whose formation was observed in the reaction mixture. Bromocyclization was investigated further to determine if Br2 or CuBr2 was the electrophile. Anisole 10 when reacted with NaBr, CuSO4, and CAN in ethanol at 72 °C for 6 h generated 70% 4-bromoanisole 11, suggesting electrophilic aromatic substitution involving Br2 (Scheme 4). Therefore, we concluded that in situ generated Br2 and I2 is the driving mechanism for the bromo-/iodocyclization reaction.
Scheme 4.
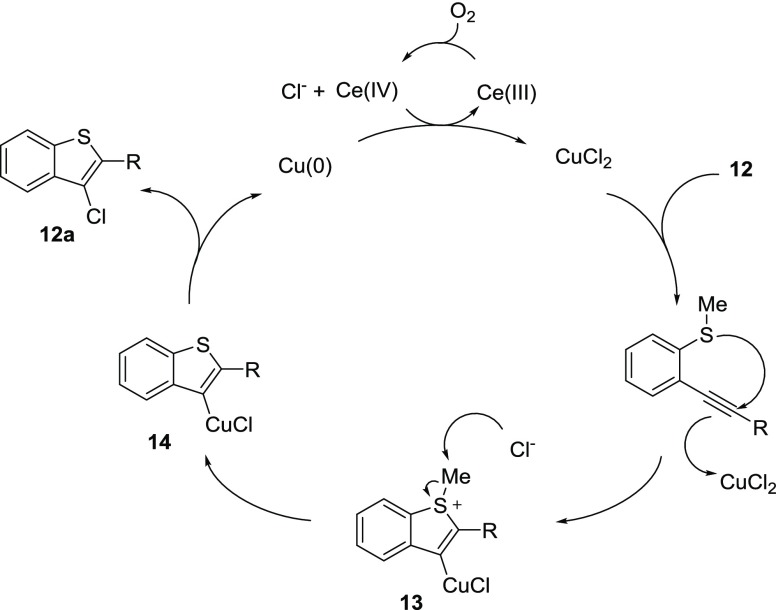
A possible mechanism of the chlorocyclization reaction is outlined in Scheme 5. Chlorocyclization occurs by the generation of CuCl2 in situ from NaCl and CuSO4. CuCl2 is very stable, and unlike CuI2 or CuBr2, it does not disproportionate. We believe that CuCl2 coordinates across the alkyne 12 providing an ideal situation for antiattack from a nearby sulfur nucleophile. The resulting cationic intermediate 13 undergoes an SN2-like displacement by a chloride anion to generate methyl chloride. Finally, copper undergoes reductive elimination to afford the chlorocyclized product 12a and copper(0). Copper(0) is readily oxidized back to Cu(II) by the Ce(IV) species present in CAN. The resulting Ce(III) is oxidized back to cerium(IV) by O2 for only a few cycles before becoming poisoned.
Scheme 5.
Conclusions
We have successfully developed the first copper-catalyzed halocyclization method with a diverse library of substrates in high yields. This method allows for considerable advancement in synthetic and medicinal chemistry by expanding the limited existing methodology to generate chloro-substituted benzo[b]thiophene derivatives. The ability to alter the conditions to achieve bromo and iodo electrophilic cyclization enhances the diversity of the method. Employment of cheap, readily available, and greener reagents while developing the first copper-catalyzed electrophilic cyclization method achieved the desired reaction conditions and outcomes needed for innovative chemistry.
General Experimental Methods
Chemicals and solvents were either purchased and employed directly from commercial suppliers. 1H NMR spectra were recorded on a Bruker 400 MHz NMR spectrometer. 13C NMR spectra were recorded on a Bruker 100 MHz NMR spectrometer. GC–MS spectra were obtained on a GCMS-QP2010 SE from an electron ionization (EI) source. A VG-70S magnetic sector mass spectrometer was employed to record the high-resolution mass spectra. Samples were introduced using a direct probe and ionized via EI. TLC was employed to monitor reactions with silica gel 60 F254 plates. Compounds were isolated via column chromatography using silica gel 60–120 mesh. Well-established literature procedures were employed to prepare alkynes 1–9.18
General Procedure for the Synthesis of Compounds 1a–8a, 1b–8b, and 1c–8c
Method A
To a vial containing 2-alkynyl thioanisole (0.3 mmol) in 3 mL of EtOH were added desired sodium halide (1.5 mmol) and CuSO4·5H2O (0.15 mmol) with an O2 balloon. The reaction mixture was allowed to stir for 30 min at 70 °C. Next, CAN (0.03 mmol) was added to the reaction mixture every 30 min until a total of 0.18 mmol had been added. The reaction was allowed to stir for 48 h. The reaction mixture was then filtered and absorbed in silica gel before purification via column chromatography using hexanes and ethyl acetate as the eluent.
Method B
To a vial containing 2-alkynyl thioanisole (0.3 mmol) in 3 mL of CH3CN were added desired sodium halide (1.5 mmol) and CuSO4·5H2O (0.15 mmol) with an O2 balloon. The reaction mixture was allowed to stir for 30 min at 70 °C. Next, CAN (0.03 mmol) was added to the reaction mixture every 2 h until a total of 0.12 mmol had been added. The reaction was allowed to stir overnight. The reaction mixture was then filtered and absorbed in silica gel before purification via column chromatography using hexanes and ethyl acetate as the eluent.
3-Chloro-2-phenylbenzo[b]thiophene (1a)
Product was isolated as a white solid, mp 64–67 °C; 1H NMR (400 MHz, chloroform-d): δ 7.39–7.51 (m, 5H), 7.79–7.87 (m, 3H), 7.88 (d, J = 7.2 Hz, 1H). Other characterization data are in good agreement with the previously reported data.17
3-Bromo-2-phenylbenzo[b]thiophene (1b)
Product was isolated as a white solid, mp 62–64 °C; 1H NMR (400 MHz, chloroform-d): δ 7.39–7.51 (m, 5H), 7.76–7.78 (m, 2H), 7.81 (d, J = 7.6 Hz, 1H), 7.89 (d, J = 8.4 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
3-Iodo-2-phenylbenzo[b]thiophene (1c)
Product was isolated as a yellow oil; 1H NMR (400 MHz, chloroform-d): δ 7.38–7.42 (m, 1H), 7.45–7.49 (m, 4H), 7.68–7.70 (m, 2H), 7.79–7.85 (m, 2H). Other characterization data are in good agreement with the previously reported data.18
4-(3-Chloro-1-benzothiophen-2-yl)phenyl Methyl Ether (2a)
Product was isolated as an off-white solid, mp 100–101 °C; 1H NMR (400 MHz, chloroform-d): δ 3.87 (s, 3H), 7.01 (d, J = 8.4 Hz, 2H), 7.38 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.73 (d, J = 8.8 Hz, 2H), 7.78 (d, J = 7.6 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
4-(3-Bromo-1-benzothiophen-2-yl)phenyl Methyl Ether (2b)
Product was isolated as an off-white solid, mp 86–88 °C; 1H NMR (400 MHz, chloroform-d): δ 3.87 (s, 3H), 7.01 (d, J = 8.4 Hz, 2H), 7.38 (t, J = 8.0 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.71 (d, J = 8.8 Hz, 2H), 7.79 (d, J = 8.0 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H). Other characterization data are in good agreement with the previously reported data.17
4-(3-Iodo-1-benzothiophen-2-yl)phenyl Methyl Ether (2c)
The product was obtained as a white solid, mp 84–86 °C; 1H NMR (400 MHz, chloroform-d): δ 3.80 (s, 3H), 6.95 (d, J = 8.6 Hz, 2H), 7.32 (t, J = 7.8 Hz, 1H), 7.41 (t, J = 7.8 Hz, 1H), 7.59 (d, J = 8.7 Hz, 2H), 7.71 (d, J = 7.8 Hz, 1H), 7.77 (d, J = 8.0 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
4-(3-Chlorobenzo[b]thiophen-2-yl)benzonitrile (3a)
Product was isolated as a white solid; 1H NMR (400 MHz, chloroform-d): δ 7.46 (td, J = 7.5, 1 Hz, 1H), 7.51 (td, J = 7.12, 1.00 Hz, 1H), 7.77 (d, J = 8.5 Hz, 2H), 7.77 (d, J = 8.5 Hz, 2H), 7.82 (d, J = 7.6 Hz, 1H), 7.77 (d, J = 8.5 Hz, 2H), 7.91 (t, J = 6.4 Hz, 3H). 13C NMR (400 MHz, chloroform-d): δ 112.10, 118.52, 118.60, 122.43, 122.71, 125.51, 126.37, 129.74, 132.42, 133.82, 137.00, 137.62 Hz. Other characterization data are in good agreement with the previously reported data.17
2-n-Butyl-3-chlorobenzo[b]thiophene (4a)
Product was isolated as a colorless oil; 1H NMR (400 MHz, chloroform-d): δ 0.97 (t, J = 7.2 Hz, 3H), 1.40–1.50 (m, 2H), 1.69–1.76 (m, 2H), 2.96 (t, J = 7.6 Hz, 2H), 7.34 (td, J = 7.6, 1.6 Hz, 1H), 7.41 (td, J = 7.6, 1.2 Hz, 1H), 7.74 (m, 2H). Other characterization data are in good agreement with the previously reported data.18
2-n-Butyl-3-bromobenzo[b]thiophene (4b)
Product was isolated as a pale yellow oil; 1H NMR (400 MHz, chloroform-d): δ 0.98 (t, J = 7.6 Hz, 3H), 1.41–1.50 (m, 2H), 1.69–1.77 (m, 2H), 2.96 (t, J = 7.6 Hz, 2H), 7.31–7.34 (m, 1H), 7.40–7.44 (m, 1H), 7.73–7.76 (m, 2H). Other characterization data are in good agreement with the previously reported data.18
2-n-Butyl-3-iodobenzo[b]thiophene (4c)
Product was isolated as a yellow oil; 1H NMR (400 MHz, chloroform-d): δ 0.98 (t, J = 7.2 Hz, 1H), 1.42–1.51 (m, 2H), 1.70–1.78 (m, 1H), 2.97 (t, J = 7.6 2H), 7.32 (t, J = 7.2 Hz, 1H), 7.40 (t, J = 7.2 Hz, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
2-tert-Butyl-3-chlorobenzo[b]thiophene (5a)
Product was isolated as a yellow oil; 1H NMR (400 MHz, chloroform-d): δ 1.57 (s, 9H), 7.34 (td, J = 8.0, 1.2 Hz, 1H), 7.41 (td, J = 6.8, 1.2 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 8.0 Hz, 1H). Other characterization data are in good agreement with the previously reported data.17
3-Bromo-2-tert-butylbenzo[b]thiophene (5b)
Product was isolated as a yellow oil; 1H NMR (400 MHz, chloroform-d): δ 1.60 (s, 9H), 7.33 (td, J = 7.2, 1.2 Hz, 1H), 7.41 (td, J = 7.2, 1.2 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.4 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
2-tert-Butyl-3-iodobenzo[b]thiophene (5c)
Product was isolated as a yellow oil; 1H NMR (400 MHz, chloroform-d): δ 1.65 (s, 9H), 7.34 (td, J = 8.0, 0.8 Hz, 1H), 7.41 (m, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.82 (d, J = 8.0 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
3-Chloro-2-(trimethylsilyl)benzo[b]thiophene (6a)
1H NMR (400 MHz, chloroform-d): δ 0.45 (s, 9H), 7.40 (dt, J = 7.6, 1.6 Hz, 1H), 7.43 (d, J = 7.6, 1H), 7.83 (t, J = 6.8 Hz, 1H), 7.85 (t, J = 7.2 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
3-Bromo-2-(trimethylsilyl)benzo[b]thiophene (6b)
δ 0.47 (s, 9H), 7.35–7.39 (t, J = 7.6 Hz, 1H), 7.41–7.45 (t, J = 7.6 Hz, 1H), 7.82–7.84 (d, J = 7.6 Hz, 2H). Other characterization data are in good agreement with the previously reported data.21
3-Iodo-2-(trimethylsilyl)benzo[b]thiophene (6c)
Product was isolated as a pale yellow oil; 1H NMR (400 MHz, chloroform-d): δ 0.51 (s, 9H), 7.37 (td, J = 8.0, 1.2 Hz, 1H), 7.44 (td, J = 7.2, 1.2 Hz, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.83 (d, J = 8.0 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
4-(3-Chlorobenzo[b]thiophene-2-yl)butanenitrile (7a)
Product was isolated as a colorless oil; 1H NMR (400 MHz, chloroform-d): δ 2.08–2.15 (m, 2H), 2.44 (t, J = 7.2 Hz, 2H), 3.12 (t, J = 7.2 Hz, 2H), 7.38 (t, J = 7.6 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
4-(3-Bromobenzo[b]thiophene-2-yl)butanenitrile (7b)
Product was isolated as a colorless oil; 1H NMR (400 MHz, chloroform-d): δ 2.08–2.16 (m, 2H), 2.44 (t, J = 7.2 Hz, 2H), 3.13 (t, J = 7.2 Hz, 2H), 7.38 (t, J = 8.0 Hz, 1H), 7.45 (t, J = 7.2 Hz, 1H), 7.75–7.78 (m, 2H). Other characterization data are in good agreement with the previously reported data.18
4-(3-Iodobenzo[b]thiophene-2-yl)butanenitrile (7c)
Product was isolated as a yellow oil; 1H NMR (400 MHz, chloroform-d): δ 2.09–2.17 (m, 2H), 2.45 (t, J = 7.2 Hz, 2H), 3.14 (t, J = 7.2 Hz, 2H), 7.35 (td, J = 8.0, 1.2 Hz, 1H), 7.42 (td, J = 8.0, 1.2 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 7.6 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
(3-Chloro-1-benzothiophen-2-yl)methanol (8a)
Product was isolated as a white solid, mp 91–92 °C; 1H NMR (400 MHz, chloroform-d): δ 1.58 (br s, 1H), 5.00 (d, J = 4.0 Hz, 2H), 7.38–7.42 (m, 1H), 7.43–7.47 (m, 1H), 7.79–7.82 (m, J = 8.0 Hz, 2H). Other characterization data are in good agreement with the previously reported data.18
(3-Bromo-1-benzothiophen-2-yl)methanol (8b)
Product was isolated as a white solid, mp 92–94 °C; 1H NMR (400 MHz, chloroform-d): δ 2.00 (br s, 1H), 4.99 (d, J = 6.0 Hz, 2H), 7.39 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 7.2 Hz, 1H), 7.80 (t, J = 8.0 Hz, 2H). Other characterization data are in good agreement with the previously reported data.18
(3-Iodo-1-benzothiophen-2-yl)methanol (8c)
The product was obtained as a white solid, mp 97–99 °C; 1H NMR (400 MHz, chloroform-d): δ 2.05 (t, J = 6.0 Hz, 1H), 4.97 (d, J = 5.8 Hz, 2H), 7.37 (t, J = 7.8 Hz, 1H), 7.43 (t, J = 6.8 Hz, 1H), 7.73 (d, J = 8.4 Hz, 1H), 7.78 (d, J = 8.2 Hz, 1H). Other characterization data are in good agreement with the previously reported data.18
3-Chloro-2,5-diphenylthiophene (9a)
Product was isolated as a white solid, mp 55–57 °C; 1H NMR (400 MHz, CDCl3): δ 7.22 (s, 1H), 7.31–7.47 (m, 6H), 7.59 (d, J = 7.6 Hz, 2H), 7.72 (d, J = 7.6 Hz, 2H). Other characterization data are in good agreement with the previously reported data.18
3-Bromo-2,5-diphenylthiophene (9b)
Product was isolated as a yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.27 (s, 1H), 7.31–7.47 (m, 6H), 7.59 (d, J = 7.2 Hz, 2H), 7.71 (d, J = 7.2 Hz, 2H). Other characterization data are in good agreement with the previously reported data.18
3-Iodo-2,5-diphenylthiophene (9c)
Product was isolated as a yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.30–7.34 (m, 2H), 7.39–7.47 (m, 5H), 7.59 (d, J = 7.2 Hz, 2H), 7.66 (d, J = 7.2 Hz, 2H). Other characterization data are in good agreement with the previously reported data.18
Acknowledgments
We are thankful to Research Corporation for Science Advancement for a Cottrell College Science Award (ID 23248). The authors are also appreciative of generous support provided by University of West Florida (UWF), UWF’s Office of Research and Sponsored Programs and Office of Undergraduate Research. The authors are also grateful to Dr. Tim Royappa and Dr. Karen Molek for their help and support throughout the project.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00300.
Copies of 1H and 13C NMR spectra of all products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wishart D. S.; Knox C.; Guo A. C.; Shrivastava S.; Hassanali M.; Stothard P.; Chang Z.; Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandes M.; Cavalcanti S. M.; Moreira D. R.; de Azevedo Junior W.; Leite A. C.; Lima A. C. Halogen Atoms in the Modern Medicinal Chemistry: Hints for the Drug Design. Curr. Drug Targets 2010, 11, 303–314. 10.2174/138945010790711996. [DOI] [PubMed] [Google Scholar]
- a Qiu D.; Mo F.; Zheng Z.; Zhang Y.; Wang J. Gold(III)-Catalyzed Halogenation of Aromatic Boronates with N-Halosuccinimides. Org. Lett. 2010, 12, 5474–5477. 10.1021/ol102350v. [DOI] [PubMed] [Google Scholar]; b Castanet A.-S.; Colobert F.; Broutin P.-E. Mild and Regioselective Iodination of Electron-Rich Aromatics with N-Iodosuccinimide and Catalytic Trifluoroacetic Acid. Tetrahedron Lett. 2002, 43, 5047–5048. 10.1016/s0040-4039(02)01010-9. [DOI] [Google Scholar]; c Jana N. K.; Verkade J. G. Phase-Vanishing Methodology for Efficient Bromination, Alkylation, Epoxidation, and Oxidation Reactions of Organic Substrates. Org. Lett. 2003, 5, 3787–3790. 10.1021/ol035391b. [DOI] [PubMed] [Google Scholar]; d Surya Prakash G. K. S.; Mathew T.; Hoole D.; Esteves P. M.; Wang Q.; Rasul G.; Olah G. A. N-halosuccinimide/BF3·H2O, Efficient Electrophilic Halogenating Systems for Aromatics. J. Am. Chem. Soc. 2004, 126, 15770–15776. 10.1021/ja0465247. [DOI] [PubMed] [Google Scholar]; e Song S.; Sun X.; Li X.; Yuan Y.; Jiao N. Efficient and Practical Oxidative Bromination and Iodination of Arenes and Heteroarenes with DMSO and Hydrogen Halide: A Mild Protocol for Late-Stage Functionalization. Org. Lett. 2015, 17, 2886–2889. 10.1021/acs.orglett.5b00932. [DOI] [PubMed] [Google Scholar]; f Du B.; Jiang X.; Sun P. Palladium-Catalyzed Highly Selective ortho-Halogenation (I, Br, Cl) of Arylnitriles via Sp2 C-H Bond Activation Using Cyano as Directing Group. J. Org. Chem. 2013, 78, 2786–2791. 10.1021/jo302765g. [DOI] [PubMed] [Google Scholar]; g Rajesh K.; Somasundaram M.; Saiganesh R.; Balasubramanian K. K. Bromination of Deactivated Aromatics: A Simple and Efficient Method. J. Org. Chem. 2007, 72, 5867–5869. 10.1021/jo070477u. [DOI] [PubMed] [Google Scholar]
- a Krasnokutskaya E.; Semenischeva N.; Filimonov V.; Knochel P. A New, One-Step, Effective Protocol for the Iodination of Aromatic and Heterocyclic Compounds via Aprotic Diazotization of Amines. Synthesis 2007, 2007, 81–84. 10.1055/s-2006-958936. [DOI] [Google Scholar]; b Hodgson H. H. The Sandmeyer Reaction. Chem. Rev. 1947, 40, 251–277. 10.1021/cr60126a003. [DOI] [PubMed] [Google Scholar]; c Sandmeyer T. Ueber Die Ersetzung Der Amidgruppe Durch Chlor in Den Aromatischen Substanzen. Ber. Dtsch. Chem. Ges. 1884, 17, 1633–1635. 10.1002/cber.18840170219. [DOI] [Google Scholar]; d Sandmeyer T. Ueber Die Ersetzung Der Amid-Gruppe Durch Chlor, Brom Und Cyan in Den Aromatischen Substanzen. Ber. Dtsch. Chem. Ges. 1884, 17, 2650–2653. 10.1002/cber.188401702202. [DOI] [Google Scholar]; e Cowdrey W. A.; Davies D. S. Sandmeyer and Related Reactions. Q. Rev., Chem. Soc. 1952, 6, 358–379. 10.1039/qr9520600358. [DOI] [Google Scholar]; f He L.; Qiu G.; Gao Y.; Wu J. Removal of Amino Groups from Anilines through Diazonium Salt-Based Reactions. Org. Biomol. Chem. 2014, 12, 6965–6971. 10.1039/c4ob01286k. [DOI] [PubMed] [Google Scholar]; g Krasnokutskaya E.; Semenischeva N.; Filimonov V.; Knochel P. A New, One-Step, Effective Protocol for the Iodination of Aromatic and Heterocyclic Compounds via Aprotic Diazotization of Amines. Synthesis 2007, 2007, 81–84. 10.1055/s-2006-958936. [DOI] [Google Scholar]; h Hubbard A.; Okazaki T.; Laali K. K. Halo- and Azidodediazoniation of Arenediazonium Tetrafluoroborates with Trimethylsilyl Halides and Trimethylsilyl Azide and Sandmeyer-Type Bromodediazoniation with Cu(I)Br in [BMIM][PF6] Ionic Liquid. J. Org. Chem. 2008, 73, 316–319. 10.1021/jo701937e. [DOI] [PubMed] [Google Scholar]; i Beletskaya I.; Sigeev A.; Peregudov A.; Petrovskii P. Catalytic Sandmeyer Bromination. Synthesis 2007, 2007, 2534–2538. 10.1055/s-2007-983784. [DOI] [Google Scholar]
- a Clososki G. C.; Rohbogner C. J.; Knochel P. Direct Magnesiation of Polyfunctionalized Arenes and Heteroarenes Using (tmp)2Mg 2LiCl. Angew. Chem., Int. Ed. 2007, 46, 7681–7684. 10.1002/anie.200701487. [DOI] [PubMed] [Google Scholar]; b Houlden C. E.; Lloyd-Jones G. C.; Booker-Milburn K. I. Facile Double-Lithiation of a Transient Urea: Vicarious Ortho-Metalation of Aniline Derivatives. Org. Lett. 2010, 12, 3090–3092. 10.1021/ol101102y. [DOI] [PubMed] [Google Scholar]; c MacNeil S. L.; Familoni O. B.; Snieckus V. Selective Ortho and Benzylic Functionalization of Secondary and Tertiary p-Tolylsulfonamides. Ipso-Bromo Desilylation and Suzuki Cross-Coupling Reactions. J. Org. Chem. 2001, 66, 3662–3670. 10.1021/jo001402s. [DOI] [PubMed] [Google Scholar]; d Menzel K.; Fisher E. L.; Dimichele L.; Frantz D. E.; Nelson T. D.; Kress M. H.; Pennsyl V.; Lincoln E. A. V. An Improved Method for the Bromination of Metalated Haloarenes via Lithium, Zinc Transmetalation: A Convenient Synthesis of 1,2-Dibromoarenes. J. Org. Chem. 2006, 71, 2188–2191. 10.1021/jo052515k. [DOI] [PubMed] [Google Scholar]; e Nguyen T.-H.; Castanet A.-S.; Mortier J. Directed ortho-Metalation of Unprotected Benzoic Acids. Methodology and Regioselective Synthesis of Useful Contiguously 3- and 6-Substituted 2-Methoxybenzoic Acid Building Blocks. Org. Lett. 2006, 8, 765–768. 10.1021/ol0530427. [DOI] [PubMed] [Google Scholar]; f Ple N.; Turck A.; Couture K.; Queguiner G. Diazines 13: Metalation without ortho-Directing Group-Functionalization of Diazines via Direct Metalation. J. Org. Chem. 1995, 60, 3781–3786. 10.1021/jo00117a033. [DOI] [Google Scholar]; g Winkle M. R.; Ronald R. C. Regioselective Metalation Reactions of Some Substituted (Methoxymethoxy)arenes. J. Org. Chem. 1982, 47, 2101–2108. 10.1021/jo00132a022. [DOI] [Google Scholar]
- a Fournier dit Chabert J.; Marquez B.; Neville L.; Joucla L.; Broussous S.; Bouhours P.; David E.; Pellet-Rostaing S.; Marquet B.; Moreau N.; et al. Synthesis and Evaluation of New Arylbenzo[b]Thiophene and Diarylthiophene Derivatives as Inhibitors of the NorA Multidrug Transporter of Staphylococcus Aureus. Bioorg. Med. Chem. 2007, 15, 4482–4497. 10.1016/j.bmc.2007.04.023. [DOI] [PubMed] [Google Scholar]; b Naganagowda G.; Petsom A. Synthesis and Antimicrobial Activity of Some New 2-(3-chloro-1-benzothiophen-2-yl)-3-(Substituted-phenyl)-4- (3H)-Quinazolinones Derivatives. J. Sulfur Chem. 2011, 32, 223–233. 10.1080/17415993.2011.575943. [DOI] [Google Scholar]
- a Witter D. J.; Belvedere S.; Chen L.; Secrist J. P.; Mosley R. T.; Miller T. A. Benzo[b]Thiophene-Based Histone Deacetylase Inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 4562–4567. 10.1016/j.bmcl.2007.05.091. [DOI] [PubMed] [Google Scholar]; b Martorana A.; Gentile C.; Perricone U.; Piccionello A. P.; Bartolotta R.; Terenzi A.; Pace A.; Mingoia F.; Almerico A. M.; Lauria A. Synthesis, Antiproliferative Activity, and in Silico Insights of New 3-Benzoylamino-Benzo[b]Thiophene Derivatives. Eur. J. Med. Chem. 2015, 90, 537–546. 10.1016/j.ejmech.2014.12.002. [DOI] [PubMed] [Google Scholar]; c Innocenti A.; Villar R.; Martinez-Merino V.; Gil M. J.; Scozzafava A.; Vullo D.; Supuran C. T. Carbonic Anhydrase Inhibitors: Inhibition of Cytosolic/Tumor-Associated Carbonic Anhydrase Isozymes I, II, and IX with Benzo[b]thiophene 1,1-Dioxide Sulfonamides. Bioorg. Med. Chem. Lett. 2005, 15, 4872–4876. 10.1016/j.bmcl.2005.04.078. [DOI] [PubMed] [Google Scholar]; d Jarak I.; Kralj M.; Šuman L.; Pavlović G.; Dogan J.; Piantanida I.; Žinić M.; Pavelić K.; Karminski-Zamola G. Novel Cyano- and N-Isopropylamidino-Substituted Derivatives of Benzo[b]Thiophene-2-Carboxanilides and Benzo[b]Thieno[2,3-c] Quinolones: Synthesis, Photochemical Synthesis, Crystal Structure Determination, and Antitumor Evaluation. J. Med. Chem. 2005, 48, 2346–2360. 10.1021/jm049541f. [DOI] [PubMed] [Google Scholar]
- Clouser C. L.; Chauhan J.; Bess M. A.; Oploo J. L. v.; Zhou D.; Dimick-Gray S.; Mansky L. M.; Patterson S. E. Anti-HIV-1 Activity of Resveratrol Derivatives and Synergistic Inhibition of HIV-1 by the Combination of Resveratrol and Decitabine. Bioorg. Med. Chem. Lett. 2012, 22, 6642–6646. 10.1016/j.bmcl.2012.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrade L.; Aisa B.; Ramirez M. J.; Galiano S.; Guccione S.; Moltzau L. R.; Levy F. O.; Nicoletti F.; Battaglia G.; Molinaro G.; et al. Novel Benzo[b]Thiophene Derivatives as New Potential Antidepressants with Rapid Onset of Action. J. Med. Chem. 2011, 54, 3086–3090. 10.1021/jm2000773. [DOI] [PubMed] [Google Scholar]
- Pinto E.; Queiroz M.-J. R. P.; Vale-Silva L. A.; Oliveira J. F.; Begouin A.; Begouin J.-M.; Kirsch G. Antifungal Activity of Synthetic Di(Hetero)Arylamines Based on the Benzo[b]Thiophene Moiety. Bioorg. Med. Chem. 2008, 16, 8172–8177. 10.1016/j.bmc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- Johnson D. S.; Ahn K.; Kesten S.; Lazerwith S. E.; Song Y.; Morris M.; Fay L.; Gregory T.; Stiff C.; Dunbar J. B.; et al. Benzothiophene Piperazine and Piperidine Urea Inhibitors of Fatty Acid Amide Hydrolase (FAAH). Bioorg. Med. Chem. Lett. 2009, 19, 2865–2869. 10.1016/j.bmcl.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhr I. M. I.; Radwan M. A. A.; el-Batran S.; Abd el-Salam O. M. E.; el-Shenawy S. M. Synthesis and Pharmacological Evaluation of 2-Substituted Benzo[b]Thiophenes as Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2009, 44, 1718–1725. 10.1016/j.ejmech.2008.02.034. [DOI] [PubMed] [Google Scholar]
- a Liu H.; Liu J.; van Breemen R. B.; Thatcher G. R. J.; Bolton J. L. Bioactivation of the Selective Estrogen Receptor Modulator Desmethylated Arzoxifene to Quinoids: 4′-Fluoro Substitution Prevents Quinoid Formation. Chem. Res. Toxicol. 2005, 18, 162–173. 10.1021/tx049776u. [DOI] [PubMed] [Google Scholar]; b Overk C. R.; Peng K.-W.; Asghodom R. T.; Kastrati I.; Lantvit D. D.; Qin Z.; Frasor J.; Bolton J. L.; Thatcher G. R. J. Structure–Activity Relationships for a Family of Benzothiophene Selective Estrogen Receptor Modulators Including Raloxifene and Arzoxifene. ChemMedChem 2007, 2, 1520–1526. 10.1002/cmdc.200700104. [DOI] [PubMed] [Google Scholar]; c Palkowitz A. D.; Glasebrook A. L.; Thrasher K. J.; Hauser K. L.; Short L. L.; Phillips D. L.; Muehl B. S.; Sato M.; Shetler P. K.; Cullinan G. J.; et al. Discovery and Synthesis of [6-Hydroxy-3-[4-[2-(1-Piperidinyl)Ethoxy]Phenoxy]-2-(4-Hydroxyphenyl)]Benzo[b]Thiophene: A Novel, Highly Potent, Selective Estrogen Receptor Modulator. J. Med. Chem. 1997, 40, 1407–1416. 10.1021/jm970167b. [DOI] [PubMed] [Google Scholar]; d Qin Z.; Kastrati I.; Chandrasena R. E. P.; Liu H.; Yao P.; Petukhov P. A.; Bolton J. L.; Thatcher G. R. J. Benzothiophene Selective Estrogen Receptor Modulators with Modulated Oxidative Activity and Receptor Affinity. J. Med. Chem. 2007, 50, 2682–2692. 10.1021/jm070079j. [DOI] [PubMed] [Google Scholar]; e Romagnoli R.; Baraldi P. G.; Carrion M. D.; Cara C. L.; Preti D.; Fruttarolo F.; Pavani M. G.; Tabrizi M. A.; Tolomeo M.; Grimaudo S.; et al. Synthesis and Biological Evaluation of 2- and 3-Aminobenzo[b]Thiophene Derivatives as Antimitotic Agents and Inhibitors of Tubulin Polymerization. J. Med. Chem. 2007, 50, 2273–2277. 10.1021/jm070050f. [DOI] [PubMed] [Google Scholar]
- Keri R. S.; Chand K.; Budagumpi S.; Balappa Somappa S.; Patil S. A.; Nagaraja B. M. An Overview of Benzo[b]Thiophene-Based Medicinal Chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. 10.1016/j.ejmech.2017.07.038. [DOI] [PubMed] [Google Scholar]
- Chen C.-C.; Chen C.-M.; Wu M.-J. Transition Metal-Catalyzed Cascade Cyclization of Aryldiynes to Halogenated Benzo[b]Naphtho[2,1-d]Thiophene Derivatives. J. Org. Chem. 2014, 79, 4704–4711. 10.1021/jo500377v. [DOI] [PubMed] [Google Scholar]
- Cao J.; Kong Y.; Yu L.; Fu L.; Lai G.; Cui Y.; Hu Z.; Wang G. Electrophilic Cyclization of o-Anisole- and o-Thioanisole-Substituted Ynamides: Synthesis of 2-Amidobenzofurans and 2-Amidobenzothiophenes. Synthesis 2013, 45, 1975–1982. 10.1055/s-0033-1338481. [DOI] [PubMed] [Google Scholar]
- Lu W.-D.; Wu M.-J. Halocyclization of 2-Alkynylthioanisoles by Cupric Halides: Synthesis of 2-Substituted 3-Halobenzo[b]Thiophenes. Tetrahedron 2007, 63, 356–362. 10.1016/j.tet.2006.10.068. [DOI] [Google Scholar]
- a Kesharwani T.; Giraudy K. A.; Morgan J. L.; Kornman C.; Olaitan A. D. Green Synthesis of Halogenated Thiophenes, Selenophenes and Benzo[b]Selenophenes Using Sodium Halides as a Source of Electrophilic Halogens. Tetrahedron Lett. 2017, 58, 638–641. 10.1016/j.tetlet.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kim S.; Dahal N.; Kesharwani T. Environmentally Benign Process for the Synthesis of 2,3-Disubstituted Benzo[b]Thiophenes Using Electrophilic Cyclization. Tetrahedron Lett. 2013, 54, 4373–4376. 10.1016/j.tetlet.2013.05.139. [DOI] [Google Scholar]; c Kesharwani T.; Kornman C.; Tonnaer A.; Hayes A.; Kim S.; Dahal N.; Romero R.; Royappa A. Sodium halides as the source of electrophilic halogens in green synthesis of 3-halo- and 3,n-dihalobenzo[b]thiophenes. Tetrahedron 2018, 74, 2973–2984. 10.1016/j.tet.2018.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. W.; Steffens L. D.; Sigman M. S.. The Wacker Oxidation. Organic Reactions; John Wiley & Sons, Inc., 2004. [Google Scholar]
- a Nair V.; Augustine A.; Suja T. D. CAN Mediated Reaction of Aryl Sulfinates with Alkenes and Alkynes: Synthesis of Vinyl Sulfones, β-Iodovinyl Sulfones and Acetylenic Sulfones. Synthesis 2002, 2259–2265. 10.1055/s-2002-34838. [DOI] [Google Scholar]; b Chen Q.-H.; Praveen Rao P. N.; Knaus E. E. Design, Synthesis, and Biological Evaluation of Linear 1-(4-, 3- or 2-Methylsulfonylphenyl)-2-Phenylacetylenes: A Novel Class of Cyclooxygenase-2 Inhibitors. Bioorg. Med. Chem. 2005, 13, 6425–6434. 10.1016/j.bmc.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Yue D.; Larock R. C. Synthesis of 2,3-Disubstituted Benzo[b]thiophenes via Palladium-Catalyzed Coupling and Electrophilic Cyclization of Terminal Acetylenes. J. Org. Chem. 2002, 67, 1905–1909. 10.1021/jo011016q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.