Abstract

The graphitic carbon nitride (g-C3N4) nanosheets decorated three-dimensional hierarchical flower-like nickel oxide (NiO) composites (NiO/g-C3N4, Ni/CN) were synthesized via a facile hydrothermal method combined with a subsequent annealing process. The structure and morphology of the as-prepared Ni/CN composites were characterized by X-ray diffraction, field-emission scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and nitrogen absorption. The gas-sensing experiments reveal that the composites with 10 wt % two-dimensional g-C3N4 (Ni/CN-10) not only exhibits the highest response of 20.03 that is almost 3 times higher than pristine NiO to 500 ppm triethylamine (TEA) at the optimal operating temperature of 280 °C but also shows a good selectivity toward TEA. The gas-sensitivity promotion mechanism is attributed to the internal charge transfer within the p–n heterojunction. Furthermore, the high specific surface area of the Ni/CN composites promotes adequate contact and reaction between the composites and triethylamine molecules. Therefore, the Ni/CN sensor has a great potential application in detecting TEA.
1. Introduction
Triethylamine (TEA), as a transparent, inflammable liquid with strong ammonia smell, is widely used as an antiseptic, organic solvent, catalyst, synthetic dyestuff, and so on.1−4 However, long-term exposure to TEA atmosphere could cause great harm to human health like nausea, headache, gastroenteritis, pulmonary edema, and even death.5 Furthermore, TEA is also secreted in dead fish and other seafood and the concentration would increase over time.6 Therefore, TEA could be used to judge the freshness of seafood. When TEA vapor is mixed with air, it forms an explosive mixture that will explode if exposed to flame. Although some traditional methods like the colorimetric method and gas/liquid/film chromatography are effective for TEA detection, the complex detection process and expensive equipment constrain the application. However, due to the low cost, easy operation, and high response of gas sensors, it is more convenient to detect TEA in the chemical and food industries.
It has been reported that there are many metal-oxide-semiconductor (MOS)-based gas sensors for detecting TEA gas, such as TiO2,7 α-Fe2O3,8 and V2O5.9 Most of these MOS materials are n-type materials, and there are few studies on TEA gas sensitivity for p-type materials, and less research on nickel oxide (NiO). However, recent experiments have shown that p-type materials have advantages in gas selectivity and lower operating temperatures. In this study, a typical p-type material (NiO) was used as the substrate for the TEA gas-sensing material, and the gas-sensing properties and mechanisms were discussed. NiO as an important p-type semiconductor material oxide with wide band gap energy (Eg = 3.6–4.0 eV)10 has received considerable attention in various fields such as catalysts,11 lithium-ion batteries,12 magnetic materials,13 gas sensors,14 etc. Many previous works have proven that NiO-based gas sensors show good gas-sensitivity response to various types of toxic gases.15,16 Lin et al. reported that uniform hexagonal NiO nanosheets were prepared by a hydrothermal method, and it shows excellent response to 50 ppm of several reducing gases (ethanol, CO, H2S, CH4, and NH3).15 Lu et al. synthesized cactus-like NiO via a simple hydrothermal approach and it has a response value of 13.51 for 100 ppm acetone at a working temperature of 260 °C.16 Dey et al. reported that coral-like NiO nanostructures were prepared by a facile hydrothermal technique, the response of the NiO sensor was found to be 292% in the presence of 190 ppm formaldehyde at 300 °C.17 In addition, the three-dimensional (3D) hierarchical structure has a higher specific surface area and faster gas diffusion rate than the conventional one-dimensional or two-dimensional (2D) structure.18−23 Ma et al. synthesized a photocatalyst of hierarchically mesoporous titanium phosphonate by a hydrothermal method and it exhibited a significantly improved photocatalytic hydrogen evolution rate.18 Cao et al. reported that the gas-sensing properties of the flower-like NiO structures are superior to the needle-like NiO structures on the basis of the research.24 Lin et al. synthesized the camellia-like NiO materials by a hydrothermal method, and they have a particular response to ethanol.25 Therefore, using 3D hierarchical flower-like NiO as the basis of the sensing materials will have better gas-sensing properties and excellent selectivity.
The sensors of pristine NiO normally have a low gas-sensitivity response. To improve the sensing properties, many scholars have compounded MOS with ultra-thin two-dimensional nanomaterials and found that two-dimensional nanomaterials can modify and sensitize MOS materials like noble metals.26,27 The graphitic carbon nitride (g-C3N4) is a two-dimensional semiconductor with a band gap energy of 2.7 eV, which has a large specific surface area and high chemical stability. Moreover, it is also an environmentally friendly material. In previous studies, there have been many reports on using it to modify various metal oxides and found that it not only prevents the aggregation of metal oxide nanoparticles but also forms a heterostructure with metal oxides to bring about new chemical and physical characteristics.28−30 Cao et al. reported that SnO2/g-C3N4 nanocomposites were synthesized by a simple hydrothermal method, and they exhibited the highest response value (S = 360) toward 500 ppm ethanol at 300 °C.31 Gong et al. synthesized Co3O4/g-C3N4 composites via a hydrothermal method, and they exhibited excellent sensing properties toward ethanol, which is 1.6 times higher than that of pure Co3O4.28 Zhang et al. reported that MgFe2O4/g-C3N4 composites were obtained by a solvothermal technique. This material not only has a gas-sensitive response to acetone of 145 times that of pure MgFe2O4 but also reduces the optimum temperature by 60 °C.32 However, the application of the NiO/g-C3N4 heterojunction in the field of gas sensors has never been reported. Therefore, in this work, we synthesized a spherical flower-like Ni/CN composites by a simple hydrothermal method for TEA-sensing application. In the TEA atmosphere, the sensitivity of the composite is much better than pure NiO. In addition, the surface morphology, structural properties, and gas-sensing mechanism of the composites were also studied and discussed in detail.
2. Results and Discussion
2.1. Morphology and Structure of Pure NiO and Ni/CN Composites
The X-ray diffraction (XRD) patterns of the as-prepared g-C3N4, NiO, and Ni/CN composites are shown in Figure 1. The peak at 27.5° in g-C3N4 is the characteristic (002) plane peak arising from the stacking of conjugated aromatic planes in g-C3N4. The peak at 13.1° could be assigned to the (100) plane, representing the interlayer structure packing motif of tri-s-triazine units. The pure NiO presents five peaks at 2θ values of 37.2, 43.3, 62.4, 75.4, and 79.1° corresponding to the (111), (200), (220), (311), and (222) planes, respectively, which are in accordance with the powder diffraction standard spectrum (JCPDS card no. 47-1049). The plane peak (002) of g-C3N4 is clearly present in the Ni/CN composite, indicating that g-C3N4 and NiO are successfully compounded. As the content of g-C3N4 in the composite increases, the (002) characteristic peak intensity becomes stronger. Comparing the XRD patterns of the composites, it can be concluded that the (002) characteristic peak is the strongest and sharpest in the Ni/CN-15 composites, while the strength is very weak in the Ni/CN-5 composites. Only in the Ni/CN-10 composites, the (002) characteristic peak intensity is relatively moderate, which may be related to the formation of the heterojunction.
Figure 1.

XRD patterns of the as-prepared g-C3N4, NiO, and Ni/CN composites with different g-C3N4 contents.
Surface chemical compositions of the pure g-C3N4, pristine NiO, and Ni/CN-10 heterostructure were further studied by X-ray photoelectron spectroscopy (XPS). As shown in the survey scan XPS spectrum (Figure 2a), C 1s and N 1s are detected in g-C3N4 and Ni/CN-10, while Ni 2p and O 1s peaks are observed in the spectra of NiO and Ni/CN-10. The C 1s peak from pure NiO is due to the adventitious carbon.33 The presence of a peak of N 1s in the spectrum of the Ni/CN-10 composite means that g-C3N4 is successfully incorporated into NiO. The corresponding high-resolution (HR) spectra of C 1s, N 1s, O 1s, and Ni 2p are also shown in Figure 2, respectively. The high-resolution O 1s spectra of NiO is presented in Figure 2b, in which the dominated peak at 529.4 eV originated from the Ni–O bond, 531.3 eV corresponds to chemically adsorbed oxygen species or OH species, and the peak at 532.7 eV may be related to the adsorbed water molecules.34−36 After calculation, the chemically adsorbed oxygen content of Ni/CN-10 is 16.2%, which is much higher than the value of NiO (10.5%). Ni/CN-10 contains a higher chemical adsorption oxygen content, which means that more oxygen anions are formed on the surface of the material, which is beneficial to improve gas sensitivity.
Figure 2.
XPS survey of g-C3N4, pristine NiO, and Ni/CN-10: (a) the general scan spectrum, (b) O 1s spectrum, (c) Ni 2p spectrum, (d) C 1s of Ni/CN-10, and (e) N 1s spectrum of Ni/CN-10.
The high-resolution Ni 2p XPS spectrum of NiO (Figure 2c) has five prominent peaks, which are typical characteristic peaks of Ni in Ni–O bonds.37 The peak with a binding energy of 854.2 eV corresponds to Ni 2p3/2 and the other peak corresponds to Ni 2p1/2, which is 872.7 eV.36,38 Other peaks were induced by shaking at 861.2 and 879.7 eV.37 In addition, the split peak of Ni 2p3/2 at 855.9 eV and the peak of O 1s at 531.3 eV indicate the coexistence of Ni2O3.39 However, a slight shift in the binding energy of O 1s and Ni 2p is observed in the spectrum of the Ni/CN-10 composite. The O 1s peak positions are changed to 529.3, 531.1, and 532.4, respectively. The Ni 2p peak positions are changed to 854.1, 855.6, 861.0, 827.8, and 879.5, respectively. This phenomenon can be attributed to the interaction of electron transfer and interface in the p–n heterojunction formed by the two phases.39 Three sub-bands located at 284.6, 286.3, and 287.9 eV (Figure 2d) can be observed in C 1s spectra of Ni/CN-10, which corresponds to sp2 C–C bonds, the combination of C–N groups and N–C=N groups, respectively. Figure 2e shows the N 1s spectra for Ni/CN-10. The prominent peak at 398.64 eV belongs to the sp2-hybridized aromatic N bond to carbon (C=N–C), while the peaks at 399.8 and 404.2 eV are ascribed to the tertiary nitrogen (N–(C)3 or H–N–(C)2) groups and π excitations.40
The morphology of pure g-C3N4, pristine NiO, and Ni/CN-10 heterojunction samples are characterized using scanning electron microscopes (SEMs). It can be observed that the as-prepared g-C3N4 exhibits a two-dimensional (2D) layered structure with many wrinkles (Figure 3a). Moreover, the pristine NiO presents a perfect 3D hierarchical flower-like morphology (Figure 3b) and it can be seen in Figure 3c that the flower particles are uniformly distributed. In Figure 3d, we can clearly see that the Ni/CN-10 composites prepared in this experiment combine the structural characteristics of both. In addition, we not only observed the samples showing the 3D layered nanostructure of NiO and the thin nanosheets of g-C3N4 but also observed that the sheet involved in the composition of NiO was filled into the nanosheet of g-C3N4. This proves that the two materials can be successfully compounded by the hydrothermal method.
Figure 3.
SEM images of (a) 2D g-C3N4, (b, c) NiO, and (d) Ni/CN-10 composites.
It can be observed from the transmission electron microscopy (TEM) image of g-C3N4 (Figure 4a) that pure g-C3N4 is curly and transparent, indicating that it is indeed a very thin nanosheet structure. Figure 4b shows a TEM image of NiO, which can be seen as a flower-like sphere assembled from NiO sheets, and the NiO sheet is also relatively thin. The TEM image of the Ni/CN-10 composite is shown in Figure 4c, and it can be seen that g-C3N4 covers the surface of NiO, and it can be seen that the g-C3N4 flake is bonded among the NiO particles. This is consistent with the SEM image. The selected-area electron diffraction (SAED) pattern of Ni/CN-10 indicates that the crystalline nature of NiO is a single particle. The appearance of a bright arc in the diffraction circle in the SAED pattern indicates that the flower sphere is formed by NiO nanocrystals in a highly preferred orientation. The HRTEM image of the Ni/CN-10 composite (Figure 4d) illustrates the inclusion of NiO nanoparticles in g-C3N4. Moreover, the HRTEM image shows that the stripe pitch of 0.21 nm can be directed to the (200) crystal plane of NiO. There were some transparent structures without clear lattice existing around the black NiO crystals, which were considered to be g-C3N4 of low crystallinity. What deserves more attention is the close contact between the two phases, indicating the formation of heterojunctions and affecting the charge transfer at the contact interface. In summary, according to the results of XRD, XPS, SEM, and TEM analyses, this experiment successfully combined 3D flower-like NiO with the g-C3N4 nanosheet by a hydrothermal method.
Figure 4.
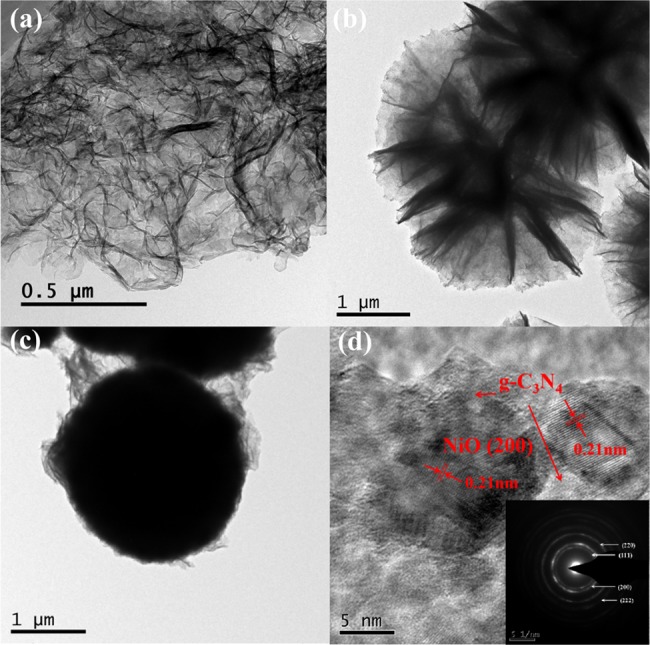
TEM images of (a) 2D g-C3N4, (b) NiO, and (c) Ni/CN-10 composites, and (d) HRTEM image and SAED pattern of Ni/CN-10.
To evaluate the specific surface area and the porosity of the Ni/CN-10 composites, N2-absorption measurements were performed. The nitrogen adsorption–desorption isotherm and the corresponding pore size distribution analysis of the Ni/CN-10 composites are shown in Figure 5. According to the IUPAC, the isotherms of the sample belong to the classical type IV pattern (Figure 5a), which reflects the typical characteristics of the mesoporous structure. The hysteresis loop of Ni/CN-10 belongs to the H3-type, demonstrating the existence of slit-like pores formed by NiO and g-C3N4 nanoflakes. Figure 5b illustrates the pore size distribution curves of the Ni/CN-10 composites. It can be seen that the pore sizes of the composites are mostly concentrated in the range of about 3–10 nm. The Brunauer–Emmett–Teller surface area of Ni/CN-10 samples was calculated to be 264.103 m2/g. Moreover, such a large surface area can provide more adsorption sites for the gas molecules, and the larger mesoporous pore size can enhance the diffusion velocity of the gas molecules, thus improving the gas sensitivity.
Figure 5.
(a) N2 adsorption–desorption isotherm curve of Ni/CN-10 and (b) pore size distribution of Ni/CN-10.
2.2. Gas-Sensing Properties
To study the performance of the prepared TEA vapor-based sensor, a series of gas-sensing tests were conducted. During the test, the operating temperature range of the laboratory was 180–320 °C. To obtain a conclusion that the composite material has better gas sensitivity to TEA, a pure NiO-based sensor was also tested. For gas-sensing tests, the operating temperature is the first consideration because temperature affects the chemical reactions occurring on the surface of the MOS materials and the adsorption and desorption of gases. Therefore, the response of the Ni/CN composite and the pure 3D layered flower-like NiO-based sensor to 500 ppm TEA vapor at different temperatures was tested. It can be seen from Figure 6a that as the temperature rises from 180 to 320 °C, all of the sensors show a trend of increasing first and then decreasing. The response of the sensors based on pure NiO, Ni/CN-5, Ni/CN-10, and Ni/CN-15 reached the highest value at 280 °C, so the optimal operating temperature of the sensor is 280 °C. The response values of pure NiO, Ni/CN-5, Ni/CN-10, and Ni/CN-15 to 500 ppm TEA at 280 °C were 6.64, 7.31, 20.04, and 16.957, respectively. Compared with the pure NiO sensor, all Ni/CN-based sensors have higher response over the entire test temperature range, indicating that the introduction of g-C3N4 in NiO can greatly enhance the response of sensors to TEA. As the content of 5–10 wt % g-C3N4 was added, the response value of the composite increased, however, the response value decreased as the content of g-C3N4 was further increased. This result indicates that the mass percentage of the best g-C3N4 in the composite system is 10%.
Figure 6.
(a) Responses of the sensors toward 500 ppm TEA at different operating acting temperatures. (b) Response curves of pure NiO and Ni/CN-10 sensors at 280 °C for different TEA concentrations.
Figure 6b shows the response of NiO and Ni/CN-10 to different TEA concentrations at 280 °C in the range of 30–1000 ppm. As the concentration of TEA increased, the response of two sensors increased gradually, but the response increased more slowly as the concentration increased. It can be seen that when the TEA concentration reaches 1000 ppm, the adsorption of the sensor on the TEA is nearly saturated. In addition, the response amplitude of Ni/CN is always higher than that of pure NiO, indicating that it is more sensitive to TEA.
Figure 7a shows the real-time response curves of the pure NiO and Ni/CN-10 sensors to different concentrations of TEA at 280 °C. It can be observed that the response values of the two samples increased as the concentration of TEA increased in the range of 30–1000 ppm. Moreover, the response amplitude of the Ni/CN-10 sensor is much higher than that of the pure NiO sensor, and the difference between the response values of the two sensors also increased with the increase of the TEA concentration, indicating that the gas-sensing performance of the composite material is improved.
Figure 7.
(a) Real-time response curves of pure NiO and Ni/CN-10 sensors to TEA in the range of 30–1000 ppm at 280 °C. (b, c) Repeatability and stability measurements of Ni/CN-10 sensors to 500 ppm TEA at 280 °C. (d) Response values of the sensors to 500 ppm different gases at 280 °C.
The repeatability and stability of the Ni/CN-10 sensor were also tested, which are key factors influencing the life of gas performances. As shown in Figure 7b, the response of the Ni/CN-10 sensor to 500 ppm TEA at 280 °C remained almost constant and remained at around 20. It can be seen that the response–recovery time of Ni/CN-10 is also stable, according to the calculation, the response time is about 157 s and the recovery time is about 350 s. Moreover, the sensor response fluctuates in a small range in a period of 30 days (Figure 7c). Therefore, the as-prepared Ni/CN-10 sensor has excellent TEA gas-sensing repeatability and stability and is very suitable for the application of the TEA sensor in practical life. Figure 7d shows the response of pure NiO and Ni/CN-10 to other 500 ppm volatile organic compounds at 280 °C, including ethanol, acetone, formaldehyde, methanol, and methylbenzene. It can be seen that the Ni/CN-10 sensor has a higher response to several other gases than the pure NiO sensor, and the response of Ni/CN-10 to TEA is higher than other gases, indicating that the sensor has a good selectivity to TEA. According to the literature,41 the bond energies of C–N, C–O, C–H, O–H, C=C, and C=O, are 307, 326, 414, 458.8, 610.3, and 798.9 kJ/mol, respectively. The higher response to TEA may be due to the low bond energy of C–N, which makes the TEA molecules more susceptible to be reduced by Ni/CN materials. Table 1 lists the gas-sensing properties of Ni/CN-10-based sensors and other reported material-based sensors toward TEA. As can be seen from Table 1, Ni/CN-10 possesses lower operating temperature and higher response value than other nanostructure pure NiO-based sensors. This analysis results indicated that the as-prepared Ni/CN-10 composite has good gas sensitivity to TEA.
Table 1. Comparison of the TEA-Sensing Performance Based on the As-prepared Ni/CN Sample and Other Literature Reported Results.
2.3. Gas-Sensing Mechanism
As a p-type semiconductor, the gas-sensing mechanism of NiO could be explained by the change of sensor resistance when the sensor is exposed to air and test gases. The degree of change in the resistance of the sensor is mainly controlled by the width of the hole accumulation layers (HALs) formed on the surface of the NiO. When the NiO sensor is exposed to the air environment, oxygen molecules will be adsorbed on the surface of NiO, transfer holes to the valence band of NiO, and then exist as substances of O–, O2–, and O2– by ionization. Moreover, the existence of oxygen anions is closely related to temperature,47 as shown in Figure 9a. In this case, the concentration of holes on the surface of NiO increases, and then a HAL is formed, resulting in a decrease in the resistance of the sensor in air.48 As shown in Figure 9b, when the sensor is exposed to a reducing gas (such as TEA vapor), the triethylamine molecule is also adsorbed onto the surface of NiO, and the oxygen anions readily react with triethylamine molecules due to their relatively low binding energy and oxidizing ability
| 1 |
| 2 |
The electrons released by the oxygen ions recombine with the holes. As a result, the concentration of holes on the surface of NiO is lowered, resulting in an increase in sensor resistance.49,50 In summary, the change in resistance of NiO sensors in different gas environments is achieved based on the adsorption and desorption of oxygen molecules. The excellent gas-sensing properties for 3D layered flower-like NiO can be attributed to its unique porous structure, and it is composed of a large number of nanosheet arrays, which are beneficial to the rapid diffusion and transportation of triethylamine molecules. For Ni/CN composites, g-C3N4 nanosheets play an important role in improving gas-sensing performance. It is generally believed that the p–n heterojunction formed on the nanosheets may be a major factor in improving the gas sensitivity of the Ni/CN composites. The p–n junction (NiO and g-C3N4) formed on the Ni/CN sheet can be seen by the HRTEM image. Since the work function of NiO (5.0 eV)51 is larger than g-C3N4 (4.3 eV),52 electrons migrate from g-C3N4 to the conduction band of NiO through the bending band, and the holes migrate in the opposite direction until their Fermi level becomes equal. A part of the holes of HAL on NiO is neutralized by electrons from g-C3N4, and as a result, a hole depletion layer is formed on the NiO side. Therefore, the thickness of HAL on the Ni/CN particles is reduced, so that the Ra of Ni/CN is higher than that of pure NiO, as illustrated in Figure 9c. It can be seen from Figure 8 that the Ra value of Ni/CN-10 is about 2.0 kΩ, which is higher than that of NiO (0.9 kΩ), and the results conform to the above inference. For p-type materials whose response is defined as Rg/Ra, a higher Ra value will result in a lower response value. However, the gas-sensitivity test results show that the response of the Ni/CN sensor is much higher than that of the pure NiO sensor. This is due to the lattice mismatch effect between NiO and g-C3N4. That is to say, defects formed near the region of the p–n junction may become potentially active sites for gas adsorption and surface reactions,53 which will allow the Ni/CN composite to provide more active sites for the surface reaction between TEA molecules and oxygen anions. After the reaction, more electrons are released back to NiO, more holes in the HAL are neutralized by electrons, and the thickness of the HAL is further reduced, resulting in a higher Rg of the Ni/CN sensor than pure NiO. At the same time, the effect of electron migration from g-C3N4 to NiO conduction band also exists, further increasing the resistance of Ni/CN, as shown in Figure 9d.
Figure 9.

Interaction of the flower-like NiO surface with oxygen at different temperatures (a), a schematic model for the flower-like NiO sensor during exposure to TEA (b), and the energy band diagram of Ni/CN when exposed to air (c) and TEA gas (d).
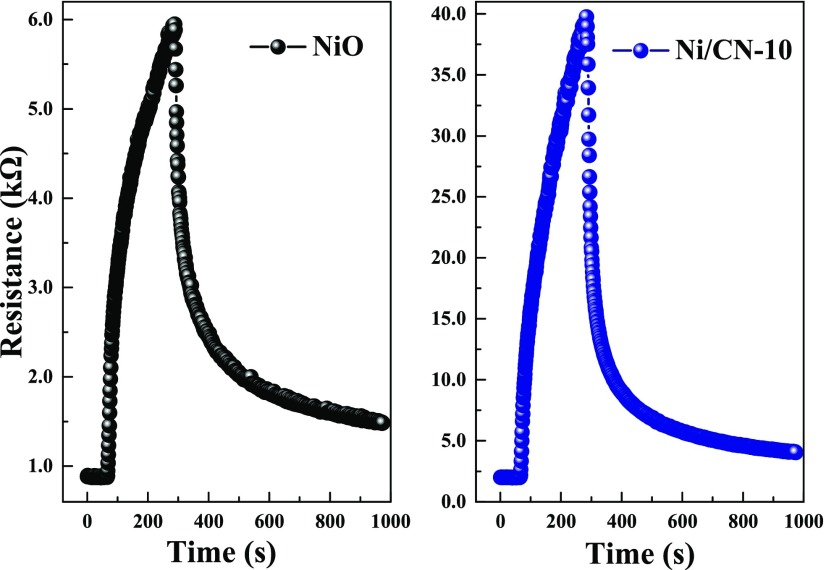
Figure 8.
Response transient of the sensors to 500 ppm TEA at 280 °C.
In addition, it was found that as the g-C3N4 content was increased from 5 to 10 wt %, the response of the Ni/CN-10 sensor was increased, and as the g-C3N4 content was further increased to 15 wt %, the response was observed to decrease. This phenomenon is also explained by the p–n junction. When the g-C3N4 content is relatively low (<10 wt %), all g-C3N4 nanosheets form (p) NiO–(n) g-C3N4 junctions with the flower-like NiO nanomaterials, which means that with the increasing contents of g-C3N4, the composite will provide more active sites for surface reactions and thus increase the response. When the content of g-C3N4 is higher than 10 wt %, excessive g-C3N4 nanosheets will accumulate on the surface of NiO, which hinders the contact of TEA molecules with the Ni/CN surface to inhibit the TEA-sensing reaction. This is consistent with the analysis of the results of XRD and SEM.
3. Conclusions
In summary, the flower-like NiO and g-C3N4 decorative flower-like NiO composites (Ni/CN) were successfully synthesized by a simple one-step hydrothermal method. The sensors based on NiO and Ni/CN materials were systematically studied by XRD, SEM, TEM, XPS, and N2-adsorption. Compared to the pure NiO sensor, the Ni/CN-10 sensor exhibits an enhanced response to triethylamine at an optimum temperature of 280 °C and a nearly 3-fold increase in response. In addition to the higher response, the Ni/CN sensor has better selectivity and stability, mainly due to the introduction of g-C3N4. The excellent gas-sensing properties of Ni/CN-10 composites indicate that this material is an ideal candidate for triethylamine gas-sensing applications.
4. Experimental Section
4.1. Sample Preparation
The chemicals of nickel chloride (NiCl2·6H2O, 98.0%, AR), urea (CO(NH2)2 99.0%, AR), and sodium dodecyl sulfonate (SDS, C12H25NaO3S, 97.0%, CP) were purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China) and used as received without further purification. Graphitic carbon nitride (g-C3N4) was synthesized by our previously reported method.54 In a typical experiment, 10 wt % g-C3N4 nanosheets decorated 3D hierarchical flower-like NiO (Ni/CN-10) were synthesized by the hydrothermal method. 0.0166 g g-C3N4 was dispersed in a 20 mL mixed solution, which contained 10 mL of deionized water and 10 mL of ethanol, with ultrasonic treatment for 2 h. Meanwhile, 0.289 g (1 mmol) of sodium dodecyl sulfonate (SDS) was dissolved in 20 mL of distilled water under magnetic stirring for 10 min to form a clear solution. Then 0.475 g (2 mmol) NiCl2·6H2O, and 0.600 g (10 mmol) of urea were dissolved into the g-C3N4 mixture solution under magnetic stirring for 20 min. Subsequently, 20 mL of SDS solution was added into the above solution dropwise and stirred continuously for 30 min at room temperature. The homogeneous mixture was transferred into a 50 mL stainless-steel Teflon-lined autoclave and heated at 120 °C for 12 h. The product was washed with deionized and ethanol several times and dried at 60 °C for 10 h. Finally, the composites were obtained by calcining the precipitate at 350 °C for 2 h under air atmosphere. According to this method, the 5 and 15 wt % g-C3N4 decorated NiO were also prepared and marked as Ni/CN-5 and Ni/CN-15. For the comparison purpose, the same method was used to synthesize the pure 3D hierarchical flower-like NiO.
4.2. Characterization
Powder X-ray diffraction (XRD) analysis was performed on a Bruker AXS D8 (Bruker, Madison, WI) Advance diffractometer to examine the purity and crystalline of the samples, with Cu Kα radiation at 40 kV and 25 mA over a range of 10–80° (2θ). The product morphologies and microstructures were observed by field-emission scanning electron microscopy (Quanta 250 FEG) (FEI, Eindhoven, The Netherlands) and high-resolution transmission electron microscopy (HRTEM JEOL, JEM-2100) (JEOL, Tokyo, Japan). X-ray photoelectron spectroscopy (XPS) measurements were tested on a Thermo ESCALAB 250XI X-ray photoelectron spectrometer (Thermo Fisher Scientific, Waltham, MA) with the Al Kα radiation, and energy calibration by fixing the binding energy of C 1s to 284.6 eV. Nitrogen adsorption–desorption isotherms were obtained on a Quantachrome Autosorb-iQ2 sorption analyzer (Quantachrome, Boynton Beach, FL).
4.3. Gas-Sensing Properties Test
The gas-sensitivity properties of pure NiO and Ni/CN composites were investigated using a static gas-sensing analysis system of CGS-4TPS (Beijing Elite Tech Co., Ltd. Beijing, China). Figure 10 shows the schematic of the gas-sensing test system and the construction of the fabricated sensor. The sample was dispersed in distilled water and made the mixture to form a paste, which was then slowly dropped onto the surface of a ceramic substrate (13.4 mm × 7 mm) with interdigitated Ag–Pd electrode and dried at 60 °C overnight to obtain the resistance type sensor. Before carrying out the measurement, the sensors were heated and degassed for more than 2 h at 300 °C in air to improve the stability and repeatability. The test gas is injected into the closed chamber of the system described above using a microsyringe. The resistance of the sensor in the test gas and air is Rg and Ra, respectively, and the ratio of the resistance between the two is defined as the response (S = Rg/Ra) of it. The response and recovery time were defined as the time taken for the sensor to reach 90% of the equilibrium value in the case of injecting and removing the test gas, respectively. During the test, the relative humidity was 18% in the test chamber. During the test, from the injection of the test gas to the end of the gas release, the response time of each sensor was controlled at 200 s.
Figure 10.
Schematic diagram of the gas-sensing measuring system and the gas-sensor substrate.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (U1704255), Zhongyuan Thousand Talent Program-Zhongyuan Young Top-notch Talents (ZYQR201810133), the Program for Science & Technology Innovation Talents in Universities of Henan Province (19HASTIT042), Research Foundation for Youth Scholars of Higher Education of Henan Province (2017GGJS053), the Program for Innovative Research Team of Henan Polytechnic University (T2018-2, T2019-1), and Foundation for Distinguished Young Scientists of Henan Polytechnic University (J2017-3).
The authors declare no competing financial interest.
References
- Sun J.; Shu X.; Tian Y.; Tong Z.; Bai S.; Luo R.; Li D.; Chen A. Preparation of polypyrrole@WO3 hybrids with p-n heterojunction and sensing performance to triethylamine at room temperature. Sens. Actuators, B 2017, 238, 510–517. 10.1016/j.snb.2016.07.012. [DOI] [Google Scholar]
- Mirzaei A.; Leonardi S. G.; Neri G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: a review. Ceram. Int. 2016, 42, 15119–15141. 10.1016/j.ceramint.2016.06.145. [DOI] [Google Scholar]
- Ju D. X.; Xu H. Y.; Qiu Z. W.; Zhang Z. C.; Xu Q.; Zhang J.; Wang J. Q.; Cao B. Q. Near room temperature, fast-response, and highly sensitive triethylamine sensor assembled with Au-loaded ZnO/SnO2 core-shell nanorods on flat alumina substrates. ACS Appl. Mater. Interfaces 2015, 7, 19163–19171. 10.1021/acsami.5b04904. [DOI] [PubMed] [Google Scholar]
- Sun G.; Chen H.; Li Y.; Ma G.; Zhang S.; Jia T.; Cao J.; Wang X.; Bala H.; Zhang Z. Synthesis and triethylamine sensing properties of mesoporous α-Fe2O3 microrods. Mater. Lett. 2016, 178, 213–216. 10.1016/j.matlet.2016.04.209. [DOI] [Google Scholar]
- Chen E. X.; Fu H. R.; Lin R.; Tan Y. X.; Zhang J. Highly selective and sensitive trimethylamine gas sensor based on cobalt imidazolate framework material. ACS Appl. Mater. Interfaces 2014, 6, 22871–22875. 10.1021/am5071317. [DOI] [PubMed] [Google Scholar]
- Woo H. S.; Na C. W.; Kim I. D.; Lee J. H. Highly sensitive and selective trimethylamine sensor using one-dimensional ZnO-Cr2O3 hetero-nanostructures. Nanotechnology 2012, 23, 245501 10.1088/0957-4484/23/24/245501. [DOI] [PubMed] [Google Scholar]
- Yang H. Y.; Cheng X. L.; Zhang X. F.; Zheng Z. K.; Tang X. F.; Xu Y. M.; Gao S.; Zhao H.; Huo L. H. A novel sensor for fast detection of triethylamine based on rutile TiO2 nanorod arrays. Sens. Actuators, B 2014, 205, 322–328. 10.1016/j.snb.2014.08.092. [DOI] [Google Scholar]
- Zhang F.; Yang H.; Xie X.; Li L.; Zhang L.; Yu J.; Zhao H.; Liu B. Controlled synthesis and gas-sensing properties of hollow sea urchin-like α-Fe2O3 nanostructures and α-Fe2O3 nanocubes. Sens. Actuators, B 2009, 141, 381–389. 10.1016/j.snb.2009.06.049. [DOI] [Google Scholar]
- Wu M.; Zhang X.; Gao S.; Cheng X.; Rong Z.; Xu Y.; Zhao H.; Huo L. Construction of monodisperse vanadium pentoxide hollow spheres via a facile route and triethylamine sensing property. CrystEngComm 2013, 15, 10123–10131. 10.1039/c3ce41471j. [DOI] [Google Scholar]
- Yang H. M.; Tao Q. F.; Zhang X. C.; Tang A. D.; Ouyang J. Solid-state synthesis and electrochemical property of SnO2/NiO nanomaterials. J. Alloys Compd. 2008, 459, 98–102. 10.1016/j.jallcom.2007.04.258. [DOI] [Google Scholar]
- Jiang S. J.; Handberg E. S.; Liu F.; Liao Y. T.; Wang H. Y.; Li Z.; Song S. Q. Effect of doping the nitrogen into carbon nanotubes on the activity of NiO catalysts for the oxidation removal of toluene. Appl. Catal., B 2014, 160–161, 716–721. 10.1016/j.apcatb.2014.06.026. [DOI] [Google Scholar]
- Zhao J. F.; Shao Y.; Zha J. C.; Wang H. Y.; Yang Y.; Ruan S. D.; Yang G.; Chen J. H. Large-scale preparation of crinkly NiO layers as anode materials for lithium-ion batteries. Ceram. Int. 2016, 42, 3479–3484. 10.1016/j.ceramint.2015.10.147. [DOI] [Google Scholar]
- Cui Y. F.; Wang C.; Wu S. J.; Liu G.; Zhang F. F.; Wang T. M. Lotus-root-like NiO nanosheets and flower-like NiO microspheres: synthesis and magnetic properties. CrystEngComm 2011, 13, 4930–4934. 10.1039/c1ce05389b. [DOI] [Google Scholar]
- Tian K.; Wang X. X.; Li H. Y.; Nadimicherla R.; Guo X. Lotus pollen derived 3-dimensional hierarchically porous NiO microspheres for NO2 gas sensing. Sens. Actuators, B 2016, 227, 554–560. 10.1016/j.snb.2015.12.104. [DOI] [Google Scholar]
- Lin L. Y.; Liu T. M.; Miao B.; Zeng W. Hydrothermal fabrication of uniform hexagonal NiO nanosheets: structure growth and response. Mater. Lett. 2013, 102–103, 43–46. 10.1016/j.matlet.2013.03.103. [DOI] [Google Scholar]
- Lu Y.; Ma Y. H.; Ma S. Y.; Jin W. X.; Yan S. H.; Xu X. L.; Jiang X. H.; Wang T. T.; Yang H. M.; Chen H.; Qiang Z. Synthesis of cactus-like NiO nanostructure and their gas-sensing properties. Mater. Lett. 2016, 164, 48–51. 10.1016/j.matlet.2015.10.117. [DOI] [Google Scholar]
- Dey S.; Santra S.; Sen S.; Burman D.; Ray S. K.; Guha P. K. Photon-Assisted Ultra-Selective Formaldehyde Sensing by Defect Induced NiO-Based Resistive Sensor. IEEE Sens. J. 2018, 18, 5656–5661. 10.1109/JSEN.2018.2839967. [DOI] [Google Scholar]
- Li H.; Sun Y.; Yuan Z. Y.; Zhu Y. P.; Ma T. Y. Titanium Phosphonate Based Metal-Organic Frameworks with Hierarchical Porosity for Enhanced Photocatalytic Hydrogen Evolution. Angew. Chem., Int. Ed. 2018, 57, 3222–3227. 10.1002/anie.201712925. [DOI] [PubMed] [Google Scholar]
- Ge H.; Cui L.; Sun Z.; Wang D.; Nie S.; Zhu S.; Matthews B.; Wu G.; Song X. M.; Ma T. Y. Unique Li4Ti5O12/TiO2 multilayer arrays with advanced surface lithium storage capability. J. Mater. Chem. A 2018, 6, 22053–22061. 10.1039/C8TA03075H. [DOI] [Google Scholar]
- Guo X.; Zhu Y.; Ma T. Lowering reaction temperature: Electrochemical ammonia synthesis by coupling various electrolytes and catalysts. J. Energy Chem. 2017, 26, 1107–1116. 10.1016/j.jechem.2017.09.012. [DOI] [Google Scholar]
- Feng D. M.; Sun Y.; Liu Z. Q.; Zhu Y. P.; Ma T. Y. Designing Nanostructured Metal-Based CO2 Reduction Electrocatalysts. J. Nanosci. Nanotechnol. 2019, 19, 3079–3096. 10.1166/jnn.2019.16648. [DOI] [PubMed] [Google Scholar]
- Li G.; Cheng Z.; Xiang Q.; Yan L.; Wang X.; Xu J. Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone. Sens. Actuators, B 2019, 283, 590–601. 10.1016/j.snb.2018.09.117. [DOI] [Google Scholar]
- Xu J.; Xue Z.; Qin N.; Cheng Z.; Xiang Q. The crystal facet-dependent gas sensing properties of ZnO nanosheets: Experimental and computational study. Sens. Actuators, B 2017, 242, 148–157. 10.1016/j.snb.2016.09.193. [DOI] [Google Scholar]
- Cao S. K.; Zeng W.; Long H. W.; Zhang H. Hydrothermal synthesis of novel flower-needle NiO architectures: structure, growth and gas response. Mater. Lett. 2015, 159, 385–388. 10.1016/j.matlet.2015.07.045. [DOI] [Google Scholar]
- Lin L. Y.; Liu T. M.; Yu W. J.; Gou Z. P.; Zeng W. Synthesis of multifarious hierarchical flower-like NiO and their gas-sensing properties. Mater. Res. Bull. 2013, 48, 2730–2736. 10.1016/j.materresbull.2013.04.004. [DOI] [Google Scholar]
- Meng F.; Zheng H.; Sun Y.; Li M.; Liu J. Trimethylamine Sensors Based on Au-Modified Hierarchical Porous Single-Crystalline ZnO Nanosheets. Sensors 2017, 17, 1478. 10.3390/s17071478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z.; Zhang J.; Meng F.; Li Y.; Li R.; Chang Y.; Zhao J.; Han E.; Wang S. Highly Sensitive Ammonia Sensors Based on Ag-Decorated WO3 Nanorods. IEEE Trans. Nanotechnol. 2018, 17, 1252–1258. 10.1109/TNANO.2018.2871675. [DOI] [Google Scholar]
- Gong Y.; Wang Y.; Sun G.; Jia T.; Jia L.; Zhang F.; Lin L.; Zhang B.; Cao J.; Zhang Z. Carbon nitride decorated ball-flower like Co3O4 hybrid composite: Hydrothermal synthesis and ethanol gas sensing application. Nanomaterials 2018, 8, 132. 10.3390/nano8030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H.; Zhang S.; Xu G.; Peng Y.; Gong L.; Li X.; Li Y.; Lin Y.; Chen G. Highly photoactive heterojunction based on g-C3N4 nanosheets decorated with dendritic zinc(ii) phthalocyanine through axial coordination and its ultrasensitive enzyme-free sensing of cholin. RSC Adv. 2014, 4, 58226–58230. 10.1039/C4RA09841B. [DOI] [Google Scholar]
- Meng F.; Chang Y.; Qin W.; Yuan Z.; Zhao J.; Zhang J.; et al. ZnO-Reduced Graphene Oxide Composites Sensitized with Graphitic Carbon Nitride Nanosheets for Ethanol Sensing. ACS Appl. Nano Mater. 2019, 2, 2734. 10.1021/acsanm.9b00257. [DOI] [Google Scholar]
- Cao J.; Qin C.; Wang Y. Synthesis of g-C3N4 nanosheets decorated flower-like tin oxide composites and their improved ethanol gas sensing properties. J. Alloys Compd. 2017, 728, 1101–1109. 10.1016/j.jallcom.2017.09.073. [DOI] [Google Scholar]
- Zhang R.; Wang Y.; Zhang Z.; Cao J. Highly sensitive acetone gas sensor based on g-C3N4 decorated MgFe2O4 porous microspheres composites. Sensors 2018, 18, 2211–2223. 10.3390/s18072211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. Y.; Guo R. T.; Zhou W. G.; Huang C. Y.; Pan W. G. Ball-flower like NiO/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction. Appl. Catal., B 2018, 237, 802–810. 10.1016/j.apcatb.2018.06.042. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Meng X. N.; Yao M. X.; Sun G.; Zhang Z. Y. Enhanced CH4 sensing properties of Pd modified ZnO nanosheets. Ceram. Int. 2019, 45, 13150–13157. 10.1016/j.ceramint.2019.03.250. [DOI] [Google Scholar]
- Xue D.; Wang Y.; Cao J.; Sun G.; Zhang Z. Improving methane gas sensing performance of flower-like SnO2 decorated by WO3 nanoplates. Talanta 2019, 199, 603–611. 10.1016/j.talanta.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Wang J.; Wan P.; Ye J.; Hussain S.; Wei H.; Hou D. Ag2O loaded NiO ball-flowers for high performances supercapacitors. Mater. Lett. 2016, 177, 71–75. 10.1016/j.matlet.2016.04.169. [DOI] [Google Scholar]
- Dai W.; Pan X.; Chen S.; Chen C.; Wen Z.; Zhang H.; Ye Z. Honeycomb-like NiO/ZnO heterostructured nanorods: photochemical synthesis, characterization, and enhanced UV detection performance. J. Mater. Chem. C 2014, 2, 4606–4614. 10.1039/c4tc00157e. [DOI] [Google Scholar]
- Xu J.; Li L.; He F.; Lv R. C.; Yang P. P. A Novel double-shelled C@NiO hollow microsphere: Synthesis and application for electrochemical capacitor. Electrochim. Acta 2014, 148, 211–219. 10.1016/j.electacta.2014.10.061. [DOI] [Google Scholar]
- Kim K. S.; Davis R. E. Electron spectroscopy of the nickel-oxygen system. J. Electron Spectrosc. Relat. Phenom. 1972, 1, 251–258. 10.1016/0368-2048(72)85014-X. [DOI] [Google Scholar]
- Thaweesak S.; Lyu M.; Peerakiatkhajohn P.; Butburee T.; Luo B.; Chen H.; Wang L. Two-dimensional g-C3N4/Ca2Nb2TaO10 nanosheet composites for efficient visible light photocatalytic hydrogen evolution. Appl. Catal., B 2017, 202, 184–190. 10.1016/j.apcatb.2016.09.022. [DOI] [Google Scholar]
- Ju D.; Xu H.; Qiu Z.; Guo J.; Zhang J.; Cao B. Highly sensitive and selective triethylamine-sensing properties of nanosheets directly grown on ceramic tube by forming NiO/ZnO P-N heterojunction. Sens. Actuators, B 2014, 200, 288–296. 10.1016/j.snb.2014.04.029. [DOI] [Google Scholar]
- Liu B.; Yang H.; Zhao H.; An L.; Zhang L.; Shi R.; Wang L.; Bao L.; Chen Y. Synthesis and enhanced gas-sensing properties of ultralong NiO nanowires assembled with NiO nanocrystals. Sens. Actuators, B 2011, 156, 251–262. 10.1016/j.snb.2011.04.028. [DOI] [Google Scholar]
- Liu B.; Wang L.; Ma Y.; Yuan Y.; Yang J.; Wang M.; Liu J.; Zhang X.; Ren Y.; Du Q.; Zhao H.; Pei C.; Liu S.; Yang H. Enhanced gas-sensing properties and sensing mechanism of the foam structures assembled from NiO nanoflakes with exposed {1 1 1} facets. Appl. Surf. Sci. 2019, 470, 596–606. 10.1016/j.apsusc.2018.11.129. [DOI] [Google Scholar]
- Yu T. T.; Zhang X. F.; Xu Y. M.; Cheng X. L.; Gao S.; Zhao H.; Huo L. H. Low concentration H2S detection of CdO-decorated hierarchically mesoporous NiO nanofilm with wrinkle structure. Sens. Actuators, B 2016, 230, 706–713. 10.1016/j.snb.2016.02.128. [DOI] [Google Scholar]
- Liu B.; Zhang L.; Zhao H.; Chen Y.; Yang H. Synthesis and sensing properties of spherical flower-like architectures assembled with SnO2 submicron rods. Sens. Actuators, B 2012, 173, 643–651. 10.1016/j.snb.2012.07.084. [DOI] [Google Scholar]
- Li W.; Xu H.; Zhai T.; Yu H.; Chen Z.; Qiu Z.; Song X.; Wang J.; Cao B. Enhanced triethylamine sensing properties by designing Au@SnO2/MoS2 nanostructure directly on alumina tubes. Sens. Actuators, B 2017, 253, 97–107. 10.1016/j.snb.2017.05.174. [DOI] [Google Scholar]
- Shaalan N. W.; Yamazaki T.; Kikuta T. Influence of morphology and structure geometry on NO2 gas-sensing characteristics of SnO2 nanostructures synthesized via a thermal evaporation method. Sens. Actuators, B 2011, 153, 11–16. 10.1016/j.snb.2010.09.070. [DOI] [Google Scholar]
- Wang C.; Cheng X.; Zhou X.; Sun P.; Hu X.; Shimanoe K.; Lu G.; Yamazoe N. Hierarchical α-Fe2O3/NiO composites with a hollow structure for a gas sensor. ACS Appl. Mater. Interfaces 2014, 6, 12031–12037. 10.1021/am501063z. [DOI] [PubMed] [Google Scholar]
- Balamurugan C.; Maheswari A. R.; Lee D. W. Structural optical, and selective ethanol sensing properties of p-type semiconducting CoNb2O6 nanopowder. Sens. Actuators, B 2014, 205, 289–297. 10.1016/j.snb.2014.08.076. [DOI] [Google Scholar]
- Barsan N.; Simion C.; Heine T.; Pokhrel S.; Weimar U. Modeling of sensing and transduction for p-type semiconducting metal oxide based sensors. J. Electroceram. 2010, 25, 11–19. 10.1007/s10832-009-9583-x. [DOI] [Google Scholar]
- Rai P.; Yoon J. W.; Jeong H. M.; Hwang S. J.; Kwak C. H.; Lee J. H. Design of highly sensitive and selective Au@NiO yolk-shell nanoreactors for gas sensor applications. Nanoscale 2014, 6, 8292–8299. 10.1039/C4NR01906G. [DOI] [PubMed] [Google Scholar]
- Ma X.; Wei Y.; Wei Z.; He H.; Huang C.; Zhu Y. Probing π-π stacking modulation of g-C3N4/graphene heterojunctions and corrsponding role of graphene on photocatalytic activity. J. Colloid Interface Sci. 2017, 508, 274–281. 10.1016/j.jcis.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Li Y.; Luo N.; Sun G.; Zhang B.; Jin H.; Lin L.; Bala H.; Cao J.; Zhang Z.; Wang Y. Synthesis of porous nanosheets-assembled ZnO/ZnCo2O4 hierarchical structure for TEA detection. Sens. Actuators, B 2019, 287, 199–208. 10.1016/j.snb.2019.02.055. [DOI] [Google Scholar]
- Cao J.; Gong Y.; Wang Y.; Zhang B.; Zhang H.; Sun G.; Bala H.; Zhang Z. Cocoon-like ZnO decorated graphitic carbon nitride nanocomposite: hydrothermal synthesis and ethanol gas sensing application. Mater. Lett. 2017, 198, 76–80. 10.1016/j.matlet.2017.03.143. [DOI] [Google Scholar]