Abstract
Inorganic nanofiltration membranes with high flux are urgently needed in water purification processes. Herein, polydopamine (PDA)-modified layer-stacked molybdenum disulfide (MoS2) nanofiltration membranes (NFMs) were fabricated via a pressure-assisted self-assembly process. The separation performance of the as-prepared membranes with various MoS2 loadings at different dopamine polymerization times was evaluated. The pure water permeance of PDA-modified MoS2 NFMs, with MoS2 loading of 0.1103 mg/cm2 at 4 h modification, could reach 135.3 LMH/bar. The rejection toward methylene blue could reach 100% with molecular weight cutoff approximately 671 Da and a high permeability of salts. Furthermore, the resultant membrane also exhibited a satisfactory long-term stability toward dye solution and antifouling property toward bovine serum albumin. This work may give inspiration to the development of inorganic membranes with high performance, especially high pure water permeance, for water-related processes.
1. Introduction
According to The United Nation World Water Development Report 2018, the global demand of clean water has been increasing at a rate of about 1% per year, owing to the economic development, population growth, and changing consumption patterns.1 Hence, clean water is limited, and there is a large requirement of treatment technology for industrial water and domestic water.
Nanofiltration, thanks to its low cost, high efficiency, low energy consumption, and facile operational process,2 has been widely used in water softening, desalination, wastewater reclamation, and industrial substance separation.3−5 In general, nanofiltration is defined as a pressure-driven membrane filtration mode intermediate between reverse osmosis and ultrafiltration,6,7 and has a high rejection of species ranging from 0.001 to 0.01 μm with a relatively high water flux.3 Polymeric nanofiltration membranes (NFMs), although widely used, could suffer from some intrinsic drawbacks such as unsatisfying mechanical strength, thermal properties, and physicochemical stability.8,9 Especially, when polymeric NFMs are used in water purification process, swelling is an unavoidable problem.4 On the other hand, although inorganic NFMs have a better performance on durability and pure water flux compared to polymeric NFMs,10,11 some drawbacks may also exist, such as brittleness and incompatibility to flexible substrates used in water purification processes.6,12,13
Recently, two-dimensional (2D) layered inorganic materials have been widely studied because of their excellent flexible mechanical properties.14,15 Particularly, 2D graphene oxide (GO) has been made for a variety of NFMs with exceptional performance.16−20 For instance, Zhang et al.21 prepared a novel GO framework composite NFM via a layer-by-layer approach, endowing it with high rejections toward heavy metal and a water permeability of 4.7 LMH/bar. Mi et al.22 fabricated GO NFMs via layer-by-layer deposition of GO nanosheets, followed by cross-linking with 1,3,5-benzenetricarbonyl trichloride. The GO NFMs exhibited a high rejection toward Rhodamine WT and a water flux ranging between 8.00 and 27.6 LMH/bar. As a typical transition-metal dichalcogenide, molybdenum disulfide (MoS2) is a new kind of 2D layered inorganic material and a laminar crystal with many unique properties, such as low cost, high surface area, and low cytotoxicity.23−26 It can also exhibit structural stability and frictionless smooth surface without oxygen-containing groups.27−29 And the layer-stacked MoS2 membranes also have the characteristics of antiswelling and rigid nanochannels.26 Thus, these properties could endow the MoS2 layer-stacked membrane with higher and steadier water permeance during water filtration process.29 The group of Mi has discovered that the interlayer spacing with 1.2 nm of fully hydrated MoS2 nanosheets could meet the requirements of moderate molecular rejection and high water permeability. And with a high pressure, the MoS2 membrane could exhibit a satisfactory neatly layer-stacked nanostructure to create a condition for stable water flux and rejection performance.26 Wang et al.30 successfully prepared ultrathin single-layered MoS2-based membranes with controlling thickness well for gas separation. Zhang et al.31 prepared a low-pressure nanofiltration membrane comprising MoS2 nanosheets as spacers in the GO layers through the forward treatment of pressure-assisted assembly and heat. This GO/MoS2 NFM exhibited a rejection of more than 95% toward different charged dyes with a pure water permeability of 10.2 LMH/bar, which was 13.6 times that of pristine GO NFM. Furthermore, the GO/MoS2 NFMs also have exhibited satisfactory antifouling properties and stability. Zhou et al.32 prepared a hybrid membrane through layer-by-layer self-assembling polyelectrolyte multilayers with incorporating MoS2 nanosheets modified by poly(diallyldimethylammonium chloride) (PDDA) (PDDA@MoS2 nanosheets). The water flux of the hybrid membrane was 2.3 times that of the pure polyelectrolyte membrane with good long-term stability. In the work of Liang et al.,33 the MoS2 was modified by zwitterion (poly(sulfobetaine methacrylate), PSBMA) to prepare MoS2–PSBMA/polyethersulfone (PES) composite membranes by the conventional phase inversion method. And the composite membrane exhibited a high rejection toward dye and a high flux of 108.3 LMH at 0.6 MPa. All of these previous studies have made it reasonable to fabricate MoS2 membranes to improve water flux in nanofiltration process.
Inspired by mussel, dopamine has been widely used to modify different kinds of membrane surfaces because it can nonspecifically adhere to various substrates under weak alkaline and oxygen-containing environment.6,34 After modification, various properties of membranes, such as tenacity, antifouling, and surface hydrophilicity, could be improved.35−39 Hence, we developed a novel layer-stacked MoS2 nanofiltration membrane via the pressure-assisted self-assembly technique. Subsequently, the surface of MoS2 membrane was modified by dopamine dip-coating method. In this process, dopamine could polymerize to highly cross-linked adhesive polydopamine (PDA) through the process of consecutive oxidation, intramolecular cyclization, and oligomerization in alkaline oxygen condition, where a conformal coating on the surface of the MoS2 membrane could be formed. MoS2 nanosheets and the as-prepared membranes were characterized by atomic force microscopy (AFM), transmission electron microscopy (TEM), scanning electron microscopy (SEM), ζ-potential, X-ray diffraction (XRD), Fourier transform infrared spectrometry (FT-IR), and water contact angles. The nanofiltration performance of PDA-modified MoS2 membranes (PDA/MoS2 NFMs) with various MoS2 loadings at different dopamine polymerization times was investigated in detail. In addition, the molecular weight cutoff (MWCO), antifouling property, and long-term stability of PDA/MoS2 NFMs were also tested.
2. Results and Discussion
2.1. Characterization of Exfoliated MoS2
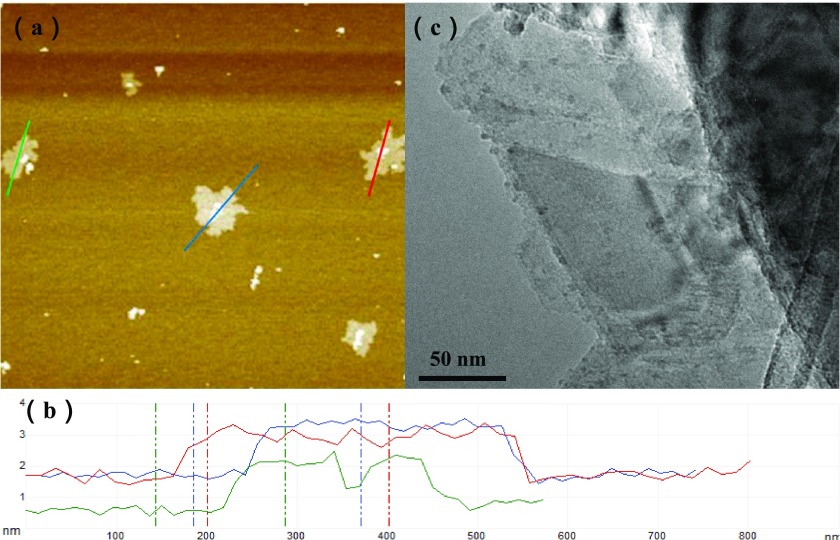
The morphology of exfoliated MoS2 nanosheets is shown in Figure 1. The AFM images (Figure 1a,b) indicated that the thickness of the exfoliated MoS2 nanosheets was about 2–3 nm. And the thickness of a single MoS2 layer is around 0.9–1.2 nm;40 therefore, the obtained MoS2 nanosheets were few-layer nanosheets that were suitable for the membrane formation. The 2D ultrathin nanosheet morphology of exfoliated MoS2 was further investigated by TEM. As can be seen in Figure 1c, the lamellar size of the individual MoS2 nanosheets was generally less than 400 nm.
Figure 1.
(a) AFM image of exfoliated MoS2. (b) Height profiles along the three lines shown in the AFM image. (c) TEM image of exfoliated MoS2.
2.2. Characterization of PDA/MoS2 NFMs
The surface morphology of the membrane was observed by SEM. As shown in Figure 2a, there was irregular bumpiness with some nanostructured papillae on the surface of the membrane at a dopamine polymerization time of 4 h. The catechol group of dopamine could be oxidized to benzoquinone with high reaction activity in a weak alkaline solution in the presence of oxygen. Then, it was self-polymerized to PDA nanoaggregates through the covalent cross-linking and noncovalent self-assembly to form a modification layer onto the MoS2 membrane surface.41,42 Therefore, the surface roughness of the membrane was ascribed to the accumulation of PDA nanoaggregates.
Figure 2.
SEM images of (a) surface and (b) cross-sectional morphology of PDA(4)/MoS2(0.1103) NFMs.
The cross-sectional morphology of the membrane is shown in Figure 2b. The active layer of PDA(4)/MoS2(0.1103) NFM was tightly coated on the porous substrate without interfacial defects. The active layer was a laminar MoS2 layer coated by the PDA nanoaggregates with a uniform thickness of about 2.2 μm.
The surface morphologies of the membranes were further observed by the three-dimensional AFM images (Figure 3). With the increase of polymerization time, more and more PDA nanoaggregates were deposited onto the surfaces of the membranes, which increased the surface roughness and tuned the membrane surface properties.
Figure 3.
AFM images of PDA(X)/MoS2(0.1103) NFMs at different polymerization times: (a) 0 h, (b) 1 h, (c) 2 h, (d) 4 h, and (e) 6 h.
The membrane surface hydrophilicity was demonstrated by the water contact angle analysis. As shown in Figure 4, water contact angles of the membranes were remarkably decreased with the extension of dopamine polymerization time. These phenomena could be explained by that the hydrophilic PDA nanoaggregates, with abundant hydrophilic amine and hydroxyl groups, were deposited gradually along with the polymerization time.43,44 On the one hand, the surface hydrophilicity was enhanced by the deposition of PDA. On the other hand, the surface structure would become more compact with increasing polymerization time. And the relatively compact surface is more beneficial for spreading of water droplets, thereby the water contact angles could be decreased.45
Figure 4.
(a) Photographs of a water droplet on PDA(X)/MoS2(0.1103) NFMs at different polymerization times and (b) water contact angles of PDA(X)/MoS2(0.1103) NFMs at different polymerization times.
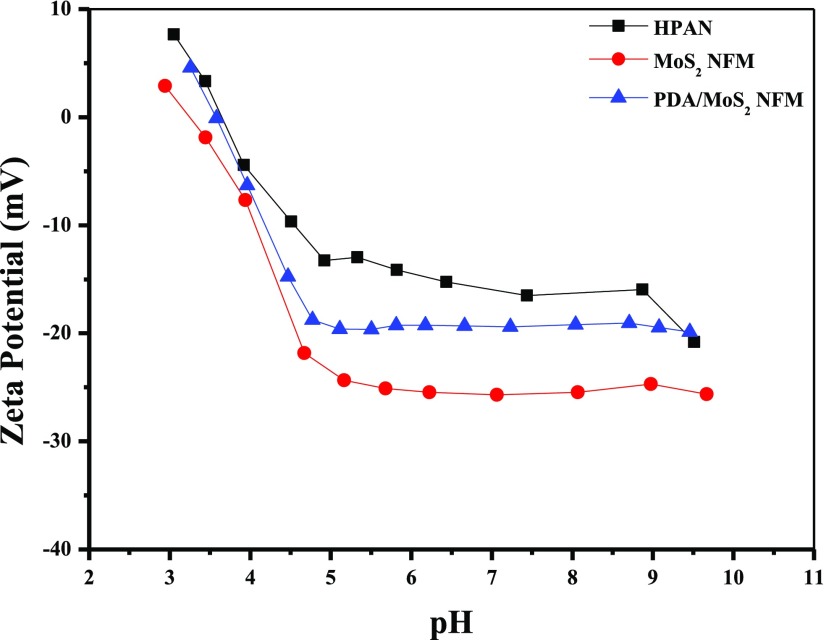
The ζ-potential of membrane surface is an important characteristic that is relevant to the separation property of membranes. According to one of the nanofiltration mechanisms, the Donnan effect, the membrane is tended to reject the molecule with the same electricity as the membrane surface and to infiltrate the molecule with opposite electricity to the membrane surface.45 The results of ζ-potential of hydrolyzed polyacrylonitrile (HPAN) substrate, MoS2 NFM, and PDA/MoS2 NFM are shown in Figure 5. These three kinds of membranes were all proved to be negatively charged at pH 4–10, especially at the neutral application conditions. The polyacrylonitrile (PAN) substrate could generate carboxyl groups (−COOH) after the hydrolyzation of nitrile groups in NaOH solution, which was the main factor of the negative electricity of the HPAN surface at pH 4–10.46,47 In Figure 5, it could also be observed that, compared with HPAN, the electronegativity of NFM loaded with MoS2 was enhanced, which could be ascribed to the electronegativity of MoS2.26 Due to the weak electronegativity of PDA, the electronegativity of the membrane surface was slightly lower after PDA modification.48
Figure 5.
Surface ζ-potentials of membranes at different pH values.
As shown in Figure 6, there was an intensive peak (002) at 2θ = 14.4° on the XRD spectra of MoS2 NFM and PDA/MoS2 NFM, which was in accordance with the typical hexagonal structure peaks of MoS2 (JCPDS card No. 77-1716).49
Figure 6.
XRD spectra of MoS2 NFM and PDA/MoS2 NFM.
FT-IR spectra of HPAN, MoS2 NFM, and PDA/MoS2 NFM samples are shown in Figure 7. The characteristic peak at around 1727 cm–1 corresponded to C=O stretching vibration, which confirmed the hydrolyzation of PAN substrate membrane. This peak became weaker and weaker after MoS2 loading and PDA modification.50,51 For the spectrum of PDA(4)/MoS2(0.1103) NFM, a new peak appeared at 1566 cm–1, which was attributed to the N–H vibration in PDA.6 And the broad band at around 3400 cm–1 was related to the catechol −OH group and N–H group from PDA, which also indicated the effectivity of PDA modification.32,50,52,53
Figure 7.
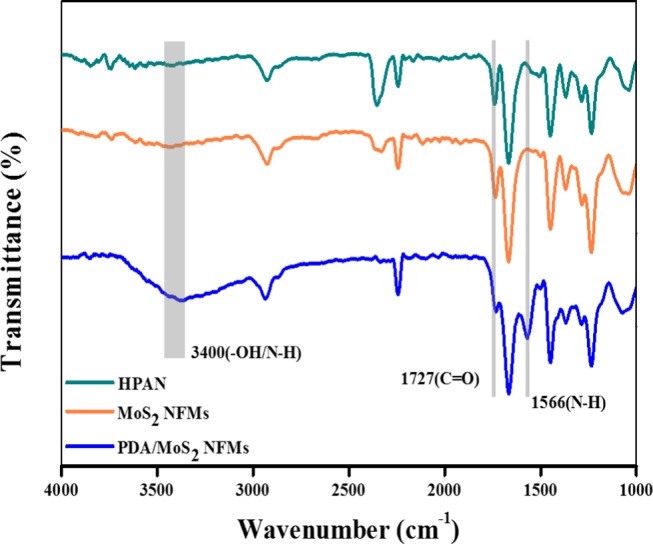
FT-IR spectra of HPAN, MoS2 NFM, and PDA(4)/MoS2(0.1103) NFM.
2.3. Membrane Performance
2.3.1. Effect of MoS2 Loading
The influence of MoS2 loading on the pure water permeance and dye rejection was investigated. As shown in Figure 8, with the increase of MoS2 loading from 0.03597 to 0.1415 mg/cm2, the membrane showed an increase of dye rejection from 45.52 to 100%. Meanwhile, the pure water permeance showed a slight decrease from 141.5 to 129.5 LMH/bar. The separation performance of the membrane, i.e., permeability and retention, was mainly dependent on the membrane material mass retained on the substrate surface.54 Therefore, the separation performance was correlated to the specific MoS2 loading. The three atomic layers of MoS2 nanosheet structure would create rigid nanochannels with stable interlaminar dimensions formed by overlapped MoS2 nanosheets, which could serve as the fluidic nanochannels for molecule separation through avoiding water channels from being further compacted under pressure.26 The lack of functional groups has endowed MoS2 nanosheets with low hydraulic resistance and smooth surface for water transportation.29 Furthermore, the smaller lateral dimensions of the exfoliated MoS2 in this work, which caused a relatively high porosity of the MoS2 layer, have effectively shortened the pathway for water transportation.26,44 With the increase of MoS2 loading, more MoS2 nanosheets were deposited on the membrane substrate, thus extending the water transport paths leading to the decrease of permeance and increase of dye rejection, known as the trade-off effect between these two properties.49 Therefore, by controlling the specific MoS2 nanosheet loading, it could meet different requirements of performance. When the MoS2 loading was 0.1103 mg/cm2, the membrane exhibited high nanofiltration performance with a pure water permeance of 135.3 LMH/bar, and the rejection toward methylene blue (MB) was 100%.
Figure 8.
Nanofiltration performance of PDA(4)/MoS2(Y) NFMs with different MoS2 loadings.
2.3.2. Effect of Dopamine Polymerization Time
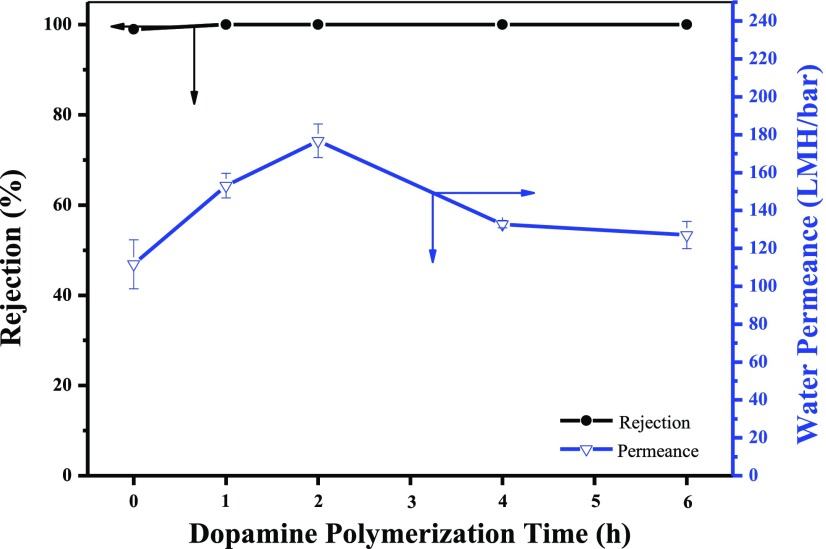
The effect of dopamine polymerization time on dye rejection and pure water permeance was also investigated. As shown in Figure 9, the dye rejection of the membrane toward MB slightly increased at first and then kept nearly 100% with the increase of dopamine polymerization time. It could be explained by that MoS2 played a dominant role in dye rejection, and the following deposition of PDA further inhibited the penetration of dyes. The water permeance increased in the first stage and then decreased with the increase of dopamine polymerization time, which was mainly ascribed to the change of the PDA polymerization layer.
Figure 9.
Nanofiltration performance of PDA(X)/MoS2(0.1103) NFMs at different dopamine polymerization times.
As shown in Figure 4, with more and more PDA deposited, a complete PDA modification layer was formed and the membrane hydrophilicity was enhanced, which led to the increase of water permeance.35,37 However, with the dopamine polymerization time prolonged further, the PDA nanoaggregates could be generated to block some passages of water transportation and the PDA modification layer also turned too dense and thick, thereby correspondingly decreasing the water permeance.51 But even at 6 h, the permeance of PDA(6)/MoS2 NFM was still higher than that of the MoS2 NFM without PDA modification because of the increased hydrophilicity. The synergy of MoS2 nanosheets and PDA modification has contributed to the high permeability of PDA/MoS2 NFMs.
2.3.3. Antifouling Property
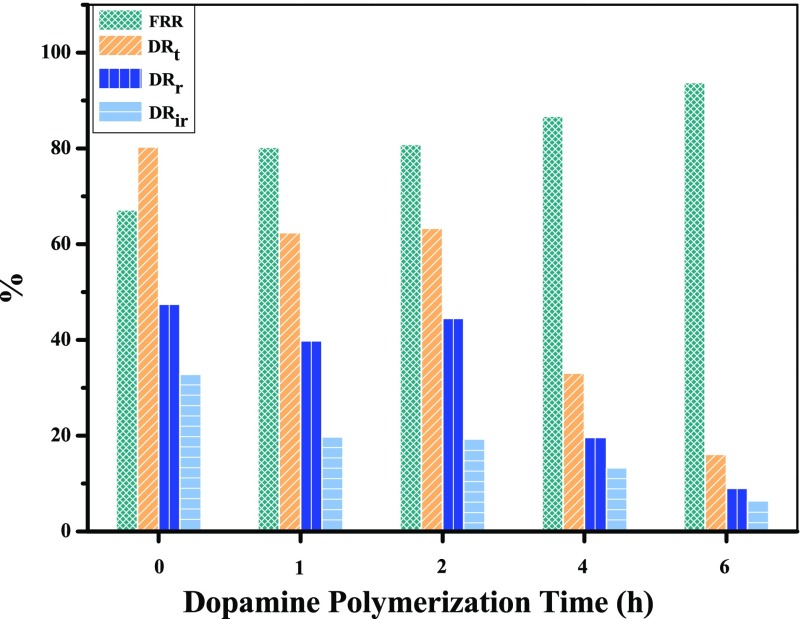
Antifouling property influences not only the separation performance but also the stability of membranes. Foulants could be adsorbed on the surface of membranes via hydrogen bonds or electrostatic interaction to form a filter cake, causing the decrease of permeance. However, with the filter cake being rinsed off, the flux could be recovered during the washing process.55 Thus, the antifouling property is an important property of membranes. In this work, the antifouling behavior of the membranes was evaluated by four fouling parameters (flux recovery ratio (FRR), total flux decline ratio (DRt), reversible flux decline ratio (DRr), and irreversible flux decline ratio (DRir)) using bovine serum albumin (BSA) as model foulant. As we all know, the lower DRt and higher FRR values mean more excellent antifouling characteristic of the membrane. For the PDA/MoS2 NFMs, the layer-stacked MoS2 nanosheets acted as a barrier and repelled the BSA molecules from the surface layers.56 And the absence of conjugated structure in MoS2 could avoid the cation−π and π–π interactions with organic fouling.29 As shown in Figure 10, with the extension of polymerization time, the FRR increased and the DRt decreased, which revealed that PDA modification could enhance the antifouling property of the membrane by decreasing BSA adhesion due to the high hydrophilicity, which could form a hydration layer, and the negative charge, which could reduce the adhesion of BSA with negative charge at neutral to alkaline pH through electrostatic repulsion.6,37 As shown in Figure 10, the membrane showed better antifouling performance at 6 h; therefore, if there is a higher requirement for antifouling performance, the polymerization time of 6 h is a good choice at the cost of longer preparation time and lower water permeance.
Figure 10.
Fouling indexes of PDA(X)/MoS2(0.1103) NFMs at different dopamine polymerization times.
2.3.4. Rejection Performance
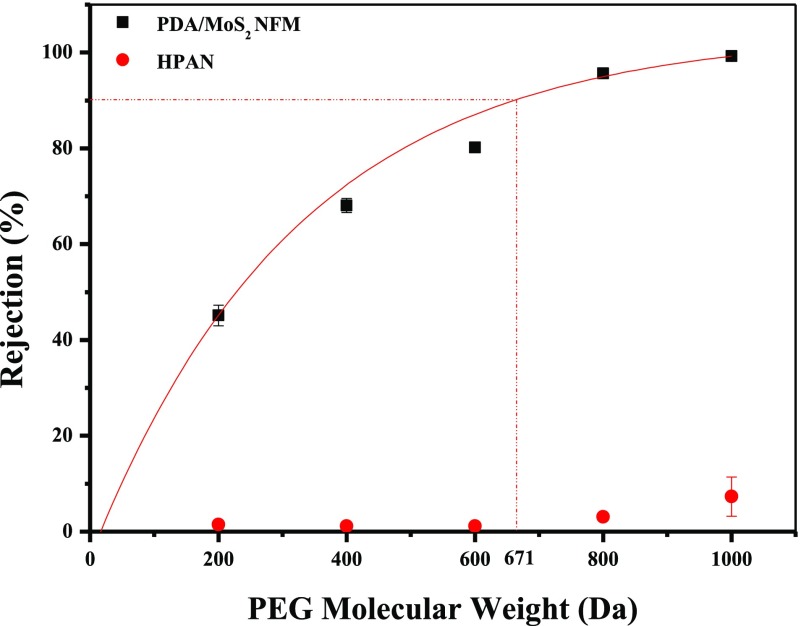
The retention performance of the PDA/MoS2 NFM was characterized through MWCO measurements using the widely used neutral molecule poly(ethylene glycol) (PEG). The MWCO was defined as the molecular weight of PEG at 90% rejection.57 As shown in Figure 11, the MWCO was estimated to be 671 Da, which was in accordance with the typical nanofiltration characteristic. As calculated by eq 3, the average transport path size of the PDA/MoS2 NFM was estimated to be ∼0.96 nm.
Figure 11.
Rejection of PDA(4)/MoS2(0.1103) NFM toward different molecular weights of PEG.
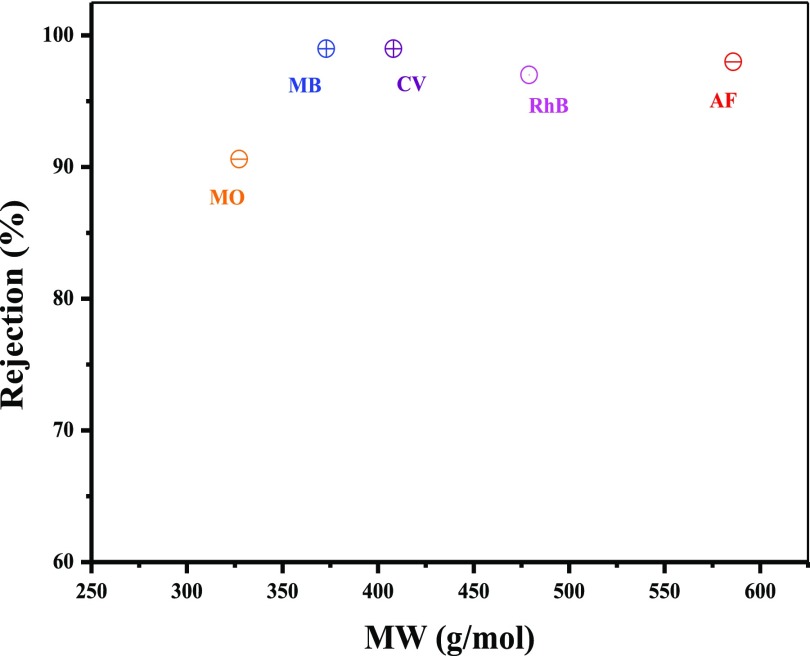
Five kinds of dyes with varying molecular weights and charges were employed to explore the separation mechanism of PDA(4)/MoS2(0.1103) NFM. The positively charged dyes are MB and crystal violet (CV), while the negatively charged dyes are methyl orange (MO) and acid fuchsin (AF), and the rhodamine B (RhB) is electroneutral. According to the extended Derjaguin–Landau–Verwey–Overbeek theory, the van der Waals attraction between MoS2 nanosheets is greater than hydration force and electrostatic repulsion, so the swelling degree of MoS2 layer is limited in water. And the maximum interlayer spacing of the fully hydrated MoS2 nanosheets could reach ∼1.2 nm.26 However, the kinetic diameter of dye molecules was generally greater than 1.1 nm.58 This makes it possible for the MoS2 nanofiltration membrane to obtain a high rejection for dye molecules.
As shown in Figure 12, the rejection toward dyes with the same electronegativity and higher molecular weights was higher, and the rejection toward RhB was as high as 97%, indicating that the steric hindrance effect played an important role in dye rejection. Because the dye molecules could be inclined to aggregate into some larger molecules via hydrophobic interaction or intermolecular hydrogen bonding in the solution, the actual size of dye molecules in the solution could be larger than theoretical values.45 So, the dye rejection was usually higher than the result from MWCO. The surface of PDA/MoS2 NFM was negatively charged (Figure 5).26,45 Thereby, except size exclusion, the capability of removing MO and AF was also contributed by the Donnan effect. The rejection toward MB and CV was higher than that of AF, due to the possible physical adsorption of dyes with positive charge onto the negatively charged membrane surface. So, the steric hindrance effect and electrostatic effect worked together for the rejection toward dyes.
Figure 12.
Rejection of PDA(4)/MoS2(0.1103) NFM toward different dyes.
The salt rejection of the PDA/MoS2 NFM is shown in Figure 13. The salt rejection was generally below 20%, which was obviously lower than the dye rejection. The contrast was partially due to the aggregation of dye molecules mentioned above. Furthermore, the inorganic MoS2 layers created rigid nanochannels, which could effectively reject the dye molecules with a large kinetic diameter and infiltrate the salt ions with a relatively lower kinetic diameter.58 The rejection toward MgSO4 and Na2SO4 was apparently higher than the rejection toward MgCl2 and NaCl, which was mainly ascribed to the Donnan effect on the negatively charged surface, which was consistent with the result of the ζ-potential measurement. And because of the steric hindrance effect, the MgSO4 and MgCl2 retentions were higher than those of Na2SO4 and NaCl.
Figure 13.
Rejection of PDA(4)/MoS2(0.1103) NFM toward different salt solutions.
2.3.5. Long-Term Stability
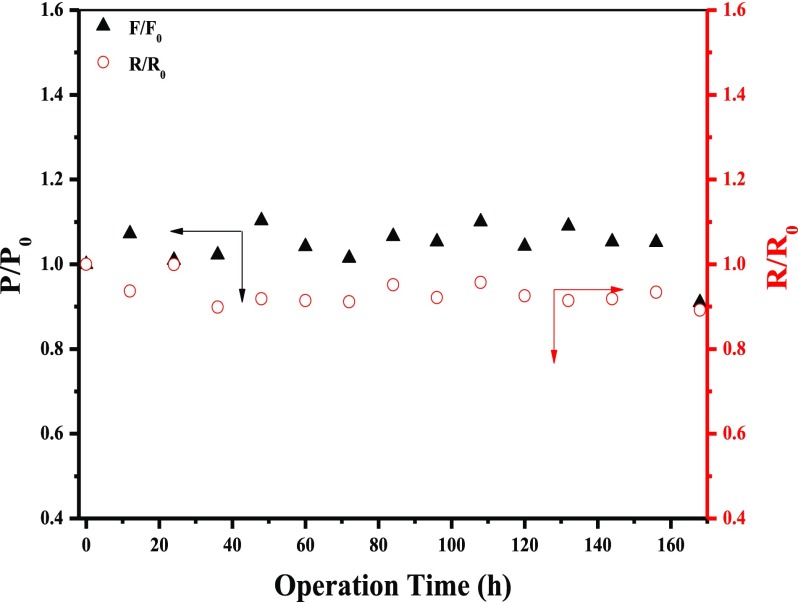
The PDA(4)/MoS2(0.1103) NFM was chosen as a representative to operate dye (MB) solution for 168 h for the long-term stability test. The flux and rejection toward MB were tested every 12 h by the methods mentioned above. Finally, P/P0 (permeance) and R/R0 (rejection) were used to express the results of long-term stability.45 From Figure 14, both permeance and rejection showed a slight fluctuation during the long-term stability testing process. This result indicated the high structural stability of PDA(4)/MoS2(0.1103) NFM. This could be on account of the excellent stability of MoS2 and PDA. MoS2 nanosheets possessed antiswelling and rigid nanochannel structure, which contributed to the stability of MoS2 layer-stacked structure during the long-term water filtration process.26,29 The PDA with the property of inherently strong adhesion also provided a stable modification layer for the NFMs.45
Figure 14.
Permeance and rejection stability of PDA(4)/MoS2(0.1103) NFM with different operation times in MB solution.
2.3.6. Comparison
The separation performance of the as-prepared membrane was compared to that of other state-of-the-art nanofiltration membranes. As shown in Table 1, the PDA(4)/MoS2(0.1103) membrane in this study showed a superior separation performance with high pure water permeance and high dye rejection.
Table 1. Separation Performance Comparison of PDA(4)/MoS2(0.1103) NFM with the State-of-the-Art NFMsa.
| membrane material | PWP (LMH bar–1) | solute | rejection (%) | applied pressure (bar) | ref |
|---|---|---|---|---|---|
| PEI–PDA/PES | 7.2 | MB | 96.5 | 2 | (56) |
| PDA + CuNPs | 25.5 | CR | 97.5 | 6 | (59) |
| Ra-PDA/PEI-1 | 26.2 | RO16 | 98.1 | 4 | (45) |
| ultrathin GO | 21.8 | MB | 99.2 | 1 | (11) |
| Ti3C2Tx–GO | 25 | MB | 99.5 | 5 | (60) |
| g-C3N4 | 29 | EB | 87 | (61) | |
| SG@GO composite | 33 | EBT | 98 | 0.5 | (62) |
| nanostrand-channeled WS2 nanosheets | 930 | EB | 83 | 3 | (63) |
| MoS2 | 245 | cyt C | 89 | 1 | (49) |
| PDA(4)/MoS2(0.1103) | 135.3 | MB | 100 | 2 | this work |
EB: Evans blue; EBT: eriochrome black T; CR: congo red; RO16: reactive orange 16; cyt C: cytochrome C.
3. Conclusions
A novel PDA/MoS2 NFM was fabricated via self-assembly of MoS2 nanosheets followed by PDA modification. The membrane was composed of a uniform MoS2 layer and a PDA layer coated on the HPAN substrate. The separation performance of PDA/MoS2 NFMs with various MoS2 loadings and dopamine polymerization times was investigated. With the increase of MoS2 loading, the dye rejection increased and the water permeance decreased. The smooth surface and rigid nanochannels of the exfoliated MoS2 nanosheets endowed the membrane with high water permeance and dye rejection. And the PDA modification effectively enhanced the membrane hydrophilicity, thus increasing the water permeance, and the dye rejection also slightly increased. Furthermore, the antifouling property of the membrane for BSA was also satisfactory with a high flux recovery ratio and a low total fouling ratio due to the increase of hydrophilicity and the electrostatic repulsion. And profited from the stability of MoS2 nanosheets and inherently strong adhesion of PDA, the PDA/MoS2 NFM also exhibited a satisfactory long-term stability. The pure water permeance of PDA(4)/MoS2(0.1103) NFM was 135.3 LMH/bar, and the rejection toward MB was 100%. The developed membrane also maintained high rejection toward various kinds of dyes (MB, CV, MO, RhB, and AF) and high permeability of salts (MgSO4, MgCl2, Na2SO4, and NaCl) with the molecular weight cutoff (MWCO) of 671 Da. This work provided a method to fabricate and modify MoS2 membranes for high-performance nanofiltration process.
4. Experimental Section
4.1. Material
Polyacrylonitrile (PAN) ultrafiltration membranes (MWCO = 50 kDa) supplied by Beijing Separate Equipment Co. Ltd. (China) were used as substrates of NFMs. MoS2 (99.5%), methylene blue (MB, 99.0%), methyl orange (MO, 97.0%), crystal violet (CV, 90.0%), lissamine rhodamine B (RhB, 99.0%), and acid fuchsin (AF, 99.0%) were provided by Shanghai Aladdin Bio-Chem Technology Co. Ltd. Magnesium sulfate (MgSO4, 99.0%), magnesium chloride (MgCl2, 98.0%), sodium sulfate (Na2SO4, 99.0%), and sodium chloride (NaCl, 99.5%) were supplied by Tianjin Fengchuan Chemical Reagent Technologies Co. Ltd. Poly(ethylene glycol) (PEG, 99.0%) with molecular weights of 200, 400, 600, 800, and 1000 Da were all from Damao Chemical Reagent Factory. Tris(hydroxymethyl)aminomethane (99.5%) was supplied by Biosharp. Bovine serum albumin (BSA, 98%) and dopamine hydrochloride (99.0%) were obtained from Beyotine Institute of Biotechnology and Merck Life Science (Shanghai) Co. Ltd., respectively. Other chemicals, including sodium hydroxide (96.0%), ethanol (99.7%), and hydrochloric acid solution (37 wt %), were offered by Tianjin Fengchuan Chemical Reagent Technologies Co. Ltd. and used without any further purification. Water used in this work was ultrapure water prepared by reverse osmosis.
4.2. Exfoliation of Multilayered MoS2
The 2D MoS2 nanosheets were prepared by a mixed-solvent mechanical exfoliation strategy.40,64 First, 10 mL of ethanol/water with an ethanol volume fraction of 45% as dispersion solvent was added into a 15 mL pressure flask with 30 mg of MoS2 powder. The dispersion was treated by ultrasound (QT3120, Ruipu, China) under 120 W for 8 h. Subsequently, the obtained dispersion was centrifuged at 3000 rpm for 20 min for removing multilayer MoS2. Then, the supernatant was centrifuged at 10 000 rpm for 10 min to obtain exfoliated MoS2 nanosheets followed by drying in vacuum.
4.3. Preparation of PDA-Modified MoS2 NFMs
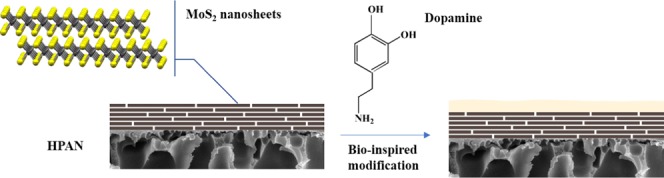
Figure 15 shows the preparation process of the PDA-modified MoS2 NFMs. First, circular pieces with 6 cm diameter of PAN substrate membranes were hydrolyzed in NaOH (1.5 M) for 60 min at 50 °C. Then, the membranes were rinsed by ultrapure water to obtain hydrolyzed PAN (HPAN) followed by storing in ultrapure water until use.
Figure 15.
Schematic of the preparation process of PDA/MoS2 NFMs.
For the loading of MoS2, the HPAN substrate was first mounted at the bottom of an Amicon cell (Millipore, Billerica, MA), which was connected to a nitrogen gas cylinder, to form a pressurized membrane filtration system. Then, MoS2 nanosheet suspension with a certain concentration was filtrated through the HPAN substrate under a pressure of 1–2 bar. The loading of MoS2 was controlled by varying the volume of filtrated MoS2 nanosheet suspension.
Next, for preparing dopamine coating solution, 80 mg of dopamine hydrochloride was dissolved in 40 mL of Tris–HCl buffer solution (50 mM, pH 8.5). Subsequently, the freshly prepared dopamine coating solution was poured into the Amicon cell loaded with MoS2 membranes for a certain period. In the end, the membranes were rinsed with ultrapure water several times and stored in it.
The as-prepared PDA-modified MoS2 NFMs were named PDA(X)/MoS2(Y) NFMs, where X is the polymerization time of dopamine (h) and Y is the loading of MoS2 (mg/cm2).
4.4. Characterization
4.4.1. Exfoliated MoS2 Characterization
The morphologies of exfoliated MoS2 nanosheets were investigated by transmission electron microscopy (TEM) analysis conducted with a JEM-2100 microscope. The thickness of exfoliated MoS2 nanosheets was measured by atomic force microscopy (AFM, BioScope Catalyst, Bruker, Germany) on a mica plate. The samples for all analyses were dispersed in ethanol with the help of ultrasound.
4.4.2. Membrane Characterization
The surface and cross-sectional morphologies of the PDA/MoS2 NFMs were observed utilizing scanning electron microscopy (SEM, Nova Nano SEM450 field emission microscope with an accelerating voltage of 10 KV). AFM was employed to examine the morphological changes of the membrane surface at difference dopamine polymerization times. The hydrophilicity of the membrane surface was investigated by water contact angle using a DAS30 video contact angle system (KRUSS, Germany). The chemical structure of the membranes was analyzed by Fourier transform infrared spectrometry (FT-IR, VERTEX 70, Bruker, Germany). The transmittance spectra were collected in the wavenumber range of 4000–400 cm–1. The X-ray diffraction (XRD) spectra of MoS2 NFMs and PDA/MoS2 NFMs were characterized by an X-ray diffractometer (D8 Discover, BRUKER AXS GMBH, Germany) with a Cu Kα anode (λ = 0.15438 nm) at 40 kV and 40 mA. And the ζ-potential of the surface of PDA/MoS2 NFM was determined by an electrokinetic analyzer (SurPASS, Anton Paar, AUS) in the pH range of 3–10 controlled by adding a certain amount of NaOH (0.1 M) or HCl (0.1 M) solution.
4.5. Membrane Flux and Rejection Tests
The nanofiltration performance in terms of pure water permeance and dye rejection was evaluated using a pressurized membrane filtration system with an Amicon cell under a certain pressure. The membrane sample with an effective area of 28.274 cm2 was precompacted with ultrapure water for 0.5 h at 2 bar to obtain a steady permeance before performance evaluation. The pure water permeance (J, L m–2 h–1 bar–1, abbreviated as LMH/bar) was tested at 1 bar using ultrapure water as the feed. Various salt and dye solutions were used to evaluate the rejection (R, %) of the membrane. The concentrations of salt and dye solution were evaluated, respectively, by an electrical conductivity meter (DDSJ-308A, Rex, CHN) and a UV–vis spectrophotometer (UV-1100, Mapada, China). The pure water permeance and rejection were calculated according to the following equations, respectively
| 1 |
where V is the total volume (L) of permeate pure water collected in a certain time t (h), and A (m2) and P (bar) represent the effective membrane area and operation pressure, respectively
| 2 |
where Cf and Cp are the solute concentrations (mg/L) of feed and permeate solutions, respectively.
Furthermore, a series of PEG solutions with molecular weights ranging from 200 to 1000 Da were used as feed solution to test the MWCO of the as-prepared membrane. According to the following equation, the Stokes radius (rs) of PEG molecule could be calculated65
| 3 |
where MW is the molecular weight of PEG.
4.6. Antifouling Property
BSA solution (500 mg/L) was used as the foulant to evaluate the antifouling property of the membrane carried on the same pressurized membrane filtration system with Amicon cell at 1 bar. The membrane sample was initially pressurized with ultrapure water for 0.5 h to ensure the membrane reaching a steady state, and the pure water permeance (P0) was measured. Then, by BSA solution replacing the ultrapure water, the foulant solution permeance (P1) was measured after 120 min and filtrated through the membrane in Amicon cell. Finally, the ultrapure water was filtrated through the membrane for 0.5 h to wash the membrane. The following pure water permeance (P2) was measured. The total flux recovery ratio (FRR), the total flux decline ratio (DRt), the reversible flux decline ratio (DRr), and the irreversible flux decline ratio (DRir), the antifouling characteristics of the membrane, were calculated according to the following equations, respectively
| 4 |
| 5 |
| 6 |
| 7 |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21576068, 21276060, 21276062, and 21306039), the Natural Science Foundation of Tianjin (16JCYBJC19 800), the Natural Science Foundation of Hebei Province (B2015202082, B2016202027, and B2017202056), the Program for Top 100 Innovative Talents in Colleges and Universities of Hebei Province (SLRC2017029), and the Hebei High Level Personnel of Support Program (A2016002027).
The authors declare no competing financial interest.
References
- WWAP (United Nations World Water Assessment Programme)/UN-Water; The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; UNESCO: Paris, 2018.
- Moran Ayala L. I.; Paquet M.; Janowska K.; Jamard P.; Quist-Jensen C. A.; Bosio G. N.; Mártire D. O.; Fabbri D.; Boffa V. Water defluoridation: Nanofiltration vs membrane distillation. Ind. Eng. Chem. Res. 2018, 57, 14740–14748. 10.1021/acs.iecr.8b03620. [DOI] [Google Scholar]
- Lee A.; Elam J. W.; Darling S. B. Membrane materials for water purification: design, development, and application. Environ. Sci.: Water Res. Technol. 2016, 2, 17–42. 10.1039/C5EW00159E. [DOI] [Google Scholar]
- Van der Bruggen B.; Mänttäri M.; Nyström M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. 10.1016/j.seppur.2008.05.010. [DOI] [Google Scholar]
- Mohammad A. W.; Teow Y. H.; Ang W. L.; Chung Y. T.; Oatley-Radcliffe D. L.; Hilal N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. 10.1016/j.desal.2014.10.043. [DOI] [Google Scholar]
- Lv Y.; Yang H. C.; Liang H. Q.; Wan L. S.; Xu Z. K. Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking. J. Membr. Sci. 2015, 476, 50–58. 10.1016/j.memsci.2014.11.024. [DOI] [Google Scholar]
- Van der Bruggen B.; Braeken L. Comparsion of methods to enhance separation characteristics in nanofiltration. Ind. Eng. Chem. Res. 2007, 46, 2236–2242. 10.1021/ie0608758. [DOI] [Google Scholar]
- Huang H.; Ying Y.; Peng X. Graphene oxide nanosheet: an emerging star material for novel separation membranes. J. Mater. Chem. A 2014, 2, 13772–13782. 10.1039/C4TA02359E. [DOI] [Google Scholar]
- Lv Y.; Du Y.; Chen Z. X.; Qiu W. Z.; Xu Z. K. Nanocomposite membranes of polydopamine/electropositive nanoparticles/polyethyleneimine for nanofiltration. J. Membr. Sci. 2018, 545, 99–106. 10.1016/j.memsci.2017.09.066. [DOI] [Google Scholar]
- Farsi A.; Jensen S. H.; Roslev P.; Boffa V.; Christensen M. L. Inorganic membranes for the recovery of effluent from municipal wastewater treatment plants. Ind. Eng. Chem. Res. 2015, 54, 3462–3472. 10.1021/acs.iecr.5b00064. [DOI] [Google Scholar]
- Han Y.; Xu Z.; Gao C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 2013, 23, 3693–3700. 10.1002/adfm.201202601. [DOI] [Google Scholar]
- Lv Y.; Du Y.; Qiu W. Z.; Xu Z. K. Nanocomposite membranes via the codeposition of polydopamine/polyethylenimine with silica nanoparticles for enhanced mechanical strength and high water permeability. ACS Appl. Mater. Interfaces 2017, 9, 2966–2972. 10.1021/acsami.6b13761. [DOI] [PubMed] [Google Scholar]
- Puthai W.; Kanezashi M.; Nagasawa H.; Tsuru T. SiO2-ZrO2 nanofiltration membranes of different Si/Zr molar ratios: Stability in hot water and acid/alkaline solutions. J. Membr. Sci. 2017, 524, 700–711. 10.1016/j.memsci.2016.11.045. [DOI] [Google Scholar]
- Dervin S.; Dionysiou D. D.; Pillai S. C. 2D nanostructures for water purification: graphene and beyond. Nanoscale 2016, 8, 15115–15131. 10.1039/C6NR04508A. [DOI] [PubMed] [Google Scholar]
- Liu G.; Jin W.; Xu N. Two-dimensional-material membranes: A new family of high-performance separation membranes. Angew. Chem., Int. Ed. 2016, 55, 13384–13397. 10.1002/anie.201600438. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Biswas P.; Fortner J. D. A review of recent developments in graphene-enabled membranes for water treatment. Environ. Sci.: Water Res. Technol. 2016, 2, 915–922. 10.1039/C6EW00187D. [DOI] [Google Scholar]
- Xu W. L.; Fang C.; Zhou F.; Song Z.; Liu Q.; Qiao R.; Yu M. Self-assembly: A facile way of forming ultrathin, high-performance graphene oxide membranes for water purification. Nano Lett. 2017, 17, 2928–2933. 10.1021/acs.nanolett.7b00148. [DOI] [PubMed] [Google Scholar]
- Akbari A.; Sheath P.; Martin S. T.; Shinde D. B.; Shaibani M.; Banerjee P. C.; Tkacz R.; Bhattacharyya D.; Majumder M. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat. Commun. 2016, 7, 10891 10.1038/ncomms10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.; Jiang Y.; Gao C. High-flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8147–8155. 10.1021/acsami.5b00986. [DOI] [PubMed] [Google Scholar]
- Lai G. S.; Lau W. J.; Goh P. S.; Ismail A. F.; Yusof N.; Tan Y. H. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. 10.1016/j.desal.2016.03.007. [DOI] [Google Scholar]
- Zhang Y.; Zhang S.; Gao J.; Chung T. S. Layer-by-layer construction of graphene oxide (GO) framework composite membranes for highly efficient heavy metal removal. J. Membr. Sci. 2016, 515, 230–237. 10.1016/j.memsci.2016.05.035. [DOI] [Google Scholar]
- Hu M.; Mi B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. 10.1021/es400571g. [DOI] [PubMed] [Google Scholar]
- Liu K.; Feng J.; Kis A.; Radenovic A. Atomically thin molybdenum disulfide nanopores with high sensitivity for DNA translocation. ACS Nano 2014, 8, 2504–2511. 10.1021/nn406102h. [DOI] [PubMed] [Google Scholar]
- Zhou L.; He B.; Yang Y.; He Y. Facile approach to surface functionalized MoS2 nanosheets. RSC Adv. 2014, 4, 32570–32578. 10.1039/C4RA04682J. [DOI] [Google Scholar]
- Tuteja S. K.; Duffield T.; Neethirajan S. Liquid exfoliation of 2D MoS2 nanosheets and their utilization as a label-free electrochemical immunoassay for subclinical ketosis. Nanoscale 2017, 9, 10886–10896. 10.1039/C7NR04307D. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Tu Q.; Zheng S.; Urban J. J.; Li S.; Mi B. Understanding the Aqueous Stability and Filtration Capability of MoS2 Membranes. Nano Lett. 2017, 17, 7289–7298. 10.1021/acs.nanolett.7b02804. [DOI] [PubMed] [Google Scholar]
- Deng M.; Kwac K.; Li M.; Jung Y.; Park H. G. Stability, molecular sieving, and ion diffusion selectivity of a lamellar membrane from two-dimensional molybdenum disulfide. Nano Lett. 2017, 17, 2342–2348. 10.1021/acs.nanolett.6b05238. [DOI] [PubMed] [Google Scholar]
- Kou J.; Yao J.; Wu L.; Zhou X.; Lu H.; Wu F.; Fan J. Nanoporous two-dimensional MoS2 membranes for fast saline solution purification. Phys. Chem. Chem. Phys. 2016, 18, 22210–22216. 10.1039/C6CP01967F. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Mi B. Environmental applications of 2D molybdenum disulfide (MoS2) Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. 10.1021/acs.est.7b01466. [DOI] [PubMed] [Google Scholar]
- Wang D.; Wang Z.; Wang L.; Hu L.; Jin J. Ultrathin membranes of single-layered MoS2 nanosheets for high-permeance hydrogen separation. Nanoscale 2015, 7, 17649–17652. 10.1039/C5NR06321C. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Gong J. L.; Zeng G. M.; Song B.; Cao W. C.; Liu H. Y.; Huan S. Y.; Peng P. Novel “loose” GO/MoS2 composites membranes with enhanced permeability for effective salts and dyes rejection at low pressure. J. Membr. Sci. 2019, 547, 112–123. 10.1016/j.memsci.2018.12.046. [DOI] [Google Scholar]
- Zhou J.; Qin Z.; Lu Y.; Li X.; An Q.; Ji S.; Wang N.; Guo H. MoS2/polyelectrolytes hybrid nanofiltration (NF) membranes with enhanced permselectivity. J. Taiwan Inst. Chem. Eng. 2018, 84, 196–202. 10.1016/j.jtice.2018.01.015. [DOI] [Google Scholar]
- Liang X.; Wang P.; Wang J.; Zhang Y.; Wu W.; Liu J.; Van der Bruggen B. Zwitterionic functionalized MoS2 nanosheets for a novel composite membrane with effective salt/dye separation performance. J. Membr. Sci. 2019, 573, 270–279. 10.1016/j.memsci.2018.12.015. [DOI] [Google Scholar]
- Lee H.; Dellatore S. M.; Miller W. M.; Messersmith P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.; Li S.; Zhao W.; Wei Q.; Nie S.; Sun S.; Zhao C. The hydrodynamic permeability and surface property of polyethersulfone ultrafiltration membranes with mussel-inspired polydopamine coatings. J. Membr. Sci. 2012, 417–418, 228–236. 10.1016/j.memsci.2012.06.045. [DOI] [Google Scholar]
- Li M.; Xu J.; Chang C. Y.; Feng C.; Zhang L.; Tang Y.; Gao C. Bioinspired fabrication of composite nanofiltration membrane based on the formation of DA/PEI layer followed by cross-linking. J. Membr. Sci. 2014, 459, 62–71. 10.1016/j.memsci.2014.01.038. [DOI] [Google Scholar]
- McCloskey B. D.; Park H. B.; Ju H.; Rowe B. W.; Miller D. J.; Chun B. J.; Kin K.; Freeman B. D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, and microfiltration membranes. Polymer 2010, 51, 3472–3485. 10.1016/j.polymer.2010.05.008. [DOI] [Google Scholar]
- Yang H. C.; Wu Q. Y.; Wan L. S.; Xu Z. K. Polydopamine gradients by oxygen diffusion controlled autoxidation. Chem. Commun. 2013, 49, 10522–10524. 10.1039/c3cc46127k. [DOI] [PubMed] [Google Scholar]
- Yang H. C.; Liao K. J.; Huang H.; Wu Q. Y.; Wan L. S.; Xu Z. K. Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation. J. Mater. Chem. A 2014, 2, 10225–10230. 10.1039/C4TA00143E. [DOI] [Google Scholar]
- Zhou K. G.; Mao N. N.; Wang H. X.; Peng Y.; Zhang H. L. A mixed-solvent strategy for efficient exfoliation of inorganic graphene analogues. Angew. Chem., Int. Ed. 2011, 50, 10839–10842. 10.1002/anie.201105364. [DOI] [PubMed] [Google Scholar]
- Hong S.; Na Y. S.; Choi S.; Song I. T.; Kim W. Y.; Lee H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. 10.1002/adfm.201201156. [DOI] [Google Scholar]
- Liu W.; Li Y.; Meng X.; Liu G.; Hu S.; Pan F.; Wu H.; Jiang Z.; Wang B.; Li Z.; Cao X. Embedding dopamine nanoaggregates into a poly(dimethylsiloxane) membrane to confer controlled interactions and free volume for enhanced separation performance. J. Mater. Chem. A 2013, 1, 3713–3723. 10.1039/c3ta00766a. [DOI] [Google Scholar]
- Xi Z. Y.; Xu Y. Y.; Zhu L. P.; Wang Y.; Zhu B. K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. 10.1016/j.memsci.2008.11.037. [DOI] [Google Scholar]
- Yang X.; Du Y.; Zhang X.; He A.; Xu Z. K. Nanofiltration membrane with a mussel-inspired interlayer for improved permeation performance. Langmuir 2017, 33, 2318–2324. 10.1021/acs.langmuir.6b04465. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhu J.; Tsehaye M. T.; Li J.; Dong G.; Yuan S.; Li X.; Zhang Y.; Liu J.; Van der Bruggen B. High flux electroneutral loose nanofiltration membranes based on rapid deposition of polydopamine/polyethyleneimine. J. Mater. Chem. A 2017, 5, 14847–14857. 10.1039/C7TA02661G. [DOI] [Google Scholar]
- Zhu J.; Uliana A.; Wang J.; Yuan S.; Li J.; Tian M.; Simoens K.; Volodin A.; Lin J.; Bernaerts K.; Zhang Y.; Van der Bruggen B. Elevated salt transport of antimicrobial loose nanofiltration membrane functionalized with copper nanoparticles via a fast bioinspired deposition. J. Mater. Chem. A 2016, 4, 13211–13222. 10.1039/C6TA05661J. [DOI] [Google Scholar]
- Han J. L.; Xia X.; Tao Y.; Yun H.; Hou Y. N.; Zhao C. W.; Lou Q.; Cheng H. Y.; Wang A. J. Shielding membrane surface carboxyl groups by covalent-binding graphene oxide to improve anti-fouling property and the simultaneous promotion of flux. Water Res. 2016, 102, 619–628. 10.1016/j.watres.2016.06.032. [DOI] [PubMed] [Google Scholar]
- Ding J.; Yang S.; Pan J.; Zheng Y.; Sotto A.; Shen J. A novel nanofiltration membrane inspired by an asymmetric porous membrane for selective fractionation of monovalent anions in electrodialysis. RSC Adv. 2018, 8, 30502–30511. 10.1039/C8RA05152F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Huang H.; Peng X. Laminar MoS2 membranes for molecule separation. Chem. Commun. 2013, 49, 10718–10720. 10.1039/c3cc46136j. [DOI] [PubMed] [Google Scholar]
- Song H.; Wang Z.; Yang J.; Jia X.; Zhang Z. Facile synthesis of copper/polydopamine functionalized graphene oxide nanocomposites with enhanced tribological performance. Chem. Eng. J. 2017, 324, 51–62. 10.1016/j.cej.2017.05.016. [DOI] [Google Scholar]
- Zhang Y.; Zhang S.; Chung T. S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environ. Sci. Technol. 2015, 49, 10235–10242. 10.1021/acs.est.5b02086. [DOI] [PubMed] [Google Scholar]
- Huang Q.; Liu M.; Chen J.; Wan Q.; Tian J.; Huang L.; Jiang R.; Wen Y.; Zhang X.; Wei Y. Facile preparation of MoS2 based polymer composites via mussel inspired chemistry and their high efficiency for removal of organic dyes. Appl. Surf. Sci. 2017, 419, 35–44. 10.1016/j.apsusc.2017.05.006. [DOI] [Google Scholar]
- Chen S.; Cao Y.; Feng J. Polydopamine as an efficient and robust platform to functionalize carbon fiber for high-performance polymer composites. ACS Appl. Mater. Interfaces 2014, 6, 349–356. 10.1021/am404394g. [DOI] [PubMed] [Google Scholar]
- Menne D.; Üzüm C.; Koppelmann A.; Wong J. E.; Foeken Cv.; Borre F.; Dähne L.; Laakso T.; Pihlajamäki A.; Wessling M. Regenerable polymer/ceramic hybrid nanofiltration membrane based on polyelectrolyte assembly by layer-by-layer technique. J. Membr. Sci. 2016, 520, 924–932. 10.1016/j.memsci.2016.08.048. [DOI] [Google Scholar]
- Wang L.; Wang N.; Li J.; Li J.; Bian W.; Ji S. Layer-by-layer self-assembly of polycation/GO nanofiltration membrane with enhanced stability and fouling resistance. Sep. Purif. Technol. 2016, 160, 123–131. 10.1016/j.seppur.2016.01.024. [DOI] [Google Scholar]
- Zhang R.; Su Y.; Zhao X.; Li Y.; Zhao J.; Jiang Z. A novel positively charged composite nanofiltration membrane prepared by bio-inspired adhesion of polydopamine and surface grafting of poly(ethylene imine). J. Membr. Sci. 2014, 470, 9–17. 10.1016/j.memsci.2014.07.006. [DOI] [Google Scholar]
- López-Muñoz M. J.; Sotto A.; Arsuaga J. M.; Van der Bruggen B. Influence of membrane, solute and solution properties on the retention of phenolic compounds in aqueous solution by nanofiltration membranes. Sep. Purif. Technol. 2009, 66, 194–201. 10.1016/j.seppur.2008.11.001. [DOI] [Google Scholar]
- You X.; Wu H.; Su Y.; Yuan J.; Zhang R.; Yu Q.; Wu M.; Jiang Z.; Cao X. Precise nanopore tuning for high-throughput desalination membrane via codepostion of dopamine and multifunctional POSS. J. Mater. Chem. A 2018, 6, 13191–13202. 10.1039/C8TA03673J. [DOI] [Google Scholar]
- Zhu J.; Qin L.; Uliana A.; Hou J.; Wang J.; Zhang Y.; Li X.; Yuan S.; Li J.; Tian M.; Lin J.; Van der Bruggen B. Elevated performance of thin film nanocomposite membranes enabled by modified hydrophilic MOFs for nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 1975–1986. 10.1021/acsami.6b14412. [DOI] [PubMed] [Google Scholar]
- Kang K. M.; Kim D. W.; Ren C. E.; Cho K. M.; Kim S. J.; Choi J. H.; Nam Y. T.; Gogotsi Y.; Jung H. T. Selective molecular separation on Ti3C2Tx-graphene oxide membranes during pressure-driven filtration: Comparison with graphene oxide and MXenes. ACS Appl. Mater. Interfaces 2017, 9, 44687–44694. 10.1021/acsami.7b10932. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li L.; Wei Y.; Xue J.; Chen H.; Ding L.; Caro J.; Wang H. Water transport with ultralow friction through partially exfoliated g-C3N4 nanosheet membranes with self-supporting spacers. Angew. Chem., Int. Ed. 2017, 56, 8974–8980. 10.1002/anie.201701288. [DOI] [PubMed] [Google Scholar]
- Shen H.; Wang N.; Ma K.; Wang L.; Chen G.; Ji S. Tuning inter-layer spacing of graphene oxide laminates with solvent green to enhance its nanofiltration performance. J. Membr. Sci. 2017, 527, 43–50. 10.1016/j.memsci.2017.01.003. [DOI] [Google Scholar]
- Sun L.; Ying Y.; Huang H.; Song Z.; Mao Y.; Xu Z.; Peng X. Ultrafast molecule separation through layered WS2 nanosheet membranes. ACS Nano 2014, 8, 6304–6311. 10.1021/nn501786m. [DOI] [PubMed] [Google Scholar]
- Shen J.; Wu J.; Wang M.; Dong P.; Xu J.; Li X.; Zhang X.; Yuan J.; Wang X.; Ye M.; Vajtai R.; Lou J.; Ajayan P. M. Surface tension components based selection of cosolvents for efficient liquid phase exfoliation of 2D materials. Small 2016, 12, 2741–2749. 10.1002/smll.201503834. [DOI] [PubMed] [Google Scholar]
- Liu S.; Wang Z.; Ban M.; Song P.; Song X.; Khan B. Chelation-assisted in situ self-assembly route to prepare the loose PAN-based nanocomposite membrane for dye desalination. J. Membr. Sci. 2018, 566, 168–180. 10.1016/j.memsci.2018.09.002. [DOI] [Google Scholar]